Introduction
Breast cancer (BC) is considered the most common
type of invasive cancer in women worldwide, with the prevalence of
cases increasing in recent decades (1). The risk of BC has been worsened by
multifactorial lifestyle and environmental factors, which can lead
to the initiation of cancer progression (2). Treatments, such as surgery,
radiotherapy and hormone therapy, have been applied in patients
with BC; however, the side effects cannot be ignored (3,4).
Screening of therapeutic agents without extensive side effects that
are effective for patients with BC has been performed, and the use
of natural products, as single agents or adjuvants, has been
suggested for cancer treatment (5,6). In a
recent study, natural medicines used as adjuvants with
chemotherapeutic drugs have been shown to have clinical use by
sensitizing cancer cells to be more responsive to the drug and to
reverse chemoresistance (7). The
small number of side effects, low toxicity and variety of bioactive
compounds are considered advantages of using a natural product for
BC treatment.
Polysaccharides, including polysaccharide-protein
complexes, are bioactive compounds, which have been highlighted for
their potential therapeutic application. Several studies have
reported on their antiproliferative, apoptosis-inducing,
antioxidative and immune-enhancing effects (8-10).
Another study reported that protein-bound polysaccharides can
promote the proliferation of peripheral blood mononuclear cells
(11). In addition, in in
vitro and in vivo experiments, polysaccharides have been
reported to enhance immune responses, with an increasing number of
cytokines, such as IL-β, IL-6 and TNF-α, and lymphocyte activation
(12,13). In addition, polysaccharides have
also been reported to induce B-cell proliferation through the
activation of MAPK and NF-κB pathways has been reported (14).
The discovery of the two most notable protein-bound
polysaccharides from Trametes versicolor (a medicinal
mushroom), polysaccharopeptide (PSP) and polysaccharide Krestin
(PSK), have been reported to act as useful adjuvants in cancer
treatment (9,15), which has prompted researchers to
explore the use of polysaccharides from other Trametes fungi
as therapeutic compounds, including T. polyzona. Based on
its physiological properties, T. polyzona is a polypore
mushroom belonging to white-rot fungi, which is similar to T.
versicolor (16). This fungus
has been reported to secrete various enzymes, such as laccase and
manganese peroxidase, which demonstrate the degradation of aromatic
compounds in a polluted environment and high tolerance for
biodegradation (17,18). In a previous study, the phenolic
extract of T. polyzona was found to have antimicrobial
abilities against various bacterial strains and antioxidant
properties against several radicals (19). However, to the best of our
knowledge, no evaluation of other bioactive compounds from this
fungus, such as polysaccharides, in cancer treatment has been
performed. Therefore, this research aimed to isolate
polysaccharides from T. polyzona CU07, which has been
successfully isolated from its natural habitat in Thailand, and to
evaluate its bioactivity on BC cells for the development of
alternative drugs for cancer and other related diseases.
Materials and methods
Chemicals and reagents
MTT membrane-permeable dye (cat. no. ab146345) and
Annexin V-DY-634 PI Apoptosis Staining/Detection Kit (cat. no.
ab214484) were purchased from Abcam. Cisplatin was obtained from
Glentham Life Sciences Ltd. Cell proliferation ELISA, BrdU
(colorimetric) was purchased from Roche Applied Science. Fetal
bovine serum (FBS), antibiotic-antimycotic (100X; containing
penicillin, streptomycin and amphotericin), 0.25% Trypsin-EDTA (1X)
and Dulbecco's modified Eagle's medium (DMEM) were purchased from
Gibco; Thermo Fisher Scientific, Inc. Standard monosaccharides
(arabinose, glucose and mannose) were purchased from Ajax Finechem
Pty Ltd.
Preparation of CTPPs
For the present study, T. polyzona CU07 was
obtained from the culture collection of Plant Biomass Utilization
Research Unit, Department of Botany, Faculty of Science,
Chulalongkorn University (Bangkok, Thailand). After cultivation for
5 days on potato dextrose agar, 10 mycelial discs of CU07
(diameter, 3 mm) were transferred into 150 ml potato dextrose
medium and cultivated at room temperature (25±2˚C) under static
conditions for 14 days. The mycelial biomass was then harvested by
paper filtration, freeze dried at -60˚C, ground and sieved to a
fine power (≤1 mm). The crude polysaccharide was obtained by hot
water extraction in a reflux refractor with solid-to-liquid ratio
of 1:40 (g/ml) at 90˚C for 4 h. The supernatant was collected and
freeze dried to obtain crude T. polyzona polysaccharides
(CTPPs).
Total carbohydrate and protein
contents
The total amount of carbohydrate in CTPPs was
determined using the anthrone assay (20). The extract (0.1 mg/ml) was mixed
with 1.25 ml anthrone reagent (MilliporeSigma) and incubated at 4˚C
for 10 min. The mixture was then heated in boiling water for 10 min
and the absorbance was observed at 630 nm. The protein content was
estimated according to Lowry's method (21) with slight modifications. Briefly,
400 µl sample was added to 2 ml Lowry reagent and incubated at room
temperature for 10 min in the dark. Subsequently, Folin reagent
(100 µl; MilliporeSigma) was added and incubated for 10 min under
the same conditions. The absorbance of mixture was observed at 750
nm. Glucose and BSA (MilliporeSigma) were used as standard
solutions for the anthrone assay and Lowry's method,
respectively.
Monosaccharide composition
To obtain the monosaccharides, CTTPs (300 mg) were
mixed with concentrated sulphuric acid [72% (v/v), 3 ml] and
incubated at room temperature for 60 min, followed by autoclaving
at 121˚C for 1 h (22). The
hydrolysate was neutralized with calcium carbonate and filtered
through a microporous membrane (pore size, 0.45 µm). The type and
content of monosaccharides were determined by high-performance
liquid chromatography (HPLC; Prominence Modular; Shimadzu
Corporation), using a refractive index detector and Shodex SUGAR
SH-1011 column (8.0x300 mm; Shodex; Resonac Corporation), at 40˚C
with 5 mM H2SO4 as the mobile phase with a
flow rate of 1.0 ml/min. Each sugar was quantified according to the
sugar standards (arabinose, glucose, mannose and xylose).
Structural analysis
The primary structure of the sample was
characterized by Fourier-transform infrared spectroscopy (FTIR)
with wave numbers in the range of 4,000 to 400 cm-1
(8). The sample was prepared by
mixing with KBr powder (10:1 by weight). The average molecular
weight analysis was performed by gel permeation chromatography
(GPC; Shimadzu HPLC 10Avp; Shimadzu Corporation) equipped with a
refractive index detector and Shodex PLgel column (7.5x300 mm;
Shodex; Resonac Corporation) that was operated at 40˚C. The sample
(10 µl) was dissolved with ultra-pure water (2 mg/ml, 10 µl) and
eluted with the same solution at a flow rate of 0.5 ml/min.
Pullulan was used as a polysaccharide standard for calibration and
molecular weights were analyzed using Lab Solutions software
(Shimadzu Corporation).
The
2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)
inhibition assay
The radical cation of ABTS+ was prepared
by mixing 7.00 mM ABTS solution (MilliporeSigma) with 2.45 mM
K2S2O8 at a ratio of 1:1 (v/v) and
was incubated at room temperature for 24 h in the dark (23). The ABTS+ solution was
then diluted with methanol to obtain the absorbance of 0.700±0.05
at 734 nm. Subsequently, 150 µl ABTS+ solution was mixed
with CTPPs dissolved in distilled water (50 µl) at different
concentrations (0.4-1.6 mg/ml). Ascorbic acid was used as a
positive control. The mixture was incubated at room temperature for
10 min in the dark and the absorbance was measured at 734 nm. The
percentage of ABTS inhibition was calculated according to the
following equation: Inhibition (%)=(A control-A sample)/A control
x100; where A control and A sample refer to the absorbances of
ABTS+ solution without and with the extract,
respectively. All tests were performed at least in triplicate. The
percentage of inhibition at each concentration of the sample was
plotted to generate the linear equation for using to calculate the
half-maximal inhibitory concentration (IC50) value.
The 2,2-diphenyl-1-picrylhydrazyl
(DPPH) inhibition assay
Samples (CTPPs) dissolved in distilled water (50 µl)
at different concentrations (1.5-10.5 mg/ml) were added to 150 µl
DPPH reagent (100 µM; MilliporeSigma) and incubated in the dark for
30 min at room temperature. The absorbance was observed at 517 nm,
and the inhibition of DPPH and IC50 value were
calculated as described for the ABTS inhibition assay. Ascorbic
acid was used as a positive control (24). All tests were performed in
triplicate and results are expressed as the mean ± SD.
Cell lines
The MCF-7 BC cell line (product no. ATCC HTB-22) was
obtained from the American Type Culture Collection, whereas the 293
control cell line were obtained from the Center of Excellence in
Molecular Genetic and Human Disease, Department of Anatomy, Faculty
of Medicine, Chulalongkorn University. The cell lines were cultured
in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic.
The four cell lines were cultured at 37˚C in a humidified
atmosphere with 5% CO2. The cells were passaged before
being used in subsequent experiments (25).
Cell treatment
The MCF-7 BC cell line and the 293 control cell line
were treated with various concentrations of CTPPs (0.3-1.6 mg/ml)
at 37˚C for 72 h. The results were compared with untreated cells,
which were considered the negative control group. Cisplatin (10-35
µM) was used as a positive control with the same treatment
conditions.
MTT assays
The MTT assay was performed as previously described
with minor modifications (25).
Briefly, the cells (5x103 cells/well) were plated in
96-well plates with medium and incubated for 24 h at 37˚C in a 5%
CO2 incubator. The medium was aspirated and fresh
complete medium was added containing different concentrations of
CTPPs. The plates were incubated for 72 h at 37˚C in a 5%
CO2 incubator, after which, MTT reagent was added. After
incubation at 37˚C for 2.5 h, the solution was removed, and DMSO
was added and mixed for 5 min. The optical density values were read
at 492 and 630 nm. The MTT assay was performed to assess cell
viability and to determine the IC50 values, which were
used to further analyze cell proliferation and apoptosis.
Cell proliferation assay
Cell proliferation was assessed using a colorimetric
immunoassay based on the measurement of BrdU incorporation during
DNA synthesis. The Cell Proliferation ELISA, BrdU (colorimetric)
assay was used according to the manufacturer's instructions. The
cells (5x103 cells/well) were treated with CTPPs
(IC50) and incubated at 37˚C for 72 h. The results were
compared to the untreated cells, which were used as a negative
control.
Detection of apoptosis
Cells (2x105 cells/well) were seeded in a
6-well plate and were incubated for 24 h at 37˚C in a 5%
CO2 incubator. Subsequently, the medium was removed, and
the cells were treated with CTPPs (IC50) for 72 h at
37˚C and 5% CO2. The treated and untreated samples were
trypsinized and centrifuged at 250 x g for 5 min, after which the
cell pellet was collected. The cells were rinsed with PBS and
stained with Annexin V-DY-634 and PI according to the
manufacturer's instructions, and flow cytometry (Beckman Coulter Dx
Flex Flow Cytometer; Beckman Coulter, Inc.). Graphs showing the
percentages of early apoptotic cells, late apoptotic cells and
necrotic cells were generated using GraphPad Prism software,
version 8 (Dotmatics).
Statistical analysis
All of the experiments were conducted in triplicate,
and the data are presented as the mean ± SD and were analyzed using
SPSS 29.0 (IBM Corp.). The statistical significance of differences
among groups was analyzed using one-way analysis of variance,
followed by Tukey's post hoc tests for cell proliferation and
apoptosis assays. Two-group comparisons were performed using
unpaired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics and structural
analysis of CTPPs
The yield and chemical properties of CTPPs,
including total carbohydrates, protein content, monosaccharide
composition and molecular weight, are described in Table I. The obtained weight of CU07
mycelia after cultivation in potato dextrose broth for 14 days was
5.04 g/ml, and the yield of CTPPs extracted by hot water extraction
was 0.15±0.02 g/g dry weight of mycelia. The total carbohydrate and
protein contents were 42.59±1.04 and 37.85±1.92%, respectively.
Glucose (97.34%) was the dominant sugar in the CTPP hydrolysate,
followed by trace amounts of arabinose (2.52%) and mannose (0.15%)
(Fig. S1). In the GPC analysis,
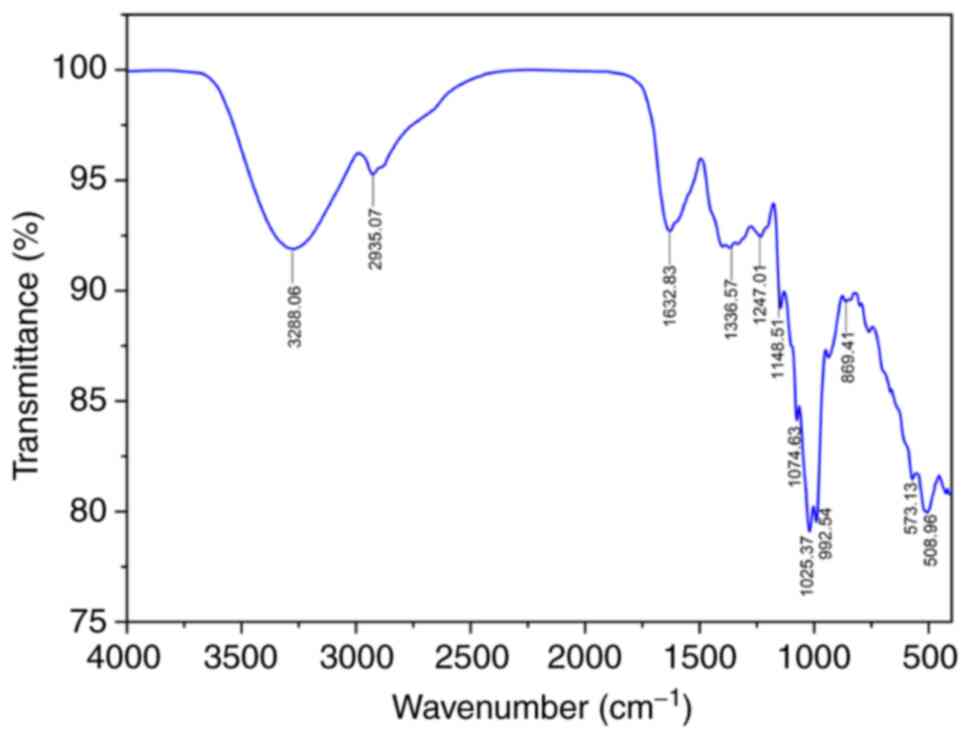
two major peaks at 0.3 and 22,528 kDa were detected (Fig. S2). The FTIR spectrum of CTPPs is
shown in Fig. 1, with typical peaks
observed within the range of 3,700 to 500 cm-1. The
broad peak around 3,288.06 cm-1 was caused by the
stretching of hydroxyl groups and the small peak in the region of
2,935.07 cm-1 was attributed to the stretching vibration
of the C-H bond (8,26,27).
The spectra peaks of CTPPs at 992.54, 1,025.37, 1,074.63 and
1,148.51 cm-1 were considered the-CO and -C-O-C-groups
in a pyranose ring, which are the fingerprint of polysaccharides
(10,28). A β-configurated peak at 869.41
cm-1 was also detected (29,30).
In addition, the existence of protein was recognized by the peak at
1,632.83 cm-1, which suggested the vibration of a
carboxyl group in the amide I band. The peaks between 1,247.01 and
1,336.57 cm-1 were possibly due to the C-N amide III
band of the protein structure (31,32).
 | Table IChemical properties and
monosaccharide composition of crude Trametes polyzona
polysaccharides. |
Table I
Chemical properties and
monosaccharide composition of crude Trametes polyzona
polysaccharides.
| Parameter | Value |
|---|
| Components, % | |
|
Yield | 15.19±1.54 |
|
Carbohydrate
content | 42.59±1.04 |
|
Protein
content | 37.85±1.92 |
|
Water
solubility | 96.11±1.62 |
|
Molecular
weight, kDa | 0.322,528.00 |
| Monosaccharide
composition, % | |
|
Glucose | 97.34 |
|
Arabinose | 2.52 |
|
Mannose | 0.15 |
Antioxidant-scavenging activities
The percentages of inhibition in response to each
concentration of CTPPs were plotted on a scatter graph (Fig. S3) to determine the IC50
values, which are presented in Table
II. The CTPPs were able to inhibit both ABTS and DPPH radicals
at IC50 values of 1.89 and 11.81 mg/ml, respectively.
The results revealed that CTPPs had stronger antioxidant activity
against the ABTS radical compared with that against the DPPH
radical.
 | Table IIIC50 (mg/ml) value of the
scavenging activities of antioxidants in the ABTS and DPPH
assays. |
Table II
IC50 (mg/ml) value of the
scavenging activities of antioxidants in the ABTS and DPPH
assays.
| | IC50,
mg/ml |
|---|
| Sample | ABTS | DPPH |
|---|
| CTPPs | 1.89±0.06 | 11.81±0.17 |
| Ascorbic acid | 0.0318±0.0003 | 0.0490±0.0005 |
Inhibitory effects of CTPPs on BC cell
viability and proliferation
The effect of each concentration of CTPPs on the
viability of MCF-7 BC cells was determined by MTT assays (Fig. S4). As shown in Table III, CTPPs decreased the viability
of MCF-7 cells compared with the untreated cells, with an
IC50 value of 0.58 mg/ml. Moreover, the CTPPs did not
show much of a cytotoxic effect on the viability of 293 cells; even
at the maximum concentration (1.6 mg/ml), the percentage of cell
viability was >80% (Fig. S4).
By contrast, cisplatin was toxic to 293 cells (Table III; Fig. S4). The IC50 values of
CTPPs on BC cell lines were used for further analysis of
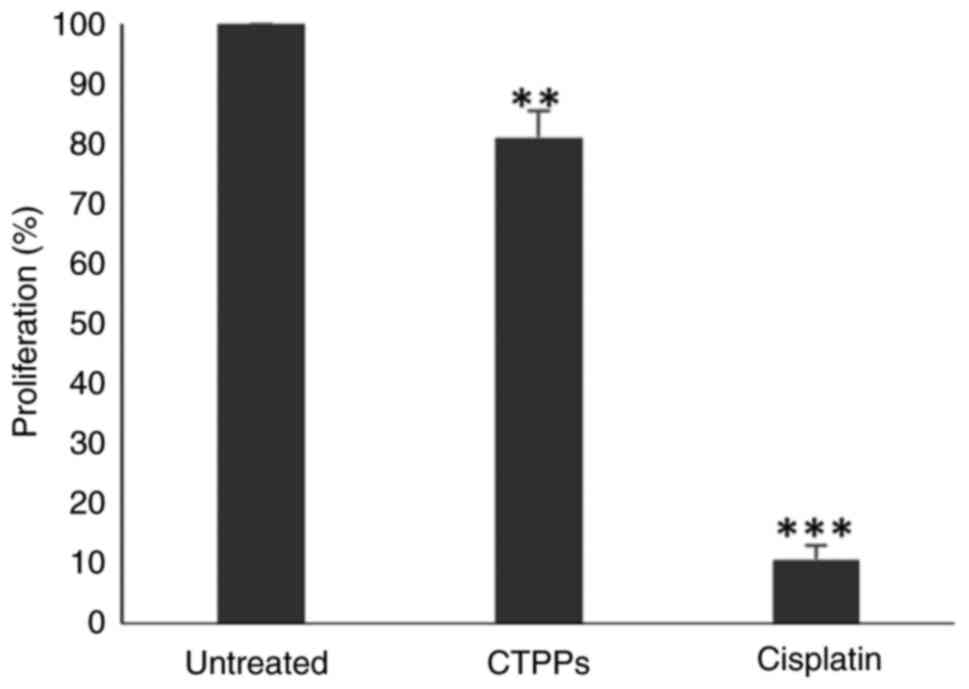
proliferation. The level of cell division was compared to that in
the control (untreated) group, as determined using the BrdU
incorporation assay. After 72 h of treatment with CTPPs
(IC50), the proliferation of MCF-7 cells was
significantly reduced by ~22.59% (Fig.
2).
 | Table IIIInhibitory effect of CTPPs on the
MCF-7 breast cancer cell line and normal human cells (293). |
Table III
Inhibitory effect of CTPPs on the
MCF-7 breast cancer cell line and normal human cells (293).
| | Cell line
IC50 |
|---|
| Sample | MCF-7 | 293 |
|---|
| CTPPs, mg/ml | 0.58±0.0260 | - |
| Cisplatin, µM | 18.75±0.8956 | 12.32±1.4460 |
Apoptosis induction in cancer cell
lines
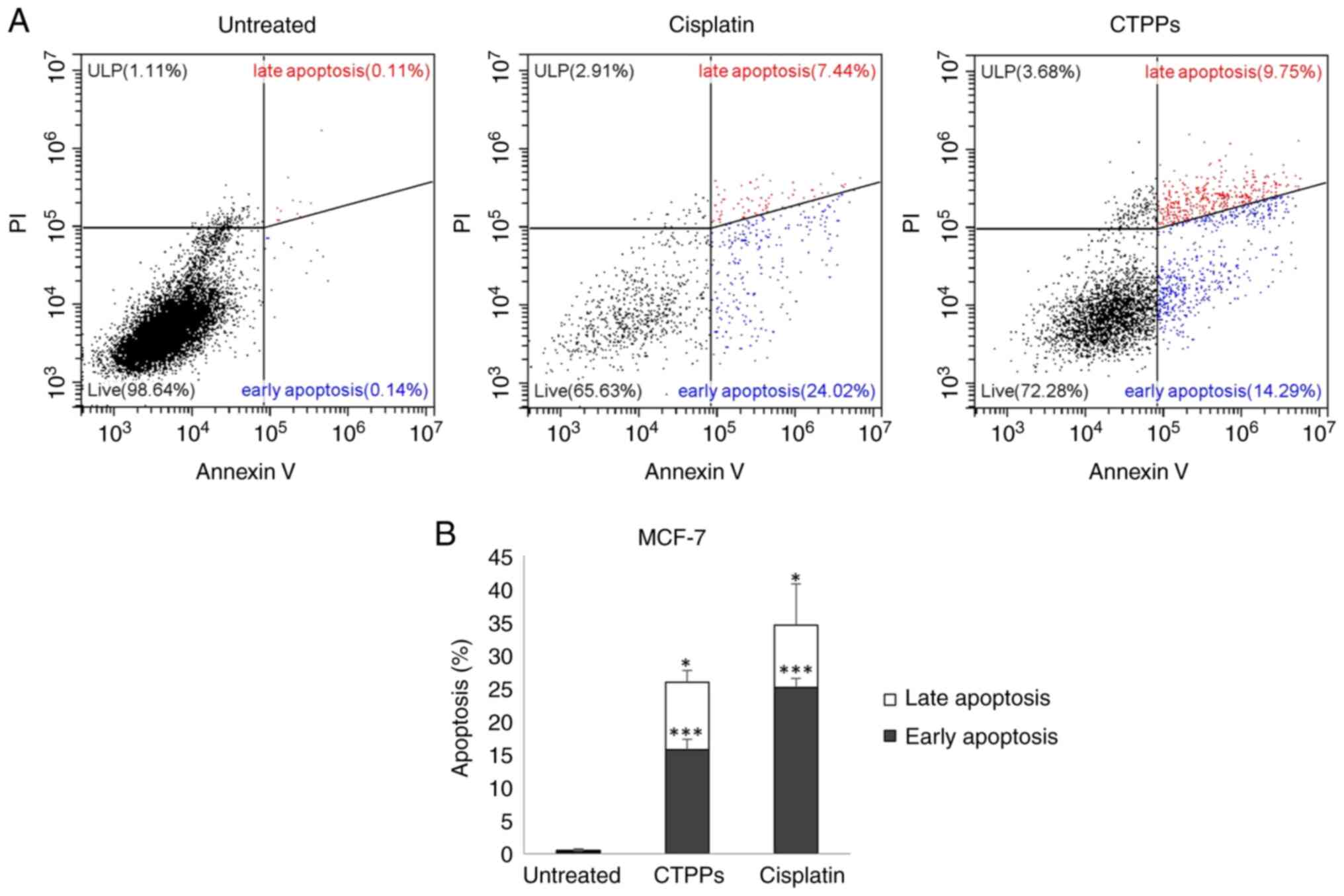
Measuring the apoptosis of MCF-7 cells treated with
CTPPs (IC50) was performed by staining the cells with
Annexin V-FITC and PI for flow cytometry. The representative flow
cytometry dot plots of cells showed fluorescence intensities
(Fig. 3A). The percentage of cells
undergoing early and late apoptosis was analyzed and compared with
the untreated control group (Fig.
3B). Treatment with CTPPs significantly accelerated early and
late apoptosis in MCF-7 cells, and the levels of induction were
13.21 and 9.21%, respectively. CTPPs also induced late apoptosis at
a higher level than cisplatin (5.90%), which was used as a positive
control.
Discussion
Based on the FTIR spectrum and sugar analysis, it
was revealed that the obtained CTPPs in this study mainly consisted
of a β-glucan, since glucose was detected as the major constituent
along with the presence of a β-configuration. In addition, CTPPs
were revealed to be protein-bound polysaccharides, which consisted
of two major components at different molecular weights. The total
sugar and protein contents of these CTPPs were similar to those of
PSP and PSK, commercial polysaccharides from T. versicolor,
which are composed of soluble carbohydrates and proteins in the
ranges of 34-46% (w/w) and 28-35% (w/w), respectively (9,12).
Although fungal polysaccharides are considered
biological response modifiers, these polysaccharides possibly
support cellular homeostasis by protecting cells from oxidative
stress and free radicals through the innate antioxidant system
(33,34). Therefore, the radical-scavenging
activity of CTPPs was evaluated, and they were revealed to scavenge
both radicals assessed, particularly ABTS. According to a previous
report, the supply of hydrogen contributes to the bioactivities of
polysaccharides (33). Meanwhile,
as polysaccharide conjugates, the amino group of proteins and
carboxyl group of polysaccharides are associated with
radical-scavenging activity, and they are capable of
proton-electron donation (35,36).
The IC50 value of CTPPs on ABTS was
comparable to the antioxidant activity of some polysaccharides from
white-rot fungi that are commercially referred to as medicinal
mushrooms and is in the average IC50 range of CTPPs on
ABTS reported in previous studies (23,37,38).
Moreover, the IC50 value of CTPPs on ABTS was higher
compared with that in some other Trametes species, for which
ethanol was used as a solvent for polysaccharide extraction of
>20 mg/ml (39). These findings
indicated that using hot water extraction for polysaccharides could
lead to an increase in the antioxidant properties of the compounds.
The present study revealed that CTTPs may have potential
antioxidant activities, particularly against the ABTS radical,
although they were shown to be inferior to the ascorbic acid
reference.
As determined using the MTT assay, CTPPs were
revealed to affect the viability of MCF-7 cells. These results
indicated that CTPPs could be considered in the treatment of
various types of BC, which is comparable to several polysaccharides
obtained from medicinal mushrooms of the Trametes species,
as described in previous reports (14,40,41).
Notably, CTPPs were revealed to be safer for normal cells than
cisplatin, which is toxic to both cancerous and normal cells.
Cisplatin has commercially been used as an antineoplastic drug with
a broad spectrum for various types of cancer, including BC
(42). In BC treatment, cisplatin
has been shown to have anticancer effects on several types of BC
cells. Thus, cisplatin has been chosen as a control in BC research
(43-45).
Furthermore, the 293 cell line was selected as a normal cell line
due to the nephrotoxic evaluation of CTPPs. Recently,
nephrotoxicity or renal toxicity of herbal medicines (HM) has been
considered as one of the main toxicities in this type of treatment;
notably, some HMs have been shown to cause kidney damage in
patients (46).
The present study also assessed the effects of CTPPs
on cell proliferation. It was shown that CTPPs suppressed the
proliferation of MCF-7 cells, which exhibited >20% inhibition
compared with untreated cells. Taken together, the present results
indicated that CTPPs had cytotoxic activity against the viability
of MCF-7 cells. The cytotoxic effects of CTPPs were probably
responsible for suppressing of the proliferating cells, as a recent
report demonstrated that cytotoxic drugs could disturb the process
of cell division (47). Even though
the results on cell proliferation were inferior compared with those
in the positive control group, this was a beneficial result in
terms of cancer treatment, because cell proliferation is a critical
process of cell division. In cancer development, the targeting of
cell proliferation is important due to the features of cancer
progression and tumorigenesis (48-50).
Another targeted feature of cancer cells is
apoptosis, a program cell death process that have therapeutic
potential for cancer therapy (51).
Notably, CTPPs induced early and late apoptosis in MCF-7 cells. It
has been indicated that CTPPs may be involved in the expression of
various apoptosis-regulating genes, including pro-apoptotic (Bax)
and anti-apoptotic (Bcl-2) genes, or in the activation of
caspase-mediated apoptosis (caspase-3, -8 and -9), through
intrinsic or extrinsic pathways (52,53).
Moreover, CTPPs could be associated with tumor suppressor signaling
pathways, such as p53, through which they may induce apoptosis and
suppress proliferation (54). A
recent report found that a Trametes polysaccharide-peptide
could promote cell apoptosis through epidermal growth factor
receptor (EGFR) pathways by suppressing the expression of EGFRs and
the level of programmed cell death-ligand 1 genes (55).
In patients with BC, the treatment is limited, and
the current gold standard is chemotherapy. However, these
treatments are associated with side effects in patients (56). Therefore, the findings of the
present study showed that CTPPs may be helpful as an alternative
adjuvant for cancer treatment. In addition, further evaluation of
gene expression related to the signalling pathway of apoptosis is
strongly suggested in order to reveal the downstream mechanism of
CTPPs for further investigation on BC cells.
Supplementary Material
High-performance liquid chromatogram
of the crude Trametes polyzona polysaccharides
hydrolysate.
Gel permeation chromatogram of crude
Trametes polyzona polysaccharides.
Dose-dependent curve of the scavenging
activity of CTPPs on ABTS and DPPH. ABTS, 2,2'-azino-bis
(3-ethylbenzothiazoline-6-sulfonic acid); CTPPs, crude Trametes
polyzona polysaccharides; DPPH,
2,2-diphenyl-1-picrylhydrazyl.
Dose-dependent curve of cell viability
in response to CTPPs, as determined by MTT assay. CTPPs, crude
Trametes polyzona polysaccharides.
Acknowledgements
The authors would like to thank the Center of
Excellence in Molecular Genetic and Human Disease (Department of
Anatomy, Faculty of Medicine, Chulalongkorn University, Bangkok,
Thailand) for providing the 293 cell line.
Funding
Funding: This research was supported by the
Ratchadaphiseksomphot Endowment Fund (Chulalongkorn University of
Plant Biomass Utilization and Research Unit), The Graduate School
Program for ASEAN and NON-ASEAN Countries (Chulalongkorn
University), and the Thailand Science Research and Innovation Fund,
Chulalongkorn University [grant no.
CU_FRB65_hea(65)_128_23_58].
Availability of data and materials
The datasets generated and/or analyzed during the
study are available from the corresponding author on reasonable
request.
Authors' contributions
SP, PY and WB were involved in study
conceptualization. BK, SP, PY and WB confirmed the authenticity of
all the raw data and designed the methodology. BK performed
experiments and wrote the original draft. SP, PY, and WB reviewed
and edited the manuscript. SP and PY acquired the funding and
supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
García MA, Fueyo R and Martínez-Balbás MA:
Chapter 10-lysine demethylases: Structure, function, and
disfunction. In: Chromatin Signaling and Diseases. Binda O and
Fernandez-Zapico ME (eds). Academic Press, Boston, pp179-194,
2016.
|
|
2
|
Ramírez K, Acevedo F, Herrera ME, Ibáñez C
and Sánchez C: Physical activity and breast cancer. Rev Med Chil.
145:75–84. 2017.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
3
|
Prihantono and Faruk M: Breast cancer
resistance to chemotherapy: When should we suspect it and how can
we prevent it? Ann Med Surg (Lond). 70(102793)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ko EY and Moon A: Natural products for
chemoprevention of breast cancer. J Cancer Prev. 20:223–231.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Noel B, Singh SK, Lillard JW Jr and Singh
R: Role of natural compounds in preventing and treating breast
cancer. Front Biosci (Schol Ed). 12:137–160. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Yang Z, Zhang Q, Yu L, Zhu J, Cao Y and
Gao X: The signaling pathways and targets of traditional Chinese
medicine and natural medicine in triple-negative breast cancer. J
Ethnopharmacol. 264(113249)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lin SR, Chang CH, Hsu CF, Tsai MJ, Cheng
H, Leong MK, Sung PJ, Chen JC and Weng CF: Natural compounds as
potential adjuvants to cancer therapy: Preclinical evidence. Br J
Pharmacol. 177:1409–1423. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
He N, Tian L, Zhai X, Zhang X and Zhao Y:
Composition characterization, antioxidant capacities and
anti-proliferative effects of the polysaccharides isolated from
Trametes lactinea (Berk.) Pat. Int J Biol Macromol. 115:114–123.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wan JMF: Chapter 27-polysaccaride krestin
(PSK) and polysaccharopeptide PSP. In: Handbook of Biologically
Active Peptides (Second Edition). Kastin AJ (ed). Academic Press,
Boston, pp180-184, 2013.
|
|
10
|
Zhang M, Yan M, Yang J, Li F, Wang Y, Feng
K, Wang S, Lin N, Wang Y and Yang B: Structural characterization of
a polysaccharide from Trametes sanguinea Lloyd with
immune-enhancing activity via activation of TLR4. Int J Biol
Macromol. 206:1026–1038. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jędrzejewski T, Pawlikowska M, Piotrowski
J and Kozak W: Protein-bound polysaccharides from Coriolus
versicolor attenuate LPS-induced synthesis of pro-inflammatory
cytokines and stimulate PBMCs proliferation. Immunol Lett.
178:140–147. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dou H, Chang Y and Zhang L: Chapter
Fifteen-coriolus versicolor polysaccharopeptide as an
immunotherapeutic in China. In: Progress in Molecular Biology and
Translational Science. Vol. 163. Zhang L (ed). Academic Press,
pp361-381, 2019.
|
|
13
|
Maehara Y, Tsujitani S, Saeki H, Oki E,
Yoshinaga K, Emi Y, Morita M, Kohnoe S, Kakeji Y, Yano T and Baba
H: Biological mechanism and clinical effect of protein-bound
polysaccharide K (KRESTIN(®)): Review of development and
future perspectives. Surg Today. 42:8–28. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kowalczewska M, Piotrowski J, Jędrzejewski
T and Kozak W: Polysaccharide peptides from Coriolus versicolor
exert differential immunomodulatory effects on blood lymphocytes
and breast cancer cell line MCF-7 in vitro. Immunol Lett.
174:37–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wan JMF, Sit WH and Louie JC:
Polysaccharopeptide enhances the anticancer activity of doxorubicin
and etoposide on human breast cancer cells ZR-75-30. Int J Oncol.
32:689–699. 2008.PubMed/NCBI
|
|
16
|
Kijpornyongpan T, Schwartz A, Yaguchi A
and Salvachúa D: Systems biology-guided understanding of white-rot
fungi for biotechnological applications: A review. iScience.
25(104640)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lueangjaroenkit P, Teerapatsakul C, Sakka
K, Sakka M, Kimura T, Kunitake E and Chitradon L: Two manganese
peroxidases and a laccase of Trametes polyzona KU-RNW027
with novel properties for dye and pharmaceutical product
degradation in redox mediator-free system. Mycobiology. 47:217–229.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wulandari R, Lotrakul P, Punnapayak H,
Amirta R, Kim SW and Prasongsuk S: Toxicity evaluation and
biodegradation of phenanthrene by laccase from Trametes
polyzona PBURU 12. 3 Biotech. 11(32)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adongbede EM, Jaiswal YS, Davis SS,
Randolph PD, Huo LN and Williams LL: Antioxidant and antibacterial
activity of Trametes polyzona (Pers.) Justo. Food Sci
Biotechnol. 29:27–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Leyva A, Quintana A, Sánchez M, Rodriguez
EN, Cremata J and Sánchez JC: Rapid and sensitive anthrone-sulfuric
acid assay in microplate format to quantify carbohydrate in
biopharmaceutical products: Method development and validation.
Biologicals. 36:134–141. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tantray JA, Mansoor S, Wani RFC and Nissa
NU: Chapter 16-protein estimation by Lowry's method. In: Basic Life
Science Methods. Tantray JA, Mansoor S, Wani RFC and Nissa NU
(eds). Academic Press, pp65-67, 2023.
|
|
22
|
Ramos-Andrés M, Aguilera-Torre B and
García-Serna J: Hydrothermal production of high-molecular weight
hemicellulose-pectin, free sugars and residual cellulose pulp from
discarded carrots. J Clean Prod. 290(125179)2021.
|
|
23
|
Wu J, Kaewnarin K, Nie X, Li Q, He N,
Huang J and Geng A: Biological activities of a polysaccharide from
the coculture of Ganoderma lucidum and Flammulina velutipes mycelia
in submerged fermentation. Process Biochem. 109:10–18. 2021.
|
|
24
|
Sridhar K and Charles AL: In vitro
antioxidant activity of Kyoho grape extracts in DPPH and ABTS
assays: Estimation methods for EC50 using advanced
statistical programs. Food Chem. 275:41–49. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hanyu X, Lanyue L, Miao D, Wentao F,
Cangran C and Hui S: Effect of Ganoderma applanatum polysaccharides
on MAPK/ERK pathway affecting autophagy in breast cancer MCF-7
cells. Int J Biol Macromol. 146:353–362. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Meng Q, Li Y, Xiao T, Zhang L and Xu D:
Antioxidant and antibacterial activities of polysaccharides
isolated and purified from Diaphragma juglandis fructus. Int J Biol
Macromol. 105:431–437. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang S, He B, Ge J, Li H, Luo X, Zhang H,
Li Y, Zhai C, Liu P, Liu X and Fei X: Extraction, chemical analysis
of Angelica sinensis polysaccharides and antioxidant activity of
the polysaccharides in ischemia-reperfusion rats. Int J Biol
Macromol. 47:546–550. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li F, Wei Y, Liang L, Huang L, Yu G and Li
Q: A novel low-molecular-mass pumpkin polysaccharide: Structural
characterization, antioxidant activity, and hypoglycemic potential.
Carbohydr Polym. 251(117090)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li F, Yuan Q and Rashid F: Isolation,
purification and immunobiological activity of a new water-soluble
bee pollen polysaccharide from Crataegus pinnatifida Bge. Carbohydr
Polym. 78:80–88. 2009.
|
|
30
|
Luo QL, Tang ZH, Zhang XF, Zhong YH, Yao
SZ, Wang LS, Lin CW and Luo X: Chemical properties and antioxidant
activity of a water-soluble polysaccharide from Dendrobium
officinale. Int J Biol Macromol. 89:219–227. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mecozzi M, Pietroletti M, Scarpiniti M,
Acquistucci R and Conti ME: Monitoring of marine mucilage formation
in Italian seas investigated by infrared spectroscopy and
independent component analysis. Environ Monit Assess.
184:6025–6036. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pavia DL, Lampman GM, Kriz GS and Vyvyan
JR: Introduction to Spectroscopy. Brooks/Cole, 2009.
|
|
33
|
Sitkoff A: 90-Medicinal mushrooms. In:
Textbook of Natural Medicine (Fifth Edition). Pizzorno JE and
Murray MT (eds). Churchill Livingstone, St. Louis (MO),
pp679-692.e676, 2020.
|
|
34
|
Wang H, Liu YM, Qi ZM, Wang SY, Liu SX, Li
X, Wang HJ and Xia XC: An overview on natural polysaccharides with
antioxidant properties. Curr Med Chem. 20:2899–2913.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ajisaka K, Agawa S, Nagumo S, Kurato K,
Yokoyama T, Arai K and Miyazaki T: Evaluation and comparison of the
antioxidative potency of various carbohydrates using different
methods. J Agric Food Chem. 57:3102–3107. 2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen Y, Lin Q, Wang J, Mu J and Liang Y:
Proteins, polysaccharides and their derivatives as macromolecular
antioxidant supplements: A review of in vitro screening methods and
strategies. International Int J Biol Macromol. 224:958–971.
2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li X and Wang L: Effect of extraction
method on structure and antioxidant activity of Hohenbuehelia
serotina polysaccharides. Int J Biol Macromol. 83:270–276.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang KF, Sui KY, Guo C and Liu CZ: Quorum
sensing molecule-farnesol increased the production and biological
activities of extracellular polysaccharide from Trametes
versicolor. Int J Biol Macromol. 104:377–383. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Knežević A, Stajić M, Sofrenić I,
Stanojković T, Milovanović I, Tešević V and Vukojević J:
Antioxidative, antifungal, cytotoxic and antineurodegenerative
activity of selected Trametes species from Serbia. PLoS One.
13(e0203064)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Amaral I, Silva C, Correia-Branco A and
Martel F: Effect of metformin on estrogen and progesterone
receptor-positive (MCF-7) and triple-negative (MDA-MB-231) breast
cancer cells. Biomed Pharmacother. 102:94–101. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Luo Z, Hu X, Xiong H, Qiu H, Yuan X, Zhu
F, Wang Y and Zou Y: A polysaccharide from Huaier induced apoptosis
in MCF-7 breast cancer cells via down-regulation of MTDH protein.
Carbohydr Polym. 151:1027–1033. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang J, Ye ZW, Tew KD and Townsend DM:
Cisplatin chemotherapy and renal function. Adv Cancer Res.
152:305–327. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vaikundamoorthy R, Krishnamoorthy V,
Vilwanathan R and Rajendran R: Structural characterization and
anticancer activity (MCF7 and MDA-MB-231) of polysaccharides
fractionated from brown seaweed Sargassum wightii. Int J Biol
Macromol. 111:1229–1237. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xie Y, Jiang Z, Yang R, Ye Y, Pei L, Xiong
S, Wang S, Wang L and Liu S: Polysaccharide-rich extract from
Polygonatum sibiricum protects hematopoiesis in bone marrow
suppressed by triple negative breast cancer. Biomed Pharmacother.
137(111338)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu H, Zou S and Xu X: The β-glucan from
Lentinus edodes suppresses cell proliferation and promotes
apoptosis in estrogen receptor positive breast cancers. Oncotarget.
8:86693–86709. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xu X, Zhu R, Ying J, Zhao M, Wu X, Cao G
and Wang K: Nephrotoxicity of herbal medicine and its prevention.
Front Pharmacol. 11(569551)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sivapatham S and Selvaraj L: Currently
available molecular analyses for personalized tumor therapy
(Review). Biomed Rep. 17(95)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Feitelson MA, Arzumanyan A, Kulathinal RJ,
Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML,
Nawroth R, Sanchez-Garcia I, et al: Sustained proliferation in
cancer: Mechanisms and novel therapeutic targets. Semin Cancer
Biol. 35 (Suppl):S25–S54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Peng Y, Li J and Zhu L: Chapter 8-cancer
and non-coding RNAs. In: Nutritional Epigenomics. Vol. 14. Ferguson
BS (ed). Academic Press, pp119-132, 2019.
|
|
50
|
Zhu YS and Zhu J: Molecular and cellular
functions of long non-coding RNAs in prostate and breast cancer.
Adv Clin Chem. 106:91–179. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Liu W, Jin W, Zhu S, Chen Y and Liu B:
Targeting regulated cell death (RCD) with small-molecule compounds
in cancer therapy: A revisited review of apoptosis,
autophagy-dependent cell death and necroptosis. Drug Discov Today.
27:612–625. 2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Carneiro BA and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014(150845)2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wan X, Jin X, Xie M, Liu J, Gontcharov AA,
Wang H, Lv R, Liu D, Wang Q and Li Y: Characterization of a
polysaccharide from Sanghuangporus vaninii and its antitumor
regulation via activation of the p53 signaling pathway in breast
cancer MCF-7 cells. Int J Biol Macromol. 163:865–877.
2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jian L, Zhicheng H and Shubai L:
Polysaccharide peptide induced colorectal cancer cells apoptosis by
down-regulating EGFR and PD-L1 expression. Iran J Pharm Res.
21(e123909)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Di Nardo P, Lisanti C, Garutti M, Buriolla
S, Alberti M, Mazzeo R and Puglisi F: Chemotherapy in patients with
early breast cancer: Clinical overview and management of long-term
side effects. Expert Opin Drug Saf. 21:1341–1355. 2022.PubMed/NCBI View Article : Google Scholar
|