Introduction
Intracytoplasmic sperm injection (ICSI) was
introduced in the early 1990s to treat severe male infertility.
However, there is still insufficient evidence to suggest that ICSI
should be used in couples without male factor infertility (1). If conventional in vitro
fertilization (IVF) cycles (co-culture of oocytes and cumulus cells
for 18-20 h) resulted in total fertilization failure (TFF),
late-ICSI would be performed. In recent years, to avoid TFF or low
fertilization in conventional IVF, short co-incubation of gametes
(4-6 h) combined with early-rescue ICSI (Re-ICSI) has been widely
practiced in numerous IVF laboratories (1,2).
Re-ICSI was provided to those oocytes with unclear
release of the second polar body, 6 h after initial insemination,
since the second polar body was reportedly released in nearly 90%
of fertilized oocytes by 6 h (2).
It should be clearly noted that the removal of cumulus cells in
ICSI procedure was presented 3-4 h after oocyte retrieval, earlier
than that in Re-ICSI group (2).
Oocytes used for direct ICSI were treated with hyaluronidase for
dispersing the cumulus cells, whereas the cumulus granulosa cells
were removed directly after 4 h of co-incubation of gametes in
Re-ICSI group (1,2).
Previous studies have indicated that short
insemination has no detrimental effects on clinical outcomes in
human IVF and that Re-ICSI can attain acceptable pregnancy outcomes
(3). However, the Re-ICSI embryos
showed adverse pregnancy outcomes (significantly lower implantation
rate) compared with those of the directly ICSI embryos in the fresh
embryo transfer (ET), as the sperm in the Re-ICSI group were
microinjected into oocytes 4-6 h later than those in the ICSI group
(2).
A meta-analysis of late-ICSI performed along with
frozen embryo transfer (FET) may overcome the technical and
biological issues associated with the fresh transfer after
late-ICSI (1). However, whether
Re-ICSI embryos would be a benefit from the FET process remains
unknown.
The purpose of the present study was to
retrospectively investigate the clinical outcomes of embryos
derived from Re-ICSI and direct ICSI insemination in fresh ET and
FET cycles.
Materials and methods
Patients
The present retrospective cohort study was carried
out at the Center for Reproductive Medicine and Infertility, the
Fourth Hospital of Shijiazhuang, from January 2016 to November
2021. Patients with primary infertility that were included in the
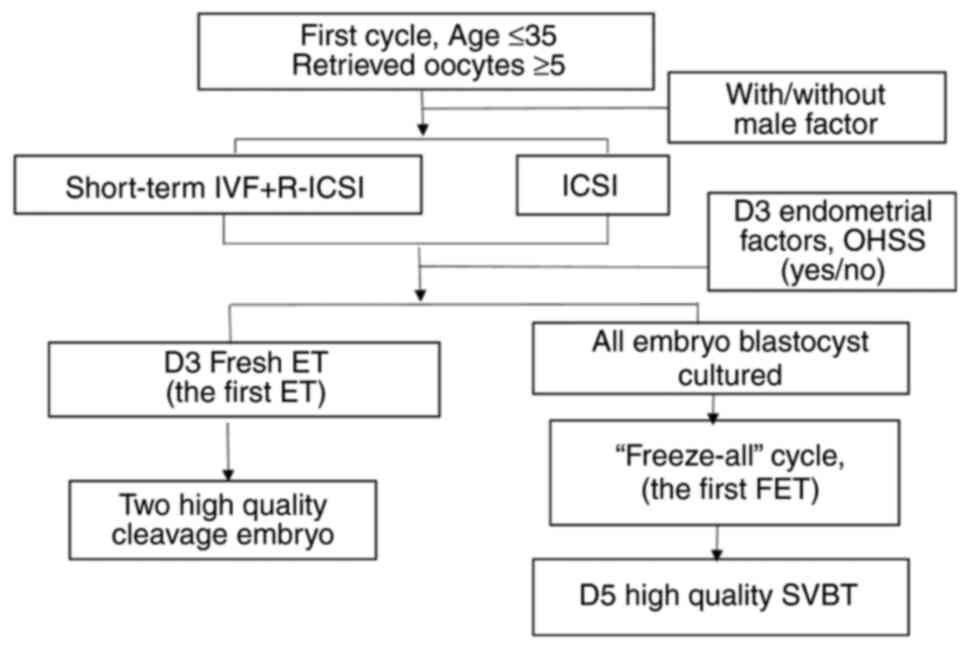
analysis were at the ages of ≤35 years, with retrieved oocytes ≥5
in their first short-term IVF + Re-ICSI cycle or directly ICSI
treatment because of severe oligospermia and underwent their first
fresh ET or FET.
The inclusion criteria were women undergoing their
first Re-ICSI/ICSI cycle with two high quality cleavage embryos in
D 3 fresh ET cycle, or single vitrified-warmed blastocyst transfer
(SVBT) with D 5 high quality blastocyst in their first FET cycle
(all embryos frozen) (Fig. 1).
The exclusion criteria included, donor eggs/sperm,
patients with oocyte maturation disorder, chromosomal
abnormalities, hyperprolactinemia, thyroid dysfunction, women with
congenital or secondary uterine abnormalities such as unicornuate
uterus, septate uterus or uterine didelphys, adenomyosis, uterine
submucosal fibroids, intrauterine adhesions and endometriosis, or
other endometrial diseases, or endometrial thickness #x003C;7 mm on
the D of embryo transfer.
Data were collected by searching electronic medical
records and entering the inclusion and exclusion criteria. A total
of 293 Re-ICSI cycles and 326 ICSI cycles were enrolled in the
present study, in which 313 were obtained from fresh ET cycles (151
Re-ICSI and 162 ICSI) and 306 from SVBT cycles (142 Re-ICSI and 164
ICSI).
The procedures that were conducted in the present
study adhered to the tenets of the Declaration of Helsinki. The
present study was approved (approval no. 20220049) by the Research
Ethics Committee of the Fourth Hospital of Shijiazhuang
(Shijiazhuang, China).
Stimulation, oocyte retrieval,
fertilization, embryo culture and scoring
The process of ovarian stimulation and oocyte
retrieval has been previously described by Jiang et al
(4). Sperm was performed using
standard IVF/ICSI insemination procedure by density gradient
centrifugation. Insemination was performed after 38~40 h of
trigger.
IVF Short co-incubation and
Re-ICSI
Each oocyte is incubated with ~20,000 sperm cells,
and the cumulus granulosa cells were removed after 4 h
co-incubation of gametes. In patients with a missing second polar
body in any of the retrieved oocytes or with a low fertilization
rate (#x003C;30%), the MII oocytes were rescued and underwent the
same ICSI method (Re-ICSI insemination) at ~6 h after fertilization
(4).
ICSI
Oocytes used for directly ICSI were treated with
bovine hyaluronidase (Sigma-Aldrich; Merck KGaA) for dispersing the
cumulus cells after 39~40 h of trigger. And the MII oocytes undergo
the ICSI insemination.
Fresh ET
‘High quality embryos’ should have 7-9 cells with no
more 20% fragments on D3, but may be a little uneven in appearance.
On D 3 two high quality embryos were selected for embryo transfer,
based on endometrial factors, the occurrence of ovarian
hyperstimulation syndrome or personal reasons.
Other embryos were transferred into G-2 culture
medium in group culture (Vitrolife). In the morning of D 5 or D 6,
blastocysts were scored by two experienced embryologists using the
system of Gardner and Schoolcraft (5). ‘High quality blastocysts’ were ≥3BB
blastocysts.
SVBT procedures
The procedure was always performed using one
blastocyst for each straw. An artificial shrinkage (AS), using a
laser pulse was performed before vitrification. The blastocyst was
then moved at room temperature (22-25˚C) to Kitazato, Corp. along
with equilibration solution (ES). After 6-8 min, the blastocyst was
quickly washed in vitrification solution (VS) for 45-60 sec and
transferred onto the straw (Kitazato) using a micropipette and
immersed vertically into liquid nitrogen (6).
A Thaw Kit (Kitazato) was used for warming. The
carrier containing the embryo was removed from the straw and placed
quickly into the dish containing the thawing medium (thawing
solution) preheated at 37˚C. The blastocysts immediately fell from
the device and could be easily identified in the medium. After 1
min, blastocysts were transferred to the DS medium (dilution
solution) for 3 min at room temperature 22-25˚C. In the last two
steps, blastocysts were placed for 5 min, in the WS1 medium and WS2
(washing solution). The embryo was then returned to G-2 medium for
culture until transfer. At this stage, an assessment was performed
on an inverted microscope to establish if the embryo survived based
on morphological integrity of the inner cell mass and
trophectoderm.
FET of SVBT
Hormone therapy cycles were used as the endometrial
preparation for the FET. After 1 or 2 h of culture the embryo was
reassessed and often the re-expansion of the blastocyst was
reported; this indicated that the embryo physiologically survived
the warming procedure. Embryo transfer was normally performed
within 2 or 3 h (6).
Clinical outcome
B ultrasound was used to observe the gestational sac
and fetal heart at 35 Ds after implantation was diagnosed as
clinical pregnancy. Implantation rate was defined as the ratio of
the number of gestational sacs and fetal heart observed under B
ultrasound and the number of transferred embryos (6). The clinical pregnancy rate,
implantation rate, ectopic pregnancy, abortion rate and live birth
rate were analyzed.
Data analysis
Statistical analyses were performed using SPSS 19.0
statistical software (SPSS Inc.). The results are presented as the
mean ± standard deviation (SD). The mean values of two groups were
compared using the independent samples unpaired t-test. The
Kolmogorov-Smirnov normality test was performed before t-test.
Percentages were compared using the χ2 test and
P#x003C;0.05 was considered to indicate a statistically significant
difference.
Results
The pregnancy outcomes of two quality
embryos between Re-ICSI and ICSI groups in fresh D 3 ET cycles
There were 151 Re-ICSI cycles and 162 ICSI cycles.
The average age and body mass index (BMI) of patients with primary
infertility in the Re-ICSI and ICSI groups were 29.0±3.2 vs.
29.1±3.1, and 23.9±4.2 vs. 23.7±4.2, respectively (P>0.05). The
rates of clinical pregnancy, implantation and live birth in Re-ICSI
group was lower than ICSI group in fresh ET cycles (P#x003C;0.05).
No significant differences were observed in the ectopic pregnancy
rate, abortion rate and sex ratio between the two groups (Table I).
 | Table IComparing outcomes of D3 embryo
between ICSI and Re-ICSI groups in fresh embryo transfer
cycles. |
Table I
Comparing outcomes of D3 embryo
between ICSI and Re-ICSI groups in fresh embryo transfer
cycles.
| | Re-ICSI | ICSI | χ2/t | P-value |
|---|
| ET 2 cleavage embryo
cycle (n) | 151 | 162 | | |
| Patient age,
years | 29.0±3.2 | 29.1±3.1 | 1.410 | 0.160 |
| Infertility duration,
years | 3.6±2.7 | 3.5±2.5 | 1.551 | 0.122 |
| Body mass index,
kg/m2 | 23.9±4.2 | 23.7±4.2 | 0.148 | 0.882 |
| Clinical pregnancy
rate, % (n) | 52.3 (79/151) | 64.2 (104/162) | 4.542 | 0.033 |
| Implantation rate, %
(n) | 34.4 (104/302) | 42.3 (137/324) | 4.065 | 0.044 |
| Ectopic pregnancy
rate, % (n) | 0 (0/90) | 1.9 (2/104) | 1.749 | 0.186 |
| Abortion rate, %
(n) | 10.0 (8/79) | 11.5 (12/104) | 0.092 | 0.762 |
| Sex ratio
(male/female) | 1.23 (49/40) | 1.09 (61/56) | 0.173 | 0.677 |
| Live birth rate, %
(n) | 58.9 (89/151) | 69.4 (117/162) | 6.128 | 0.013 |
The pregnancy outcomes of SVBT between
Re-ICSI and ICSI groups in D 5 FET cycles
In 306 FET cycles, Re-ICSI was 142 cycles and ICSI
was 164 cycles. The survival rate of D 5 high quality blastocyst
was 100% in SVBT cycles. The average age and BMI of patients in two
groups were 29.1±3.3 vs. 28.9±3.0, and 23.4±3.9 vs. 23.2±3.8,
respectively (P>0.05). There were no significant differences in
rates of clinical pregnancy, implantation, ectopic pregnancy,
abortion, sex ratio and live birth between the two groups in SVBT
cycle (Table II).
 | Table IIComparing outcomes of D5 SVBT between
ICSI and Re-ICSI groups in frozen embryo transfer cycles. |
Table II
Comparing outcomes of D5 SVBT between
ICSI and Re-ICSI groups in frozen embryo transfer cycles.
| | Re-ICSI | ICSI | χ2/t | P-value |
|---|
| SVBT cycle | 142 | 164 | | |
| Patient age,
years | 29.1±3.3 | 28.9±3.0 | 1.490 | 0.138 |
| infertility duration,
years | 3.5±2.7 | 3.4±2.4 | 1.141 | 0.255 |
| Body Mass Index
(kg/m2) | 23.4±3.9 | 23.2±3.8 | 1.774 | 0.078 |
| Clinical pregnancy
rate, % (n) | 68.3 (97/142) | 68.3 (112/164) | 0.000 | 1.000 |
| Implantation rate, %
(n) | 70.4 (100/142) | 70.1 (115/164) | 0.003 | 0.954 |
| ectopic pregnancy, %
(n) | 1.0 (1/97) | 0 (0/112) | 1.160 | 0.281 |
| Abortion rate, %
(n) | 17.5 (17/97) | 17.9 (20/112) | 0.004 | 0.950 |
| Sex ratio
(male/female) | 1.45 (48/33) | 1.19 (51/43) | 0.443 | 0.505 |
| Live birth rate, %
(n) | 57.0 (81/142) | 57.3 (94/164) | 0.002 | 0.961 |
Discussion
Previous pregnancy history, duration of infertility,
forward-moving sperm counts, and abnormal sperm, serve as a
precursor in predicting fertilization failure; thus, whether a
patient should conduct short-time insemination should be advised
based on the aforementioned indexes (7). During the procedures of short
co-incubation, patients with primary infertility, presented
#x003C;30% fertilization rate and were obliged to undergo Re-ICSI
treatment. Re-ICSI was carried out to reduce the occurrence of TFF
and near-total FF (NFF) (1). The
incidence of NFF was at 6.49% (864/13,317), whereas TFF was at
4.21% (561/13,317) (2).
The important question needed to be asked in the
present study is when exactly is the right time, for the RE-ICSI to
be performed to successfully achieve fertilization (2). For the second polar body performing
Re-ICSI after 6 h of co-incubation can salvage cases of IVF
fertilization failure. Ηigher fertilization rate after Re-ICSI
indicated that all oocytes without signs of fertilization after 6 h
of co-incubation should undergo Re-ICSI (8).
However, the effectiveness of Re-ICSI remains
controversial. In a previous study of long-term (20 h) and
short-term (4 h) insemination combined with Re-ICSI of sibling
oocytes, there were 11 cycles in which Re-ICSI was performed
because of TFF occurring in the oocytes with the short-term
insemination. In six of these cycles, fertilization occurred in
patients of the 20 h insemination group (9).
Early cumulus cell removal alone (4 h) had similar
pregnancy outcomes compared with 20 h after insemination (10). However, the pregnancy outcomes of
Re-ICSI embryos in fresh cycles remained controversial. Zeng et
al (2) showed that Re-ICSI
embryos had lower implantation rates than ICSI embryos in fresh ET.
Jiang et al (11) showed
that the rates of implantation, clinical pregnancy, and live births
were similar between the Re-ICSI and ICSI groups; however, it was
not emphasized if all the transferred embryos came from Re-ICSI,
and that only seven cycles were included. Pregnancy outcomes of
Re-ICSI and ICSI embryos during FET cycles are less reported.
In the present study, primary infertility patients
were selected with ≤35 years old and ≥5 oocytes in their first
Re-ICSI/ICSI cycle to minimize the influence of age and oocytes
factors. In the Re-ICSI cycles, especially part of oocytes IVF and
part of oocytes Re-ICSI cycles, the embryos came from short-term
insemination embryos and Re-ICSI embryos together (2). In the present study, the embryos
transferred all from Re-ICSI fertilization.
A total of two D3 high quality embryos were selected
in the first fresh cleavage-stage ET cycles, or SVBT (all embryos
frozen) with D5 high quality blastocyst in their first FET cycle to
reduce the effects of embryo quality and ET frequency. Another
reason to choose high quality D5 blastocysts is that they have a
100% survival rate during the SVBT cycle.
In the present study, it was revealed that the
clinical pregnancy rate, implantation rate and live birth rate of
Re-ICSI embryos were lower than the ICSI embryos in fresh ET
cycles. Whereas there was no significant difference between Re-ICSI
and ICSI embryos in the FET cycles.
There were two differences between the Re-ICSI and
ICSI groups, the cumulus cell removal method and fertilization
time. In the Re-ICSI group, sperms were microinjected into oocytes
4-6 h later than in the ICSI group. The differences in Re-ICSI/ICSI
pregnancy outcomes may be related to oocyte aging and subsequent
embryonic development (2).
The results of the present study indicated that
Re-ICSI/ICSI embryos present similar pregnancy outcomes during the
FET cycle, which proves that Re-ICSI/ICSI embryos have similar
developmental abilities. The pregnancy outcome of Re-ICSI embryos
in the fresh ET cycle was lower, possibly because the fertilization
time was 4-6 h later than that of the ICSI embryos, resulting in an
asynchronous endometrium.
Late-ICSI (18-20 h) leads to poor clinical pregnancy
outcome resulting from oocyte aging (12) and asynchronization between
endometrial growth and embryo development (13). However, frozen embryo transfer
appeared to improve with pregnancy rates and implantation rate in
late-ICSI patients (1). The
strategy of cryopreservation could overcome the loss of
synchronization between endometrial growth and late-ICSI embryo
development (1). In Re-ICSI cycles,
unfertilized oocytes are fertilized by ICSI only 6 h later instead
of nearly 1-day-old in late-ICSI. Therefore, the correlation of
oocyte aging and asynchronization between endometrial growth and
embryo development was minor compared with late-ICSI. Nevertheless,
the results of the present study about embryos from Re-ICSI were
similar with late-ICSI.
Fresh ET cycles can negatively affect clinical
outcomes after Re-ICSI. In the Re-ICSI cycles, if a patient had
both IVF and Re-ICSI embryos, IVF embryos should be preferred
transfer in fresh ET, whereas Re-ICSI embryos should be preferred
cryopreservation with the similar embryo score.
The limitations to the present study were that, the
analysis was centered around the D3 embryo in fresh ET and D5 SVBT
in FET cycles. There were also some patients who transferred
blastocyst in fresh cycle and cleavage embryo in FET cycle.
Analysis on these cases was not implemented because the number of
these patients was relatively small. Another limitation is that
frozen and fresh Re-ICSI were not compared in the present study, as
differences between embryo development Ds (D3 vs. D5) and number
(two cleavage embryos and one blastocyst) lacked comparability
between the groups.
ICSI was used as golden standard procedure. Re-ICSI
had lower pregnancy outcomes than ICSI in fresh ET cycles whereas
Re-ICSI and ICSI had similar outcomes in FET cycles. The results
clearly showed that the transfer of FET Re-ICSI embryos may
overcome the impaired outcomes in fresh ET. Cryopreservation can
overcome the technical and biological issues associated with the
loss of synchronization between endometrial growth and embryonic
development resulting from Re-ICSI. The favorable FET pregnancy
outcomes may explain the discrepancy between the results derived
from fresh ET and FET cycles.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Hebei Medical
Science Research Key Project (grant no. 20231650).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ, XHW and JCY made substantial contributions to
conception and design and were involved in drafting the manuscript
or revising it critically for important intellectual content.. GS,
XHZ and SBM made substantial contributions to acquisition of data,
analysis and interpretation of data. All authors reviewed the
manuscript and given final approval of the version to be published.
All authors have participated sufficiently in the work to take
public responsibility for appropriate portions of the content. YJ
and XHW confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
20220049) by The Fourth Hospital of Shijiazhuang Ethics Committee
(Shijiazhuang, China). The procedures used adhered to the tenets of
the Declaration of Helsinki. All experiments were performed in
accordance with relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Paffoni A, Reschini M, Pisaturo V,
Guarneri C, Palini S and Viganò P: Should rescue ICSI be
re-evaluated considering the deferred transfer of cryopreserved
embryos in in-vitro fertilization cycles? A systematic review and
meta-analysis. Reprod Biol Endocrinol. 19(121)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zeng J, Yao Z, Zhang Y, Tian F, Liao T, Wu
L and Li Y: Fertilization and neonatal outcomes after early rescue
intracytoplasmic sperm injection: A retrospective analysis of
16,769 patients. Arch Gynecol Obstet. 306:249–258. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
He Y, Liu H, Zheng H, Li L, Fu X and Liu
J: Effect of early cumulus cells removal and early rescue ICSI on
pregnancy outcomes in high-risk patients of fertilization failure.
Gynecol Endocrinol. 34:689–693. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jiang Y, Cao Q, Zhao X, Li L, Li S and Gao
F: Percutaneous epididymal sperm aspiration and short time
insemination in the treatment of men with obstructive azoospermia.
J Assist Reprod Genet. 30:1175–1179. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gardner DK and Schoolcraft WB: Culture and
transfer of human blastocysts. Curr Opin Obstet Gynecol.
11:307–311. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jiang Y, Song G, Zhang XH, Miao SB and Wu
XH: Frozen blastocysts: Assessing the importance of Day 5/Day 6
blastocysts or blastocyst quality. Exp Ther Med.
23(333)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guo H, Yang J, Zhang C, Li H, Yin B, Gu B,
Xie J and He Q: Analysis of clinical data of patients with
different outcomes after short-time insemination. Andrologia. 44
(Suppl 1):S667–S671. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shiraiwa Y, Enatsu N, Yamagami K,
Furuhashi K, Iwasaki T, Otsuki J and Shiotani M: Clinical outcomes
of rescue intracytoplasmic sperm injection at different timings
following in vitro fertilization. J Reprod Infertil. 22:251–257.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu J, Zhang X, Yang Y, Zhao J, Hao D,
Zhang J, Liu Y, Wu W and Wang X: Long-time vs. short-time
insemination of sibling eggs. Exp Ther Med. 12:3756–3760.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kong P, Yin M, Tang C, Zhu X, Bukulmez O,
Chen M and Teng X: Effects of early cumulus cell removal on
treatment outcomes in patients undergoing in vitro fertilization: A
retrospective cohort study. Front Endocrinol (Lausanne).
12(669507)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiang L, Qian Y, Chen X, Ji X, Ou S, Li R,
Yang D and Li Y: Effect of early rescue ICSI and split IVF-ICSI in
preventing low fertilization rate during the first ART cycle: A
real-world retrospective cohort study. Reprod Med Biol.
21(e12420)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lombardi E, Tiverón M, Inza R, Valcárcel
A, Young E and Bisioli C: Live birth and normal 1-year follow-up of
a baby born after transfer of cryopreserved embryos from rescue
intracytoplasmic sperm injection of 1-Day-old oocytes. Fertil
Steril. 80:646–648. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sermondade N, Hugues JN, Cedrin-Durnerin
I, Poncelet C, Benzacken B, Lévy R and Sifer C: Should all embryos
from Day 1 rescue intracytoplasmic sperm injection be transferred
during frozen-thawed cycles? Fertil Steril. 94:1157–1158.
2010.PubMed/NCBI View Article : Google Scholar
|