1. Introduction
Gastric cancer (GC) is an aggressive disease,
numerous patients are diagnosed at advanced stages and some are
inoperable (1,2). It consists of several subtypes; their
relative incidence is influenced by genetic and environmental
factors, thus, prevalence of each subtype may vary significantly in
different populations (3).
Conventional treatment is of limited success (4-8).
Recent advances in personalised treatment improve outcomes but, for
this to be effective, distinct subtypes need to be recognised.
Different morphological and molecular subtypes have been
highlighted by numerous classifications, but no unifying
classification is currently in use. In the present study, the main
classifications were reviewed in order to provide a workable scheme
that includes the important elements of each, so they can be
delivered with on-slide tests in the diagnostic routine and
prognostic and predictive data can be provided. It is important
that a working molecular classification is structured to include
present companion diagnostic biomarkers necessary for selection for
biological therapies. Since new biological therapies will
inevitably emerge, this classification needs to be able to
accommodate new companion diagnostics. Finally, for a working
classification to have a significaticant impact on patients
outcomes worldwide, it needs to be implementable in histopathology
departments using available resources.
2. The Laurén Classification
The Finnish pathologist Pekka Laurén first
recognised GC as a heterogenous group of diseases and, in 1965,
published his reductive histological classification consisting of
only three subtypes that associated with the biology of the disease
(9). He described two main types:
intestinal and diffuse, both with no direct counterpart in previous
morphologic classifications. As a general principle, the intestinal
type had recognisable morphological counterparts in colorectal
cancer whilst the diffuse type did not. The intestinal type is
characterised by cells resembling those found in the small
intestine. It tends to be slower growing and less aggressive than
the diffuse type, which is characterised by cells that infiltrate
more widely and are less organised. Laurén also recognised a minor
group (~15% of cases) that did not sit within these two types and
later termed ‘atypical’ or ‘indeterminate’ (9). In 1995, Carneiro and colleagues
divided Laurén's indeterminate category into mixed tumours (having
at least 5% of both intestinal and diffuse components) and solid
tumours (10).
There was little understanding of the molecular and
biological significance of loss of cell-to-cell adhesion in 1965.
More recently, this has been linked with epithelial-to-mesenchymal
transition (EMT). Nevertheless, Laurén recognised that tumours with
loss of cell-to-cell adhesion represented a separate entity.
Authors' opinion suggest that loss of cell-to-cell adhesion
(11,12) is the linchpin of the Laurén
classification.
3. The World Health Organisation (WHO)
classification
This classification recognises molecular subgroups
(see below, intrinsic classifications) but even in its latest
(2019) edition, remains fixed on taxonomy and has dozens of
different morphological types. It describes five main subtypes of
adenocarcinoma: i) tubular (the most common), ii) papillary, iii)
poorly cohesive (this includes signet ring cell and other
subtypes), iv) mucinous and v) mixed (13). Adenocarcinoma represents 95% of all
malignant epithelial tumours of the stomach (14). The World Health Organisation (WHO)
classification does not provide sufficient details to drive
personalised treatment and, notably, the Laurén classification
continues being described, even in the current WHO publication, due
to its direct relevance for prognosis and treatment.
4. Intrinsic classifications
The Laurén and WHO classifications are focused on
the morphology of the tumour cell compartment. ~20 years ago, the
intrinsic properties of the tissue (rather than of the tumour cells
alone) were investigated with a view to provide important clues for
tumour classification, tumour behaviour and response to treatment.
Such classifications represented a significant step forward in
improving outcomes for patients with GC. Since then, the intrinsic
properties of tumours have been scrutinised by genomics,
transcriptomics and proteomics of the whole tumour tissue and
intrinsic classifications have been proposed for numerous tumour
types, including ovarian, colon, breast, endometrium and lung
(15-19).
The two major intrinsic molecular classifications
for GC are those of The Cancer Genome Atlas and the Asian Cancer
Research Group. Both are large scale, multi-institutional studies
that characterise the changes that occur in GC using multi-omics
techniques. Both aim at understanding the biology of the disease to
identify new therapeutic targets (20-22).
The Cancer Genome Atlas (TCGA) and the ACRG classifications are
briefly described below.
TCGA intrinsic classification
In 2014, TCGA proposed a classification of gastric
carcinoma based on the genetic and molecular characteristics of the
tumour (20). It was developed by
TCGA research network as part of a large-scale effort to understand
the underlying basis of gastric carcinoma. Tissues from 295 cases
of primary gastric carcinoma with no prior chemotherapy or
radiotherapy were studied using six different molecular platforms,
including single nucleotide polymorphism array, somatic copy-number
analysis, whole-exome sequencing, mRNA sequencing, miRNA
sequencing, array-based DNA methylation profiling and reverse-phase
protein arrays. A subset (77%) was also tested by whole genome
sequencing (Next-generation sequencing).
This work resulted in the grouping of GC into four
major types: i) GC associated with Epstein-Barr virus (EBV), ii) GC
with mismatch repair deficiency (dMMR), iii) genomically stable
(GS) GC and iv) GC with chromosome instability (CIN).
GC-EBV(+). GC-EBV(+) (9%) is more common in
younger individuals, predominately male patients and has an
improved prognosis compared with other subtypes. These tumours are
mainly in fundus or body (62%), have hypermethylation of CDKN2A
(p16INK4a) promoter, have the highest rate of
phosphoinosytol-3 kinase (PIK3CA) mutations (80%) and overexpress
programmed death-ligand (PD-L)1/2 (15%) due to amplification at
9p24.1, a locus containing genes encoding for JAK2, PD-L1 and
PD-L2.
GC-dMMR. GC-dMMR (22%) is typically more
aggressive than other subtypes and affects older patients (median
age, 72 years). It is characterised by hypermethylation of MLH1
promoter. Mutations in PIK3CA are also common in this subtype
(42%).
GC-GS. GC-GS (20%) is characterised by low
levels of genetic instability and tends to be slower-growing. It is
predominantly of diffuse histology (73%) and is associated with
CDH1 (E-cadherin) mutations (37%), RHOA mutations (30%) as well as
CLDN18-ARHGAP (Claudin-18) rearrangements (30%). The latter two
mutations are mutually exclusive; they affect key molecules in
cell-to-cell adhesion and are probably responsible for the diffuse
growth pattern.
GC-CIN. GC-CIN (50%) subtype is characterised
by amplification of receptor tyrosine kinases, has a high
percentage of TP53 mutations (73% of CIN tumours) and corresponds
to the intestinal type of Laurén. Numerous genes are affected in
this subtype, including VEGFA (7%), ERBB2 (24%), ERBB3 (8%), ERBB1
(10%), FGFR2 (8%) as well as c-Met (8%).
ACRG intrinsic classification
There are significant differences in GC arising in
the Asian population, possibly related to a combination of genetic
and environmental factors. In 2014, ACRG published a different
molecular classification for GC. Initially, they performed whole
genome sequencing on tissue from 49 gastric tumours (22), and later added gene expression
profiling, genome-wide copy number, microarrays and targeted gene
sequencing from a further 251 cases (21).
ACRG divide GC into MMR-proficient (pMMR) and
MMR-deficient (dMMR) types. The pMMR GC is further divided into
three subtypes. Their proposed four molecular subtypes have some
overlap with TCGA groups and are GC-dMMR, GC with EMT (GC-EMT), CG
with intact p53 (GC-p53wt) and GC with functional loss of p53 due
to mutation (GC-p53m).
GC-dMMR. The GC-dMMR (23%) group contains
tumours that are mainly located in the antrum, are usually
diagnosed at early stages (clinical stage I/II), have the lowest
frequency of recurrence and, when recurrence occurs, this is
usually in liver. These tumours are predominantly intestinal type
(60%) and have the best prognosis. In this group, there are GC with
mutations in KRAS (23%), PI3K-PTEN-mTOR (42%), ALK (16%) and ARID1A
(44.2%). This subtype has an overlap with the GC-dMMR of TCGA
classification.
GC-EMT. The GC-EMT (15%) subtype is
characterised by loss of CDH1 (E-cadherin) and is predominantly
observed in young patients. These tumours correspond to the diffuse
type of Laurén, they are diagnosed at late stages (80% are clinical
stages III/IV) and therefore have worse prognosis with high
recurrence rates (mainly in peritoneal cavity). This subtype has
the lowest number of mutational events and has overlaps with the
GC-GS of TCGA classification.
GC-p53wt. The GC-p53wt (26%) group is found
more frequently in male patients, is mostly of the Laurén's
intestinal type and has intermediate prognosis. Numerous of these
patients are diagnosed in early stages (clinical I/II). This
subtype has a higher mutation rate in APC, ARID1A, KRAS, PIK3CA and
SMAD4. In addition, this group contains the highest proportion of
integrated EBV and therefore may overlap with the GC-EBV(+) of TCGA
classification.
GC-p53m. The GC-p53m (36%) subtype is
identified more frequently in male patients and is of Laurén's
intestinal type. It is diagnosed at advanced stages and has
intermediate prognosis. This group is characterised by high
prevalence (60%) of TP53 mutation, is associated with amplification
in ERBB2, ERBB1, CCNE1 and CCND1 genes and may overlap with the
GC-CIN of TCGA classification.
It is recognised that while there is some overlap
with TCGA, a major difference is that the ACRG lacks a category
that relies solely on EBV status, whilst TCGA does not have a
category reliant solely on p53 status.
5. Singapore-Duke classification
Numerous other classifications of GC have been
proposed. Some focus on clinical or surgical parameters, which have
less relevance to the pathologist, whilst others target the
cellular biology of GC using in vitro models. It is worth
describing one such classification, the Singapore-Duke
classification, which highlights the current gap in knowledge of
the intrinsic classifications. This is focused on molecular in
vitro studies and is based on different biological properties
and response to chemotherapy and targeted therapy (23). It identifies three GC subtypes
i) Mesenchymal subtype. The mesenchymal GC
has highly activated EMT molecular pathways, low rates of TP53
mutation and low level of CDH1 (E-cadherin) expression. This
subtype has cancer stem cell-like properties, corresponds to the
Laurén's diffuse type and is more sensitive to PIK3CA and mTOR
inhibitors.
ii) Proliferative subtype. The proliferative
subtype has high rates of TP53 mutation, more extensive gene
amplification, high levels of genomic instability and DNA
hypomethylation and corresponds to Laurén's intestinal type.
iii) Metabolic subtype. The metabolic type
has low rates of TP53 mutation, expresses genes characteristic of
normal gastric mucosa and has no intuitive counterpart in the
Laurén's classification. These tumours respond well to
5-fluorouracil associated with surgery (23).
6. The role of on-slide tests: Molecular
classifications using surrogate on-slide tests
The precise molecular landscape continues to be in
evolution and while the most effective and reliable method for
characterising the various subtypes remains the subject of debate,
it is now desirable to devise a working molecular classification
for GC that can be adopted widely using the current diagnostic
histopathology framework. It should be noted that any GC
classification is just one of a number of factors that the
multidisciplinary teams/tumour boards consider when determining the
best treatment for a patient. The stage of the disease, overall
health of the patient and availability of different treatment
options are all key elements in the decision making process
(24).
Molecular stratification of GC can provide insights
about the underlying biology, which may have important implications
for treatment decisions. However, it is not currently possible to
deliver worldwide molecular classification using the tools employed
by TCGA or ACRG. Such tools are costly, not widely available, and
there is a lack of capacity for the rapid turnaround time required
for critically-ill patients. A fall-back position is to devise a
series of on-slide tests that histopathologists can implement more
widely and can be performed on formalin-fixed paraffin-embedded
(FFPE) tissue.
Setia et al (25) provided an excellent example of such
an approach. In 2016, they published a GC classification using only
on-slide tests [immunohistochemical (IHC) and in situ
hybridization (ISH)]. Initially, they evaluated 15 biomarkers using
FFPE tissue from a cohort of 146 cases of GC and ultimately
condensed GC into five clusters using only four on-slide tests. The
authors adopted a hierarchical approach, similar to that of the
molecular classification of endometrial carcinoma now ratified by
the WHO (26).
Cluster 1. GC-EBV(+) (5%). This group is
associated with marked lymphoid infiltrate, high PD-L1 expression
and has an improved survival rate.
Cluster 2. GC-dMMR (16%). This is
characterised by loss of MLH1 and PMS2 in 96% of cases, has lower
rate of nodal metastasis and improved survival rate.
Cluster 3. GC-E-cadherin(-) (21%). These
tumours have aberrant E-cadherin expression and contain
predominately tumour of Laurén's diffuse type. This group is
associated with low rate of p53 mutation compared with the other
clusters. It is further divided into Cluster 3A (40%) showing
complete loss of E-cadherin and Cluster 3B (60%) showing granular
cytoplasmic staining of E-cadherin. Cluster 3B contains more
elderly patients than 3A.
Cluster 4. GC-p53m (51%). This has aberrant
p53 expression, contains predominantly tumours of Laurén's
intestinal type, is associated with high Her2 expression, has more
often carcinoma within lymphatics and lymph node positivity. Based
on IHC expression of MUC and CD10, Cluster 4 is subdivided into
four subgroups.
Cluster 5. GC-p53wt (7%). This has normal p53
expression, includes tumours that lack EBV or dMMR and has no
defect of E-cadherin expression. All these tumours are of the
Laurén's intestinal type.
More recently, others have used the same portfolio
of on-slide tests and the same subclassification. For example,
Ramos et al (27)
demonstrated that such an approach is viable in a prospective
study. Importantly, they highlighted the potential difficulty in
classifying tumours when expression of these four markers is
heterogeneous. They raised the issue of sampling bias, thus
recognising the importance of correctly interpreting mixed
profiles. Ahn et al (28)
tested a retrospective cohort of GC patients using tissue
microarrays (TMAs) and showed similar correlation with prognosis.
Zhao et al (29) used
retrospective tissue in TMAs stained by IHC for mismatch repair
proteins (PMS2, MLH1, MSH2 and MSH6), E-cadherin and p21 to
classify CG into four subtypes, which associate with different
prognoses.
7. Additional companion diagnostic
biomarkers useful in GC
The adoption of a working classification would be
greatly helped by providing clear links to specific treatments and
recommendations on when additional tests should be performed, with
a particular emphasis on biomarkers relevant for treatment
selection. Some of these biomarkers are already in clinical routine
use and others not yet in mainstream use for GC.
Her2. The frequency of HER2 mutation in GC
has been reported to be as high as 7.7% in a previous study
(30); however, a lower average of
~4.5% is more often reported (31-33).
HER2 gene amplification represents a major proportion of these
mutations, probably ~50%, and results in Her2 protein
overexpression. In these patients, humanised anti-Her2 monoclonal
antibodies, including trastuzumab, can be used successfully either
to block Her-2 function, or to approach a toxic payload, including
trastuzumab deruxtecan (1,34,35).
Other HER2 mutations are putative resistance mechanisms to
trastuzumab in Her2-positive GC. Some HER2 mutations within the
active tyrosine kinase (TK)-domain, such as Ex20 insertions, are
targetable by specific TK inhibitors (TKIs) (36). While a small amount of data is
available on the exact incidence of HER2 activating mutations in GC
(37), they are likely to represent
at least 25% of total HER2 mutations (38).
EGFR. This protein is overexpressed in 27-64%
of GC and can be targeted by humanised anti-EGFR monoclonal
antibodies, including cetuximab or nimotuzumab (39,40).
Overexpression is not always associated with gene amplification.
Sensitising and resistance mutations in EGFR are known to exist in
other tumour sites however no data is available on their incidence
in GC.
PD-L1. PD-L1 selects for eligibility to
immune checkpoint inhibitors (anti-PD-1, anti-PD-L1 or anti-CTLA-4)
such as pembrolizumab, durvalumab, nivolumab or ipilimumab
(41-43).
PIK3CA. Mutations in the catalytic domain of
PIK3CA are the third most frequent mutations in GC. These mutations
are associated with more aggressive behaviour and are present in
9-12% of non-hypermutated and 32% of hypermutated tumours (44). PIK3CA mutations are associated with
GC-EBV(+) and GC-dMMR clusters. The most common mutation is H1047R
in Ex20, which has a predilection for the GC-dMMR cluster (45). Tumour harbouring PIK3KA activating
mutation can be targeted by TKIs.
KRAS. In TCGA study, KRAS mutations occur in
23% of all cases, although other studies report different
penetrance, from 4 to 23% (21,46).
Regardless of their incidence, there are strong correlations
between KRAS mutations and the GC-dMMR cluster. At present, the
only targetable KRAS mutation is G12C; this has a low prevalence,
between 0.33% or 2/595 patients and 0.6% or 9/1401 patients
(47-49).
ALK. The incidence of ALK rearrangement in GC
is low and possibly <1% (50,51)
and may be associated with dMMR. Carcinomas with ALK translocation
respond well to TKIs.
MET. Amplification of MET is probably
frequent in GC (52,53). Its prevalence differs in various
studies, from 50% in vitro (52,54) to
20% in vivo (54-57).
However, in a small cohort of 38 locally advanced GC, polysomy of
Ch 7 rather than gene amplification was the reason for the
increased number of MET genes per cell (58).
Claudin18.2. Part of a large family of
transmembrane proteins involved in tight junctions, claudin18.2
arises from differential splicing of mRNA and is overexpressed is
some GC cases, particularly those of diffuse Laurén type (59,60).
It is targeted by antibody-drug conjugates, bispecific antibodies
(zolbetuximab) and cell therapies such as chimeric antigen receptor
T-cells (61). The mechanism of
action of zolbetuximab is either via antibody-dependent cellular
cytotoxicity or complement-dependent cytotoxicity. The
identification of GC with overexpression of claudin18.2 is likely
to become soon mandatory (62).
8. An inclusive working classification
Recent advances in personalised treatment have
provided renewed pressure to abandon traditional taxonomic
classifications in favour of molecular classifications. GC has
unfulfilled needs with a large proportion of patients potentially
eligible for treatments that could improve outcomes significantly.
It is important to define parameters for a classification of GC
that enables improved access to such treatments and harmonises
diagnostic categories and nomenclature. The longevity of the Laurén
classification is testament to the strength inherent to the
taxonomic approach and should be retained. The majority of the
classifications using on-slide tests only inform some of the
oncological treatments; there is a need for additional tests to
select the appropriate therapy. A working classification should
therefore incorporate all the currently required biomarkers and
make provision for any future companion diagnostics.
In the present study, minor modifications of the
nomenclature were proposed in order to reflect in an improved way
current knowledge underlying these subgroups; some annotation
regarding the potential therapeutic approaches was provided and
possible additional companion diagnostic tests that should be
considered were indicated. This proposal is summarised in Fig. 1. Through acquired knowledge from
other tumour sites (e.g. endometrium), it is considered that for
all cases with controversial or uninterpretable test results an
indeterminate category should be added (63). The present study's classification
used EBV-ISH, MMR status by IHC, E-cadherin and beta-catenin IHC
and p53 IHC, which are all tests that can be delivered by most
histopathology laboratories.
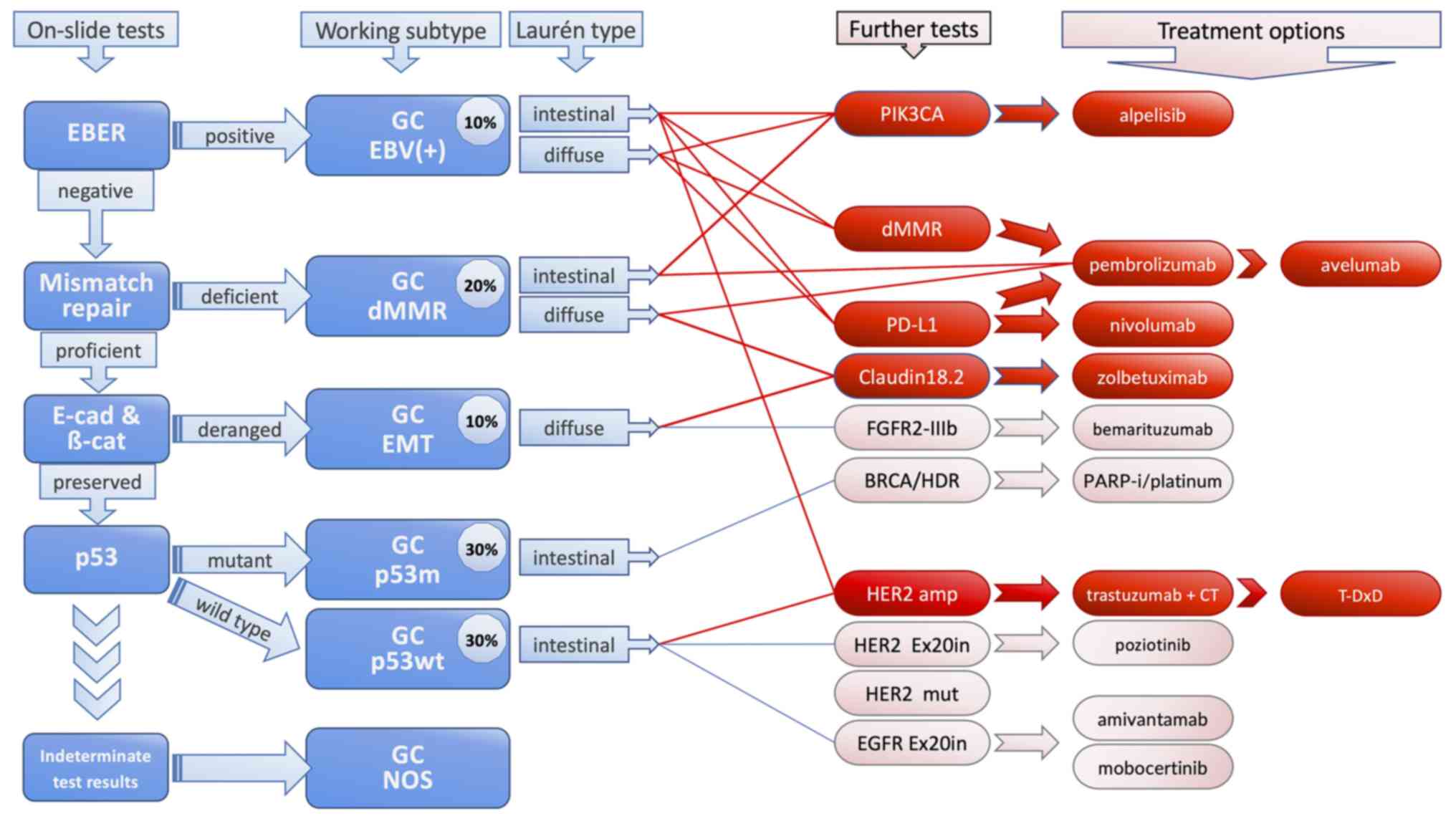 | Figure 1Proposed working classification. On
the left side, the five on-slide tests required for classification
are presented. The working classification comprises of 6 subtypes.
The gastric cancer ‘not otherwise specified’ subtype is reserved
for all cases with indeterminate test results. Further companion
diagnostics may be selected after the Laurén's types have been
considered. The therapeutic options which are more widely available
at present are in red, while the others (in pink with blue lines)
are mostly in pipelines. EBER, Epstein-Barr encoding region; E-cad,
E-cadherin; β-cat, beta-catenin; EBV, Epstein-Barr virus; dMMR,
mismatch repair deficiency; EMT, epithelial-mesenchymal transition;
p53m, mutant p53; p53wt, wild-type p53; NOS, not otherwise
specified; T-DxD, trastuzumab deruxtecan. |
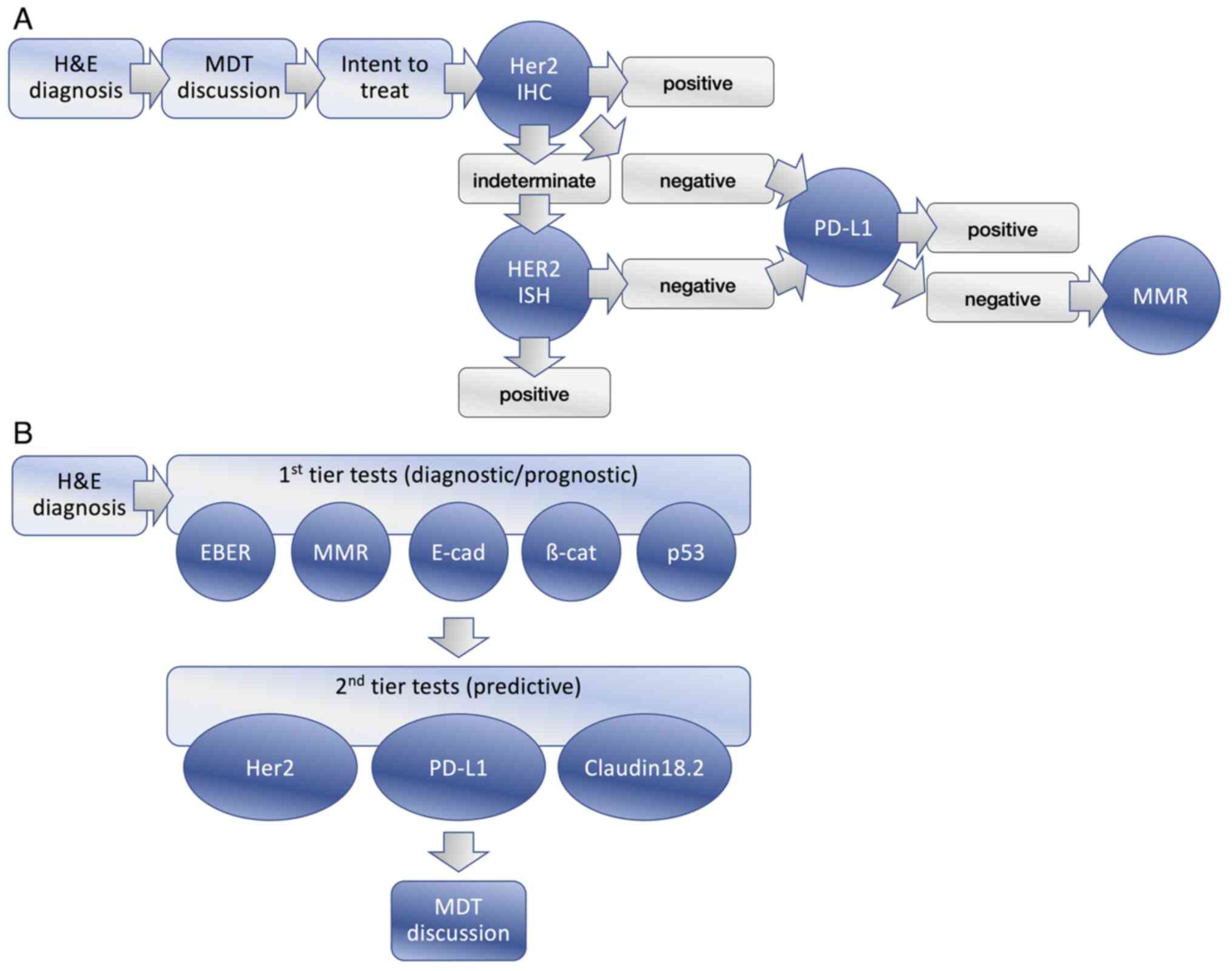
An important consideration for large-scale
implementation is cost and impact on laboratory capacity. While a
step-wise approach would have reduced the number of tests required,
it would have a negative impact on TAT and would have increased the
indirect costs of the laboratory to the extent that these would
have exceeded any savings in reagents. It was demonstrated in other
tumour types that performance of all the necessary tests up fronted
results in considerable savings and allowed clinicians to have all
the data within a few days (64). A
first tier of on-slide biomarkers would be likely to provide
prognostic data and instruct a second tier of predictive biomarkers
for therapy (Fig. 2). As part of
the proposed evolution of this classification, the feasibility of
its implementation on a cohort of GC using FFPE have been
assessed.
9. Discussion
There are a number of GC classifications based on
different tests. Some of the diagnostic categories have
considerable overlap, however this is not always clear, since they
use different terminology. The authors consider that a first step
towards widespread implementation of a working classification is
the characterization of a terminology that associates the subgroups
with the test results. The ‘cascade’ approach that has been used
successfully in the WHO classification of endometrial carcinoma was
adopted (26).
The modified working classification proposed here
has a major focus on treatment options to help histopathologists as
well as oncologists. Similar to the classification of endometrial
carcinoma, there is aspiration that working molecular
classification will become established in the diagnostic routine.
The limited capacity for further companion diagnostic tests is well
known; the working classification recognises associations between
diagnostic groups and specific targeted treatments and therefore is
focused on further tests regarding subgroups that are more likely
to benefit. In this classification, it is relatively easy for
further tests to be added as new targeted treatments become
available. In Fig. 1, thin blue
lines were used for treatment options that may emerge from clinical
trials to exemplify how new tests can be added to the model.
Recent studies using artificial intelligence
revealed that systems can be built to recognise molecular changes
from H&E sections (65,66). Therefore, it is plausible that
pathologists may eventually be able to recognise various molecular
subtypes of GC from unique morphological features without the
adjunctive help of the molecular data. This would certainly be
useful for all those clinicians who do not have access to molecular
tools. As demonstrated in other tumour sites, subclassification by
molecular changes using surrogate on-slide markers, is a step
towards this (63).
The proposed classification of the present study is
a pragmatic approach to aid current GC patients. A major limitation
is the relatively small number of available on-slide tests when
compared with the multi-omics approach. However, whilst other
classifications that use multi-omics may be more accurate in
identifying relevant subgroups, their widespread implementation is
currently not possible.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SC searched the literature for similar work and
articles. SC, MS, SW and CD contributed to writing the manuscript.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnston FM and Beckman M: Updates on
management of gastric cancer. Curr Oncol Rep. 21(67)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA A Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention. Prz
Gastroenterol. 14:26–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wagner AD, Syn NL, Moehler M, Grothe W,
Yong WP, Tai BC, Ho J and Unverzagt S: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev.
8(CD004064)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Park SR, Chun JH, Kim YW, Lee JH, Choi IJ,
Kim CG, Lee JS, Bae JM and Kim HK: Phase II study of low-dose
Docetaxel/Fluorouracil/Cisplatin in metastatic gastric carcinoma.
Am J Clin Oncol. 28:433–438. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sun DS, Jeon EK, Won HS, Park JC, Shim BY,
Park SY, Hong YS, Kim HK and Ko YH: Outcomes in elderly patients
treated with a single-agent or combination regimen as first-line
chemotherapy for recurrent or metastatic gastric cancer. Gastric
Cancer. 18:644–652. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Narahara H, Iishi H, Imamura H, Tsuburaya
A, Chin K, Imamoto H, Esaki T, Furukawa H, Hamada C and Sakata Y:
Randomized phase III study comparing the efficacy and safety of
irinotecan plus S-1 with S-1 alone as first-line treatment for
advanced gastric cancer (study GC0301/TOP-002). Gastric Cancer.
14:72–80. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van De Velde CJH, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Laurén P: The two histological main types
of gastric carcinoma: Diffuse and So-called Intestinal-type
carcinoma: An attempt at a Histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Carneiro F, Seixas M and Sobrinho-Simões
M: New elements for an updated classification of the carcinomas of
the stomach. Pathol Res Pract. 191:571–584. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lei ZN, Teng QX, Tian Q, Chen W, Xie Y, Wu
K, Zeng Q, Zeng L, Pan Y, Chen ZS, et al: Signaling pathways and
therapeutic interventions in gastric cancer. Signal Transduct
Target Ther. 7(358)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Monster JL, Kemp LJS, Gloerich M and Van
Der Post RS: Diffuse gastric cancer: Emerging mechanisms of tumor
initiation and progression. Biochim Biophys Acta Rev Cancer.
1877(188719)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Organisation mondiale de la santé and
Centre international de Recherche Sur le cancer: Digestive system
tumours. 5th edition. International agency for research on cancer,
Lyon, 2019.
|
|
14
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netws. 20:167–192. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Davidson B and Tropé CG: Ovarian cancer:
Diagnostic, biological and prognostic aspects. Womens Health
(Lond). 10:519–533. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Roepman P, Schlicker A, Tabernero J,
Majewski I, Tian S, Moreno V, Snel MH, Chresta CM, Rosenberg R,
Nitsche U, et al: Colorectal cancer intrinsic subtypes predict
chemotherapy benefit, deficient mismatch repair and
epithelial-to-mesenchymal transition. Int J Cancer. 134:552–562.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J
and Shi B: Breast cancer intrinsic subtype classification, clinical
use and future trends. Am J Cancer Res. 5:2929–2943.
2015.PubMed/NCBI
|
|
18
|
Talhouk A and McAlpine JN: New
classification of endometrial cancers: The development and
potential applications of genomic-based classification in research
and clinical care. Gynecol Oncol Res Pract. 3(14)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
West L, Vidwans SJ, Campbell NP, Shrager
J, Simon GR, Bueno R, Dennis PA, Otterson GA and Salgia R: A novel
classification of lung cancer into molecular subtypes. PLoS One.
7(e31906)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
The Cancer Genome Atlas Research Network.
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Wong SS, Kim KM, Ting JC, Yu K, Fu J, Liu
S, Cristescu R, Nebozhyn M, Gong L, Yue YG, et al: Genomic
landscape and genetic heterogeneity in gastric adenocarcinoma
revealed by whole-genome sequencing. Nat Commun.
5(5477)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chia NY and Tan P: Molecular
classification of gastric cancer. Ann Oncol. 27:763–769.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alsina M, Arrazubi V, Diez M and Tabernero
J: Current developments in gastric cancer: From molecular profiling
to treatment strategy. Nat Rev Gastroenterol Hepatol. 20:155–170.
2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Setia N, Agoston AT, Han HS, Mullen JT,
Duda DG, Clark JW, Deshpande V, Mino-Kenudson M, Srivastava A,
Lennerz JK, et al: A protein and mRNA expression-based
classification of gastric cancer. Modern Pathol. 29:772–784.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Organisation mondiale de la santé and
Centre international de Recherche Sur le cancer: Female genital
tumours. 5th edition. International agency for research on cancer,
Lyon, 2020.
|
|
27
|
Ramos MFKP, Pereira MA, Amorim LC, Mello
ES, Faraj SF, Ribeiro U, Hoff PMG, Cecconello I and Castria TB:
Gastric cancer molecular classification and adjuvant therapy: Is
there a different benefit according to the subtype? J Surg Oncol.
121:804–813. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ahn S, Lee SJ, Kim Y, Kim A, Shin N, Choi
KU, Lee CH, Huh GY, Kim KM, Setia N, et al: High-throughput Protein
and mRNA Expression-based classification of gastric cancers can
identify clinically distinct subtypes, concordant with recent
molecular classifications. Am J Surg Pathol. 41:106–115.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao C, Feng Z, He H, Zang D, Du H, Huang
H, Du Y, He J, Zhou Y and Nie Y: Protein expression-based
classification of gastric cancer by immunohistochemistry of tissue
microarray. PLoS One. 15(e0238836)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Park S, Ahn S, Kim DG, Kim H, Kang SY and
Kim KM: High frequency of juxtamembrane domain ERBB2 mutation in
gastric cancer. Cancer Genomics Proteomics. 19:105–112.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Robichaux JP, Elamin YY, Vijayan RSK,
Nilsson MB, Hu L, He J, Zhang F, Pisegna M, Poteete A, Sun H, et
al: Pan-cancer landscape and analysis of ERBB2 mutations identifies
poziotinib as a clinically active inhibitor and enhancer of T-DM1
activity. Cancer Cell. 36:444–457.e7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Karczewski KJ, Francioli LC, Tiao G,
Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A,
MacArthur DG, et al: The mutational constraint spectrum quantified
from variation in 141,456 humans. Nature. 581:434–443.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discovery. 2:401–404.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697.
2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wilding B, Scharn D, Böse D, Baum A,
Santoro V, Chetta P, Schnitzer R, Botesteanu DA, Reiser C, Kornigg
S, et al: Discovery of potent and selective HER2 inhibitors with
efficacy against HER2 exon 20 insertion-driven tumors, which
preserve wild-type EGFR signaling. Nat Cancer. 3:821–836.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu Y, Zhu X, Wei X, Tang C and Zhang W:
HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev
Cancer. 1876(188549)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kahraman S and Yalcin S: Recent advances
in systemic treatments for HER-2 positive advanced gastric cancer.
Onco Targets Ther. 14:4149–4162. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Adua D, Di Fabio F, Rojas Llimpe FL, Pini
S and Pinto C: Long-term survival in an advanced gastric cancer
patient treated with cetuximab in association with FOLFIRI: A case
report. J Gastrointest Oncol. 5:E13–E17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Satoh T, Lee KH, Rha SY, Sasaki Y, Park
SH, Komatsu Y, Yasui H, Kim TY, Yamaguchi K, Fuse N, et al:
Randomized phase II trial of nimotuzumab plus irinotecan versus
irinotecan alone as second-line therapy for patients with advanced
gastric cancer. Gastric Cancer. 18:824–832. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Muro K, Chung HC, Shankaran V, Geva R,
Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al:
Pembrolizumab for patients with PD-L1-positive advanced gastric
cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial.
Lancet Oncol. 17:717–726. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Janjigian YY, Bendell J, Calvo E, Kim JW,
Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, et al:
CheckMate-032 study: Efficacy and safety of nivolumab and nivolumab
plus ipilimumab in patients with metastatic esophagogastric cancer.
J Clin Oncol. 36:2836–2844. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kim JW, Lee HS, Nam KH, Ahn S, Kim JW, Ahn
SH, Park DJ, Kim HH and Lee KW: PIK3CA mutations are associated
with increased tumor aggressiveness and Akt activation in gastric
cancer. Oncotarget. 8:90948–90958. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Te Paske IBAW, Garcia-Pelaez J, Sommer AK,
Matalonga L, Starzynska T and Jakubowska A: Solve-RD-GENTURIS
group. van der Post RS, Lubinski J, Oliveira C, et al: A mosaic
PIK3CA variant in a young adult with diffuse gastric cancer: Case
report. Eur J Hum Genet. 29:1354–1358. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Polom K, Das K, Marrelli D, Roviello G,
Pascale V, Voglino C, Rho H, Tan P and Roviello F: KRAS mutation in
gastric cancer and prognostication associated with microsatellite
instability status. Pathol Oncol Res. 25:333–340. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Salem M, El-Refai S, Sha W, Grothey A,
Puccini A, George T, Hwang J, Kadakia K, Musselwhite L, Van Cutsem
E, et al: O-3 Characterization of KRAS mutation variants and
prevalence of KRAS-G12C in gastrointestinal malignancies. Ann
Oncol. 32(S218)2021.
|
|
48
|
Osterlund E, Ristimäki A, Kytölä S, Kuopio
T, Heervä E, Muhonen T, Halonen P, Kallio R, Soveri LM, Sundström
J, et al: KRAS-G12C mutation in one Real-life and three
population-based Nordic cohorts of metastatic colorectal cancer.
Front Oncol. 12(826073)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chida K, Kotani D, Masuishi T, Kawakami T,
Kawamoto Y, Kato K, Fushiki K, Sawada K, Kumanishi R, Shirasu H, et
al: The prognostic impact of KRAS G12C mutation in patients with
metastatic colorectal cancer: A multicenter retrospective
observational study. Oncologist. 26:845–853. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ambrosini M, Del Re M, Manca P, Hendifar
A, Drilon A, Harada G, Ree AH, Klempner S, Mælandsmo GM, Flatmark
K, et al: ALK inhibitors in patients with ALK Fusion-positive GI
cancers: An international data set and a molecular case series. JCO
Precis Oncol. 6(e2200015)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chon HJ, Kim HR, Shin E, Kim C, Heo SJ,
Lee C, Park JK, Noh SH, Chung HC and Rha SY: The Clinicopathologic
features and prognostic impact of ALK positivity in patients with
resected gastric cancer. Ann Surg Oncol. 22:3938–3945.
2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Smolen GA, Sordella R, Muir B, Mohapatra
G, Barmettler A, Archibald H, Kim WJ, Okimoto RA, Bell DW, Sgroi
DC, et al: Amplification of MET may identify a subset of cancers
with extreme sensitivity to the selective tyrosine kinase inhibitor
PHA-665752. Proc Natl Acad Sci USA. 103:2316–2321. 2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bachleitner-Hofmann T, Sun MY, Chen CT,
Tang L, Song L, Zeng Z, Shah M, Christensen JG, Rosen N, Solit DB,
et al: HER kinase activation confers resistance to MET tyrosine
kinase inhibition in MET oncogene-addicted gastric cancer cells.
Mol Cancer Ther. 7:3499–3508. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kuniyasu H, Yasui W, Kitadai Y, Yokozaki
H, Ito H and Tahara E: Frequent amplification of the c-met gene in
scirrhous type stomach cancer. Biochem Biophys Res Commun.
189:227–232. 1992.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Seruca R, Suijkerbuijk RF, Gärtner F,
Criado B, Veiga I, Olde-Weghuis D, David L, Castedo S and
Sobrinho-Simões M: Increasing levels of MYC and MET
co-amplification during tumor progression of a case of gastric
cancer. Cancer Genet Cytogenet. 82:140–145. 1995.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tsujimoto H, Sugihara H, Hagiwara A and
Hattori T: Amplification of growth factor receptor genes and DNA
ploidy pattern in the progression of gastric cancer. Virchows
Archiv. 431:383–389. 1997.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Sakakura C, Mori T, Sakabe T, Ariyama Y,
Shinomiya T, Date K, Hagiwara A, Yamaguchi T, Takahashi T, Nakamura
Y, et al: Gains, losses, and amplifications of genomic materials in
primary gastric cancers analyzed by comparative genomic
hybridization. Genes Chromosom Cancer. 24:299–305. 1999.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Janjigian YY, Tang LH, Coit DG, Kelsen DP,
Francone TD, Weiser MR, Jhanwar SC and Shah MA: MET expression and
amplification in patients with localized gastric cancer. Cancer
Epidemiol Biomarkers Prev. 20:1021–1027. 2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xu B, Chen F, Zhang X, Wang Z, Che K, Wu
N, Yu L, Fan X, Liu B and Wei J: Antigen-Specific T cell
immunotherapy targeting Claudin18.2 in gastric cancer. Cancers.
14(2758)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Grizzi G, Venetis K, Denaro N, Bonomi M,
Celotti A, Pagkali A, Hahne JC, Tomasello G, Petrelli F, Fusco N,
et al: Anti-Claudin treatments in gastroesophageal adenocarcinoma:
Mainstream and upcoming strategies. J Clin Med.
12(2973)2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Qi C, Gong J, Li J, Liu D, Qin Y, Ge S,
Zhang M, Peng Z, Zhou J, Cao Y, et al: Claudin18.2-specific CAR T
cells in gastrointestinal cancers: Phase 1 trial interim results.
Nat Med. 28:1189–1198. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sahin U, Türeci Ö, Manikhas G, Lordick F,
Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B,
et al: FAST: A randomised phase II study of zolbetuximab (IMAB362)
plus EOX versus EOX alone for first-line treatment of advanced
CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma.
Ann Oncol. 32:609–619. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Alexa M, Hasenburg A and Battista MJ: The
TCGA molecular classification of endometrial cancer and its
possible impact on adjuvant treatment decisions. Cancers.
13(1478)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wedden S, Miller K, Frayling IM, Thomas T,
Chefani A, Miller K, Hamblin A, Taylor JC and D'Arrigo C:
Colorectal cancer stratification in the routine clinical pathway: A
district general hospital experience. Appl Immunohistochem Mol
Morphol. 27:e54–e62. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Fremond S, Andani S, Barkey Wolf J,
Dijkstra J, Melsbach S, Jobsen JJ, Brinkhuis M, Roothaan S,
Jurgenliemk-Schulz I, Lutgens LCHW, et al: Interpretable deep
learning model to predict the molecular classification of
endometrial cancer from haematoxylin and Eosin-stained Whole-slide
images: A combined analysis of the PORTEC randomised trials and
clinical cohorts. Lancet Digital Health. 5:e71–e82. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Fell C, Mohammadi M, Morrison D,
Arandjelović O, Syed S, Konanahalli P, Bell S, Bryson G, Harrison
DJ and Harris-Birtill D: Detection of malignancy in whole slide
images of endometrial cancer biopsies using artificial
intelligence. PLoS One. 18(e0282577)2023.PubMed/NCBI View Article : Google Scholar
|