1. Morphology
The vertebral column is the structural base of the
human body. It comprises complex bony elements (vertebrae) and soft
fibrous elements (intervertebral discs and ligaments). The key
anatomical parts of the vertebrae are the vertebral body, the
pedicle, lamina, the spinous process and the transverse process in
the thoracic and lumbar parts of the spine. The intervertebral disc
is located between the vertebral bodies. The zygapophyseal and
Luschka joints are the primary joints that contribute to the
maintenance of the vertebral column's architecture during static
position and motion. Finally, various fibrous ligaments, such as
the anterior and posterior longitudinal ligaments and the
ligamentum flavum, contribute to the maintenance of spinal cord
structure. Each part of the vertebral column has unique biochemical
and functional characteristics; however, they all articulate with
each other in order to for the body to be able to make complex and
delicate movements (1,2).
The intervertebral disc is the most critical and
extensively investigated structure of the soft tissues of the
vertebral column. It is placed between the un-elastic and
non-compressed bodies of the vertebrae and sustains multi-direction
compressive, bending, or shearing forces (1,2) during
body motion or posture sustenance. However, the acting forces over
the spine are not distributed equally over the intervertebral
discs, leading to more significant wear of the most stressed parts
of the disc. The reasons for that are some anatomical
characteristics of intervertebral disc components (e.g., eccentric
location of nucleus pulposus in the disc) and the fact that the
spine sustains multi-direction loads (3).
The intervertebral disc is separated into two parts:
The outer part is the annulus fibrosus, while the inner part is the
nucleus pulposus. Furthermore, the annulus fibrosus is subdivided
into an external zone consisting of complex collagen type I fibers
and an internal area composed of soft collagen type II fibers
(1,2). The external zone of the annulus
fibrosus bridges two successive vertebral bodies. In addition, due
to its architecture and biochemical characteristics, the annulus
fibrosus functions similar to a diffusion filter that controls the
crossing of fluids, ions and macromolecules between articular
plates and intervertebral discs (4).
The nucleus pulposus is a gel-like formation
composed mainly of glycosaminoglycans and water (1,2). It is
located approximately in the middle of the distance between the
central and posterior parts of the intervertebral disc (5). In the case that a static compressive
load is forced on the intervertebral disc, the nucleus pulposus
loses some of its water content and its height is reduced. When the
pressure from this load is terminated, the nucleus pulposus retains
the lost moisture and regains its original size. In the case that a
shear load is inflicted on the intervertebral disc, the nucleus
pulposus can move inside the annulus fibrosus, consuming the load.
The nucleus pulposus retains its original location inside the disc
when the pressure of the load is terminated.
This difference in the biochemical structure of the
annulus fibrosus and nucleus pulposus is fundamental to their
unique functionality. Thus, the annulus fibrosus, with its high
content of fibers, serves to stand tension, shear and torsion,
while the nucleus pulposus, with its high content of proteoglycans,
serves to stand compression forces (6-8).
In conclusion, the intervertebral disc acts functions as an elastic
jolt absorber under multi-axial loads.
Two continuous vertebrae are linked with a pair of
joints known as the zygapophyseal. These are accurate joints
containing articular plates, articular cartilage and synovial
tissue, and bridge the faceting process of two continuous
vertebrae. Apart from their connecting role, zygapophyseal joints
participate in the motions of the spine and sustain a part of the
loads that act over the spine. Similar to intervertebral discs,
zygapophysial joints are designed to sustain multi-axial
compressive (9) and shear (10,11)
loads. Additionally, zygapophyseal joints stabilize other parts of
the spine's soft tissues, particularly the upper vertebral column
(12).
On the lateral side of the cervical intervertebral
disc, the annulus fibrosus is subdivided by transverse clefts
(13,14). These clefts are not anatomical
formations that exist in the fetus, but develop later in the
child's life and become more profound in adulthood (15,16).
Later on in adult life, a joint pseudocapsule is formed inside the
fissures (1,2), and the formed joint is known as the
uncovertebral or Luschka joint (13,14).
However, the exact formation mechanism of these fissures remains to
be determined. In various models, it has been found that the clefts
are formed in the intervertebral disc area, where the highest load
pressure acts (12,17,18).
On the contrary, the role of Luschka joints is well known. They
cooperate with facet joints to perform lateral bending and axial
spine rotation (19). Furthermore,
Luschka joints restrict extreme movements of the spine (7,20),
avoiding possible damage.
Finally, various fibrous ligaments connect two or
more continuous elements of the vertebral column. These ligaments
are generally high-percentage elastin and collagen structures and
are designed to resist tensile and destructive loads (7). Their exact function depends on their
biochemical characteristics and the spinal parts they connect.
Ligaments with a high concentration of elastin have a more elastic
function (21,22), whereas ligaments with a high
percentage of collagen have a more stabilizing role. Furthermore,
the complex entheses of spine ligaments render them capable of
resisting multiple loads, although they are most effective when
distracted along the direction of the fibers (23). The critical ligaments of the spine
are described below:
The anterior longitudinal ligament is located on the
ventral side of the spinal cord and binds the bodies of the
vertebrae. Due to its location, the anterior longitudinal ligament
limits the extension of the spine (23).
The posterior longitudinal ligament binds the dorsal
part of the vertebral bodies. Due to its caudal location, it limits
the flexion of the spine. On the other hand, as the posterior
longitudinal ligament is located close to the center of rotation,
it is not as effective against loads during rotation (12).
Interspinous ligaments connect the vertebral
processes of the spine. Mainly, they are composed of collagen
fibers, as 5-20% of them are comprised of collagen (24,25).
Therefore, their main role is to limit the flexion of the vertebral
column (25). Additionally, these
ligaments cooperate with the anterior longitudinal ligament and
resist the applied forces during rotation.
The ligamentum flavum is located in the posterior
part of the laminae. It is the most elastic tissue of the body,
with a collagen/elastin ratio of 1/4 (26,27).
Its main role is to maintain the vertical posture of the spine and
assist the vertebral column in resuming it after flexion.
Capsular ligaments connect the inferior articular
process of a vertebra with the superior process of the lower
vertebra (28). They serve as local
stabilizers of the zygapophyseal joint (28,29),
particularly during rotation (28).
2. Pathophysiology
Intervertebral discs, joints and ligaments have a
poor or absent feeding vascular network. As a result, the nutrition
of the intervertebral discs is covered mainly through a vascular
network that penetrates only to the outer zone of the annulus
fibrosus and through diffusion from vertebral end plates (30). Additionally, fluids and elements of
nutrition enter the intervertebral disc during the movements of the
spine. When the disc is compressed, it loses water through a
mechanism which is discussed below. When the compression stops, the
disc retains its original height, absorbing fluids and nutrients
with a mechanism similar to a pump.
This lack of blood vessels inside the nucleus
pulposus, the inner part of the annulus fibrosus and the articular
cartilage does not only have negative effects. The architecture of
the aforementioned structures appears to be more solid without
penetrating vessels, rendering them more effective in resisting
loads (31).
The harmonic cooperation of the vertebrae, the
intervertebral discs, the small joints and the ligaments renders
the vertebral column capable of sustaining multiple external
forces, such as compression, shear and rotation during the static
or dynamic posture of the body. Additionally, it contributes to
maintaining the architectural integrity and functionality of the
vertebral column during and after the force stops acting.
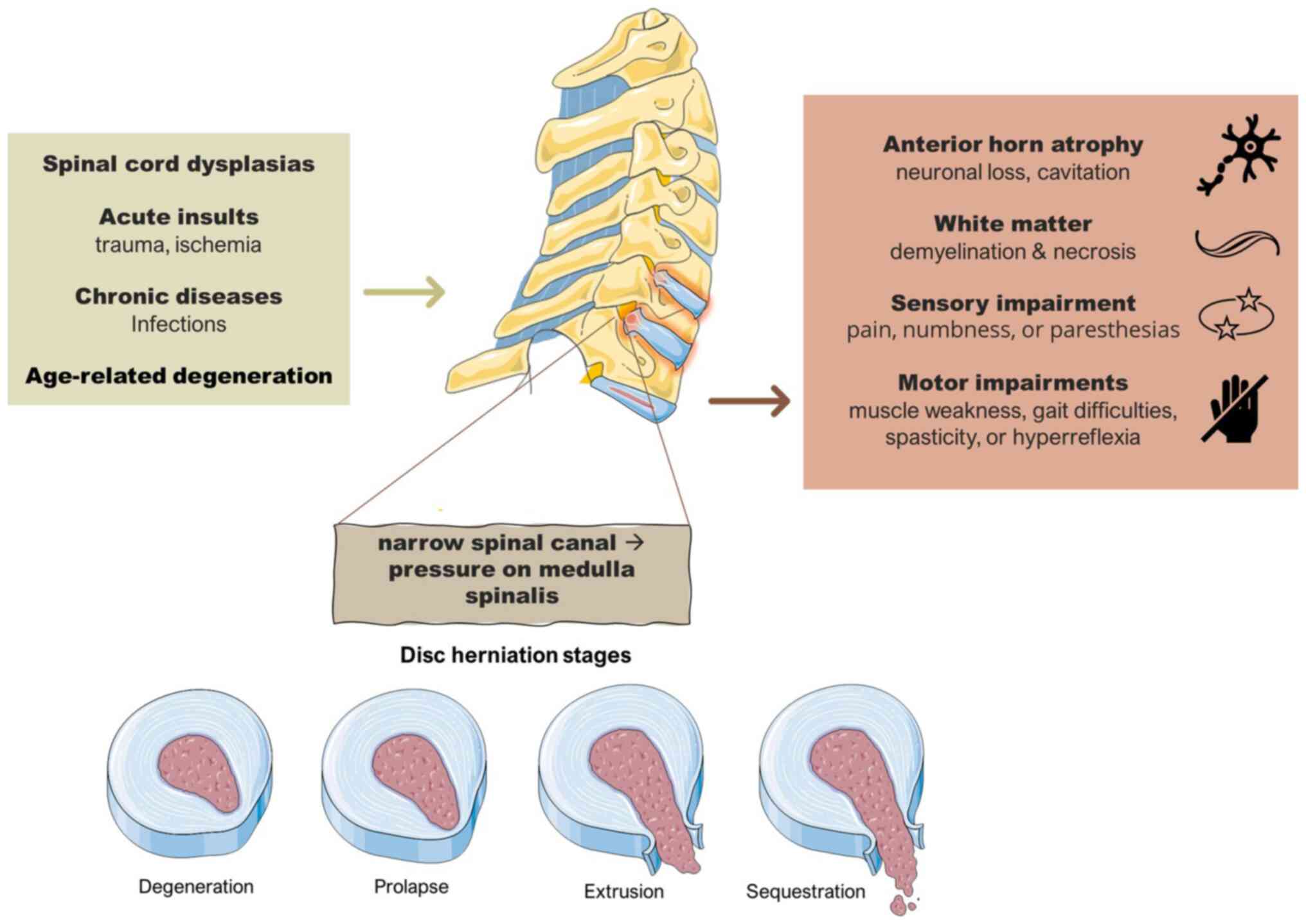
Cervical spondylotic myelopathy
(CSM)
Cervical myelopathy is a well-described medulla
spinalis syndrome characterized by sensory disorders, such as pain,
numbness, or paresthesia in the limbs, as well as motor disorders,
such as muscle weakness, gait difficulties, spasticity, or
hyperreflexia. Pathologically, myelopathy is characterized by
atrophy of the anterior horn (32)
and loss of the neurons in the gray matter, with accompanying
cavity formation within the gray matter. By contrast, in white
matter, demyelination, necrosis (33), myelin pallor,and atrophy can be
encountered (33,34). A summary of the underlying causes,
mechanisms and consequences of this condition is illustrated in
Fig. 1.
Cervical myelopathy can result from transgene
dysplasias of the spinal cord, acute insults, such as trauma or
ischemia, and chronic issues such as infections and age-related
degeneration of the spinal cord. Spondylosis is a multifactorial
(genetic deformation, aging deterioration and loading history)
(35) cause of cervical myelopathy
and affects various elements of the spine like vertebrae,
intervertebral discs, joints and ligaments. Spondylosis is
characterized by multi-type vertebral column deformations, such as
the formation of bony spurs, the degeneration of facet and Luschka
joints (32), the calcification of
soft tissues and ligaments, and the degeneration of the
intervertebral disc. The outcome of all these deformations is a
profoundly narrow spinal canal (36,37),
which causes direct pressure on the medulla spinalis. Additionally,
it has been shown that the chronic degeneration of the spine causes
the static compression of the spinal medulla. The dynamic
compression that occurs during the movements of the spine can cause
cervical myelopathy (38,39). Finally, ischemic deterioration
appears to be induced during aging and contributes to the
development of myelopathy (33,34).
Spondylosis is widely considered a condition
affecting middle-aged individuals; 95% of asymptomatic males and
70% of asymptomatic males by the age of 60-65 years have signs of
degeneration in cervical radiography (38), and 57% of asymptomatic individuals
>40 years of age have disc degeneration, while 40% of the
individuals in the same age group have bone spurs in a cervical MRI
(40), whereas only 10% of
individuals by the age of 25 have spondylotic deformations
(41).
Age deterioration
During the first years of life, the vertebral column
is at the peak of its morphological integrity and functionality. As
the years progress, a number of age-related changes occur in the
spine. These changes, along with spinal deformations which occur
due to acting loads, disrupt the architecture of the vertebral
column and deteriorate its functionality.
The intervertebral disc, as aforementioned, is an
avascular structure that meets its needs for fluids and
macromolecules through the vascular network of the outer annulus
fibrosus and diffusion from the surrounding tissues. More
specifically, when a load compresses the disc, water is drained
out, increasing the osmotic pressure inside the disc and decreasing
the height of the disc. When the load stops acting, the high
osmotic pressure in the disc drives the lost amount of water back
to the nucleus pulposus and the disc back to its original height.
The disc of a young individual contains an increased number of
proteoglycans and only a small amount of fiber, and thus it has an
enhanced ability to absorb water. In summary, during youth, the
biochemical structure and the proper function of the intervertebral
disc, in combination with the integrity of the annulus fibrosus
vascular network, guarantee the proper supplementation of the disc
(31).
During aging, the biochemical composition (42) and the architecture of the
intervertebral disc change significantly. The biochemical changes
involve the shift of chondroitin-4-sulfate, chondroitin-6-sulfate
and keratan sulfate, which are the main glycosaminoglycans in the
intervertebral disc of a young individual, to dermatan sulfate
(31). The changes which occur in
the glycosaminoglycans, and amounts and quality of proteins I the
disc during aging reduce the quantity of water inside the disc.
As a result, the disc height is reduced (1) and it becomes an unelastic and fibrous
structure (41). Additionally,
melanin-like molecules are collected inside the nucleus pulposus,
and the disc thus acquires a dark brown shade (43). Architectural changes occur in the
first years of adult life. They involve multiple tears and fissures
that develop on the lateral surface of the annulus fibrosus and
progressively extend to the nucleus pulposus (1,44). Due
to its biochemical and structural changes, these gap formations
result from the reduced capacity of the disc to carry loads
(44).
Furthermore, nutrient supply to the avascular disc
becomes less efficient in a spine from an older individual. As a
result, the poorly supplied intervertebral disc has a low
regeneration rate. This fact reduces the ability of the disc to
repair the damage from mechanical loads (45).
The aging procedure affects the intervertebral disc
and the surrounding cartilage formations. Namely, the amount of
proteoglycans in cartilage is reduced over time (46), reducing the ability of the cartilage
to maintain an adequate amount of water, and making it less
elastic. Additionally, the connection between the collagen fibers
alone (47) and the collagen and
sugar molecules become tighter, increasing the inelasticity of the
spine.
In summary, during the first years of adulthood,
intervertebral discs and the surrounding cartilage domains become
stiffer, the height of the disc decreases, and the amount of water
inside the disc is reduced, rendering the poorly supplied disc
unable to sustain multi-direction loads (35). The changes described above, which
are early deteriorations observed over the spine, are known as
intervertebral chondrosis (1).
In the following years of adult life, more
deteriorated detriments accumulate over the spinal cord. During
this stage, the percentage of intra-disc water is further reduced,
causing a significant downside to the intervertebral disc compared
with the stage of intervertebral chondrosis (1). In addition, the nucleus pulposus and
the internal part of the annulus fibrosus are the most affected
during this stage. By contrast, the outer part of the annulus
fibrosus is less affected. As a result, internal part of the disc
prolapses through the healthier outer part of the annulus fibrosus
(1).
Apart from the intervertebral disc, cartilage and
spongiosa are affected at this stage. The reason is that the
degenerated disc does not function sufficiently, and some acting
loads are forced onto the adjacent structures of the disc. This
causes a disorder of the natural architecture of the cartilage
endplates and the formation of ossification, while the vertebral
spongiosa becomes sclerotic and thicker (1). This second stage of spinal
degeneration is termed intervertebral osteochondrosis.
As aforementioned, the intervertebral disc is an
avascular formation. Nonetheless, during the aging procedure, newly
formed blood vessels penetrate the nucleus pulposus through the
tears of the annulus fibrosus or the end plates of the vertebrae
(48,49). The exact mechanism of the deployment
of blood vessels remains to be determined. High-quantity
glycosaminoglycan formations, such as intervertebral discs resist
the deployment of new vessels. During aging, the quantity of
glycosaminoglycans is reduced in the intervertebral disc, allowing
them to penetrate new vessels. Angiogenesis may be a potential
repair mechanism of the spine for age-related degeneration
(31) or the outcome of reduced
levels of glycosaminoglycans. The only confirmed fact is that the
penetration of blood vessels inside the nucleus pulposus changes
its structure. The expression of metalloproteinases near the newly
developed vessels of the intervertebral disc can contribute to
these changes (50).
All the age-related changes in the architecture of
the vertebral column described above affect its stability and
efficiency in resisting forces during standing or body movements.
The degenerated intervertebral disc cannot stand the loads during
acting, inflicting an increased load stress on the adjacent
articular cartilage of vertebrae and their end plates (44). To reduce the instability of the
spine, multiple bony particles (osteophytes) are formed (51) at the edge of the vertebrae. Lamellar
bone covers osteophytes, which have spongiosa similar to that of
the vertebrae (52). These
osteophytes increase the area of the area that sustains the
compression and make the arthrosis more stable. Spondylosis
deformations enhance this effect, and osteophytes are common in the
more mobile cranial part of the cervical spine. At the same time,
they are uncommon in the caudal part (53,54).
While the formation of osteophytes is a well-known
defense mechanism to stabilize degenerated arthrosis, the exact
mechanism of osteophyte formation is controversial. Schmorl's first
model postulates that the fissures and tears in the outer zone of
the annulus fibrosus make the intervertebral disc complex, and the
nearby vertebral bodies more unstable and susceptible to
pathological movements. The outcome of these movements is that the
anterior longitudinal ligament sustains an increasing load, which
is transferred to ligament insertions on the surface of vertebral
bodies. Additionally, the intervertebral disc presses the anterior
longitudinal ligament during these movements, increasing the
tension at the ligament insertions. The outcome of this continuous
stress is the formation of osteophytes at the insertions of the
anterior longitudinal ligament (55). The second model, described by
Collins (56), proposes that the
fissures and tears in the outer zone of the annulus fibrosus are
the ports through which tissue from the degenerated disc penetrates
out of the nucleus puplosus. During this time, the collected
penetrating disc tissue near the vertebrae edges is ossified,
resulting in the formation of vertebrae body osteophytes (56). In summary, both models propose that
the anterior longitudinal ligament plays a key role in the
formation of osteophytes. This is unusual, considering that the
common location where osteophytes are formed is the ventral surface
of the vertebrae just caudally to the vertebrae edges, a location
where the anterior longitudinal ligament is not sufficiently strong
(53).
The sum of all age-related spine deformations
affects not only the intervertebral disc, but also the joints of
the spinal cord. Osteochondrosis of the vertebral end plates and
intervertebral discs alters the segmentation of the acting loads
(44). In addition, the joint is
forced to participate in a greater range of movements (57) due to the instability caused by the
degeneration of the spine (51).
These structural changes, combined with hypermobility, are believed
to induce the formation of tears in cartilage and
osteoarthritis-like deformation of the facet joints. Additionally,
intense sclerosis is found in the subchondral formations, while the
final step of degeneration is the hyalinization of the
zygapophyseal joints and the formation of osteophytes (52).
Furthermore, due to the change in load segmentation,
uncovertebral processes and Luschka joints are forced to resist
higher forces (44), resulting in
the flattening of uncovertebral processes (1,44). The
load segmentation change, in combination with the flattening of
uncovertebral processes, increasing the load on the articular
cartilage and the adjacent end plate of the vertebrae (44), inflicting further damage to these
structures. Additionally, the flatter uncovertebral processes are a
potential place for osteophyte formation (52). These osteophytes can grow in the
direction of the transverse foramen and compress the vertebral
artery, particularly during extreme neck movements, causing severe
hypoperfusion to the cervical part of the medulla spinalis
(58).
Finally, the overgrowing osteophytes can compress
the ligamentum flavum, bending it and making it harder (59). This bent ligamentum flavum can
inflict direct pressure on the medulla spinalis and the vertebral
artery, causing lesions to the spinal cord due to pressure or
hypoperfusion.
Canal size
The medulla spinalis is a delicate neural formation
inside a protective cage known as the spinal canal of the vertebral
column. The size of the spinal canal differs along the spinal cord
or among the sexes (males appear to have a wider canal in all the
cervical segments compared with females) (60). The wider part is located in the
lumbar spine, while the diameter of the spinal canal is reduced
when during cranial movements. The anteroposterior diameter of the
canal between C3 and C7 segments has been reported to be 17-18 mm
(61,62), while other reports have demonstrated
a decrease to the considered normal sagittal diameter of the canal
to 14.1±1.6 and 13.73±1.37 mm (60,63).
Various researchers have reported that spinal canal
stenosis is a key factor predisposing to the development of the
direct compression of the medulla spinalis and cervical myelopathy
(38,63,64).
The fact that individuals with congenital canal stenosis are more
susceptible to cervical myelopathy (65,66)
supports this theory. Moreover, the size of the cervical canal is
considerably reduced in patients with cervical myelopathy compared
with healthy individuals (37,67).
By contrast, myelopathy symptoms are more severe in patients with a
considerably decreased canal size (63). Direct measurements in patients and
cadavers have demonstrated that a compromise of the canal's acreage
<60 mm2 (68) or the
canal's sagittal diameter <13 mm (69) is associated with an increased
possibility of developing cervical myelopathy (37,44).
On the other hand, individuals with a canal diameter between 13 and
17 mm have a reduced possibility of developing myelopathy. However,
they can still present signs of cervical spondylosis, and
individuals with a canal diameter >17 mm will not develop
cervical spondylosis (37).
Spondylosis, as aforementioned, is the spinal cord's
normal aging procedure and includes a group of changes, such as the
deterioration of the intravertebral disc, the hypertrophy and
ossification of the spine's ligaments, and the formation of
osteophytic spurs (32,70). These changes compromise the size of
the spinal canal, inflicting direct pressure on the medulla.
Chronic pressure is a predisposing factor for developing CSM
(32,44,70).
Moreover, when spondylosis and congenitally narrowed canals
coexist, the possibility of developing CSM increases (44).
Dynamic compression
Although the model described above appears
sufficient, it fails to explain the onset of myelopathy in patients
with minimal compromise of the spinal canal and the absence of
symptoms in healthy individuals with spinal canal stenosis
(71,72). Moreover, cervical myelopathy
increases in incidence in individuals with extreme or
unphysiological neck movements (64,71,73-75).
Subsequently, static compression of the medulla from spondylotic
formations does not appear to be the unique pathophysiological
model that describes spondylotic myelopathy.
To explain that paradox, the motion physiology of
the spinal cord needs to be studied. During normal flexion and
extension, the morphology of the spine is altered, affecting the
diameter of the spinal canal (76,77).
In flexion, the spinal canal is elongated, and the spinal cord is
stretched, inducing axial tension (72,76).
Typically, the cervical and lumbar spine are the most mobile parts
of the spinal cord; thus, it is logical that the white and grey
matter of these spine parts are stressed the most (78,79).
In extension, the spinal canal is narrowed due to the shingling of
the laminae and buckling of the ligamentum flavum, while the spinal
cord itself becomes shorter and thicker (42,76).
Additionally, during a shift from flexion to extension, the bulging
of the intervertebral discs and ligamentum flavum decreases the
diameter of the spinal canal (80).
Moreover, the canal is compressed by intervertebral discs and
ligamentum flavum bulging when a load is inflicted upon the spinal
cord. These modifications of the architecture of the spine during
motion inflict direct pressure on the cervical medulla (81), and are predisposing factors for the
development of CSM. The observation advocates the theory that
extreme cervical spine movements are associated with progressive
CSM (73-75).
An additional supporting argument is that surgical decompression
and stabilization of the spine, which decrease the pressure over
the cord and eliminate the abnormal motion, improve the clinical
status of patients with CSM (82-85).
Of note, age-related degenerative changes in the
spine exacerbate the dynamic compression of the cord. In flexion,
the spinal cord can be farther stretched over anterior osteophytes
or calcified herniated discs, while in extension, the buckling
ligamentum flavum compresses the cord (69,86).
As previously demonstrated in a clinical protocol, the cord's
compression during motion by ventral osteophytes can induce chronic
stretching and shear injury to the dorsal cord (87). This fact supports the theory that
dynamic compression, in combination with spondylosis, is a
predisposal factor for developing CSM. Moreover, age-related
changes in the spine can induce cord pressure and, consequently,
CSM through local tethering action. In individuals with no
spondylosis, the strain during motion of the vertebral column is
split over the entire spinal cord. By contrast, in individuals with
spondylotic deformation, the strain is focused adjacent to
age-related formations (72). A
potential explanation is that spine ligaments induce tethering
stress over the cord in areas near spondylotic deformations during
flexion and extension (72).
Ischemia
The notion that ischemia contributes to the
development of CSM is not a new one, but remains controversial.
Numerous protocols support this theory. Specifically, the anterior
spinal artery and parenchymal arterioles present pathological
changes, such as vessel wall thickening and hyalinization (88,89).
By contrast, radicular artery diameter is affected by the fibrosis
of intervertebral foramina in patients with CSM (90). Additionally, histopathological clues
of ischemic injury over the grey and white matter of the spine have
been observed in patients with CSM (76,91).
A pathophysiologic explanation is that time-related
degenerative formations of the cervical spine can compress major
feeding arteries such as the vertebral arteries (33), the anterior spinal artery and its
ventral branches, or the radicular arteries of the neuroforamina
(73,89). As a result, the blood flow velocity
within the vertebral artery can be abnormally reduced (92), while blood perfusion to vital parts
of the spinal cord is compromised (93). Moreover, spondylotic deformities can
compress the venous outflow of the spine, reducing blood drainage
from the spine (73,89). Various studies on humans and animals
support this hypothesis. The outcome of a canine study where
terminal branches of the anterior spinal artery and penetrating
branches of the lateral pial plexus are curved and stretched around
degenerative formations of the spine was a decrease in blood flow
to corticospinal tracts (94).
Additionally, angiography studies on animal models suffering from
CSM have revealed signs of ischemia (95,96).
Other researchers have examined the simultaneous insult of direct
compression and ischemia to the cord. In detail, ischemia appears
to enhance the injury due to the anterior compression over the
medulla (94), changing blood flow
to the spinal cord (97). In this
protocol, corticospinal tracts are the most affected part of the
medulla (94), which has also been
found in patients with CSM (69).
In another experimental protocol, the direct compression of
specific spine arteries causes a decrease in blood flow to the
respective artery's feeding part of the spine (98).
On the other hand, there are some clinical and
experimental protocols that fail to associate ischemia with CSM. In
detail, patients or laboratory animals with moderate CSM have no
(99) or only mild signs of
ischemia (100,101). By contrast, pathological evidence
of ischemia has only been found when severe canal stenosis coexists
(102,103). Moreover, some experimental studies
have only found minor changes in blood flow during compression and
decompression (79,104).
3. Conclusion
Spondylosis is a multi-factor cause of cervical
myelopathy. The onset of CSM-related symptoms is insidious and if
left untreated, it can cause severe disability in affected
patients. Given the fact that spondylotic changes take time to be
developed and that the population is gradually becoming older, CSM
will be one of the most common health issues among elderly patients
in the future. A better understanding of the mechanism that drives
to the formation of spondylotic changes will aid in the development
of more effective treatment and preventive strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
GF and KF conceptualized the study. IGL, VEG, PP,
NT, PS, GF, KF and DAS made a substantial contribution to the
interpretation and analysis of the literature data to be include in
the review, and wrote and prepared the draft of the manuscript. GF
and KF analyzed the data from the literature for inclusion in the
review and provided critical revisions. All authors contributed to
manuscript revision, and have read and approved the final version
of the manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Prescher A: Anatomy and pathology of the
aging spine. Eur J Radiol. 27:181–195. 1998.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nguyen C, Sanchez K, Roren A, Palazzo C,
Falcou L, Drapé JL, Rannou F, Poiraudeau S and Lefèvre-Colau MM:
Anatomical specificities of the degenerated cervical spine: A
narrative review of clinical implications, with special focus on
targeted spinal injections. Ann Phys Rehabil Med. 59:276–281.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Maiman DJ and Yoganandan N: Biomechanics
of cervical spine trauma. In: Clinical neurosurgery. Black P (ed).
Vol 97. Williams & Wilkins, Baltimore, MD, pp543-570, 1991.
|
|
4
|
Neidlinger-Wilke C, Würtz K, Liedert A,
Schmidt C, Börm W, Ignatius A, Wilke HJ and Claes L: A
three-dimensional collagen matrix as a suitable culture system for
the comparison of cyclic strain and hydrostatic pressure effects on
intervertebral disc cells. J Neurosurg Spine. 2:457–465.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Seipelt H, Griefahn B and Wiersbitzky H:
Calcinosis of intervertebral disks-relatively rare, heterogenous
and mostly benign. Z Arztl Fortbild (Jena). 81:603–605.
1987.PubMed/NCBI(In German).
|
|
6
|
Yasuma T, Suzuki F, Koh S and Yamauchi Y:
Pathological changes in the cartilaginous plates in relation to
intervertebral disc lesions. Acta Pathol Jpn. 38:735–750.
1988.PubMed/NCBI View Article : Google Scholar
|
|
7
|
White AA and Panjabi MM: Clinical
biomechanics of the spine, 2nd edition. J.B. Lippincott,
Philadelphia, PA, 1990.
|
|
8
|
Ratish S, Gao ZX, Prasad HM, Pei Z and
Bijendra D: Percutaneous endoscopic lumbar spine surgery for lumbar
disc herniation and lumbar spine stenosis: Emphasizing on clinical
outcomes of transforaminal technique. Surg Sci. 9:63–84. 2018.
|
|
9
|
Kumaresan S, Yoganandan N and Pintar FA:
Posterior complex contribution to the axial compressive and
distraction behavior of the cervical spine. J Musculoskeletal Res.
2:257–265. 1998.
|
|
10
|
Onan OA, Heggeness MH and Hipp JA: A
motion analysis of the cervical facet joint. Spine (Phila Pa 1976).
23:430–439. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jonas R, Demmelmaier R and Wilke HJ:
Influences of functional structures on the kinematic behavior of
the cervical spine. Spine J. 20:2014–2024. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yoganandan N, Kumaresan S and Pintar FA:
Biomechanics of the cervical spine Part 2. Cervical spine soft
tissue responses and biomechanical modeling. Clin Biomech (Bristol,
Avon). 16:1–27. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kumaresan S, Yoganandan N and Pintar FA:
Methodology to quantify the uncovertebral joint in the human
cervical spine. J Musculoskeletal Res. 1:1–9. 1997.
|
|
14
|
Sherk HH, Dunn EJ, Eismont FJ, Fielding
JW, Long DM, Ono K, Penning L and Raynor R: The cervical spine, 2nd
edition. Philadelphia, PA: Lippincott, 1989.
|
|
15
|
Bland JH: Luschka's Joint. Arch Intern
Med. 116(635)1965.
|
|
16
|
Hayashi K and Yabuki T: Origin of the
uncus and of Luschka's joint in the cervical spine. J Bone Joint
Surg Am. 67:788–791. 1985.PubMed/NCBI
|
|
17
|
Hattori S, Oda H and Kawai S: Cervical
intradiscal pressure in movements and traction of the cervical
spine. Z Orthop. 119:568–569. 1981.
|
|
18
|
Pospiech J, Stolke D, Wilke HJ and Claes
LE: Intradiscal pressure recordings in the cervical spine.
Neurosurgery. 44:379–385. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nagamoto Y, Ishii T, Iwasaki M, Sakaura H,
Moritomo H, Fujimori T, Kashii M, Murase T, Yoshikawa H and
Sugamoto K: Three-dimensional motion of the uncovertebral joint
during head rotation. J Neurosurg Spine. 17:327–333.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wolfla CE: Adult and child and neck
anatomy. In: Yoganandan N, Pintar FA, Larson SJ and Sances A (eds).
Frontiers in head and neck trauma: Clinical and biomechanical. IOS
Press, Amsterdam, pp18-33, 1998.
|
|
21
|
Yoganandan N, Pintar F, Butler J, Reinartz
J, Sances A Jr and Larson SJ: Dynamic response of human cervical
spine ligaments. Spine (Phila Pa 1976). 14:1102–1110.
1989.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yoganandan N, Kumaresan S and Pintar FA:
Geometric and mechanical properties of human cervical spine
ligaments. J Biomech Eng. 122:623–629. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Myklebust JB, Pintar F, Yoganandan N,
Cusick JF, Maiman D, Myers TJ and Sances A Jr: Tensile strength of
spinal ligaments. Spine (Phila Pa 1976). 13:526–531.
1988.PubMed/NCBI
|
|
24
|
Ohara Y: Ossification of the ligaments in
the cervical spine, including ossification of the anterior
longitudinal ligament, ossification of the posterior longitudinal
ligament, and ossification of the ligamentum flavum. Neurosurg Clin
N Am. 29:63–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Barros EMKP, Rodrigues CJ, Rodrigues NR,
Oliveira RP, Barros TEP and Rodrigues AJ Jr: Aging of the elastic
and collagen fibers in the human cervical interspinous ligaments.
Spine J. 2:57–62. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yahia LH, Garzon S, Strykowski H and
Rivard CH: Ultrastructure of the human interspinous ligament and
ligamentum flavum. A preliminary study. Spine (Phila Pa 1976).
15:262–268. 1990.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yong-Hing K, Reilly J and Kirkaldy-Willis
WH: The ligamentum flavum. Spine. 1:226–234. 1976.
|
|
28
|
Goel VK, Clark CR, McGowan D and Goyal S:
An in-vitro study of the kinematics of the normal, injured and
stabilized cervical spine. J Biomech. 17:363–376. 1984.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zdeblick TA, Abitbol JJ, Kunz DN, McCabe
RP and Garfin S: Cervical stability after sequential capsule
resection. Spine (Phila Pa 1976). 18:2005–2008. 1993.PubMed/NCBI View Article : Google Scholar
|
|
30
|
De Geer CM: Intervertebral disk nutrients
and transport mechanisms in relation to disk degeneration: A
narrative literature review. J Chiropr Med. 17:97–105.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Walsh DA: Angiogenesis in osteoarthritis
and spondylosis: Successful repair with undesirable outcomes. Curr
Opin Rheumatol. 16:609–615. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tracy JA and Bartleson JD: Cervical
spondylotic myelopathy. Neurologist. 16:176–187. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
McCormack BM and Weinstein PR: Cervical
spondylosis. An update. West J Med. 165:43–51. 1996.PubMed/NCBI
|
|
34
|
Ito T, Oyanagi K, Takahashi H, Takahashi
HE and Ikuta F: Cervical spondylotic myelopathy. Clinicopathologic
study on the progression pattern and thin myelinated fibers of the
lesions of seven patients examined during complete autopsy. Spine
(Phila Pa 1976). 21:827–833. 1996.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Adams MA and Dolan P: Spine biomechanics.
J Biomech. 38:1972–1983. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Benneker LM, Heini PF, Anderson SE, Alini
M and Ito K: Correlation of radiographic and MRI parameters to
morphological and biochemical assessment of intervertebral disc
degeneration. Eur Spine J. 14:27–35. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Morishita Y, Naito M, Hymanson H, Miyazaki
M, Wu G and Wang JC: The relationship between the cervical spinal
canal diameter and the pathological changes in the cervical spine.
Eur Spine J. 18:877–883. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gore DR: Roentgenographic findings in the
cervical spine in asymptomatic persons: A ten-year follow-up. Spine
(Phila Pa 1976). 26:2463–2466. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kuwazawa Y, Pope MH, Bashir W, Takahashi K
and Smith FW: The length of the cervical cord: effects of postural
changes in healthy volunteers using positional magnetic resonance
imaging. Spine (Phila Pa 1976). 31:E579–E583. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Boden SD, McCowin PR, Davis DO, Dina TS,
Mark AS and Wiesel S: Abnormal magnetic-resonance scans of the
cervical spine in asymptomatic subjects. A prospective
investigation. J Bone Joint Surg Am. 72:1178–1184. 1990.PubMed/NCBI
|
|
41
|
Shedid D and Benzel EC: Cervical
spondylosis anatomy: Pathophysiology and biomechanics.
Neurosurgery. 60 (1 Supp1 1):S7–S13. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rao R: Neck pain, cervical radiculopathy,
and cervical myelopathy: Pathophysiology, natural history, and
clinical evaluation. J Bone Joint Surg Am. 84:1872–1881.
2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tang X, Jing L, Richardson WJ, Isaacs RE,
Fitch RD, Brown CR, Erickson MM, Setton LA and Chen J: Identifying
molecular phenotype of nucleus pulposus cells in human
intervertebral disc with aging and degeneration. J Orthop Res.
34:1316–1326. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Baptiste DC and Fehlings MG:
Pathophysiology of cervical myelopathy. Spine J. 6 (6
Suppl):190S–197S. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Horner HA and Urban JP: 2001 Volvo award
winner in basic science studies: Effect of nutrient supply on the
viability of cells from the nucleus pulposus of the intervertebral
disc. Spine (Phila Pa 1976). 26:2543–2549. 2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bayliss MT, Hutton S, Hayward J and
Maciewicz RA: Distribution of aggrecanase (ADAMts 4/5) cleavage
products in normal and osteoarthritic human articular cartilage:
The influence of age, topography, and zone of tissue.
Osteoarthritis Cartilage. 9:553–560. 2001.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Duance VC, Crean JK, Sims TJ, Avery N,
Smith S, Menage J, Eisenstein SM and Roberts S: Changes in collagen
cross-linking in degenerative disc disease and scoliosis. Spine
(Phila Pa 1976). 23:2545–2551. 1998.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Brown MF, Hukkanen MV, McCarthy ID,
Redfern DR, Batten JJ, Crock HV, Hughes SP and Polak JM: Sensory
and sympathetic innervation of the vertebral endplate in patients
with degenerative disc disease. J Bone Joint Surg Br. 79:147–153.
1997.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Freemont AJ, Watkins A, Le Maitre C, Baird
P, Jeziorska M, Knight MT, Ross ER, O'Brien JP and Hoyland JA:
Nerve growth factor expression and innervation of the painful
intervertebral disc. J Pathol. 197:286–292. 2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Roberts S, Caterson B, Menage J, Evans EH,
Jaffray DC and Eisenstein SM: Matrix metalloproteinases and
aggrecanase: Their role in disorders of the human intervertebral
disc. Spine (Phila Pa 1976). 25:3005–3013. 2000.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Carette S and Fehlings MG: Clinical
practice. Cervical radiculopathy. N Engl J Med. 353:392–399.
2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Llopis E, Belloch E, León JP, Higueras V
and Piquer J: The degenerative cervical spine. Radiologia. 58
(Suppl 1):S13–S25. 2016.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
53
|
Pesch HJ, Becker T, Bischoff W and Seibold
H: ‘Physiological osteoporosis’ and ‘osteoblast insufficiency’ in
old age. Comparative radiological-morphometric and statistical
studies on the spongy bone of lumbar and cervical vertebral bodies.
Arch Orthop Trauma Surg. 110:1–14. 1990.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ferrara LA: The biomechanics of cervical
spondylosis. Adv Orthop. 2012(493605)2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Palanca M, Ruspi ML, Cristofolini L,
Liebsch C, Villa T, Brayda-Bruno M, Galbusera F, Wilke HJ and La
Barbera L: The strain distribution in the lumbar anterior
longitudinal ligament is affected by the loading condition and bony
features: An in vitro full-field analysis. PLoS One.
15(e0227210)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Collins DH: The pathology of the articular
and spinal diseases. Edward Arnold, London, 1949.
|
|
57
|
Arlet V and Aebi M: Junctional spinal
disorders in operated adult spinal deformities: Present
understanding and future perspectives. Eur Spine J. 22 (Suppl
2):S276–S295. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Braun IF, Pinto RS, De Filipp GJ,
Lieberman A, Pasternack P and Zimmerman RD: Brain stem infarction
due to chiropractic manipulation of the cervical spine. South Med
J. 76:1507–1510. 1983.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Muthukumar N: Ossification of the
ligamentum flavum as a result of fluorosis causing myelopathy:
Report of two cases. Neurosurgery. 56(E622)2005.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lee MJ, Cassinelli EH and Riew KD:
Prevalence of cervical spine stenosis. Anatomic study in cadavers.
J Bone Joint Surg Am. 89:376–380. 2007.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Matsunaga S, Nakamura K, Seichi A,
Yokoyama T, Toh S, Ichimura S, Satomi K, Endo K, Yamamoto K, Kato
Y, et al: Radiographic predictors for the development of myelopathy
in patients with ossification of the posterior longitudinal
ligament: A multicenter cohort study. Spine (Phila Pa 1976).
33:2648–2650. 2008.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Bohlman HH: Cervical spondylosis and
myelopathy. Instr Course Lect. 44:81–97. 1995.PubMed/NCBI
|
|
63
|
Edwards WC and LaRocca H: The
developmental segmental sagittal diameter of the cervical spinal
canal in patients with cervical spondylosis. Spine (Phila Pa 1976).
8:20–27. 1983.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hayashi H, Okada K, Hamada M, Tada K and
Ueno R: Etiologic factors of myelopathy. A radiographic evaluation
of the aging changes in the cervical spine. Clin Orthop Relat Res.
200–209. 1987.PubMed/NCBI
|
|
65
|
Schmidt MH, Quinones-Hinojosa A and
Rosenberg WS: Cervical myelopathy associated with degenerative
spine disease and ossification of the posterior longitudinal
ligament. Semin Neurol. 22:143–148. 2002.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Houten JK and Cooper PR: Laminectomy and
posterior cervical plating for multilevel cervical spondylotic
myelopathy and ossification of the posterior longitudinal ligament:
Effects on cervical alignment, spinal cord compression, and
neurological outcome. Neurosurgery. 52:1081–1078. 2003.PubMed/NCBI
|
|
67
|
Miura J, Doita M, Miyata K, Marui T,
Nishida K, Fujii M and Kurosaka M: Dynamic evaluation of the spinal
cord in patients with cervical spondylotic myelopathy using a
kinematic magnetic resonance imaging technique. J Spinal Disord
Tech. 22:8–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Cooper PR and Epstein F: Radical resection
of intramedullary spinal cord tumors in adults. Recent experience
in 29 patients. J Neurosurg. 63:492–499. 1985.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Fehlings MG and Skaf G: A review of the
pathophysiology of cervical spondylotic myelopathy with insights
for potential novel mechanisms drawn from traumatic spinal cord
injury. Spine (Phila Pa 1976). 23:2730–2737. 1998.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Debois V, Herz R, Berghmans D, Hermans B
and Herregodts P: Soft cervical disc herniation. Influence of
cervical spinal canal measurements on development of neurologic
symptoms. Spine (Phila Pa 1976). 24:1996–2002. 1999.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Albert TJ and Vacarro A: Postlaminectomy
kyphosis. Spine (Phila Pa 1976). 23:2738–2745. 1998.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Henderson FC, Geddes JF, Vaccaro AR,
Woodard E, Berry KJ and Benzel EC: Stretch-associated injury in
cervical spondylotic myelopathy: New concept and review.
Neurosurgery. 56:1101–1113. 2005.PubMed/NCBI
|
|
73
|
Matz PG, Pritchard PR and Hadley MN:
Anterior cervical approach for the treatment of cervical
myelopathy. Neurosurgery. 60 (1 Supp1 1):S64–S70. 2007.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ferguson RJ and Caplan LR: Cervical
spondylitic myelopathy. Neurol Clin. 3:373–382. 1985.PubMed/NCBI
|
|
75
|
Muhle C, Metzner J, Weinert D, Schön R,
Rautenberg E, Falliner A, Brinkmann G, Mehdorn HM, Heller M and
Resnick D: Kinematic MR imaging in surgical management of cervical
disc disease, spondylosis and spondylotic myelopathy. Acta Radiol.
40:146–153. 1999.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kimura M, Ito K, Onizuka J, Hirayama M and
Kuriyama M: Cervical spinal cord infarction in a patient with
unilateral internal carotid artery occlusion and cervical
spondylosis. Rinsho Shinkeigaku. 37:927–929. 1997.(In
Japanese).
|
|
77
|
Otani K, Sato K, Yabuki S, Iwabuchi M and
Kikuchi S: A segmental partial laminectomy for cervical spondylotic
myelopathy: Anatomical basis and clinical outcome in comparison
with expansive open-door laminoplasty. Spine (Phila Pa 1976).
34:268–273. 2009.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Polak-Kraśna K, Robak-Nawrocka S, Szotek
S, Czyż M, Gheek D and Pezowicz C: The denticulate ligament-tensile
characterisation and finite element micro-scale model of the
structure stabilising spinal cord. J Mech Behav Biomed Mater.
91:10–17. 2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Ichihara K, Taguchi T, Sakuramoto I,
Kawano S and Kawai S: Mechanism of the spinal cord injury and the
cervical spondylotic myelopathy: New approach based on the
mechanical features of the spinal cord white and gray matter. J
Neurosurg. 99 (3 Suppl):278–285. 2003.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zeng C, Xiong J, Wang JC, Inoue H, Tan Y,
Tian H and Aghdasi B: The evaluation and observation of ‘Hidden’
hypertrophy of cervical ligamentum flavum, cervical canal, and
related factors using kinetic magnetic resonance imaging. Global
Spine J. 6:155–163. 2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Chen CJ, Hsu HL, Niu CC, Chen TY, Chen MC,
Tseng YC, Wong YC and Wang LJ: Cervical degenerative disease at
flexion-extension MR imaging: Prediction criteria. Radiology.
227:136–142. 2003.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Maurer PK, Ellenbogen RG, Ecklund J,
Simonds GR, van Dam B and Ondra SL: Cervical spondylotic
myelopathy: Treatment with posterior decompression and Luque
rectangle bone fusion. Neurosurgery. 28:680–684. 1991.PubMed/NCBI
|
|
83
|
Lau D, Winkler EA, Than KD, Chou D and
Mummaneni PV: Laminoplasty versus laminectomy with posterior spinal
fusion for multilevel cervical spondylotic myelopathy: Influence of
cervical alignment on outcomes. J Neurosurg Spine. 27:508–517.
2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Park Y, Maeda T, Cho W and Riew KD:
Comparison of anterior cervical fusion after two-level discectomy
or single-level corpectomy: Sagittal alignment, cervical lordosis,
graft collapse, and adjacent-level ossification. Spine J.
10:193–199. 2010.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kim PK and Alexander JT: Indications for
circumferential surgery for cervical spondylotic myelopathy. Spine
J. 6 (Suppl 6):299S–307S. 2006.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Panjabi M and White A III: Biomechanics of
nonacute cervical spinal cord trauma. Spine (Phila Pa 1976).
13:838–842. 1988.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Smart KM, Blake C, Staines A, Thacker M
and Doody C: Mechanisms-based classifications of musculoskeletal
pain: Part 2 of 3: Symptoms and signs of peripheral neuropathic
pain in patients with low back (± leg) pain. Man Ther. 17:345–351.
2012.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Tu J, Vargas Castillo J, Das A and Diwan
AD: Degenerative cervical myelopathy: Insights into its
pathobiology and molecular mechanisms. J Clin Med.
10(1214)2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Dohle E, Beardall S, Chang A, Mena KPC,
Jovanović L, Nath U, Lee KS, Smith AH, Thirunavukarasu AJ, Touzet
AY, et al: Human spinal cord tissue is an underutilised resource in
degenerative cervical myelopathy: Findings from a systematic review
of human autopsies. Acta Neurochir (Wien). 165:1121–1131.
2023.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Lestini WF and Wiesel SW: The pathogenesis
of cervical spondylosis. Clin Orthop Relat Res. 69–93.
1989.PubMed/NCBI
|
|
91
|
Lee WH, Lee SU and Jung SH: Ischemic
cervical myelopathy caused by vertebral artery dissection: The
clinical utility of a motor-evoked potential study. Neurologist.
21:8–10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Strek P, Reroń E, Maga P, Modrzejewski M
and Szybist N: A possible correlation between vertebral artery
insufficiency and degenerative changes in the cervical spine. Eur
Arch Otorhinolaryngol. 255:437–440. 1998.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Hashizume Y, Iijima S, Kishimoto H and
Yanagi T: Pathology of spinal cord lesions caused by ossification
of the posterior longitudinal ligament. Acta Neuropathol.
63:123–130. 1984.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Gooding MR, Wilson CB and Hoff JT:
Experimental cervical myelopathy. Effects of ischemia and
compression of the canine cervical spinal cord. J Neurosurg.
43:9–17. 1975.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Wilson CB, Bertan V, Norrell HA Jr and
Hukuda S: Experimental cervical myelopathy. II. Acute ischemic
myelopathy. Arch Neurol. 21:571–589. 1969.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Hukuda S and Wilson CB: Experimental
cervical myelopathy: Effects of compression and ischemia on the
canine cervical cord. J Neurosurg. 37:631–652. 1972.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Gooding MR, Wilson CB and Hoff JT:
Experimental cervical myelopathy: Autoradiographic studies of
spinal cord blood flow patterns. Surg Neurol. 5:233–239.
1976.PubMed/NCBI
|
|
98
|
Pavlov PW: Anterior decompression for
cervical spondylotic myelopathy. Eur Spine J. 12 (Suppl
2):S188–S194. 2003.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Pranteda G, Magri F, Moliterni E, Pranteda
G and Quaglino P: Sannino-barduagni-bottoni syndrome. G Ital
Dermatol Venereol. 154:732–734. 2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
al-Mefty O, Harkey HL, Marawi I, Haines
DE, Peeler DF, Wilner HI, Smith RR, Holaday HR, Haining JL, Russell
WF, et al: Experimental chronic compressive cervical myelopathy. J
Neurosurg. 79:550–561. 1993.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Hoff JNM, Pitts L, Vilnis V, Tuerk K and
Lagger R: The role of ischemia in the pathogenesis of cervical
spondylotic myelopathy: A review and new microangiopathic evidence.
Spine (Phila Pa 1976). 2:100–108. 1977.
|
|
102
|
Ono K, Ota H, Tada K and Yamamoto T:
Cervical myelopathy secondary to multiple spondylotic protrusions;
a clinicopathologic study. Spine. 2:109–125. 1977.
|
|
103
|
Ogino H, Tada K, Okada K, Yonenobu K,
Yamamoto T, Ono K and Namiki H: Canal diameter, anteroposterior
compression ratio, and spondylotic myelopathy of the cervical
spine. Spine (Phila Pa 1976). 8:1–15. 1983.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Carlson GD, Warden KE, Barbeau JM, Bahniuk
E, Kutina-Nelson KL, Biro CL, Bohlman HH and LaManna JC:
Viscoelastic relaxation and regional blood flow response to spinal
cord compression and decompression. Spine (Phila Pa 1976).
22:1285–1291. 1997.PubMed/NCBI View Article : Google Scholar
|