Introduction
Human induced pluripotent stem (iPS) cells can
differentiate into somatic cells (1). Hepatocytes derived from iPS cells are
suitable for transplantation into patients with liver insufficiency
and for use in toxicity tests (2).
These methods have been previously used to obtain hepatocytes from
iPS cells.
Protocols for hepatocyte differentiation from iPS
cells have been studied. One such approach involves the use of
growth factors (3-6).
Another method involves the introduction of transcription factors
(6-8).
Human liver organoids are formed from the assembly of
hepatocyte-like cells differentiated from iPS cells, human
umbilical vascular endothelial cells and mesenchymal stem cells
(9). Human liver organoids are
expected to be used as a ‘mini-liver’ instead of resected liver
fragments (10). However,
hepatocytes, including organoids, remain immature following the
above protocols (11,12). Therefore, methods for obtaining
hepatocytes from iPS cells should be further investigated.
Glucose is essential for cell survival and is
metabolized to pyruvate (13).
Pyruvate enters the tricarboxylic acid cycle to generate energy.
Under low-oxygen conditions, pyruvate does not enter the
tricarboxylic acid cycle, but is instead metabolized to lactate.
Cancer cells continue to produce lactate via glycolysis when oxygen
is abundant (the Warburg effect) (14). Human embryonic stem cells also
exhibit the Warburg effect (15).
Cells die in glucose-deprived media (16). Under glucose-free conditions,
galactose is converted to galactose-1-phosphate and used as an
energy source (17). This cycle
involves gluconeogenesis and is performed solely by hepatocytes. A
Hepatocyte selection medium (HSM) was developed to enrich
hepatocytes from co-cultures with iPS cells (18). The HSM does not contain glucose, and
galactose is added. In the HSM, iPS cells died within three days;
however, hepatocytes survived. After two days of culture in HSM,
the expression levels of α-fetoprotein (AFP) and albumin, which are
hepatocyte markers, were upregulated, suggesting that hepatocyte
differentiation was initiated (19). It was hypothesized that the addition
of glucose and galactose promoted hepatocyte differentiation of iPS
cell. The HSM was modified into a hepatocyte differentiation
inducer (HDI). HDI is based on an HSM with the addition of
oncostatin M and an apoptosis inhibitor (20). iPS cells showing elevated
expressions survived for 7 days, but eventually died.
It was hypothesized that iPS cells would
differentiate into hepatocytes in a medium without glucose for
longer periods. Metabolic changes were focused in search for a
novel approach for iPS cells to survive under glucose deprivation.
Metabolic alterations need to be clarified to overcome the
short-term survival of iPS cells in HDI. Therefore, a metabolome
analysis was performed.
Materials and methods
Cell culture
A human iPS cell line, 201B7 (Riken Cell Bank), was
cultured on 10 cm dishes (Asahi Glass Co., Ltd.), six-well plates
(Asahi Glass Co., Ltd.) or 96-well plates (Asahi Glass Co., Ltd.)
coated with Matrigel® (Corning, Inc.) in ReproFF
(ReproCell) at 37˚C with 5% carbon dioxide in a humidified chamber.
When cells reached confluence, they were rinsed with PBS and
harvested using Acutase (Innovative Cell Technologies). The
cultured cells were observed under a microscope (CKX41N-31PHP;
Olympus Corp.).
Reagents
Calcium lactate was purchased from Nacalai Tesque
Inc. Sodium lactate was obtained from Sigma-Aldrich (Merck KGaA).
Lactic acid was purchased from Kozakai Pharmaceutical Co., Ltd.
Metabolome analysis
The metabolome analysis was performed by Human
Metabolome Technologies. Cells were cultured in 10-cm dishes at
confluency. The cells were processed according to the
manufacturer's instructions. In brief, culture medium was aspirated
and cells were rinsed with 10 ml of 5% mannitol solution (Wako Pure
Chemical Industries, Ltd.) in water. The rinse was repeated with 2
ml of 5% mannitol solution. After aspiration of 5% mannitol
solution, the entire dish was covered with 800 µl of 100% methanol
(Wako Pure Chemical Industries, Ltd.) and left for 30 sec.
Subsequently, 550 µl of Internal Standard Solution (Human
Metabolome Technologies) was added and slowly pipetted up and down
three times. The dishes were then incubated for 30 sec at room
temperature. A total of 1,000 µl was transferred from the total
1,350 µl of the supernatant to a 1.5 ml tube and kept on ice. The
transferred samples were centrifuged at 1,300 x g at 4˚C for 5 min.
Subsequently, 350 µl of the supernatant was transferred to a tube
with a filter cup supplied by the company and centrifuged at 1,100
x g, at 4˚C for 2 h. The filter cup was removed and the tube
containing the sample was sealed and frozen at 80˚C. The samples
were packed on dry ice, sent to Human Metabolome Technologies and
subjected to C-SCOPE, which measures metabolites related with
energy metabolism.
Cell proliferation assay
After 72 h, an MTS assay (Promega Corporation) was
performed according to the manufacturer's instructions. MTS was
bio-reduced by the cells into a colored formazan product with
reduced absorbance at 490 nm. The absorbance was analyzed at a
wavelength of 490 nm with an iMark microplate reader (Bio-Rad
Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA (5 mg), isolated with Isogen (Nippon Gene
Co., Ltd.), was used for first-strand cDNA synthesis with
SuperScript III reverse transcriptase and oligo (dT) primers
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Real-time qPCR was performed using Fast SYBR Green
Master Mix (Thermo Fisher Scientific, Inc.) and the results were
analyzed using a Mini Opticon system (Bio-Rad Laboratories, Inc.).
qPCR was performed for 40 cycles, with 5 sec for denaturation
(95˚C) and 5 sec for annealing-extension (60˚C), using an MJ Mini
Cycler (Bio-Rad Laboratories, Inc.). The primer sequences are
listed in Table I. Ribosomal
protein L19 (RPL19) was used as an endogenous control to monitor
the amount of mRNA because it is a constitutively expressed
house-keeping gene (21). Gene
expression levels were automatically analyzed using the Mini
Opticon system based on the 2-∆∆Cq method (22). The relative expression level of a
gene was calculated as the gene expression level divided by that of
RPL19.
 | Table IPrimers used for qPCR. |
Table I
Primers used for qPCR.
| GenBank
ID/description | Primer name | Sequence (5' to
3') | Product size, bp |
|---|
| NM_001134 | | | 147 |
|
qPCR, hAFP,
forward | OMC317 |
ACACAAAAAGCCCACTCCAG | |
|
qPCR, hAFP,
reverse | OMC318 |
GGTGCATACAGGAAGGGATG | |
| BC000530 | | | 157 |
|
qPCR,
hRPL19, forward | OMC321 |
CGAATGCCAGAGAAGGTCAC | |
|
qPCR,
hRPL19, reverse | OMC322 |
CCATGAGAATCCGCTTGTTT | |
| NM_000477 | | | 114 |
|
qPCR,
hAlbumin, forward | OMC329 |
GCTCGTGAAACACAAGCCCAAG | |
|
qPCR,
hAlbumin, reverse | OMC330 |
GCAAAGCAGGTCTCCTTATCGTC | |
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation. One-way analysis of variance was performed
using JMP 10.0.2 software (SAS Institute, Inc.). Tukey's test was
used as the post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Metabolome analysis
To analyze the differences in the metabolite
concentrations between the undifferentiated state and that of cells
cultured in HDI, metabolome analysis was performed. iPS cells were
cultured on 10-cm dishes in HDI for 2 days and the samples were
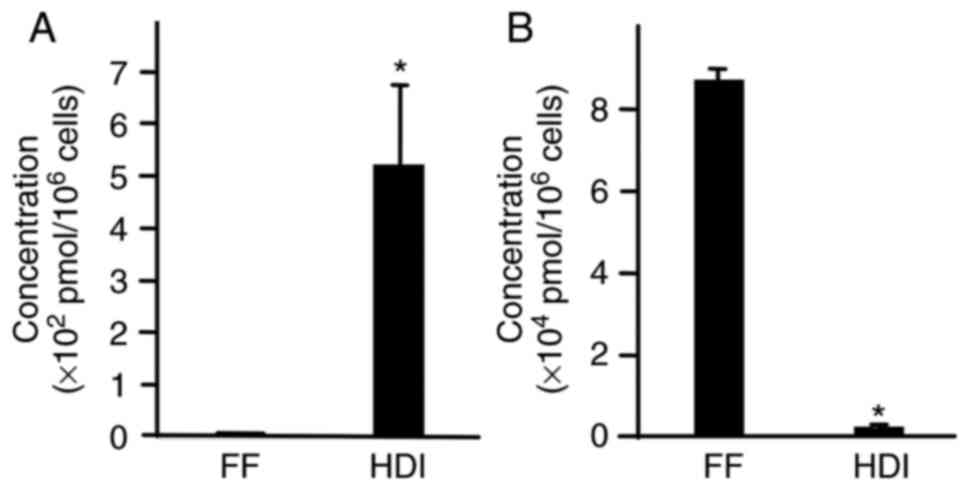
subjected to metabolome analysis. Among the metabolites,
galactose-1-phosphate was applied in order to confirm that
galactose entered gluconeogenesis. Lactate was also focused on
because it is a typical glycolytic metabolite (23). The galactose-1-phosphate
concentration, as measured by Human Metabolome Technologies, was
5.2±1.6x102 pmol/106 cells in HDI and
0.5±0.0x102 pmol/106 cells in ReproFF
(Fig. 1A). The lactate
concentration, as measured by Human Metabolome Technologies, was
2.1±0.4x103 pmol/106 cells in HDI and
8.7±0.3x104 pmol/106 cells in ReproFF
(Fig. 1B). As expected, galactose
in HDI was metabolized to galactose-1-phosphate. Unexpectedly, the
lactate concentration was significantly lower in cells cultured in
HDI than in those cultured in ReproFF. Lower lactate levels were
speculated to be related to limited survival.
Cell proliferation
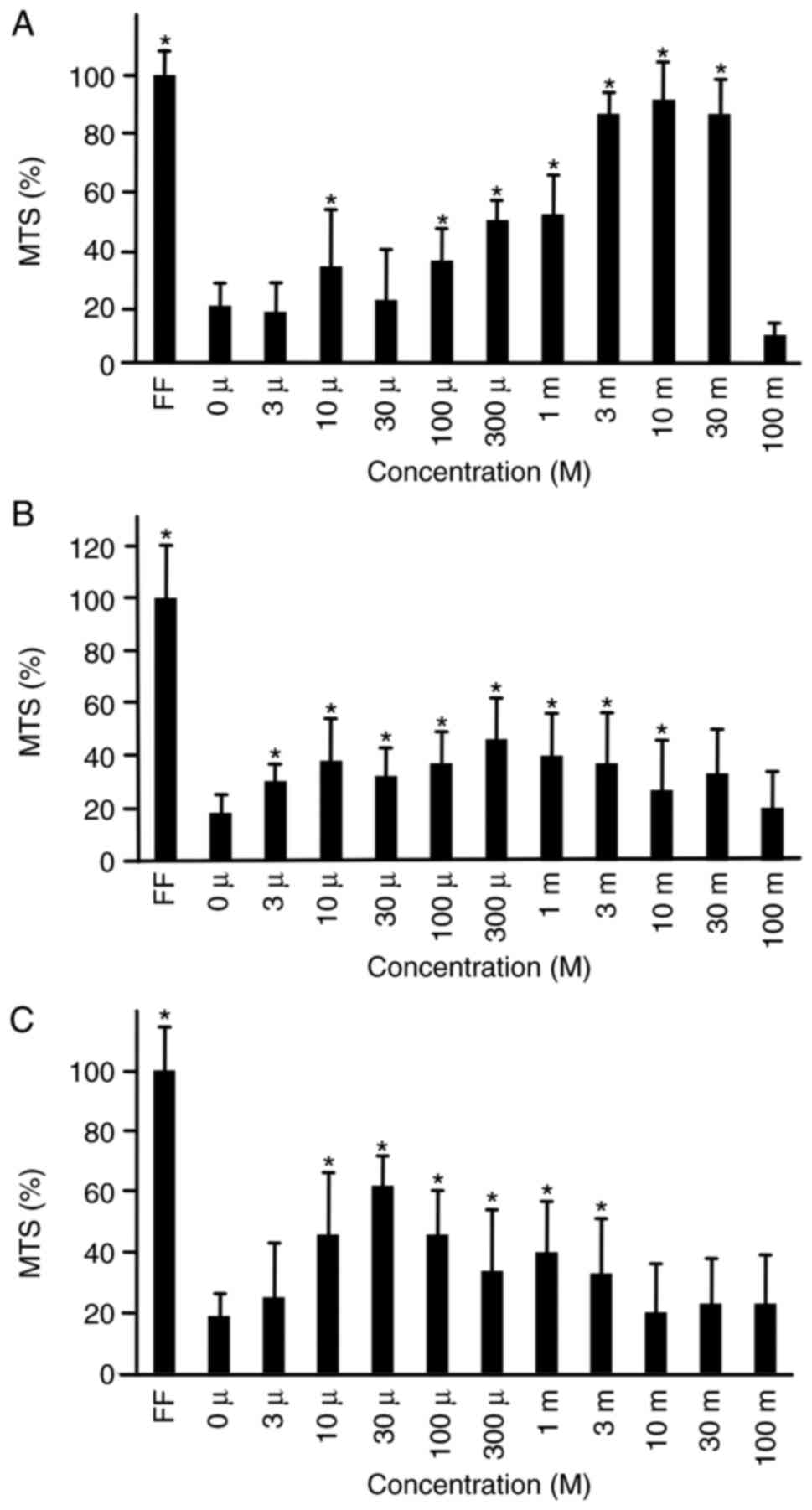
To analyze whether lactate prolongs iPS cell
survival in HSM, a cell proliferation assay was performed. Three
types of lactate were used: Calcium lactate, sodium lactate and
lactic acid. iPS cells were cultured in HSM supplemented with
calcium lactate (Fig. 2A), sodium
lactate (Fig. 2B) or lactic acid
(Fig. 2C) at concentrations of 0,
3, 10, 100 or 300 µM, and 1, 3, 10, 30 or 100 mM. After 72 h of
culture, cells were subjected to a cell proliferation assay. Cell
proliferation was 92±13, 87±7 and 52±14% in comparison to ReproFF,
at 10, 3 and 1 mM of calcium lacatate, respectively. Cells cultured
in calcium lactate showed the highest proliferation potential
compared to those cultured in sodium lactate and lactic acid.
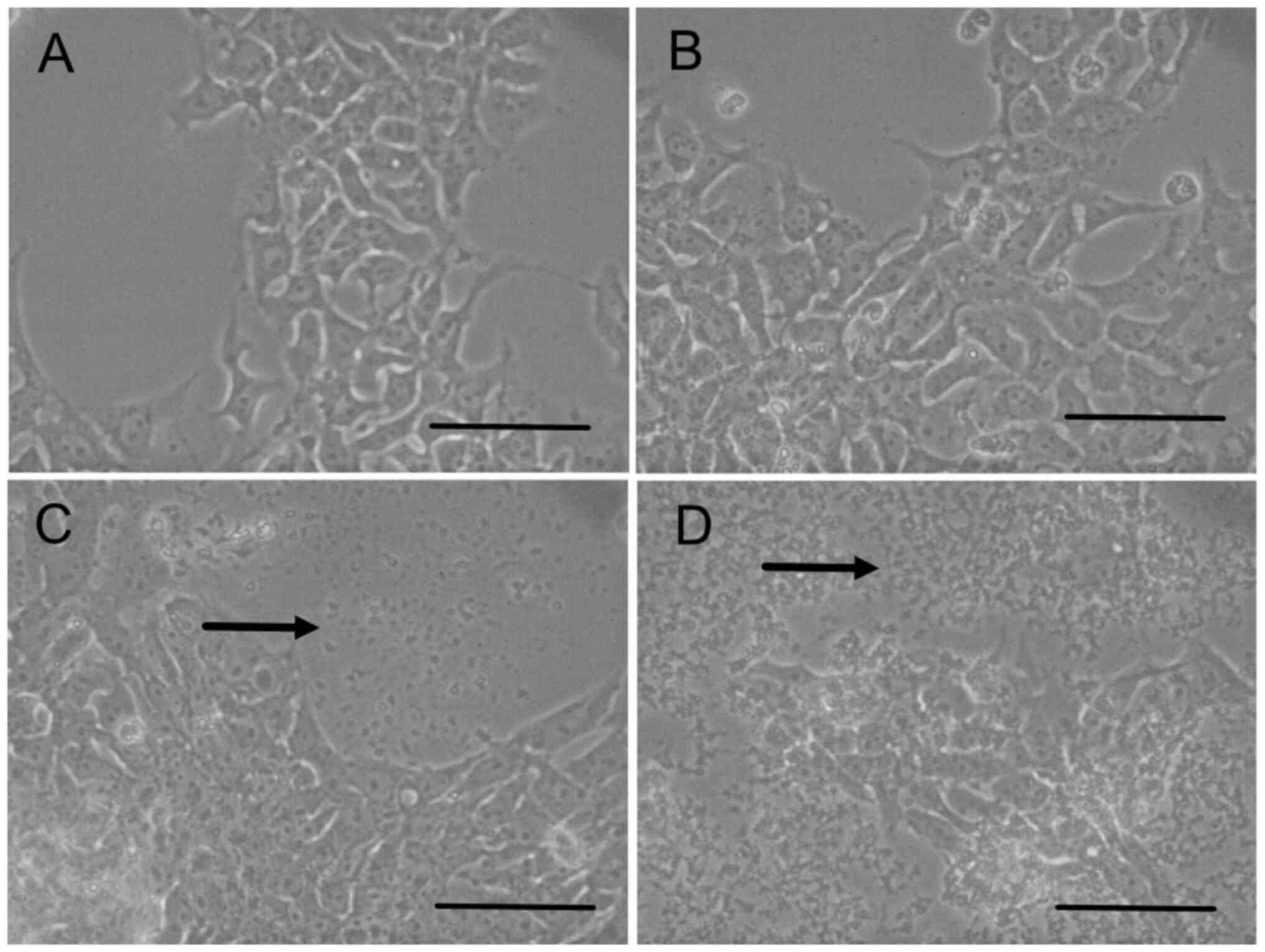
Precpitation
In the cell proliferation assay, cultured cells were
observed under a microscope. Precipitation was found at the bottom
of the dishes after 72 h of culture in HSM supplemented with >3
mM of calcium lactate. The medium was transferred to another dish
and cultured, and no bacteria or fungi grew. iPS cells were
cultured in HSM supplemented with calcium lactate at 0 mM (Fig. 3A), 1 mM (Fig. 3B), 3 mM (Fig. 3C) and 10 mM (Fig. 3D). Precipitation was observed at
concentrations of 3 and 10 mM. The precipitation was denser at 10
than at 3 mM. It was speculated that precipitation was formed with
calcium from calcium lactate and carbonate in the medium because
calcium carbonate is insoluble (24). Combined with the results of the cell
proliferation assay, these results suggested that 1 mM of calcium
lactate was suitable for further experiments.
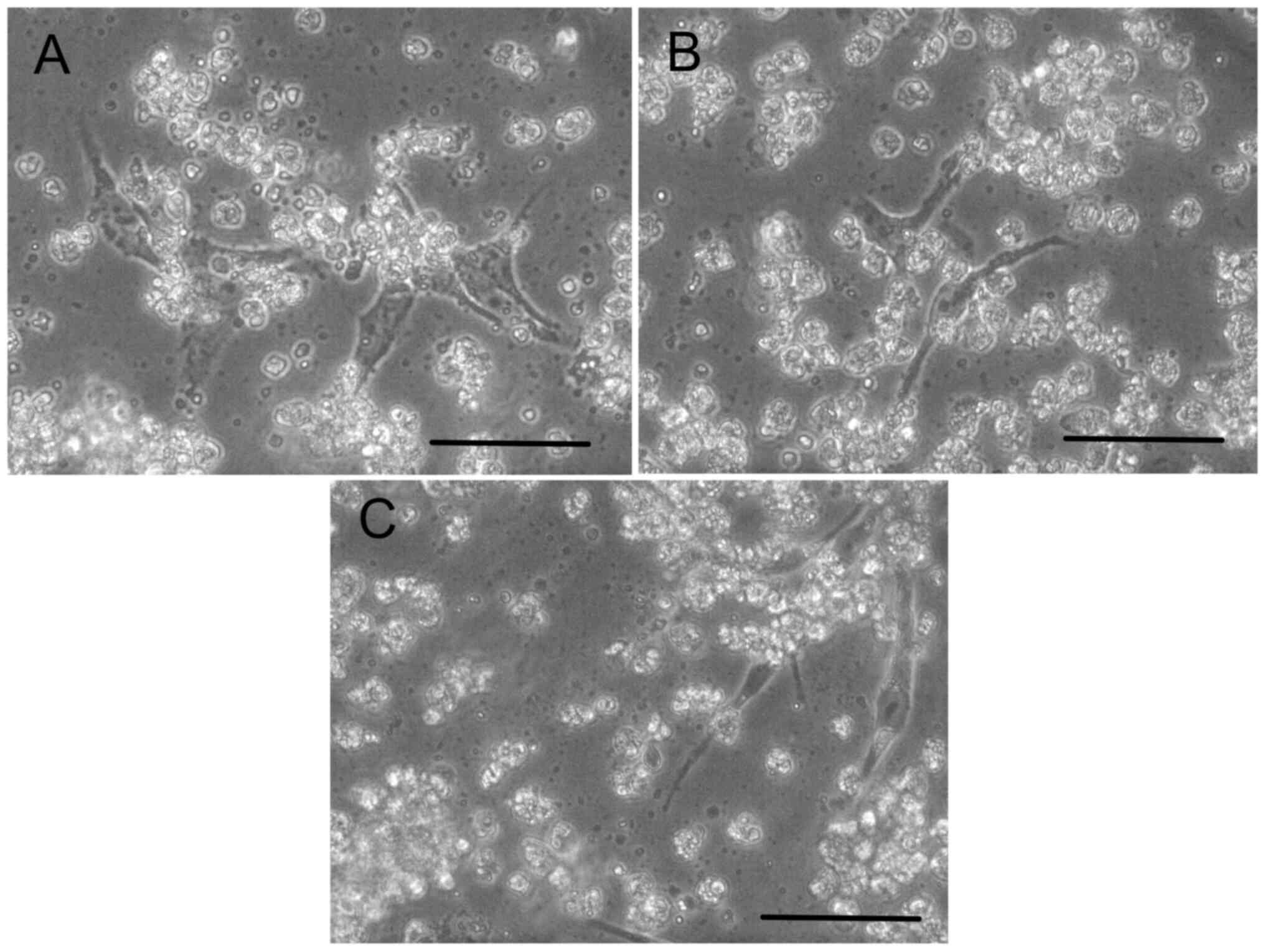
Survival with calcium lactate
To observe iPS cell survival, they were cultured in
HSM supplemented with calcium lactate (Fig. 4A), sodium lactate (Fig. 4B) or lactic acid (Fig. 4C). After 7 days of culture, the
cultured cells had survived. The floating cells were dead cells or
debris because the medium did not contain enough glucose.
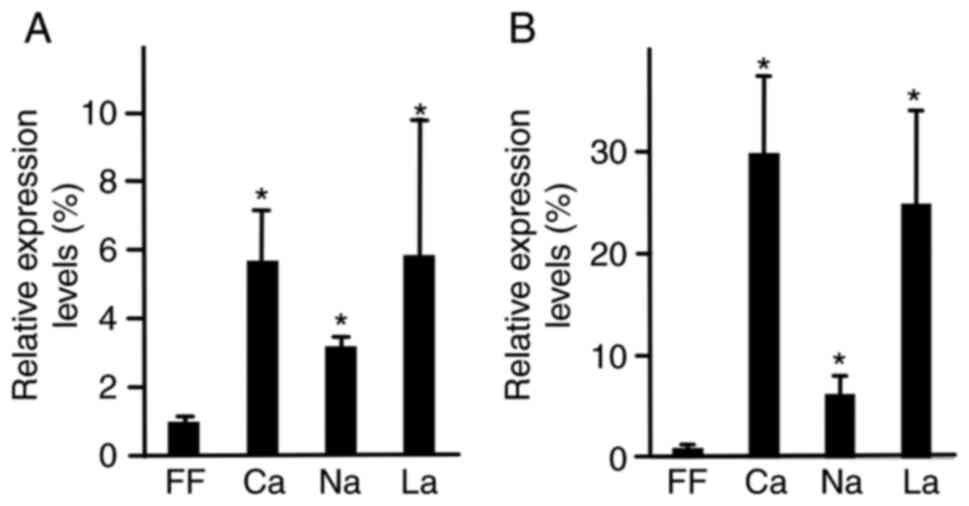
RT-qPCR
To clarify the differentiation into the hepatocyte
lineage, RT-qPCR analysis of specific markers was performed. iPS
cells were cultured in HSM supplemented with 1 mM of calcium
lactate. After 7 days, RNA was isolated and subjected to qPCR. In
the presence of calcium lactate, sodium lactate and lactic acid,
relative AFP expression levels were 5.7±1.4, 3.2±0.3 and 5.8±4.0,
respectively (Fig. 5A). In the
presence of calcium lactate, sodium lactate and lactic acid,
relative albumin expression levels were 30.0±7.5, 6.3±1.8, and
25.0±9.0, respectively. AFP and albumin expression levels were
higher in the calcium lactate, sodium lactate and lactic acid
groups than in the ReproFF group (P<0.05).
Discussion
iPS cells die within 3 d in HSM because the medium
is deprived of glucose (18). In
the current study, the cells survived for >7 d in HSM
supplemented with lactate. It has been suggested that iPS cell
survival was promoted by lactate. The present metabolome analysis
showed that lactate was produced by iPS cells in conventional
media, which is consistent with the Warburg effect (15). Galactose is metabolized to
galactose-1-phosphate, which then enters glycolysis (17). The present data clearly showed that
galactose-1-phosphate was produced in iPS cells from galactose in
the HDI medium. The lactate concentration was low in iPS cells
cultured in HDI medium. The reason for this is unknown; however,
one speculation is that gluconeogenesis was immature. It may be
hypothesized that the addition of lactate to HSM would promote iPS
cell survival. In a previous study, high lactate levels (up to 28
mM) decreased cell proliferation of human embryonic stem cells
(25). This discrepancy was due to
the concentration of glucose. In the present study, HSM deprived of
glucose was used. Odenwelder et al (26) reported that 13C-labeled
lactate enters the tricarboxylic acid cycle via pyruvate. In this
study, lactate was converted to pyruvate and used as the energy
substrate (27). This metabolic
pathway is called the Cori cycle and is executed solely by
hepatocytes (27). In addition to
survival, lactate promotes cell proliferation (23).
The present RT-qPCR results indicated that AFP and
albumin levels were upregulated in cells in HSM with calcium
lactate. It was suggested that hepatocyte differentiation was
promoted by HSM with lactate, as these two genes are hepatocyte
markers (28,29). Previous studies by our group showed
that iPS differentiation toward the hepatocyte lineage was promoted
in medium without glucose and supplemented with galactose (18,19).
Hepatocyte differentiation is promoted by inhibiting glycolysis
with 3-bromopyruvate and 2-deoxy-d-glucose, pyruvate and glucose
analogs, respectively (29). The
current data are consistent with those of the previous studies by
our group. One major issue with HSM and HDI is that the cultured
cells do not survive long enough to differentiate into hepatocytes.
It may be possible to promote the differentiation of iPS cells into
hepatocytes if the cells survive for longer. In the present study,
cultured cells survived for >7 d and exhibited an upregulation
of hepatocyte markers. The previous studies by our group and the
current study indicate that hepatocyte differentiation of iPS cells
is promoted in media without glucose and supplemented with
galactose. Lactate may promote the differentiation of iPS cells to
hepatocytes, while the Cori cycle is activated in hepatocytes.
Sinton et al (30) reported
that steatosis is induced in hepatocyte-like cells differentiated
from iPS cells when added with lactate, pyruvate and octanoate.
Hepatocyte differentiated from iPS cells would be useful for
research on liver diseases.
Lactate was added to the HSM and galactose was added
to the medium without glucose. Thus, it is possible that lactate
affects iPS cell differentiation. Human embryonic stem cells show
decreased pluripotency in media containing 11 mM of lactate
(25). It is possible that lactate
promotes hepatocyte differentiation; however, this remains to be
further clarified.
One major limitation of the present study was that
endodermal and hepatocyte markers other than AFP and albumin were
not analyzed. It is not known what role the obtained cells had in
the differentiation toward a hepatocyte lineage.
Our next step would be to determine the
differentiation state of cells cultured in HSM supplemented with
lactate. In the future, metabolome analysis will be performed using
lactate labeled with carbon 13.
In conclusion, lactate promoted the survival of iPS
cells cultured in a medium without glucose and supplemented with
galactose. Under these conditions, iPS cells began differentiating
into the hepatocyte lineage. Lactate may be a novel approach to
produce hepatocytes from iPS cells.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by a Grant-in-Aid for
Scientific Research (C) from the Japan Society for the Promotion of
Science (grant no. 22K08022).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MT performed the experiments and wrote the
manuscript. FS and TM performed the statistical analysis. HT and MS
performed the MTS assay and took photographs of cells. All authors
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Deguchi S, Takayama K and Mizuguchi H:
Generation of human induced pluripotent stem cell-derived
hepatocyte-like cells for cellular medicine. Biol Pharm Bull.
43:608–615. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
DeLaForest A, Nagaoka M, Si-Tayeb K, Noto
FK, Konopka G, Battle MA and Duncan SA: HNF4A is essential for
specification of hepatic progenitors from human pluripotent stem
cells. Development. 138:4143–4153. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Si-Tayeb K, Noto FK, Nagaoka M, Li J,
Battle MA, Duris C, North PE, Dalton S and Duncan SA: Highly
efficient generation of human hepatocyte-like cells from induced
pluripotent stem cells. Hepatology. 51:297–305. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo
S, Song X, Guo Y, Zhao Y, Qin H, et al: Efficient generation of
hepatocyte-like cells from human induced pluripotent stem cells.
Cell Res. 19:1233–1242. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takayama K, Inamura M, Kawabata K,
Katayama K, Higuchi M, Tashiro K, Nonaka A, Sakurai F, Hayakawa T,
Furue MK and Mizuguchi H: Efficient generation of functional
hepatocytes from human embryonic stem cells and induced pluripotent
stem cells by HNF4α transduction. Mol Ther. 20:127–137.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tashiro K, Kawabata K, Inamura M, Takayama
K, Furukawa N, Sakurai F, Katayama K, Hayakawa T, Furue MK and
Mizuguchi H: Adenovirus vector-mediated efficient transduction into
human embryonic and induced pluripotent stem cells. Cell Reprogram.
12:501–507. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Transcription factors and
medium suitable for initiating the differentiation of human-induced
pluripotent stem cells to the hepatocyte lineage. J Cell Biochem.
117:2001–2009. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takebe T, Sekine K, Enomura M, Koike H,
Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, et al:
Vascularized and functional human liver from an iPSC-derived organ
bud transplant. Nature. 499:481–484. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shinozawa T, Kimura M, Cai Y, Saiki N,
Yoneyama Y, Ouchi R, Koike H, Maezawa M, Zhang RR, Dunn A, et al:
High-fidelity drug-induced liver injury screen using human
pluripotent stem cell-derived organoids. Gastroenterology.
160:831–846.e10. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Roy-Chowdhury N, Wang X, Guha C and
Roy-Chowdhury J: Hepatocyte-like cells derived from induced
pluripotent stem cells. Hepatol Int. 11:54–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Olgasi C, Cucci A and Follenzi A:
iPSC-derived liver organoids: A journey from drug screening, to
disease modeling, arriving to regenerative medicine. Int J Mol Sci.
21(6215)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Finley LWS: What is cancer metabolism?
Cell. 186:1670–1688. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Abu Dawud R, Schreiber K, Schomburg D and
Adjaye J: Human embryonic stem cells and embryonal carcinoma cells
have overlapping and distinct metabolic signatures. PLoS One.
7(e39896)2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Y, Lei J, Zhang S, Wang X, Jin J, Liu
Y, Gan M, Yuan Y, Sun L, Li X, et al: 4EBP1 senses extracellular
glucose deprivation and initiates cell death signaling in lung
cancer. Cell Death Dis. 13(1075)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Adeva-Andany MM, Pérez-Felpete N,
Fernández-Fernández C, Donapetry-García C and Pazos-García C: Liver
glucose metabolism in humans. Biosci Rep. 36(e00416)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Survival of primary human
hepatocytes and death of induced pluripotent stem cells in media
lacking glucose and arginine. PLoS One. 8(e71897)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: An optimal medium
supplementation regimen for initiation of hepatocyte
differentiation in human induced pluripotent stem cells. J Cell
Biochem. 116:1479–1489. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Oncostatin M in William's E
medium is suitable for initiation of hepatocyte differentiation in
human induced pluripotent stem cells. Mol Med Rep. 15:3088–3092.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Davies B and Fried M: The L19 ribosomal
protein gene (RPL19): Gene organization, chromosomal mapping, and
novel promoter region. Genomics. 25:372–380. 1995.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tam S, Clavijo A, Engelhard EK and
Thurmond MC: Fluorescence-based multiplex real-time RT-PCR arrays
for the detection and serotype determination of foot-and-mouth
disease virus. J Virol Methods. 161:183–191. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ordoño J, Pérez-Amodio S, Ball K, Aguirre
A and Engel E: The generation of a lactate-rich environment
stimulates cell cycle progression and modulates gene expression on
neonatal and hiPSC-derived cardiomyocytes. Biomater Adv.
139(213035)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen X, Chen A, Woo TL, Choo ABH, Reuveny
S and Oh SKW: Investigations into the metabolism of two-dimensional
colony and suspended microcarrier cultures of human embryonic stem
cells in serum-free media. Stem Cells Dev. 19:1781–1792.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khan AA, Allemailem KS, Alhumaydhi FA,
Gowder SJT and Rahmani AH: The biochemical and clinical
perspectives of lactate dehydrogenase: An enzyme of active
metabolism. Endocr Metab Immune Disord Drug Targets. 20:855–868.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Odenwelder DC, Lu X and Harcum SW: Induced
pluripotent stem cells can utilize lactate as a metabolic substrate
to support proliferation. Biotechnol Prog. 37(e3090)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cori CF: The glucose-lactic acid cycle and
gluconeogenesis. Curr Top Cell Regul. 18:377–387. 1981.PubMed/NCBI
|
|
28
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: 2-Deoxy-D-glucose initiates
hepatocyte differentiation in human induced pluripotent stem cells.
Mol Med Rep. 15:3083–3087. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Differentiation of human
induced pluripotent stem cells in William's E initiation medium
supplemented with 3-bromopyruvate and 2-deoxy-d-glucose. Mol Med
Rep. 15:3719–3723. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sinton MC, Meseguer-Ripolles J,
Lucendo-Villarin B, Wernig-Zorc S, Thomson JP, Carter RN, Lyall MJ,
Walker PD, Thakker A, Meehan RR, et al: A human pluripotent stem
cell model for the analysis of metabolic dysfunction in hepatic
steatosis. iScience. 24(101931)2020.PubMed/NCBI View Article : Google Scholar
|