1. Introduction
The discovery of the Lin-4 gene in the nematode,
Caenorhabditis elegans, in 1993 by Lee et al
(1) marked a major milestone in the
field of RNA biology. Lin-4 encodes a small RNA molecule that was
later found to belong to a larger family of RNA molecules known as
microRNAs (miRNAs) (2). miRNAs are
short (18-25 nucleotides), non-coding RNA molecules that play a
crucial role in post-transcriptional regulation of gene expression
by binding to the complementary mRNA at the 3'-untranslated region
(UTR) and silencing the expression of the target gene (2). The binding of miRNAs to their target
mRNA can lead to mRNA degradation or translational repression,
depending on the degree of complementarity between the miRNA and
the target mRNA. The 3'-UTR is the most common site for miRNA
binding, but miRNAs can also interact with other regions of the
target mRNA, such as the 5'-UTR, gene promoters and the coding
region (2).
In terms of molecular mechanisms, gene expression is
highly regulated through transcriptional, post-transcriptional and
translational processes during development. miRNAs play a critical
role in this complex regulatory network, requiring various feedback
and feedforward mechanisms to ensure proper gene expression
(3). miRNAs are versatile and
important regulators of various cellular processes that influence
the development and function of living organisms, and play crucial
roles in fundamental biological events, such as cell
differentiation (4), tissue
regeneration (5),
epithelial-mesenchymal transition (6), embryogenesis (7), proliferation, apoptosis and metabolism
(8). A substantial body of clinical
and preclinical evidence supports the role of miRNAs in various
diseases, including cancer (9) and
infectious (10), neurodegenerative
(11), cardiovascular and
immune-related diseases (12,13).
Hence, miRNAs have emerged as promising biomarkers and therapeutic
targets for a variety of diseases. Analyzing miRNA expression
patterns is a promising method for detecting cancer, predicting
disease prognosis and evaluating treatment efficacy (9,14). A
key advantage of miRNAs is their high stability in bodily fluids
and tissues. In the bloodstream, miRNAs are found in RNA-binding
complexes and/or exosomes, and their short length makes them
resistant to degradation, improving their stability in various
storage conditions (9,14). Studies have shown that miRNAs are
markedly resilient in plasma and serum, maintaining their integrity
despite exposure to RNase activity, extreme pH and multiple rounds
of freezing-thawing (15,16).
The present review focuses on miR-367, a member of
the miR-302/367 cluster found in the intronic region on the 4q25
locus of human chromosome 4(17).
This cluster, which includes miR-302a/b/c/d and miR-367, has been
extensively studied and is involved in diverse neoplastic diseases
through various modes of gene regulation. The miR-302/367 cluster
is transcribed as a 1,974-nucleotide miRNA precursor for eight
miRNAs, from a polycistronic miRNA gene containing the classical
TATA box and polyadenylation signal (18). Regulated expression of the
miR-302/367 cluster in the nucleus, cytoplasm and outside the cell,
in addition to the exchange of its members between cells via
exosomes, results in the differential expression and biological
importance of the cluster (17,18).
mir-367 has poor sequence homology with the other members of the
miR-302/367 cluster and thus has mostly different mRNA targets and
mechanisms of action compared with miR-302a/b/c/d (19). miR-302 family members were
previously recognized as essential components of somatic cell
reprogramming to generate pluripotent stem cells (20). In addition, the involvement of the
miR-302/367 cluster in colorectal cancer is mostly mediated by the
miR-302 family members (21).
Despite the differences in sequences, targets and functions between
miR-302a/b/c/d and miR-367, these miRNAs were commonly considered
as a general miR-302/367 cluster in various contexts (17,18,20,21).
Hence, it is crucial to examine the roles of miR-367 more
specifically in cancer and other physiological processes due to its
distinct targets and functions from miR-302.
Overall, the present review highlights the role of
miR-367 in various physiological processes and cancer biology. A
comprehensive understanding of miR-367 function may contribute to
the development of new diagnostic and therapeutic strategies for a
range of diseases. The potential of miR-367 as a therapeutic target
warrants further investigation, and ongoing studies may reveal
opportunities for its use in clinical settings.
2. miRNA biogenesis
The biogenesis of miRNAs begins with the processing
of RNA transcripts either post- or co-transcriptionally (22). According to de Rie et al
(23), most miRNAs are intragenic
and originate from introns of protein-coding genes. By contrast,
intergenic miRNAs are located between genes, therefore lacking a
host gene, and are transcribed via their own polymerase II (Pol II)
or polymerase III (Pol III) promoters (24).
In animals, miRNA biogenesis involves a series of
steps, starting with the transcription of primary miRNA (pri-miRNA)
from the DNA sequence and progressing to the generation of mature
miRNA (22). Drosha, a class 2
ribonuclease III enzyme (25),
plays a central role in this process by processing pri-miRNAs into
precursor-miRNAs (pre-miRNAs) within the nucleus (26). After Drosha cleaves the pri-miRNA
transcript, the resulting pre-miRNA is transported to the cytoplasm
for further processing (27). This
transport is facilitated by the Ras-related nuclear protein
GTPase-dependent nuclear transport receptor, exportin-5(28). In the cytoplasm, Dicer, a
ribonuclease III enzyme, further processes the pre-miRNAs into
functional 21 or 22-nucleotide miRNAs. These miRNAs are then
incorporated into Argonaute proteins to form miRNA-induced
silencing complexes (miRISCs), which regulate gene expression
through post-transcriptional silencing of complementary target
mRNAs (2). miRISC recruitment to
target mRNAs can result in their degradation or translational
repression, leading to gene silencing (29).
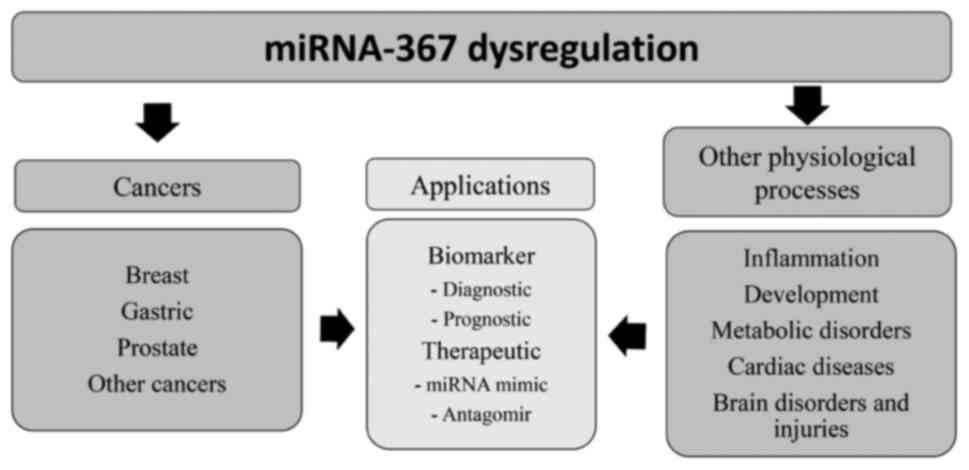
3. Roles of miR-367 in disease
pathogenesis
miR-367 in cancer
Extensive cancer research has focused on miR-367,
which has demonstrated tumor-suppressing activity through the
targeting of oncogenes. In multiple types of cancer, including
breast (30-32),
gastric (33,34) and prostate cancer (35), higher levels of miR-367 have been
associated with improved prognoses and survival rates. In addition,
miR-367 has been recognized as a possible biomarker for diagnosing
various types of cancer including breast (30,31)
and gastric (33) cancer (Fig. 1).
Breast cancer. Breast cancer is a common
cancer mainly found in women, accounting for 11.7% of all cancer
diagnoses and 6.9% of cancer-related deaths globally in women
(36). Studies have shown that
miRNAs can be either upregulated (37) or downregulated (30,31) in
patients with breast cancer and different levels can distinguish
these patients from healthy individuals. Studies have shown that
miR-367 is involved in the development and progression of breast
cancer, and its dysregulation has been implicated in various
aspects of breast cancer biology, including cell proliferation,
apoptosis, invasion and metastasis (32,38).
In a recent study, Yang et al (30) discovered that miR-367-3p expression
was significantly downregulated in the whole blood samples of
patients with breast cancer with axillary lymph node metastasis
(ALNM). This finding was particularly significant as it highlighted
the potential diagnostic value of miR-367-3p in distinguishing
between patients with breast cancer with or without ALNM. The
presence of ALNM is a critical factor in determining the prognosis
of patients with breast cancer and ALNs are the most common site
for breast cancer metastasis (39).
The results of the study demonstrated that low expression of
miR-367-3p was associated with positive ALNM, larger tumor size and
a more advanced tumor-node-metastasis stage (30). In addition, a study conducted by Liu
et al (31) revealed a
strong association between miR-367 expression and the
clinicopathologic features and prognosis of breast cancer. It was
found that the miR-367 expression level in the serum of patients
with breast cancer was significantly lower than that of the control
group. Moreover, the study demonstrated that the downregulation of
miR-367 in patients with breast cancer was strongly correlated with
advanced tumor stage, larger tumor size and lymph node metastasis.
Furthermore, the study demonstrated that patients with breast
cancer with low levels of miR-367 had significantly shorter
disease-free and overall survival times compared with the control
group. These findings suggest that miR-367 may play a critical role
in the progression and prognosis of breast cancer and that
circulating miR-367 has potential as a non-invasive biomarker for
breast cancer.
A recent study investigating the involvement of
miR-367-3p in breast cancer using the MCF-7 cell line also
demonstrated its potential as a therapeutic target (32). The study found that miR-367-3p could
induce apoptosis and suppress migration of MCF-7 cells by
downregulating the expression of human choline kinase α, a key
enzyme involved in breast cancer progression. Furthermore,
bioinformatics analysis demonstrated that miR-367-3p was related to
numerous potential targets, such as metal regulatory transcription
factor 1 and phosphatase and tensin homolog, and multiple
tumor-related pathways, including cell adhesion molecules,
mammalian target of rapamycin and cellular senescence pathways,
suggesting that it may play a crucial role in the development and
progression of breast cancer (30).
Overall, these findings highlight the potential clinical utility of
miR-367-3p as a diagnostic marker and its potential role in breast
cancer pathology.
Gastric cancer. According to GLOBOCAN 2020,
an online database providing global cancer statistics and estimates
of incidence and mortality rates in 185 countries for 36 types of
cancer, gastric cancer is the fifth most common cancer and the
fourth most common cause of cancer-related death worldwide
(36). This disease remains
difficult to cure, primarily due to most patients presenting with
advanced disease, when prognosis is poor and treatment options have
become limited (40). Thus, it is
important to develop a method to identify and predict gastric
cancer prognosis in clinical settings at the earlier stages of
disease. miRNAs play a significant role in gastric cancer,
influencing cell cycle, proliferation, apoptosis, migration and
invasion (41). In addition,
miR-367-3p acts as a tumor suppressor in gastric cancer (34). A study by Bin et al (33) found that miR-367 expression is
downregulated in gastric cancer tissues and is linked to disease
progression. The study also revealed that overexpression of miR-367
in gastric cancer cells inhibited migration and invasion by
targeting Rab23, and that Rab23 overexpression reversed the
inhibitory effect of miR-367. Thus, miR-367 is a crucial negative
regulator of gastric cancer invasion and metastasis, indicating its
potential as a therapeutic tool for treating this disease.
Tao et al (34) reported that miR-367-3p has the
potential to bind to the 3'-UTR of high-mobility group AT-hook 2
(HMGA2) mRNA, an oncogene in gastric cancer. The study also
demonstrated that miR-367-3p negatively regulated the expression of
HMGA2 at both the mRNA and protein level in gastric cancer cells,
as confirmed by luciferase reporter assays. Additionally, the
levels of miR-367-3p and HMGA2 mRNA exhibited a negative
correlation in gastric cancer tissues. By contrast, in a 2018 study
by Liu et al (42), it was
discovered that miR-367-5p functions as an oncogene in gastric
cancer cells and targets p27Kip1, which is a CDK
inhibitor that regulates cell proliferation, cell motility and
apoptosis. In addition, low expression of p27Kip1 was
associated with poor prognosis and high-grade tumors in gastric
cancer. The study also found that the expression of circular RNA of
Yes-associated protein 1 (circYAP1), which acted as a sponge for
miR-367-5p, was significantly downregulated in gastric cancer
tissues, and this low circYAP1 expression was associated with poor
patient prognosis. circYAP1 upregulated the expression of
p27Kip1, but this effect was inhibited by miR-367-5p.
Therefore, the study demonstrated that miR-367-5p targeted
p27Kip1 and that circYAP1 sponged miR-367-5p to
upregulate p27Kip1 expression in gastric cancer
cells.
Moreover, overexpression of miR-367-3p was found to
promote growth, migration and epithelial-mesenchymal transition of
gastric cancer cells, while inhibition of this miRNA led to the
opposite effect (43). Based on the
contrasting effects of miR-367 (possibly due to whether it is
miR-367-3p or 5p) on the pathogenesis of gastric cancer, the use of
this miRNA as a therapeutic target or biomarker is dependent on the
pathway involved.
Prostate cancer. Prostate cancer is a common
type of cancer in males. Siegel et al (44) reported that >248,530 cases of
prostate cancer will be diagnosed in 2021, resulting in 34,130
deaths. While prostate-specific antigen is commonly used as a
marker to diagnose and predict the progress of prostate cancer, its
effectiveness is limited due to false-positive outcomes, which can
result in the unnecessary treatment of non-aggressive cancer and
incorrect decisions for treating cases of relapse (45). Consequently, there is a significant
need for new approaches to identify and predict prostate cancer
prognosis in clinical settings at earlier stages of disease.
Studies have shown that miR-367 is dysregulated in
prostate cancer and plays a role in prostate cancer pathogenesis
(46,47). A recent study by Du et al
(35) demonstrated that miR-367-3p
inhibited the expression of Rab23 and the Hedgehog signaling
pathway in prostate cancer cells. Rab23 is a GTPase family member
and is known to play a critical role in tumor progression (48). The Hedgehog signaling pathway is a
downstream pathway of Rab23, and its overactivation has been
implicated in oncogenic occurrence and development. Notably,
miR-367-3p is downregulated in prostate cancer, and overexpression
of miR-367-3p has been found to impede cell proliferation,
invasion, and migration as demonstrated by in vitro and
in vivo experiments (35).
Furthermore, Du et al (35)
found that miR-367-3p downregulates the Hedgehog pathway, leading
to the regulation of key transcription factors, Gli1 and Gli2,
which are responsible for cell proliferation, differentiation and
angiogenesis. These findings suggest that miR-367-3p exerts
tumor-suppressive effects by targeting critical genes and cell
signaling pathways, making it a promising target for further
investigation of prostate cancer treatment.
Other cancer types. Kaid et al
(49) discovered that miR-367 may
be a therapeutic target and a marker in aggressive embryonal
central nervous system tumors, and it may facilitate prognosis and
early diagnosis of this disease. Circulating miR-367 is also
upregulated in acute lymphoblastic leukemia (ALL), making it a
potential therapeutic target and a distinguishing factor between
patients with ALL and healthy individuals (50).
In endometrial cancer, miR-367-3p expression levels
are correlated with the International Federation of Gynecology and
Obstetrics stage and lymph node metastasis (51). High expression of miR-367-3p results
in high survival rates, suppression of cancer cell metastasis and
inhibition of malignant tumor behaviors. This is achieved through
the downregulation of HMGA2 by miR-367-3p, as shown by Ma et
al (51). The study also
suggested that the miR-302a-5p/367-3p-HMGA2 axis could be used as a
diagnostic biomarker for endometrial carcinoma metastasis and
prognosis, and as a therapeutic target.
miR-367 may play a crucial role in the progression
of cutaneous melanoma and may be a promising target for the
treatment of this disease. A study by Long et al (52) demonstrated that miR-367 enhanced the
proliferation and invasion of cutaneous malignant melanoma. The
upregulation of miR-367 was observed in both melanoma tissues and
cell lines, and its level in tumor tissues was found to be
positively correlated with tumor thickness, stage, lymph node
involvement, distant metastasis and patient survival rate.
Moreover, a higher expression of miR-367 promoted the growth,
migration and invasion of melanoma cells, while lower levels of
miR-367 had an inhibitory effect on cell proliferation and
invasion.
miR-367 has also been implicated in non-small cell
lung cancer (NSCLC). Yu et al (53) found that overexpression of
miR-367-3p lead to the downregulation of serum and glucocorticoid
regulated kinase 3, resulting in the inhibition of NSCLC cell
proliferation and migration.
Roles of miR-367 in various
physiological processes. miR-367 and other diseases
miR-367 has traditionally been studied for its
potential as a tumor suppressor and diagnostic biomarker in cancer
research. However, previous studies have highlighted its
significance in regulating essential genes involved in inflammation
(54), development (55,56),
metabolic disorders (57), cardiac
diseases (12,58), brain disorders (59,60)
and pregnancy disorders (61).
These findings demonstrate the multifaceted role of miR-367 in
controlling diverse physio-logical processes beyond cancer
(Fig. 1).
Inflammation. miR-367 has been found to play
a crucial role in regulating inflammation in various diseases. Yuan
et al (54) demonstrated
that miR-367 negatively regulated the inflammatory response of
microglia by targeting interleukin 1 receptor-associated kinase 4
(IRAK4) in intracerebral brain hemorrhage (ICH). The study found
that ICH downregulated the expression of miR-367 and upregulated
the expression of IRAK4 in primary microglia. It was also
demonstrated that miR-367 suppressed IRAK4 expression by directly
binding to its 3'-UTR region. Furthermore, miR-367 inhibited NF-κB
activation and the production of downstream proinflammatory
mediators including interleukin 6 (IL-6), IL-1b and tumor necrosis
factor α (TNF-α). Knocking down IRAK4 expression in microglia
inhibited both NF-κB activation and the production of
proinflammatory mediators. Additionally, the study found that
miR-367 could reduce brain oedema, improve neurological functions
and inhibit the expression of proinflammatory cytokines in ICH
mice. In addition, miR-367 has also been found to regulate the
inflammatory response of microglia by targeting the transcription
factor, CCAAT enhancer binding protein α, which promotes microglia
M2 polarization (62). The same
study also demonstrated that miR-367 attenuated ICH-induced
inflammatory injury and could potentially be applied for the
management of ICH. The administration of miR-367 has also been
shown to ameliorate neurological function by reducing neurological
injury and brain oedema (54). The
microglia-mediated inflammatory response plays a critical role in
the pathogenesis of ICH, which is the most severe cerebrovascular
disease and is characterized by high mortality and disability rates
(62). Thus, controlling the
production of pro-inflammatory mediators represents a promising
strategy to prevent brain injury following ICH.
Stroke is a severe disease that can be categorized
into ischemic or hemorrhagic stroke. Ischemic stroke is
particularly burdensome, accounts for 85% of strokes and currently
lacks effective treatment strategies, especially for reducing
neuroinflammation that leads to cell death (63). miRNAs may provide new therapeutic
options for neuroinflammation associated with ischemic stroke
(64). In a study by Tabet et
al (65), miR-367 was found to
be associated with ischemic stroke. The study found that miR-367-3p
was reduced in brain homogenates following sustained ischemia and G
protein-coupled receptor class C group 5 member A (GPRC5A)
expression, a target gene of miR-367-3p, was increased.
Furthermore, miR-367-3p and GPRC5A were co-expressed in human
cortical neurons (HCN-2 cell line) and inhibition of miR-367-3p led
to increased expression of GPRC5A in mouse primary neurons,
indicating that the loss of miR-367-3p suppression of GPRC5A (due
to the reduced levels of this miRNA during ischemia) may contribute
to neuroinflammation in ischemic stroke. As for hemorrhagic stroke,
miR-367 has been found to attenuate neurobehavioral and
neuropathological changes in hemorrhagic stroke by improving
blood-brain barrier integrity, suppressing neuroinflammation and
reducing neuronal apoptosis, as demonstrated by Xu et al
(66).
Myocardial infarction (MI) is a common and serious
ischemic cardiomyopathy with high morbidity and mortality rates
globally (67). A recent study by
Wang et al (68)
demonstrated that miR-367-5p was directly involved in the
pathogenesis of MI through targeting and inhibiting the expression
of cyclooxygenase 2 (COX2). This study confirmed that miR-367-5p
downregulation or COX2 overexpression leads to cellular dysfunction
of cardiomyocytes. Moreover, knockdown of miR-367-5p has been found
to promote the secretion of inflammatory cytokines, such as IL-1β,
IL-6 and TNF-α, which exacerbate the inflammatory response in
patients with MI (68). These
findings suggest that miR-367-5p could be relevant for the
treatment of MI.
Development. During neurulation, the neural
plate, which is predominantly composed of neural progenitor cells
(NPCs), undergoes a series of complex and highly dynamic
morpho-logical changes, including bending, bilateral lifting,
folding and fusion, to form the closed neural tube (69). Precise control of NPC behaviors,
such as proliferation, differentiation and survival, is essential
for coordinating this process and preventing open neural tube
defects (NTDs). miRNAs are ideal regulators for orchestrating these
cellular behaviors for normal neural tube (NT) formation by binding
to multiple targets that regulate the planar cell polarity pathway
(70). A mouse development study
demonstrated that miR-302/367 expression started in the embryonic
region after implantation and became restricted to the anterior
neural tube by embryonic day 8.5(55). Furthermore, Yang et al
(56) conducted a study that
demonstrated the significance of miR-302/367 in mammalian NT
formation and the survival of developing embryos. The results
showed that miR-302/367 regulated NPC proliferation,
differentiation and perseverance by inhibiting cyclin D1/D2 and
fibroblast growth factor 15 gene expression at the
post-transcriptional level. Depletion of miR-302/367 leads to early
differentiation, increased cell proliferation and cell death, which
could cause NTD.
Research has revealed that mammalian primed
pluripotent stem cells are particularly vulnerable to cell death
stimuli as they possess a low apoptotic threshold. A key mechanism
that regulates apoptosis in the post-implantation epiblast is the
miRNA-mediated pathway (55).
Research conducted on cultured cells indicates that the miR-302/367
cluster has crucial functions in regulating pluripotency,
differentiation, cell cycle progression and apoptosis in embryonic
stem cells or primed pluripotent stem cells (55). This study also indicated that three
miRNA families, namely miR-20, miR-92 and miR-302/367, played a
crucial role in controlling the mitochondrial apoptotic machinery
by precisely regulating the levels of the proapoptotic protein,
Bcl-2-like 11. These miRNA families act as a necessary buffer for
sustaining the survival of stem cells that are primed for both
differentiation and cell death.
Cardiac diseases. Atrial fibrillation (AF) is
a heart rhythm disorder characterized by irregular and fast
heartbeats that can affect blood flow and oxygen delivery (71). According to the 2019 Global Burden
of Disease study, there was an estimated 59.7 million (95%
uncertainty interval, 45.7 million to 75.3 million) cases of atrial
flutter (AF) in 2019, an ~2-fold increase compared with the number
of cases in 1990 (72,73). Since AF can lead to serious
complications, such as stroke, heart failure and dementia, there is
a growing interest in preventing this condition. Certain studies
have suggested that miRNAs may play a role in the development of AF
(74,75), and have shown that miRNAs found in
the bloodstream could be used as biomarkers to identify AF
(76,77). The potential of miR-367 as a
diagnostic biomarker for AF was highlighted in a recent study by
Cao and Cui (58). The
identification of differentially expressed miRNAs in AF revealed
that miR-367 exhibited greater diagnostic utility than other
miRNAs. The aberrant expression of miR-367 in the atrial tissues of
patients with AF suggests its use as a non-invasive diagnostic tool
for distinguishing between healthy individuals and those with AF
(58).
In addition, Hu et al (12) validated the role of miR-367-3p in
regulating the development of cardiac fibrosis, a condition
characterized by the accumulation of extracellular matrix proteins
in the cardiac interstitium, which can lead to cardiac dysfunction
(78). The study (12) found that the expression of
miR-367-3p was lower in patients with cardiac fibrosis compared
with the non-fibrosis controls, and further analysis revealed that
CD69, a protein involved in the immune response, was a target of
miR-367-3p. Downregulation of CD69 by miR-367-3p mimics and CD69
small interfering RNA indicated that CD69 expression was associated
with a decrease in cytokine levels and the upregulation of T helper
17 cells. These findings indicate that miR-367-3p plays a critical
role in regulating cardiac fibrosis by targeting CD69 and
modulating the immune response.
Coronary artery disease refers to constriction of
the arteries that supply blood and oxygen to the heart (79). Off-pump coronary artery bypass
(OPCAB) surgery is a technique that involves grafting veins or
arteries to bypass the narrowed or blocked areas, thus improving
blood flow and reducing the risk of death from coronary artery
disease (80). However, individuals
who undergo this procedure may experience complications related to
coagulation and hemostasis (81).
Thus, there is a need to investigate the molecular mechanisms
underlying the impact of OPCAB. Sun et al (13) conducted a comprehensive
investigation into the molecular mechanisms underlying the efficacy
of OPCAB surgery. Through the analysis of differentially expressed
genes (DEGs) and miRNAs, miR-367 was identified as a crucial
regulatory factor in the immune response following OPCAB surgery.
Specifically, miR-367 was shown to modulate the expression of early
growth response (EGR) 2, a key regulator of the Toll-like receptor
signaling pathway and immune inflammation feedback. Thus, the
miR-367 expression level in patients post-OPCAB may serve as an
informative marker for monitoring immune responses. These findings
provided valuable insights into the molecular mechanisms underlying
the effectiveness of OPCAB surgery and have important implications
for improving patient outcomes in the treatment of coronary heart
disease.
Brain disorders or injuries. Schizophrenia is
a multifaceted neurodevelopmental disorder for which the underlying
mechanisms remain unclear. As such, there is a pressing need to
understand the molecular mechanisms of schizophrenia, identify
potential biomarkers and devise new treatment strategies. A recent
study identified miR-367 as a promising biomarker for
schizophrenia, with evidence indicating its involvement in the
regulation of DEGs, such as SMAD7 and EGR1(59). The study suggested that the
miR-367-SMAD7-EGR1 and miR-367-SMAD7-aryl hydrocarbon receptor
nuclear translocator axis could serve as potential biomarkers for
schizophrenia. Thus, understanding the function of miR-367 in the
molecular mechanisms of schizophrenia could aid in the development
of diagnostic biomarkers and effective therapeutic strategies for
this complex neurodevelopmental disorder.
In addition, Svenningsen et al (60) highlighted the significance of
miR-367 in depression. In this study, rats exposed to the learned
helplessness rat model of depression displayed alterations in miRNA
expression levels in the medial and lateral habenula, with miR-367
being one of the four miRNAs significantly regulated in the lateral
habenula. This finding suggests that miR-367 may play a role in
depression, as changes in its expression were observed in a
relevant brain region associated with the disorder. Additionally,
it was found that the target genes of miR-367 are involved in
neurotransmission, specifically neutrophin and ErbB signaling,
further supporting its role in depression (60). Understanding the precise role of
miR-367 in depression could provide valuable insights into the
pathogenesis of the disease and lead to the development of novel
treatment approaches.
4. Conclusion and future perspectives
miR-367 is a versatile miRNA involved in various
physiological processes, including cell proliferation,
differentiation, self-renewal and reprogramming. miR-367
dysregulation has been implicated in numerous diseases, including
cancer, and recent studies have indicated that miR-367 could be a
therapeutic target and biomarker of disease. The investigation of
miR-367 regulation and its potential use in the prognosis and
treatment of various diseases offers encouraging opportunities for
future clinical applications. As aforementioned, miR-367 may either
act as an oncogene or tumor suppressor depending on the type of
cancer. In future, anti-miR-367 oligonucleotides (antagomirs) or
miR-367 mimics could be used as therapeutics to either block or
restore the activity of miR-367 for tumor suppression. The level of
miR-367 in circulating serum has been used as a prognostic marker
for breast (31) and testicular
germ cell (82,83) cancer. However, more studies to
investigate the correlations between miR-367 serum levels and
various stages of diseases would enhance the potential of this
miRNA as a biomarker. The combined tumor suppression effects of
miR-367 and other miRNAs or drugs, such as aspirin (84), should also be explored in the
future. However, further research is necessary to fully understand
the complex regulatory mechanisms involving miR-367 in different
contexts and to identify potential off-target effects of
miR-367-targeted therapies. Studies to verify the targets of
miR-367 are also required to elucidate the pathways involved in
disease development.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by The Malaysia Ministry of
Higher Education Fundamental Research Grant Scheme [grant no.
FRGS/1/2021/SKK0/USM/02/34 (203/PPSK/6171299)].
Availability of data and materials
Not applicable.
Authors' contributions
SM drafted the manuscript. LLF and WCST verified the
contents and revised the manuscript. SAH, GBYT and BYK critically
revised and edited the manuscript. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Use of artificial intelligence tools
During the preparation of this work, ChatGPT was
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee RC, Feinbaum RL and Ambros V: The C.
Elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993.PubMed/NCBI View Article : Google Scholar
|
|
2
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of MicroRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9(402)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shenoy A and Blelloch RH: Regulation of
microRNA function in somatic stem cell proliferation and
differentiation. Nat Rev Mol Cell Biol. 15:565–576. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Galagali H and Kim JK: The multifaceted
roles of microRNAs in differentiation. Curr Opin Cell Biol.
67:118–140. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hutchins ED, Eckalbar WL, Wolter JM,
Mangone M and Kusumi K: Differential expression of conserved and
novel microRNAs during tail regeneration in the lizard Anolis
carolinensis. BMC Genomics. 17(339)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Peng F, Fan H, Li S, Peng C and Pan X:
MicroRNAs in epithelial-mesenchymal transition process of cancer:
Potential targets for chemotherapy. Int J Mol Sci.
22(7526)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lin Y, Zeng Y, Zhang F, Xue L, Huang Z, Li
W and Guo M: Characterization of microRNA expression profiles and
the discovery of novel microRNAs involved in cancer during human
embryonic development. PLoS One. 8(e69230)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ambros V and Ruvkun G: Recent molecular
genetic explorations of Caenorhabditis elegans MicroRNAs. Genetics.
209:651–673. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chakrabortty A, Patton DJ, Smith BF and
Agarwal P: miRNAs: Potential as biomarkers and therapeutic targets
for cancer. Genes (Basel). 14(1375)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou X, Li X and Wu M: miRNAs reshape
immunity and inflammatory responses in bacterial infection. Signal
Transduct Target Ther. 3(14)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Roy B, Lee E, Li T and Rampersaud M: Role
of miRNAs in neurodegeneration: From disease cause to tools of
biomarker discovery and therapeutics. Genes (Basel).
13(425)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu H, Li J and Zhang J: Dysregulation of
CD69 by overexpression of microRNA-367-3p associated with
post-myocardial infarction cardiac fibrosis. Mol Med Rep.
18:3085–3092. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun Y, Gao Y, Sun J, Liu X, Ma D, Ma C and
Wang Y: Expression profile analysis based on DNA microarray for
patients undergoing off-pump coronary artery bypass surgery. Exp
Ther Med. 11:864–872. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ho PTB, Clark IM and Le LTT:
MicroRNA-based diagnosis and therapy. Int J Mol Sci.
23(7167)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matias-Garcia PR, Wilson R, Mussack V,
Reischl E, Waldenberger M, Gieger C, Anton G, Peters A and
Kuehn-Steven A: Impact of long-term storage and freeze-thawing on
eight circulating microRNAs in plasma samples. PLoS One.
15(e0227648)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Glinge C, Clauss S, Boddum K, Jabbari R,
Jabbari J, Risgaard B, Tomsits P, Hildebrand B, Kääb S, Wakili R,
et al: Stability of circulating blood-based MicroRNAs-pre-analytic
methodological considerations. PLoS One.
12(e0167969)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu J, Wang Y, Ji P and Jin X: Application
of the microRNA-302/367 cluster in cancer therapy. Cancer Sci.
111:1065–1075. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao Z, Zhu X and Dou Y: The miR-302/367
cluster: A comprehensive update on its evolution and functions.
Open Biol. 5(150138)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo M, Gan L, Si J, Zhang J, Liu Z, Zhao
J, Gou Z and Zhang H: Role of miR-302/367 cluster in human
physiology and pathophysiology. Acta Biochim Biophys Sin
(Shanghai). 52:791–800. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kuo CH, Deng JH, Deng Q and Ying SY: A
novel role of miR-302/367 in reprogramming. Biochem Biophys Res
Commun. 417:11–16. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pidíkova P, Reis R and Herichova I: miRNA
clusters with down-regulated expression in human colorectal cancer
and their regulation. Int J Mol Sci. 21(4633)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014.
|
|
23
|
de Rie D, Abugessaisa I, Alam T, Arner E,
Arner P, Ashoor H, Åström G, Babina M, Bertin N, Burroughs AM, et
al: An integrated expression atlas of miRNAs and their promoters in
human and mouse. Nat Biotechnol. 35:872–878. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Filippov V, Solovyev V, Filippova M and
Gill SS: A novel type of RNase III family proteins in eukaryotes.
Gene. 245:213–221. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wahid F, Shehzad A, Khan T and Kim YY:
MicroRNAs: Synthesis, mechanism, function, and recent clinical
trials. Biochim Biophys Acta. 1803:1231–1243. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III Drosha initiates microRNA processing. Nature. 425:415–419.
2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bohnsack MT, Czaplinski K and Gorlich D:
Exportin 5 is a RanGTP-dependent dsRNA-binding protein that
mediates nuclear export of pre-miRNAs. RNA. 10:185–191.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jonas S and Izaurralde E: Towards a
molecular understanding of microRNA-mediated gene silencing. Nat
Rev Genet. 16:421–433. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang B, Wang YW, Qian LH, Xu Y, Chen X,
Chen YD, Liu C, Tian YR and Zhang K: Downregulated miR-367-3p,
miR-548aq-5p, and miR-4710 in human whole blood: Potential
biomarkers for breast cancer with axillary lymph node metastasis.
Clin Breast Cancer. 23:189–198. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu B, Pan J and Fu C: Correlation of
microRNA-367 in the clinicopathologic features and prognosis of
breast cancer patients. Medicine (Baltimore).
100(e26103)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Raikundalia S, Sa'Dom SAFM, Few LL and See
Too WCS: MicroRNA-367-3p induces apoptosis and suppresses migration
of MCF-7 cells by downregulating the expression of human choline
kinase α. Oncol Lett. 21(183)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bin Z, Dedong H, Xiangjie F, Hongwei X and
Qinghui Y: The microRNA-367 inhibits the invasion and metastasis of
gastric cancer by directly repressing Rab23. Genet Test Mol
Biomarkers. 19:69–74. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tao Y, Wan X, Fan Q, Wang Y, Sun H, Ma L,
Sun C and Wu Y: Long non-coding RNA OIP5-AS1 promotes the growth of
gastric cancer through the miR-367-3p/HMGA2 axis. Dig Liver Dis.
52:773–779. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Du W, Li D, Xie J and Tang P: miR-367-3p
downregulates Rab23 expression and inhibits Hedgehog signaling
resulting in the inhibition of the proliferation, migration, and
invasion of prostate cancer cells. Oncol Rep.
46(192)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
O'Bryan S, Dong S, Mathis JM and Alahari
SK: The roles of oncogenic miRNAs and their therapeutic importance
in breast cancer. Eur J Cancer. 72:1–11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pan B, Liu B, Pan J, Xin J and Fu C:
MicroRNA-367 inhibits breast cancer and promotes apoptosis by
targeting AT-rich interactive domain-containing protein 1B. J
Biomater Tissue Eng. 12:717–723. 2022.
|
|
39
|
Wu JL, Tseng HS, Yang LH, Wu HK, Kuo SJ,
Chen ST and Chen DR: Prediction of axillary lymph node metastases
in breast cancer patients based on pathologic information of the
primary tumor. Med Sci Monit. 20:577–581. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Necula L, Matei L, Dragu D, Neagu AI,
Mambet C, Nedeianu S, Bleotu C, Diaconu CC and Chivu-Economescu M:
Recent advances in gastric cancer early diagnosis. World J
Gastroenterol. 25:2029–2044. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ishiguro H, Kimura M and Takeyama H: Role
of microRNAs in gastric cancer. World J Gastroenterol.
20:5694–5699. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu H, Liu Y, Bian Z, Zhang J, Zhang R,
Chen X, Huang Y, Wang Y and Zhu J: Circular RNA YAP1 inhibits the
proliferation and invasion of gastric cancer cells by regulating
the miR-367-5p/p27Kip1 axis. Mol Cancer.
17(151)2018.
|
|
43
|
Jing Y, Zhang L, Zhang Y, Wei GJ, Yang HJ
and Huang LZ: miR-367-3p enhanced gastric cancer progression by
targeting Smad7 to regulate the transforming growth factor-1/Smad3
pathway. Res Sq, 2020. DOI: https://doi.org/10.21203/rs.2.24158/v1.
|
|
44
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ali HEA, Gaballah MSA, Gaballa R, Mahgoub
S, Hassan ZA, Toraih EA, Drake BF and Abd Elmageed ZY: Small
extracellular vesicle-derived microRNAs stratify prostate cancer
patients according to gleason score, race and associate with
survival of African American and Caucasian men. Cancers (Basel).
13(5236)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Guo Y, Cui J, Ji Z, Cheng C, Zhang K,
Zhang C, Chu M, Zhao Q, Yu Z, Zhang Y, et al: miR-302/367/LATS2/YAP
pathway is essential for prostate tumor-propagating cells and
promotes the development of castration resistance. Oncogene.
36:6336–6347. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zenner ML, Baumann B and Nonn L: Oncogenic
and tumor-suppressive microRNAs in prostate cancer. Curr Opin
Endocr Metab Res. 10:50–59. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang W, Yu F, Wang Y, Zhang Y, Meng L and
Chi Y: Rab23 promotes the cisplatin resistance of ovarian cancer
via the Shh-Gli-ABCG2 signaling pathway. Oncol Lett. 15:5155–5160.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kaid C, Jordan D, Bueno HMDS, Araujo BHS,
Assoni A and Okamoto OK: miR-367 as a therapeutic target in
stem-like cells from embryonal central nervous system tumors. Mol
Oncol. 13:2574–2587. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hosseinpour-Soleimani F, Khamisipour G,
Derakhshan Z and Ahmadi B: Expression analysis of circulating
miR-22, miR-122, miR-217 and miR-367 as promising biomarkers of
acute lymphoblastic leukemia. Mol Biol Rep. 50:255–265.
2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ma J, Li D, Kong FF, Yang D, Yang H and Ma
XX: miR-302a-5p/367-3p-HMGA2 axis regulates malignant processes
during endometrial cancer development. J Exp Clin Cancer Res.
37(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Long J, Luo J and Yin X: miR-367 enhances
the proliferation and invasion of cutaneous malignant melanoma by
regulating phosphatase and tensin homolog expression. Mol Med Rep.
17:6526–6532. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yu Q, Luo J, Zhang J, Chen Y, Chen K, Lin
J, Sun S and Lin X: Oxymatrine inhibits the development of
non-small cell lung cancer through miR-367-3p upregulation and
target gene SGK3 downregulation. Am J Transl Res. 12:5538–5550.
2020.PubMed/NCBI
|
|
54
|
Yuan B, Shen H, Lin L, Su T, Zhong L and
Yang Z: MicroRNA367 negatively regulates the inflammatory response
of microglia by targeting IRAK4 in intracerebral hemorrhage. J
Neuroinflammation. 12(206)2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pernaute B, Spruce T, Smith KM,
Sánchez-Nieto JM, Manzanares M, Cobb B and Rodríguez TA: MicroRNAs
control the apoptotic threshold in primed pluripotent stem cells
through regulation of BIM. Genes Dev. 28:1873–1878. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yang SL, Yang M, Herrlinger S, Liang C,
Lai F and Chen JF: MiR-302/367 regulate neural progenitor
proliferation, differentiation timing, and survival in neurulation.
Dev Biol. 408:140–150. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li DD, Liu Y, Xue L, Su DY and Pang WY:
Up-regulation of microRNA-367 promotes liver steatosis through
repressing TBL1 in obese mice. Eur Rev Med Pharmacol Sci.
21:1598–1603. 2017.PubMed/NCBI
|
|
58
|
Cao Y and Cui L: Identifying the key
microRNAs implicated in atrial fibrillation. Anatol J Cardiol.
25:429–436. 2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xie M, Li Z, Li X, Ai L, Jin M, Jia N,
Yang Y, Li W, Xue F, Zhang M and Yu Q: Identifying crucial
biomarkers in peripheral blood of schizophrenia and screening
therapeutic agents by comprehensive bioinformatics analysis. J
Psychiatr Res. 152:86–96. 2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Svenningsen K, Venø MT, Henningsen K,
Mallien AS, Jensen L, Christensen T, Kjems J, Vollmayr B and Wiborg
O: MicroRNA profiling in the medial and lateral habenula of rats
exposed to the learned helplessness paradigm: Candidate biomarkers
for susceptibility and resilience to inescapable shock. PLoS One.
11(e0160318)2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zhang H, Xue L, Lv Y, Yu X, Zheng Y, Miao
Z and Ding H: Integrated microarray analysis of key genes and a
miRNA-mRNA regulatory network of early-onset preeclampsia. Mol Med
Rep. 22:4772–4782. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pei H, Peng Q, Guo S, Gu Y, Sun T, Xu D,
Jiang Y, Xie J, Zhang L and Zhu Z: MiR-367 alleviates inflammatory
injury of microglia by promoting M2 polarization via targeting
CEBPA. In Vitro Cell Dev Biol Anim. 56:878–887. 2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jayaraj RL, Azimullah S, Beiram R, Jalal
FY and Rosenberg GA: Neuroinflammation: Friend and foe for ischemic
stroke. J Neuroinflammation. 16(142)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Todoran R, Falcione SR, Clarke M, Joy T,
Boghozian R and Jickling GC: MicroRNA as a therapeutic for ischemic
stroke. Neurochem Int. 163(105487)2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Tabet F, Lee S, Zhu W, Levin MG, Toth CL,
Cuesta Torres LF, Vinh A, Kim HA, Chu HX, Evans MA, et al:
microRNA-367-3p regulation of GPRC5A is suppressed in ischemic
stroke. J Cereb Blood Flow Metab. 40:1300–1315. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Xu W, Gao L, Zheng J, Li T, Shao A, Reis
C, Chen S and Zhang J: The roles of MicroRNAs in stroke: Possible
therapeutic targets. Cell Transplant. 27:1778–1788. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
de Lemos JA, Newby LK and Mills NL: A
proposal for modest revision of the definition of type 1 and type 2
myocardial infarction. Circulation. 140:1773–1775. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wang B, Zhang Y, Fang S and Wang H: Role
of circRNA circ_0000080 in myocardial hypoxia injury.
Bioengineered. 13:10902–10913. 2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mukhopadhyay P, Seelan RS, Greene RM and
Pisano MM: MicroRNA-mediated regulation of BMP signaling in the
developing neural tube. Microrna. 12:63–81. 2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Mukhopadhyay P, Greene RM and Pisano MM:
MicroRNA targeting of the non-canonical planar cell polarity
pathway in the developing neural tube. Cell Biochem Funct.
38:905–920. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Harada M, Melka J, Sobue Y and Nattel S:
Metabolic considerations in atrial fibrillation-mechanistic
insights and therapeutic opportunities. Circ J. 81:1749–1757.
2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Roth GA, Mensah GA, Johnson CO, Addolorato
G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ,
Benziger CP, et al: Global burden of cardiovascular diseases and
risk factors, 1990-2019: Update from the GBD 2019 study. J Am Coll
Cardiol. 76:2982–3021. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Zulkifly H, Lip GYH and Lane DA:
Epidemiology of atrial fibrillation. Int J Clin Pract.
72(e13070)2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Lv X, Li J, Hu Y, Wang S, Yang C, Li C and
Zhong G: Overexpression of miR-27b-3p targeting Wnt3a regulates the
signaling pathway of Wnt/β-catenin and attenuates atrial fibrosis
in rats with atrial fibrillation. Oxid Med Cell Longev.
2019(5703764)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Reilly SN, Liu X, Carnicer R, Recalde A,
Muszkiewicz A, Jayaram R, Carena MC, Wijesurendra R, Stefanini M,
Surdo NC, et al: Up-regulation of miR-31 in human atrial
fibrillation begets the arrhythmia by depleting dystrophin and
neuronal nitric oxide synthase. Sci Transl Med.
8(340ra74)2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Liu Z, Zhou C, Liu Y, Wang S, Ye P, Miao X
and Xia J: The expression levels of plasma micoRNAs in atrial
fibrillation patients. PLoS One. 7(e44906)2012.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Menezes Junior ADS, Ferreira LC, Barbosa
LJV, Silva DME, Saddi VA and Silva AMTC: Circulating MicroRNAs as
specific biomarkers in atrial fibrillation: A meta-analysis.
Noncoding RNA. 9(13)2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Berk BC, Fujiwara K and Lehoux S: ECM
remodeling in hypertensive heart disease. J Clin Invest.
117:568–575. 2007.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Worthley SG, Osende JI, Helft G, Badimon
JJ and Fuster V: Coronary artery disease: Pathogenesis and acute
coronary syndromes. Mt Sinai J Med. 68:167–181. 2001.PubMed/NCBI
|
|
80
|
Nalysnyk L, Fahrbach K, Reynolds MW, Zhao
SZ and Ross S: Adverse events in coronary artery bypass graft
(CABG) trials: A systematic review and analysis. Heart. 89:767–772.
2003.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Kim NY, Shim JK, Bang SO, Sim JS, Song JW
and Kwak YL: Effects of ulinastatin on coagulation in high-risk
patients undergoing off-pump coronary artery bypass graft surgery.
Korean J Anesthesiol. 64:105–111. 2013.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Rosas Plaza X, van Agthoven T, Meijer C,
van Vugt MATM, de Jong S, Gietema JA and Looijenga LHJ:
miR-371a-3p, miR-373-3p and miR-367-3p as serum biomarkers in
metastatic testicular germ cell cancers before, during and after
chemotherapy. Cells. 8(1221)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Syring I, Bartels J, Holdenrieder S,
Kristiansen G, Müller SC and Ellinger J: Circulating serum miRNA
(miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as biomarkers
in patients with testicular germ cell cancer. J Urol. 193:331–337.
2015.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Rezania MA, Eghtedari A, Taha MF, Ardekani
AM and Javeri A: A novel role for aspirin in enhancing the
reprogramming function of miR-302/367 cluster and breast tumor
suppression. J Cell Biochem. 123:1077–1090. 2022.PubMed/NCBI View Article : Google Scholar
|