Introduction
The abrupt loss of cardiac function in patients
diagnosed with heart disease is defined as cardiac arrest. The
reduction of blood flow or the termination of the coronary artery
of the heart, causing damage to the heart muscle, may result in
myocardial infarction (MI). The high risk of the short- and
long-term recurrent MI events is noteworthy, in spite of the recent
development of novel therapeutic approaches for heart disease.
Acute MI (AMI) involves myocardial necrosis caused by acute and
persistent ischemia, and hypoxia of the coronary arteries (1). AMI is accompanied by an increased
serum myocardial enzymatic activity and progressive changes in an
electrocardiogram (ECG), which can be complicated by arrhythmia,
shock or heart failure, and it is mainly life-threatening (2). In recent decades, the pathogenesis of
atherothrombosis and coronary heart disease, including acute
coronary syndrome, has been frequently studied (3). The development of AMI may be
attributed to an imbalance between pro-inflammatory and
anti-inflammatory responses, and cardiac function can be promoted
via the post-MI intramyocardial injection of bone marrow-derived
mononuclear cells in an interleukin (IL)-10-dependent manner
(4). Moreover, a notable reduction
in the number of immune-suppressive regulatory T-cells (Tregs) has
been noted in patients with AMI (5), and alleviating cardiac damage, and
inhibiting cardiac hypertrophy and fibrosis despite sustained
angiotensin II-induced hypertension in mice could result from the
adoptive transfer of Tregs (6).
In healthy individuals, the rapid differentiation of
immature myeloid cells (IMCs) generated in bone marrow into mature
granulocytes, macrophages or dendritic cells has been reported
(7). Partially, the suppression of
the differentiation of IMCs into mature myeloid cells could lead to
an expansion of this population under pathological conditions
(e.g., cancer, infectious diseases, sepsis, etc.) (8,9).
Myeloid-derived suppressor cells (MDSCs) have exhibited potent
immunosuppressive functions, inducing tumor progression,
angiogenesis and immune escape (10). A previous study demonstrated that
the immunophenotype of MDSCs in tumor-bearing mice was protein
gamma response 1 (Gr-1)+CD11b+ cells
(11). The MDSCs can be classified
into two subtypes: Monocyte like-MDSCs (M-MDSCs), involving
CD11b+Ly6ChighLy6G- cells with a
monocyte-like morphology, and granulocyte like-MDSCs (G-MDSCs),
including CD11b+Ly6ClowLy6G- cells
with a granulocyte-like morphology (12). However, in humans, MDSCs are mainly
identified by CD11b and CD33 antibodies, with low levels of the
major histocompatibility complex class II molecule human leukocyte
antigen-D-related (HLA-DR). The MDSCs inhibit immune responses by
expressing high levels of arginase 1 and producing nitric oxide
(NO) and reactive oxygen species (13), as well as by inducing Tregs
(14). MDSCs exert their
suppressive effects via the expression or secretion of copious
amounts of immunosuppressive mediators [e.g., programmed
death-ligand 1 (PD-L1)] (14). The
suppression of T-cell proliferation and the promotion of T-cell
apoptosis may be attributed to MDSCs, facilitating the induction of
Tregs by the release of interleukin (IL)-10 and transforming growth
factor-β, which may result in reducing cell-mediated immunity
(15).
The accumulation of MDSCs has been found to be
associated with occurrence of cancer and chronic inflammatory and
autoimmune diseases (16).
Nevertheless, a few studies have concentrated on the role of MDSCs
in AMI. Notably, the accumulation and activation of MDSCs can be
induced by inflammation-associated factors, vascular endothelial
growth factor and prostaglandin E2, and pro-inflammatory cytokines
(17). The present study aimed to
indicate whether the recruitment of MDSCs and Tregs is associated
with the occurrence of AMI. The results may further assist
cardiologists in the treatment of heart diseases, particularly
AMI.
Materials and methods
Participants
A total of 34 patients who were diagnosed with AMI
and admitted to the Department of Cardiology of the Beijing
Tsinghua Changgung Hospital (Beijing, China) between December, 2019
and December, 2020 were enrolled in the study, including 19 males
and 15 females, with an average age of 62.23±8.54 years. The
diagnostic criteria of AMI were based on the Fourth Edition of the
Universal Definition of MI (18).
These diagnostic criteria include the rise and/or fall of cardiac
troponin T with at least one value above the 99th percentile upper
reference limit and satisfying at least one of the following
criteria: i) The presence of symptoms of myocardial ischemia; ii)
presenting new ischemic ECG changes; iii) developing pathological Q
waves; iv) imaging evidence of the loss of viable myocardium or
regional wall motion abnormalities; v) the identification of a
coronary thrombus by coronary angiography. The diagnosis was
confirmed by coronary angiography at the admission, and all
patients with AMI underwent reperfusion by percutaneous coronary
intervention without any delay. Additionally, 37 healthy
individuals without any clinical signs of myocardial ischemia were
included as the healthy controls (HCs), consisting of 21 males and
16 females, with an average age of 58.23±10.27 years. AMI patients
and HCs were age- and sex-matched. The present study was conducted
in accordance with the Declaration of Helsinki. The study was
approved by the Ethics Committee of the Beijing Tsinghua Changgung
Hospital (Approval no. 18190-0-01). All patients and HCs were
informed of the study objective, and they signed informed consent
forms prior to enrollment.
Isolation of peripheral blood
mononuclear cells (PBMCs)
Peripheral blood samples were collected in
heparin-contained tubes at 5-7 days following the onset of AMI for
patients with AMI or during the physical examination for the HCs.
The blood samples were immediately placed in ice and were then
centrifuged at 1,200 x 6 g for 5 min at room temperature. The PBMCs
were then isolated using the Ficoll-Hypaque density gradient
centrifugation method (reagents used: Histopaque, 1077,
Sigma-Aldrich; PBS buffer solution, SH30256.01B, HyClone;
Cytiva).
Phenotypic analysis
Two subsets of MDSCs, including G-MDSCs
(CD15+CD33+CD11b+CD14-HLA-DRlow)
and M-MDSCs
(CD14+CD15-CD11b+HLA-DRlow),
and Tregs
(CD3+CD4+CD25highCD127low
T-cells) were characterized by fluorescence-activated cell sorting
(FACS) using a CytoFLEX flow cytometer (Beckman Coulter, Inc.) with
a panel of anti-human-specific antibodies labeled with fluorescein
isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll
protein (PerCP)-cy5.5, allophycocyanin (APC) and PE-Cy7. Human
antibodies specific for surface markers of T-cells included
anti-CD3 (cat. no. MHCD0327; eBioscience), anti-CD4 (cat. no.
25-0049-42; eBioscience), anti-CD25 (cat. no. 12-0257-42;
eBioscience), anti-CD127 (cat. no. 17-1278-42; eBioscience),
anti-CTLA-4 anti-programmed cell death 1 (PD-1; cat. no.
85-46-1529-42; Invitrogen; Thermo Fisher Scientific, Inc.), and
surface makers of MDSCs included anti-CD15 (cat. no. 11-0159-42;
eBioscienc), anti-CD33 (cat. no. 56-0338-42; eBioscience),
anti-CD14 (cat. no. 61-0149-42; eBioscience), anti-CD45 (cat. no.
47-0459-42; eBioscience), anti-CD11b (cat. no. 46-0118-42;
eBioscience), anti-HLA-DR (cat. no. 25-9952-42; eBioscience),
anti-PD-L1 (cat. no. 85-12-5888-42; eBioscience) and anti-PD-L2
(cat. no. 25-9952-42; eBioscience). The dilutions used for the
antibodies (1:20) and the incubation conditions (at 4˚C for 30 min)
were as per the manufacturer's instructions.
MDSC-inducible Tregs
The PBMCs derived from patients with AMI and HCs
were classified as phenotypic types of G-MDSCs
(CD15+CD33+CD11b+CD14-HLA-DR-/low)
and M-MDSCs
(CD14+CD15-CD11b+CD33+HLA-DR-/low)
using flow cytometry. Well-characterized G-MDSCs and M-MDSCs were
defined as to be purified >90%. The naive CD4+
T-cells derived from the HCs were characterized as
CD45RA+CD4+ using a commercial kit (Miltenyi
Biotec GmbH) by DxFLEX flow cytometry (Beckman Coulter).
Co-culture system of MDSCs and naive
CD4+ T-cells
The naive CD4+ T-cells were co-cultured
with M-MDSCs and G-MDSCs, respectively, and they were isolated from
patients with AMI and HCs, with soluble anti-CD3 (1 µg/ml; cat. no.
16-0037-81; eBioscience) and anti-CD28 antibodies (1 µg/ml; cat.
no. 16-0289-81; eBioscience) (incubation conditions: at 37˚C in a
CO2 incubator for 4 h) that were added to achieve T-cell
receptor (TCR) stimulation via the TCR/CD3 complex. The co-culture
system used a was Roswell Park Memorial Institute (RPMI)-1640
medium supplemented with 10% fetal calf serum under the humid
condition plus 5% CO2 at 37˚C for 5 days. The Tregs
(CD4+FoxP3+) were characterized using DxFLEX
flow cytometry (Beckman Coulter).
Statistical analysis
GraphPad Prism 8.0 software (GraphPad Software,
Inc.) was utilized to perform statistical analysis. Continuous
variables are expressed as the mean ± standard deviation and
compared using a t-test or one-way analysis of variance followed by
Tukey's post hoc test where appropriate. P<0.05 was considered
statistically significant.
Results
Recruitment of G-MDSCs in the
peripheral blood following AMI
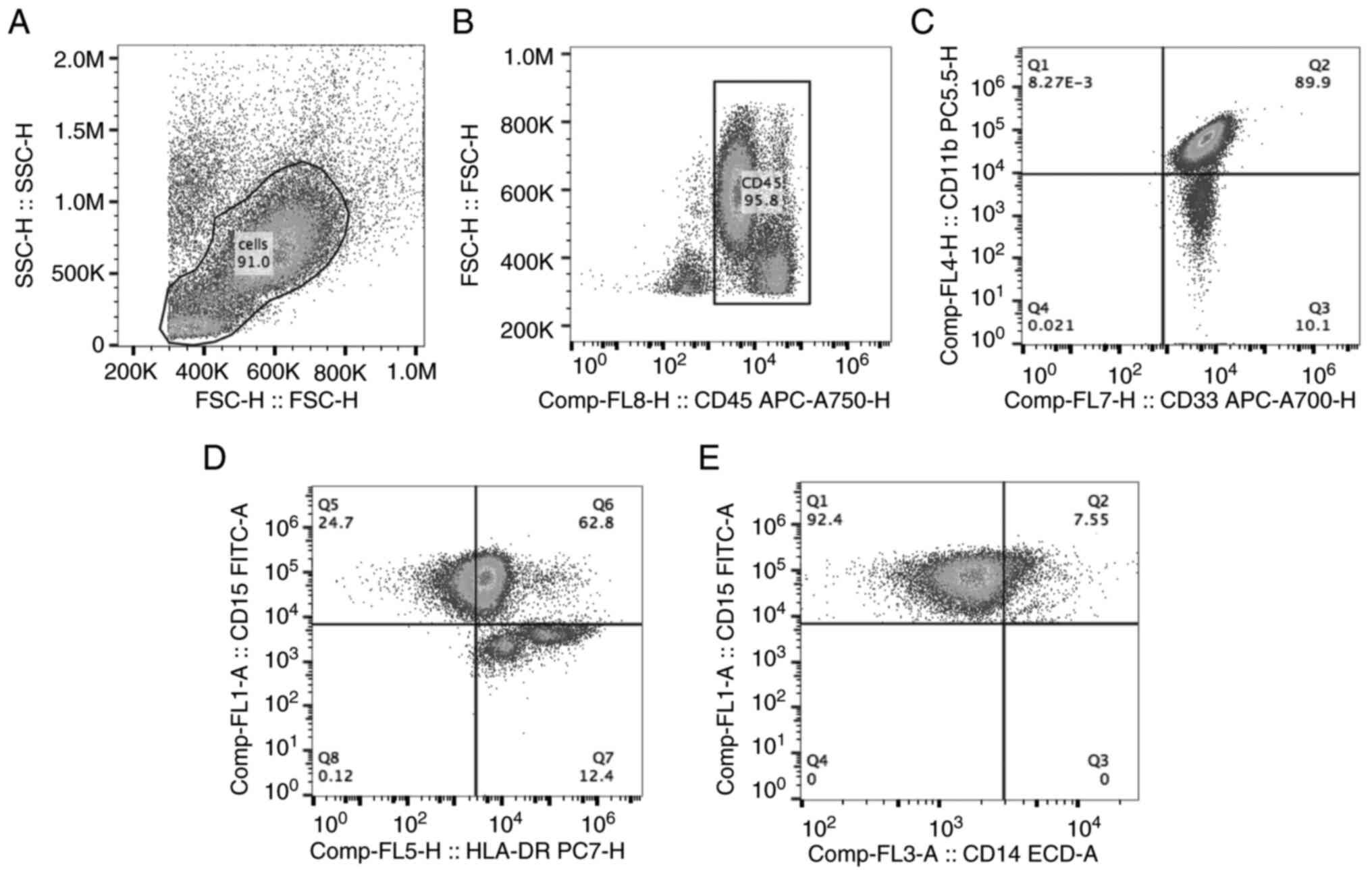
The gating strategy for G-MDSCs is illustrated in
Fig. 1. Firstly, leukocytes in
PBMCs were gated based on forward scatter height (FSC-H) and side
scatter height (SSC-H) (Fig. 1A).
FSC-H and CD45 were used to gate CD45+ cells (Fig. 1B), in which
CD11b+CD33+ cells (Q2; Fig. 1C) and
CD15+HLA-DRlow cells were gated (Q5; Fig. 1D). Finally,
CD15+CD14- cells were analyzed and sorted as
G-MDSCs (Q1 in Fig. 1E). The
percentages of G-MDSCs in patients with AMI and the HCs were
compared using flow cytometry (Fig.
2). It was found that the mean fluorescence intensity (MFI) of
the G-MDSCs was higher in the peripheral blood of patients with AMI
than in the HCs (P<0.05, Table
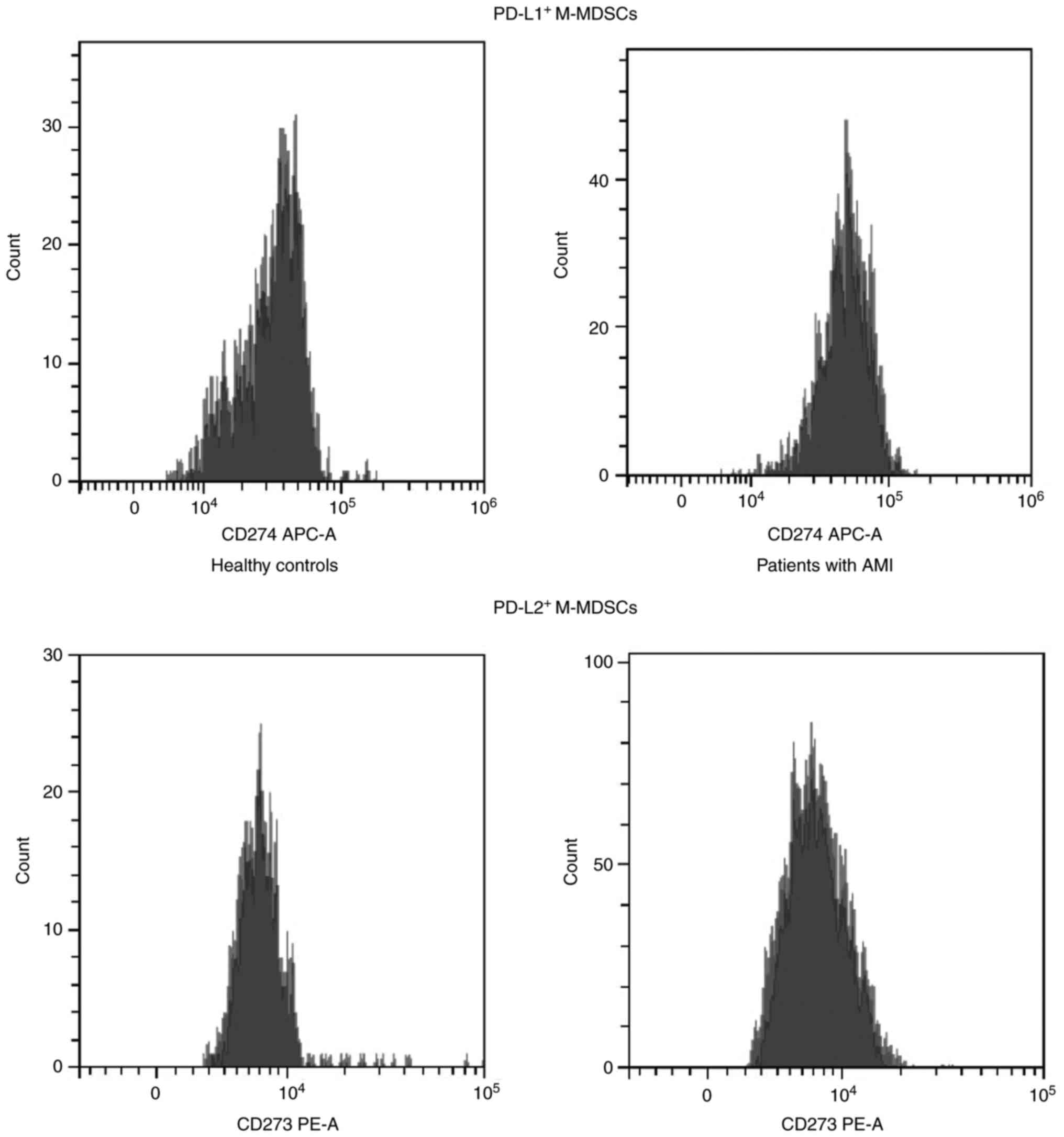
I). MDSCs exert their suppressive effects via the expression or
secretion of copious amounts of immunosuppressive mediators,
including PD-L1. PD-L1 and PD-L2 both are ligands of PD-1 and
inhibit T-cell activation (19,20).
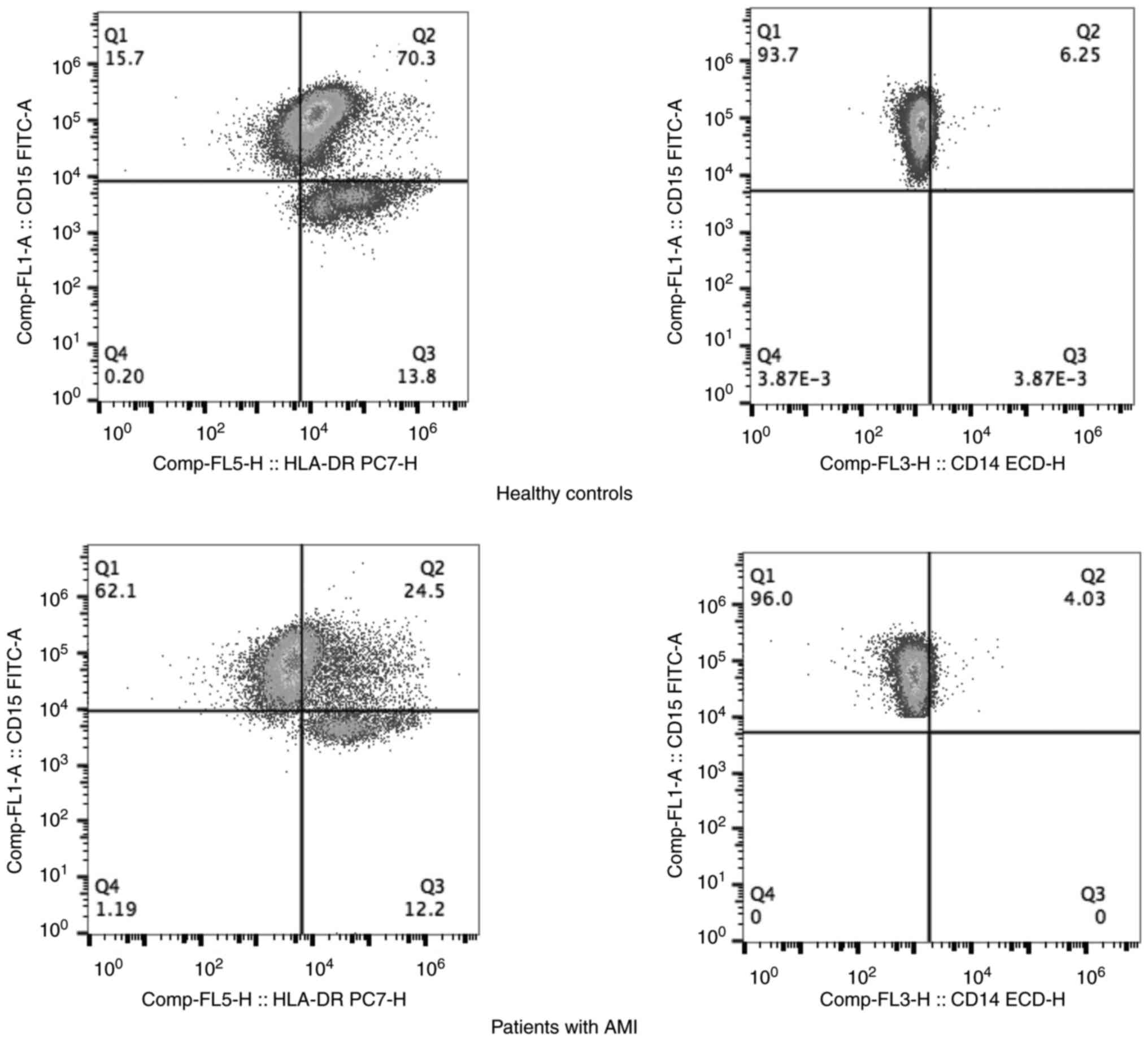
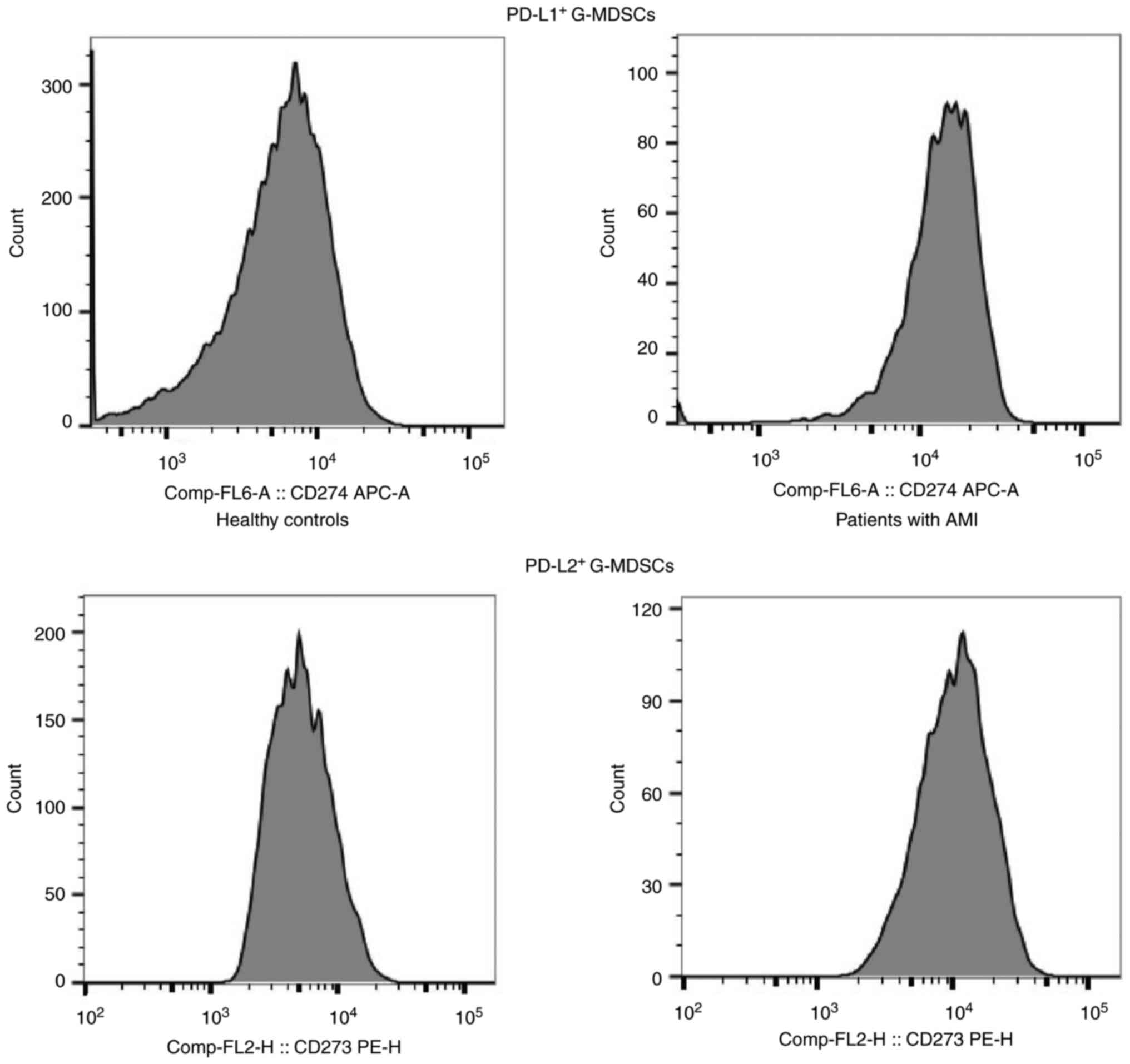
To explore the interaction among MDSCs, PD-L1 and PD-L2 in AMI,
flow cytometry of G-MDSCs was performed using anti-PD-L1 and
anti-PD-L2 antibodies in the peripheral blood of patients with AMI
and the HCs. Flow cytometry of the G-MDSCs using anti-PD-L1 and
anti-PD-L2 revealed that the patients with AMI had higher
proportions of PD-L1+ G-MDSCs and PD-L2+
G-MDSCs than the HCs (P<0.05) (Fig.
3 and Table I). These data
suggested the accumulation of G-MDSCs following AMI, as well as
that these express PD-L1 and PD-L2.
 | Table IThe mean fluorescence intensity of
G-MDSCs and M-MDSCs in the peripheral blood of AMI patients and
healthy controls. |
Table I
The mean fluorescence intensity of
G-MDSCs and M-MDSCs in the peripheral blood of AMI patients and
healthy controls.
| Cell type | HC (n=37) | Patients with AMI
(n=34) | P-value (HC vs.
patients with AMI) |
|---|
| G-MDSCs (%) | 16.47±13.69 |
33.90±18.72a | <0.001 |
| PD-L1+
G-MDSCs |
7,485.62±1,618.75 |
9,007.53±2,026.47 | <0.001 |
| PD-L2+
G-MDSCs |
6,110.62±1,490.44 |
11,408.65±5,272.43b | <0.001 |
| M-MDSCs (%) | 8.81±2.89 | 18.08±7.63 | <0.001 |
| PD-L1+
M-MDSCs |
27,379.68±12,855.26 |
29,696.24±10,572.92c | 0.45 |
| PD-L2+
M-MDSCs |
10,661.59±3,801.90 |
13,063.97±3,726.12 | 0.011 |
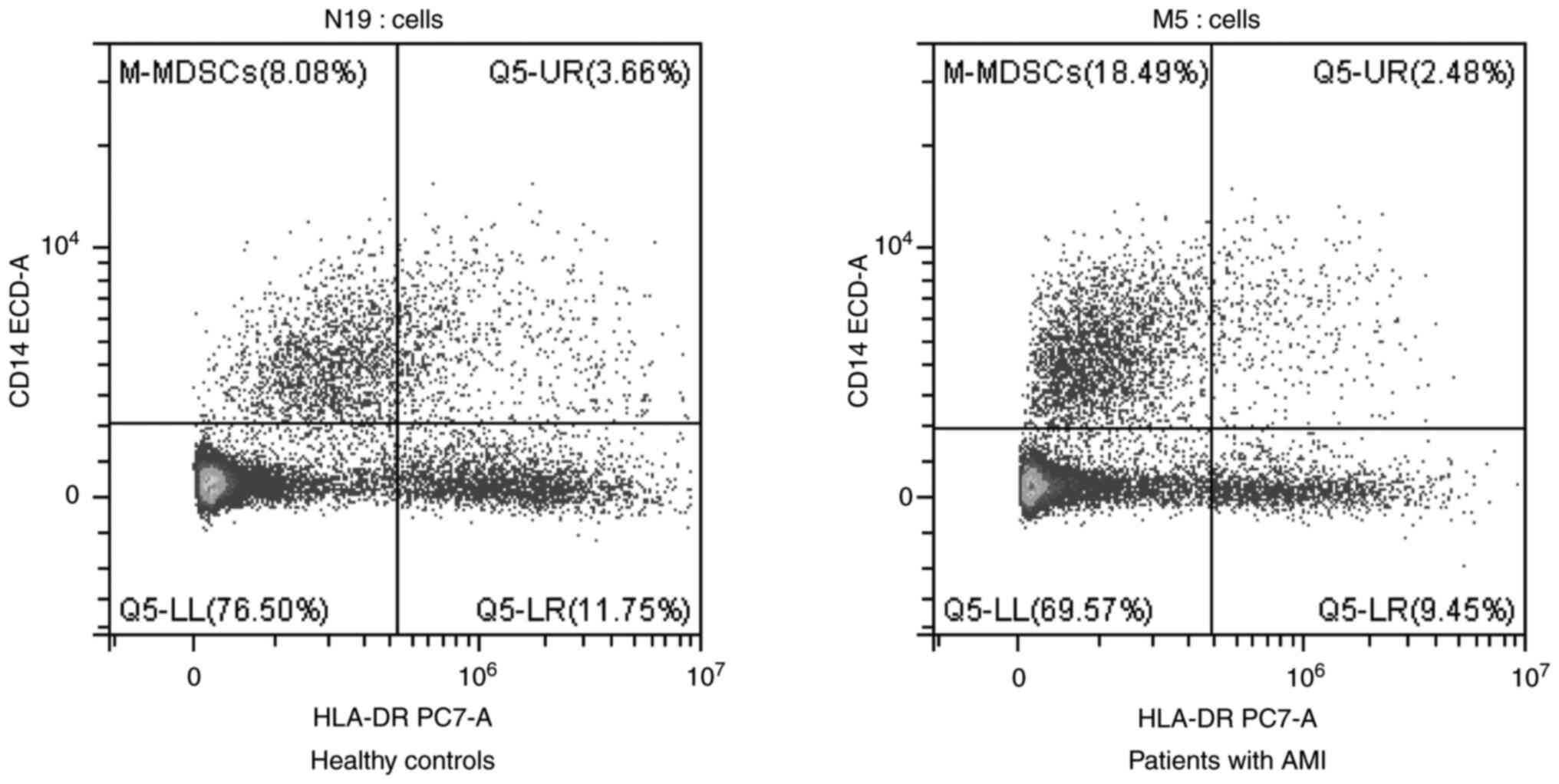
Recruitment of M-MDSCs in the
peripheral blood following AMI
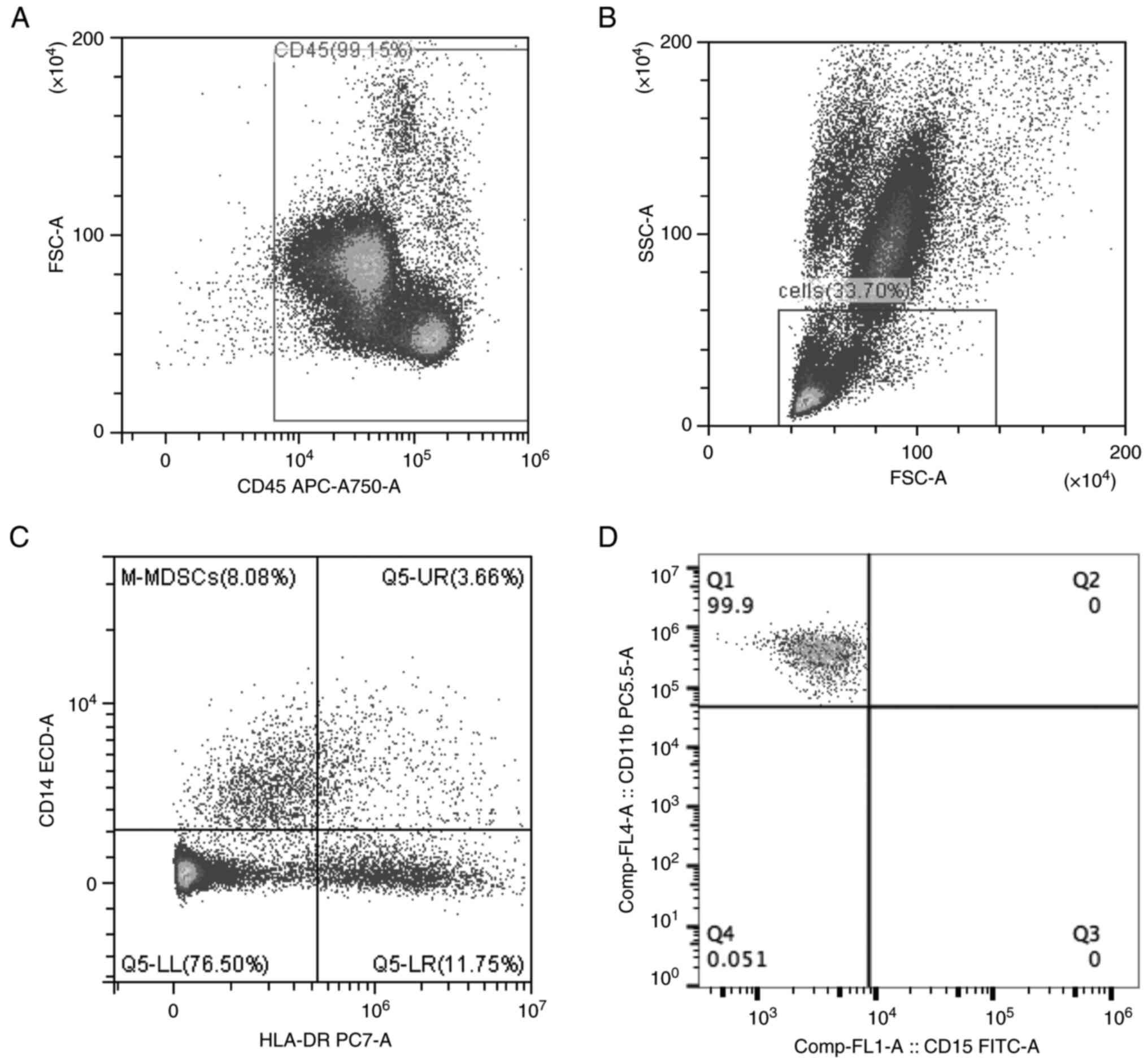
The gating strategy for the M-MDSCs is illustrated
in Fig. 4. The forward scatter area
(FSC-A) and CD45 were used to gate CD45+ cells (Fig. 4A). Neutrophil granulocytes were
excluded, and lymphocytes and monocytes were sorted based on FSC-A
and SSC-A (Fig. 4B). Finally,
CD14+HLA-DRlow cells were analyzed in
lymphocytes and monocytes (Fig.
4C). Further analysis revealed that the
CD14+HLA-DRlow cells were
CD11b+CD15- cells, which were also known as
M-MDSCs (Fig. 4D). The percentages
of M-MDSCs in the patients with AMI and HCs were sorted using flow
cytometry (Fig. 5). The results
revealed that the patients with AMI exhibited a higher MFI of
M-MDSCs in the peripheral blood than the HCs (P<0.05; Table II). Flow cytometry of the M-MDSCs
using anti-PD-L1 and anti-PD-L2 antibodies indicated that the
patients with AMI had higher proportions of PD-L1+
M-MDSCs and PD-L2+ M-MDSCs than the HCs (P<0.05)
(Fig. 6 and Table II). These results demonstrated that
the M-MDSCs could be aggregated following AMI, and that they
expressed PD-L1 and PD-L2.
 | Table IIRatios of Tregs to CD4+ T
cells, PD-1+CD4+ T-cells,
CTLA-4+CD4+ T-cells,
PD-1+CD8+ T-cells,
CTLA-4+CD8+ T cells, CTLA-4+ Tregs
and PD-1+ Tregs in the peripheral blood of patients with
AMI and healthy controls. |
Table II
Ratios of Tregs to CD4+ T
cells, PD-1+CD4+ T-cells,
CTLA-4+CD4+ T-cells,
PD-1+CD8+ T-cells,
CTLA-4+CD8+ T cells, CTLA-4+ Tregs
and PD-1+ Tregs in the peripheral blood of patients with
AMI and healthy controls.
| Cell type | Healthy controls
(n=37) | AMI patients
(n=34) | t | P-value |
|---|
|
Tregs/CD4+ T | 6.90±2.36 | 8.52±1.75 | 3.26 | 0.002 |
|
PD-1+CD4+ T | 23.62±10.49 | 24.69±7.08 | 0.50 | 0.619 |
| CTLA-4+
CD4+ T | 17.08±6.78 | 18.56±5.59 | 1.00 | 0.322 |
|
PD-1+CD8+ T | 27.22±10.58 | 29.45±8.26 | 0.98 | 0.329 |
| CTLA-4+
CD8+ T | 20.76±7.42 | 22.05±5.83 | 0.81 | 0.421 |
| CTLA-4+
Tregs | 13.78±5.00 | 15.99±5.17 | 1.83 | 0.072 |
| PD-1+
Tregs | 23.73±7.81 | 28.03±6.03 | 2.61 | 0.011 |
MDSCs acquire a granulocytic phenotype
following AMI
The proportions of G-MDSCs and M-MDSCs in the
peripheral blood of patients with AMI were compared, and higher
proportions of G-MDSCs were found in the peripheral blood of
patients with AMI than those of M-MDSCs. It was revealed that more
PD-L2+ G-MDSCs were detected than PD-L1+
G-MDSCs in the peripheral blood of patients with AMI, and more
PD-L1+ M-MDSCs were identified than the
PD-L2+ M-MDSCs in the peripheral blood of patients with
AMI. The aforementioned findings indicated that the MDSCs acquire a
granulocytic phenotype following AMI, and the G-MDSCs and M-MDSCs
are more likely to express PD-L2 and PD-L1, respectively.
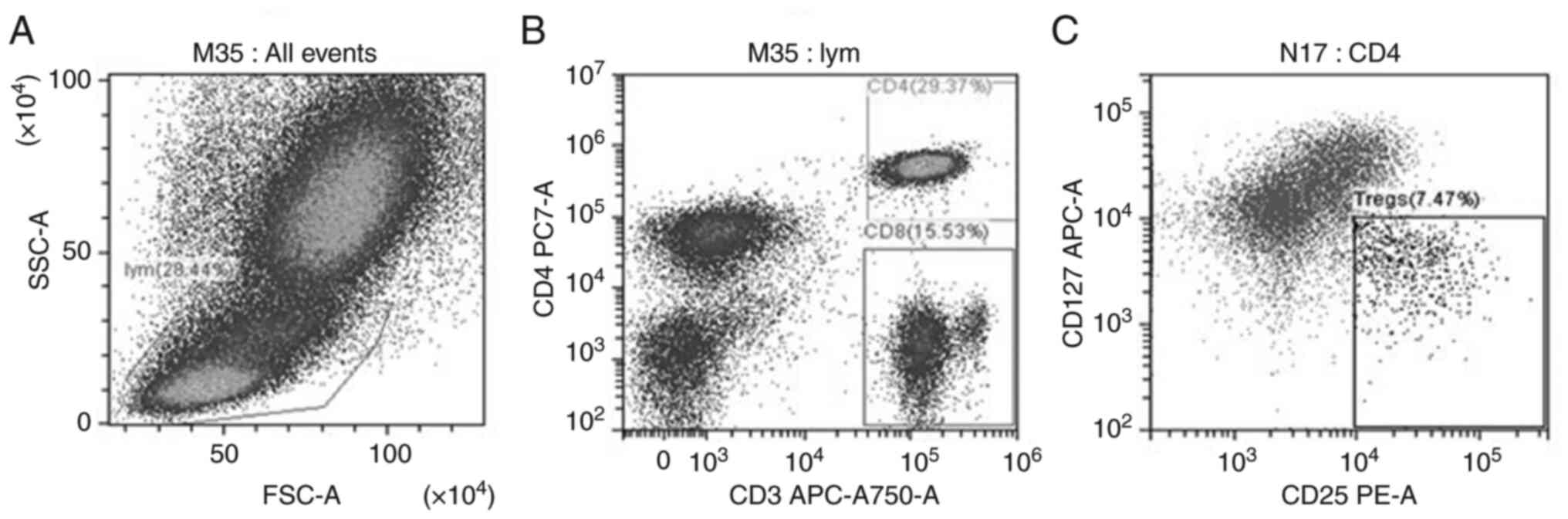
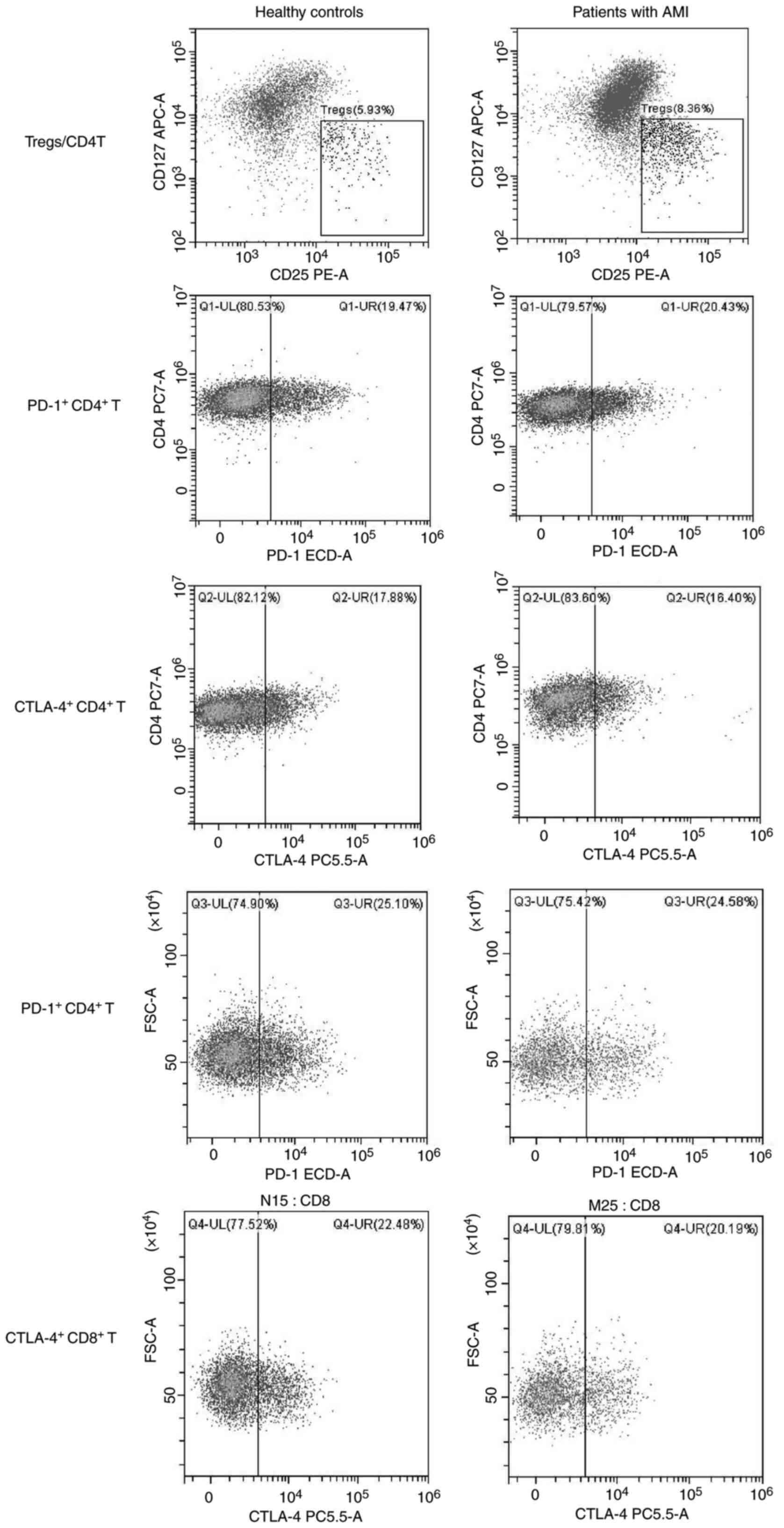
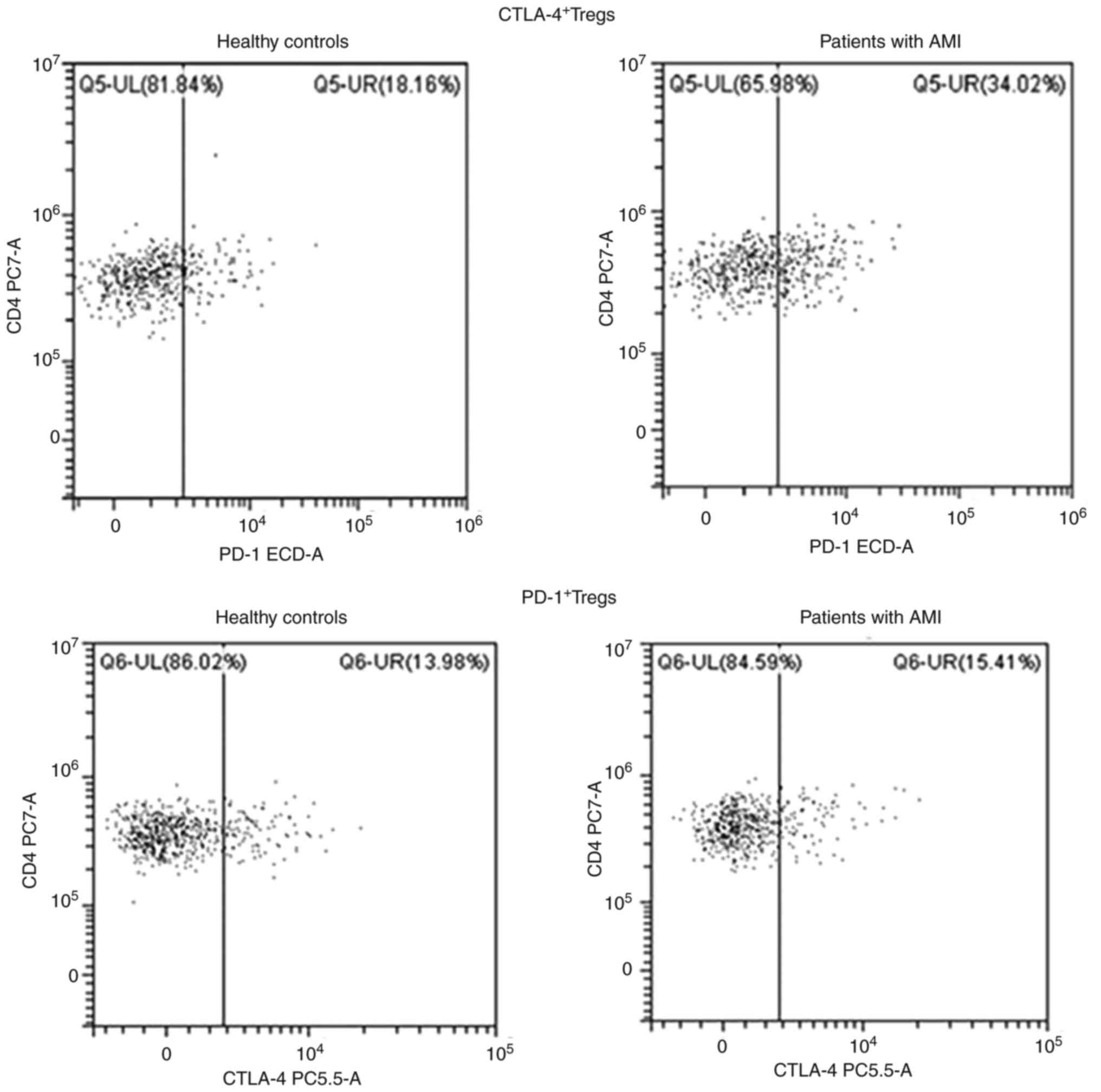
Treg accumulation in peripheral blood
following AMI
The gating strategy for Tregs is displayed in
Fig. 7. Initially, lymphocytes in
PBMCs were gated based on FSC-A and SSC-A (Fig. 7A). Cells were gated using CD3 and
CD4 antibodies, and CD4+ and CD8+ T-cells
were sorted (Fig. 7B). Tregs were
finally analyzed in CD4+ T cells, namely
CD3+CD4+CD25highCD127low
T-cells (Fig. 7C). The ratios of
Tregs to CD4+ T-cells, PD-1+CD4+
T-cells, CTLA-4+ CD4+ T-cells,
PD-1+CD8+ T-cells,
CTLA-4+CD8+ T-cells, CTLA-4+ Tregs
and PD-1+ Tregs (Figs. 8
and 9) in the peripheral blood of
patients with AMI and HCs were analyzed using flow cytometry. As
presented in Table II, the ratios
of Tregs to CD4+ T-cells and PD-1+ Tregs in
the peripheral blood of patients with AMI were higher than those in
the HCs (P<0.05). However, no significant differences were found
in the numbers of PD-1+CD4+ T-cells,
CTLA-4+CD4+ T-cells,
PD-1+CD8+ T-cells, CTLA-4+
CD8+ T-cells and CTLA-4+ Tregs in the
peripheral blood between patients with AMI and the HCs (P>0.05).
These results revealed the expansion of Tregs following AMI and a
high PD-1 expression level on the surface of Tregs.
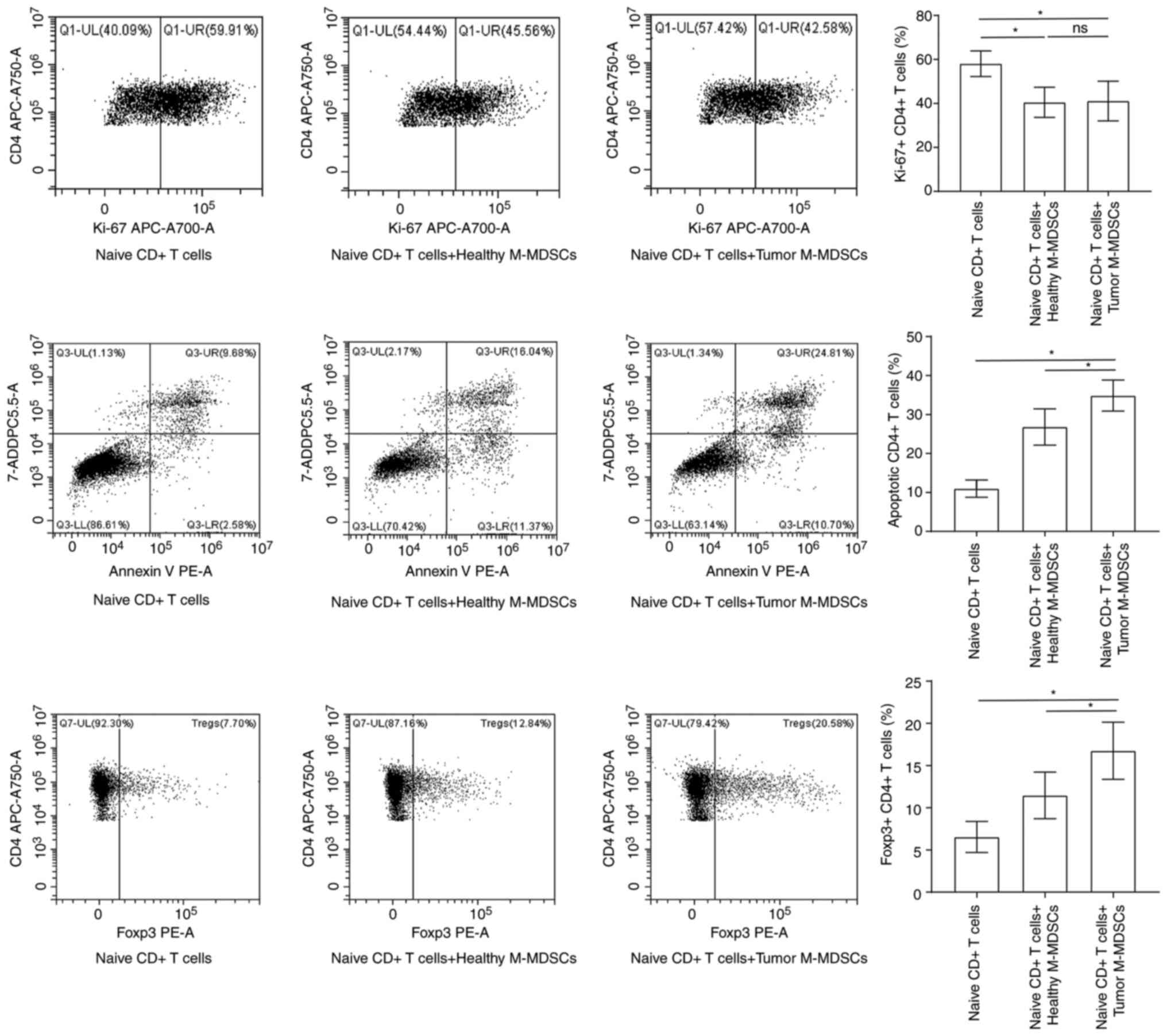
G-MDSCs derived from patients with AMI
induce the production of Tregs
The results of flow cytometry demonstrated an
increase in the numbers of inducible Tregs in the co-culture system
with M-MDSCs derived from patients with AMI compared with those
from the HCs (Fig. 10). However,
no significant effect of the M-MDSCs from patients with AMI on the
differentiation to Tregs was found.
Discussion
To date, rapid progress has been made in the
understanding of MDSCs, a particular type of suppressor cells, in
tumor progression and antitumor response. Previous findings have
demonstrated that in addition to tumor cells, PD-L1+
MDSCs may be another major source of PD-L1, inhibiting
tumor-infiltrating cytotoxic T-lymphocyte activation and function
in the tumor microenvironment (21). However, the role of MDSCs in AMI has
not yet been fully clarified. In the present study, it was found
that the proportions of G-MDSCs and M-MDSCs in patients with AMI
were significantly elevated vs. those in HCs. These two subtypes of
MDSCs, expressing significantly high expression levels of PD-L1 and
PD-L2, could induce the expansion of Tregs by binding PD-1 on the
surface of Treg, playing a pivotal role in AMI.
The aggregation and activation of MDSCs are
regulated by a variety of cytokines, such as granulocyte macrophage
colony stimulating factor (22),
vascular endothelial growth factor, IL-1β (23), IL-6(24), prostaglandin E2(25) and S100A8/A9(26). Under the action of a series of
cytokines, MDSCs are recruited, migrated and expanded ~10-fold in
the peripheral blood and tumor. Zhou et al (27) assessed the cardioprotective role of
MDSCs in heart failure, and found a cardioprotective role of MDSCs
in heart failure by their anti-hypertrophic effects on
cardiomyocytes and anti-inflammatory effects through IL-10 and NO.
The pharmacological targeting of MDSCs by rapamycin constituted a
promising therapeutic strategy for heart failure (27). According to the results of the
present study, the proportions of G-MDSCs and M-MDSCs were higher
in the peripheral blood of patients with AMI than in the HCs. The
patients with AMI had higher numbers of PD-L1- and PD-L2-positive
G-MDSCs and M-MDSCs than the HCs. The findings suggested that the
aggregation and recruitment of MDSCs following AMI may be
associated with the upregulation of PD-L1 and PD-L2 on the surface
of MDSCs. In tumors, hypoxia-induced factor-1α-mediated hypoxia
could upregulate the PD-L1 expression level on the surface of
MDSCs, resulting in the promotion of the immunosuppressive
functions of MDSCs (28). The PD-L1
expression level in MDSCs is also regulated by interferon-γ
(IFN-γ). IFN-γ activates p-STAT1 to directly regulate interferon
regulatory factor-1 (IRF1) transcription, and IRF1 directly binds
to an IRF-binding consensus element to upregulate PD-L1 expression
level in MDSCs (14). A previous
study reported that the MDSC transfer facilitated immune tolerance
and prevented type 1 diabetes mellitus (29). It is possible that MDSCs not only
promote the resolution of inflammation, but also strengthen heart
functions by unknown mechanisms in the context of AMI. Sun et
al (30) explored the effects
of G-MDSCs on the aging heart, and they found the mechanism by
which G-MDSCs promote cardiac fibrosis via the secretion of
S100A8/A9 and the regulation of FGF2-SOX9 signaling in fibroblasts
during aging. A number of scholars have concentrated on cells of
the immune system in cardiac remodeling, including main players in
resolution of inflammation and repair after myocardial infarction
(31). MDSCs also exhibit
anti-hypertrophic and anti-inflammatory effects when co-cultured
with cardiomyocytes, through the secretion of IL-10 and NO. The
numbers of MDSCs are elevated in patients with AMI, and their
depletion aggravates heart function (27). The fact that MDSCs from either
patients with AMI or mice with AMI suppress ex vivo T-cell
proliferation and IFN-γ production demonstrates their suppressive
activities. Previous studies have indicated that circulating levels
of inflammatory factors exhibit prognostic importance in the
setting of AMI (32-34).
Pro-inflammatory cytokines, such as IL-6, can recruit and activate
MDSCs (35); therefore, it is
unsurprising that the MDSC proportion is positively associated with
levels of these pro-inflammatory cytokines in patients with AMI.
More specifically, the present study indicated that MDSCs acquired
a granulocytic phenotype following AMI, and G-MDSCs and M-MDSCs
would be more likely to express PD-L2 and PD-L1, respectively.
Another finding of the present study was that Tregs
were accumulated in the peripheral blood of patients following AMI,
which was also supported by the co-culture system of MDSCs derived
from patients with AMI with naive CD4+ T-cells, which
was consistent with previously reported findings. For instance, Xia
et al (36) found that
cardiac Tregs, which are mainly thymus-derived Tregs, were
recruited from circulation that reached the peak at 7 days
following AMI and lasted for 28 days. Ke et al (37) demonstrated that pre-treatment with
rosuvastatin promoted Treg accumulation in the myocardium during
myocardial ischemia-reperfusion injury, suggesting that the
Treg-negative modulation of the inflammatory response could serve
as therapeutic target in cardiovascular diseases. Tregs, which were
traditionally considered as potent suppressors of immune response,
have increasingly attracted the attention of researchers as they
reside in parenchymal tissues and maintain local homeostasis.
Multiple studies have demonstrated cardioprotection conferred by
Tregs after MI by alleviating local inflammation, protecting
cardiomyocytes against apoptosis, as well as regulating macrophage
differentiation and myofibroblast activation (38-40).
Hence, the clarification of the characteristic of Tregs in the
context of MI is of utmost importance. Specifically, whether Tregs
have local adaption with unique phenotype, how the populations
accumulate after MI and what factors drive their accumulation
remain to be elucidated. Park et al (18) demonstrated that PD-1 was upregulated
on Tregs during chronic virus infection and promoted the
suppression of the CD8+ T-cell immune response via the
interaction with PD-L1 expressed on CD8+ T-cells,
indicating that PD-1 may be a mediator of the immunosuppressive
function of Tregs. Turnquist et al (41) demonstrated that IL-33 expanded
functional MDSCs, in which CD11b(+) cells exhibited intermediate
levels of Gr-1 and Tregs, including
ST2L+Foxp3+ cells, and they mediated the
Treg-dependent promotion of cardiac allograft survival, leading to
an interaction between Tregs and MDSCs. Previous studies have
indicated that the accumulation of MDSCs, in addition to their
ability to inhibit T-cell proliferation, can trigger the
development of Tregs (42,43). In human immunodeficiency virus
disease, the expansion of MDSCs has been found to promote the
differentiation of Tregs and inhibit T-cell function, which is a
hallmark of several chronic infectious diseases (44). A previous study indirectly indicated
that Tregs contribute to rosuvastatin-induced cardioprotection
against AMI (34). In the present
study, the comparative analysis revealed that patients with AMI had
higher numbers of PD-L1- and PD-L2-positive G-MDSCs and M-MDSCs
than the HCs, and the numbers of PD-1+ Tregs in the
peripheral blood of patients AMI were higher than those in the HCs.
The upregulation of PD-L1 and PD-L2 in MDSCs could bind with PD-1
on the surface of Tregs and then promote the differentiation of
initial CD4+ T-cells into Tregs following AMI. The
interaction between PD-1 and PD-L1 can phosphorylate two structural
motifs, an immunoreceptor tyrosine-based inhibitory motif and an
immunoreceptor tyrosine-based switch motif, in the cytoplasmic
region of PD-1, recruit src-homology 2 domain-containing tyrosine
phosphatase-2, inhibit signaling downstream of T-cell receptor by
dephosphorylation, and block T-cell activation (45,46).
Similarly, Jaworska et al (47) found that Tregs protected the kidneys
from ischemia reperfusion-induced inflammation and injury, and PD-1
on the surface of Tregs, prior to adoptive transfer, attenuated
their renoprotective effects against ischemic injury. More
specifically, they confirmed that the administration of PD-L1 or
PD-L2 blocking antibodies markedly exacerbated the loss of renal
function, renal inflammation and acute tubular necrosis during
acute kidney injury, which revealed that the renoprotective role of
Tregs was achieved by its immunomodulator PD-1 binding to the known
ligands, PD-L1 and PD-L2(47).
The present study has some limitations. Firstly, the
sample size was not sufficient, hindering the generalization of the
findings. Secondly, the absence of dynamic changes in MDSC subsets
and the number of Tregs following AMI, particularly at 12, 24 and
48 h following the onset of symptoms of AMI, needs to be
determined. Finally, no cellular and animal models were used to
demonstrate the role of MDSCs in the promotion of the expansion of
Tregs via the PD-1/PD-L1/PD-L2 interaction. Thus, further
large-scale multi-center studies are required to eliminate the
aborementioned limitations and to verify the findings of the
present study.
In conclusion, the present study demonstrated that
both subtypes of MDSCs, G-MDSCs and M-MDSCs, were accumulated
following AMI and expressed high levels of PD-L1 and PD-L2. MDSCs
promoted the expansion of Tregs by binding PD-1 on the surface of
Tregs, playing a pivotal role in AMI. Although immunosuppression
caused by the involvement of MDSCs and Tregs could benefit tumor
immune escape and penalize immunotherapy for diverse types of
cancer, on the basis of inflammatory responses compromising
microcirculation during reperfusion, it is essential to determine
whether the accumulation of MDSCs and Tregs in the myocardium from
circulation can confer cardioprotective effects against AMI; thus,
further studies are warranted. The exploration of a potent
signaling mechanism is also suggested to manipulate cellular
heterogeneity in the immune response during AMI. Molecular
AMI-related studies will be advantageous to obtain a time-dependent
equilibrium in immune regulation through metabolic and epigenetic
rearrangement in the future. Further research is also required to
determine whether the accumulation of MDSCs and Tregs in the
myocardium from the circulation can confer cardioprotective effects
against AMI, particularly in the lymph nodes, bone marrow and tumor
sites, as well as in the survival of cardiac allografts.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially supported by the
Natural Sciences Foundation of China (grant no. 81900021), the
Innovation Fund for Medical Sciences of Chinese Academy of Medical
Sciences (grant no. 2017-I2M-1-009) and the Beijing Clinical Key
Specialty (grant no. XKB2022B1002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ was involved in the design of the study and in
the drafting of the manuscript. XS performed the experiments. JZhao
was involved in data analysis. WG was involved in the study design,
manuscript revision and grammatical corrections. JZhou conceived
and supervised the study. MZ and JZhou confirm the authenticity of
all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Beijing Tsinghua Changgung Hospital (Approval no.
18190-0-01). All patients and healthy controls signed informed
consent forms.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Anderson JL and Morrow DA: Acute
myocardial infarction. N Engl J Med. 376:2053–2064. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vogel B, Claessen BE, Arnold SV, Chan D,
Cohen DJ, Giannitsis E, Gibson CM, Goto S, Katus HA, Kerneis M, et
al: ST-segment elevation myocardial infarction. Nat Rev Dis
Primers. 5(39)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alwi I: Targeting inflammation and immune
system in acute myocardial infarction. Acta Med Indones.
51:287–289. 2019.PubMed/NCBI
|
|
4
|
Meng D, Han S, Jeong IS and Kim SW:
Interleukin 10-secreting MSCs via TALEN-mediated gene editing
attenuates left ventricular remodeling after myocardial infarction.
Cell Physiol Biochem. 52:728–741. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Luo L, Zeng X, Huang Z, Luo S, Qin L and
Li S: Reduced frequency and functional defects of
CD4+CD25highCD127low/- regulatory
T cells in patients with unexplained recurrent spontaneous
abortion. Reprod Biol Endocrinol. 18(62)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Malko D, Elmzzahi T and Beyer M:
Implications of regulatory T cells in non-lymphoid tissue
physiology and pathophysiology. Front Immunol.
13(954798)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ridker PM, Everett BM, Thuren T, MacFadyen
JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker
SD, et al: Antiinflammatory therapy with canakinumab for
atherosclerotic disease. N Engl J Med. 377:1119–1131.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lutgens E, Atzler D, Döring Y, Duchene J,
Steffens S and Weber C: Immunotherapy for cardiovascular disease.
Eur Heart J. 40:3937–3946. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Keskinov AA and Shurin MR: Myeloid
regulatory cells in tumor spreading and metastasis. Immunobiology.
220:236–242. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gabrilovich DI: Myeloid-derived suppressor
cells. Cancer Immunol Res. 5:3–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Groth C, Hu X, Weber R, Fleming V,
Altevogt P, Utikal J and Umansky V: Immunosuppression mediated by
myeloid-derived suppressor cells (MDSCs) during tumour progression.
Br J Cancer. 120:16–25. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7(12150)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Talmadge JE and Gabrilovich DI: History of
myeloid-derived suppressor cells. Nat Rev Cancer. 13:739–752.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chang AL, Miska J, Wainwright DA, Dey M,
Rivetta CV, Yu D, Kanojia D, Pituch KC, Qiao J, Pytel P, et al:
CCL2 produced by the glioma microenvironment is essential for the
recruitment of regulatory T cells and myeloid-derived suppressor
cells. Cancer Res. 76:5671–5682. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu C, Redd PS, Lee JR, Savage N and Liu K:
The expression profiles and regulation of PD-L1 in tumor-induced
myeloid-derived suppressor cells. Oncoimmunology.
5(e1247135)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang JC and Sun L: PD-1/PD-L1, MDSC
pathways, and checkpoint inhibitor therapy in Ph(-)
myeloproliferative neoplasm: A review. Int J Mol Sci.
23(5837)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bahrami A, Fereidouni M, Pirro M, Bianconi
V and Sahebkar A: Modulation of regulatory T cells by natural
products in cancer. Cancer Lett. 459:72–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park HJ, Park JS, Jeong YH, Son J, Ban YH,
Lee BH, Chen L, Chang J, Chung DH, Choi I and Ha SJ: . PD-1
upregulated on regulatory T cells during chronic virus infection
enhances the suppression of CD8+ T cell immune response
via the interaction with PD-L1 expressed on CD8+ T
cells. J Immunol. 194:5801–5811. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Prima V, Kaliberova LN, Kaliberov S,
Curiel DT and Kusmartsev S: COX2/mPGES1/PGE2 pathway regulates
PD-L1 expression in tumor-associated macrophages and
myeloid-derived suppressor cells. Proc Natl Acad Sci USA.
114:1117–1122. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Teng MW, Ngiow SF, Ribas A and Smyth MJ:
Classifying cancers based on T-cell infiltration and PD-L1. Cancer
Res. 75:2139–2145. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dolcetti L, Peranzoni E, Ugel S, Marigo I,
Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati
A, et al: Hierarchy of immunosuppressive strength among
myeloid-derived suppressor cell subsets is determined by GM-CSF.
Eur J Immunol. 40:22–35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tu S, Bhagat G, Cui G, Takaishi S,
Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl
O, Fox JG and Wang TC: Overexpression of interleukin-1beta induces
gastric inflammation and cancer and mobilizes myeloid-derived
suppressor cells in mice. Cancer Cell. 14:408–419. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bunt SK, Yang L, Sinha P, Clements VK,
Leips J and Ostrand-Rosenberg S: Reduced inflammation in the tumor
microenvironment delays the accumulation of myeloid-derived
suppressor cells and limits tumor progression. Cancer Res.
67:10019–10026. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rodriguez PC, Hernandez CP, Quiceno D,
Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J and Ochoa AC: Arginase
I in myeloid suppressor cells is induced by COX-2 in lung
carcinoma. J Exp Med. 202:931–939. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sinha P, Okoro C, Foell D, Freeze HH,
Ostrand-Rosenberg S and Srikrishna G: Proinflammatory S100 proteins
regulate the accumulation of myeloid-derived suppressor cells. J
Immunol. 181:4666–4675. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhou L, Miao K, Yin B, Li H, Fan J, Zhu Y,
Ba H, Zhang Z, Chen F, Wang J, et al: Cardioprotective role of
myeloid-derived suppressor cells in heart failure. Circulation.
138:181–197. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang S, Tan Q, Hou Y and Dou H: Emerging
roles of myeloid-derived suppressor cells in diabetes. Front
Pharmacol. 12(798320)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Epelman S, Liu PP and Mann DL: Role of
innate and adaptive immune mechanisms in cardiac injury and repair.
Nat Rev Immunol. 15:117–129. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun SN, Ni SH, Li Y, Li Y, Liu X, Deng JP,
Chen ZX, Li H, Feng WJ, Huang YS, et al: G-MDSCs promote
aging-related cardiac fibrosis by activating myofibroblasts and
preventing senescence. Cell Death Dis. 12(594)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oprescu N, Micheu MM, Scafa-Udriste A,
Popa-Fotea NM and Dorobantu M: Inflammatory markers in acute
myocardial infarction and the correlation with the severity of
coronary heart disease. Ann Med. 53:1041–1047. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Karthikeyan T, Raja M, Radha D, Gaur TA,
Geetha J and Sakthivadivel V: Risk factors and inflammatory markers
in acute coronary syndrome-ST elevation myocardial infarction
(STEMI). Horm Mol Biol Clin Investig: Mar 20, 2023 (Epub ahead of
print).
|
|
33
|
Jiang Y, Li X, Xu H, Gu Y, Shi F, Wang F
and Zhang X: Tumour necrosis factor receptor-associated factors:
Interacting protein with forkhead-associated domain inhibition
decreases inflammatory cell infiltration and cardiac remodelling
after acute myocardial infarction. Interact Cardiovasc Thorac Surg.
31:85–92. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mukherjee S, Ghosh S, Sengupta A, Sarkar
S, Keswani T, Chatterjee R and Bhattacharyya A: IL-6 dependent
expansion of inflammatory MDSCs (CD11b+ Gr-1+) promote Th-17
mediated immune response during experimental cerebral malaria.
Cytokine. 155(155910)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lou X, Gao D, Yang L, Wang Y and Hou Y:
Endoplasmic reticulum stress mediates the myeloid-derived immune
suppression associated with cancer and infectious disease. J Transl
Med. 21(1)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xia N, Lu Y, Gu M, Li N, Liu M, Jiao J,
Zhu Z, Li J, Li D, Tang T, et al: A unique population of regulatory
T cells in heart potentiates cardiac protection from myocardial
infarction. Circulation. 142:1956–1973. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ke D, Fang J, Fan L, Chen Z and Chen L:
Regulatory T cells contribute to rosuvastatin-induced
cardioprotection against ischemia-reperfusion injury. Coron Artery
Dis. 24:334–341. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Weirather J, Hofmann UD, Beyersdorf N,
Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T and Frantz S: Foxp3+
CD4+ T cells improve healing after myocardial infarction by
modulating monocyte/macrophage differentiation. Circ Res.
115:55–67. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sharir R, Semo J, Shimoni S, Ben-Mordechai
T, Landa-Rouben N, Maysel-Auslender S, Shaish A, Entin-Meer M,
Keren G and George J: Experimental myocardial infarction induces
altered regulatory T cell hemostasis, and adoptive transfer
attenuates subsequent remodeling. PLoS One.
9(e113653)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Feng Q, Li Q, Zhou H, Sun L, Lin C, Jin Y,
Wang D and Guo G: The role of major immune cells in myocardial
infarction. Front Immunol. 13(1084460)2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Turnquist HR, Zhao Z, Rosborough BR, Liu
Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX, et
al: IL-33 expands suppressive CD11b+ Gr-1(int) and regulatory T
cells, including ST2L+ Foxp3+ cells, and mediates regulatory T
cell-dependent promotion of cardiac allograft survival. J Immunol.
187:4598–4610. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang B, Pan PY, Li Q, Sato AI, Levy DE,
Bromberg J, Divino CM and Chen SH: Gr-1+CD115+ immature myeloid
suppressor cells mediate the development of tumor-induced T
regulatory cells and T-cell anergy in tumor-bearing host. Cancer
Res. 66:1123–1131. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yaseen MM, Abuharfeil NM and Darmani H:
Myeloid-derived suppressor cells and the pathogenesis of human
immunodeficiency virus infection. Open Biol.
11(210216)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Guo H, Cao A, Chu S, Wang Y, Zang Y, Mao
X, Wang H, Wang Y, Liu C, Zhang X and Peng W: Astragaloside IV
attenuates podocyte apoptosis mediated by endoplasmic reticulum
stress through upregulating sarco/endoplasmic reticulum
Ca2+-ATPase 2 expression in diabetic nephropathy. Front
Pharmacol. 7(500)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Guo X, Zhang Y, Jiao H and Miao X: The
prognostic significance of PD-L1 expression in patients with
glioblastoma: A meta-analysis. Front Oncol.
12(925560)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tang L, Bai J, Chung CS, Lomas-Neira J,
Chen Y, Huang X and Ayala A: Programmed cell death receptor ligand
1 modulates the regulatory T cells' capacity to repress
shock/sepsis-induced indirect acute lung injury by recruiting
phosphatase SRC homology region 2 domain-containing phosphatase 1.
Shock. 43:47–54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jaworska K, Ratajczak J, Huang L, Whalen
K, Yang M, Stevens BK and Kinsey GR: Both PD-1 ligands protect the
kidney from ischemia reperfusion injury. J Immunol. 194:325–333.
2015.PubMed/NCBI View Article : Google Scholar
|