Introduction
In 2020 a study reported a decline in peripheral
mucosal-associated invariant T (MAIT) cells in patients with active
Coronavirus disease 2019 (COVID-19) (1). The same study indicated significant
MAIT cell enrichment and IL-17 production in the airways with
normalized levels of MAIT cells in the convalescent phase,
indicating that these cells are engaged in the immune response
against severe acute respiratory syndrome-coronavirus-2
(SARS-CoV-2) and may be involved in COVID-19 immunopathogenesis. A
year later, it was demonstrated that altered MAIT cell functions
could contribute to the severity of COVID-19 and that the
therapeutic manipulation of MAIT cells may prevent disease
aggravation (2). More recently,
another study indicated that severe COVID-19 is associated with a
reduced frequency of peripheral MAIT cells in convalescent
individuals (3). However, this
issue has not been adequately explored, and the possible role of an
induced immunoglobulin G (IgG)-mediated immune response has not
been evaluated. The present research group has been investigating
the role of the IgG repertoire in the induction, regulation and
modulation of the development of human diseases, and has proposed
the ‘hooks without bait’ hypothesis (4). In this research program, evidence has
been found to indicate that the human naturally produced or induced
IgG repertoire, obtained from different immune backgrounds, can
mediate some functional and phenotypic modulations of healthy human
thymic and peripheral T and B cells, including CD4+,
CD8+ and γδ T-cell receptor (γδ TCR)+ T
cells, and that the modulation profile may be associated with the
development or control of atopic (5-12)
or infectious diseases (13). These
approaches may contribute to the development of immune signatures
associated with the development or severity of several diseases.
Based on those evidence, the present study aimed to evaluate if the
development of mild or severe COVID-19 induces the establishment of
an IgG repertoire that can, at some point, be involved in the
development of a disease-related immune phenotype.
Materials and methods
Samples
Blood samples from the Central Laboratory Division
of the Clinics Hospital of the Faculty of Medicine of the
University of São Paulo (São Paulo, Brazil) were used. Serum
samples were isolated and kept at -20˚C until used for IgG
purification. As an inclusion criterion, the diagnosis of COVID-19
was confirmed via the detection of SARS-CoV-2 RNA by reverse
transcription-polymerase chain reaction. Patients aged >75 years
and those who did not test positive for SARS-CoV-2 were excluded
from the study. As IgG donor controls, 40 samples from healthy
individuals [17 males and 23 females; age (mean ± SE): 28.5±2,3
years] collected prior to the COVID-19 pandemic from March to July
2019 were used.
The cohort of 79 patients infected with COVID-19
included 39 males and 40 females. Patients were categorized based
on the World Health Organization classification of 2020 (https://apps.who.int/iris/handle/10665/332196):
Hospitalized patients who did not receive oxygen therapy or who
received oxygen by a mask or nasal cannula were considered to be
mild cases (n=39); patients admitted under non-invasive ventilation
or high-flow oxygen were considered severe cases (n=40); and
patients admitted under invasive ventilation without or with
additional support for another organ, for example, extracorporeal
membrane oxygenation or replacement therapy, were considered
critical cases. In the present study severe and critical cases were
evaluated together and termed as severe. The samples from patients
with COVID-19 were obtained from May to July 2020.
Blood samples from 10 volunteers (2 males and 8
females) were collected in EDTA and used for the isolation of
peripheral blood mononuclear cells (PBMCs) on the day of
collection. As an inclusion criterion, it was confirmed that these
volunteers had not tested positive for SARS-CoV-2 and had no
clinical or laboratory COVID-19 diagnosis history. The samples were
obtained from PBMCs donors from February to May 2022 and PBMCs were
isolated by via centrifugation in a density gradient using
Ficoll-Paque (GE Healthcare Bio Science) at 540 x g for 20 min at
21˚C. Detailed information about the patients with COVID-19 and the
PBMC donors is presented in Tables
SI and SII, respectively. The
Ethics Committee at the School of Medicine at the University of São
Paulo approved the study [Certificado de Apresentação de Apreciação
Ética (CAAE): 63361622.7.0000.0068 and 70823623.0.0000.0068], and
written consent was obtained from all participants.
IgG purification
IgG was purified from pooled serum using a
Melon™ Gel IgG Spin Purification Kit (Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
Purified IgG was collected, sterilized using 0.20-µm filters
(Corning Life Sciences), and stored at -80˚C for use in cell
culture experiments. IgG concentrations were determined using
Coomassie Protein Assay Reagent (Pierce; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturer's instructions. The
purity of the IgG, as evaluated by SDS-PAGE, was >95%. All pools
were evaluated for the presence of IgA, IgM and IgE antibodies, all
of which were undetectable.
Cell culture and flow cytometry
Suspensions of PBMCs were washed and resuspended in
RPMI-1640 medium containing 10% FetalClone™ III (FC-III;
HyClone; Cytiva). Using a Neubauer chamber, an aliquot of the cell
suspension was diluted in trypan blue (Sigma-Aldrich; Merck KGaA)
to evaluate the cell viability and number. Then, 1x106
viable PBMCs were placed in each well of a 96-well culture plate
(Costar; Corning, Inc.) and cultured with 100 µg/ml IgG purified
from the pooled serum samples of patients with mild or severe
COVID-19 in RPMI-1640 medium containing 10% FC-III. As controls,
the mock condition (absence of IgG), the addition of 100 µg/ml
therapeutic intravenous IgG (IVIg; Baxter International Inc.) or
the addition of IgG purified from the serum of healthy controls
were used. The culture plates were incubated for 3 days at 37˚C in
5% CO2, and 1 µg/ml brefeldin A (Sigma-Aldrich; Merck
KGaA) was added in the last 12 h for intracellular staining. Cell
staining was performed and cell labeling was evaluated via flow
cytometry. For cell viability analysis, the cells were incubated
with LIVE/DEAD™ (PE-Texas red) fluorescent reagent
(Thermo Fisher Scientific, Inc.). All extracellular and
intracellular analyses were performed using viable cells.
To perform extracellular staining, PBMCs were
transferred to test tubes, and 1 µg each antibody was added to the
cells, with the exception of the unlabelled tubes. The samples were
then incubated for 30 min at 4˚C while protected from light. After
that, 500 µl PBS solution was added, and the tubes were centrifuged
at 400 x g for 5 min at 21˚C. The supernatant was discarded by
inverting each tube. Then, PBS was added, followed by fixation in
200 µl 1% formaldehyde for ≥10 min at 8˚C. The PBMCs were then
incubated with mouse anti-human CD3 (BV421; cat. no. 555412), CD19
(FITC; cat. no. 555412), CD14 (PerCP-Cy5.5; cat. no. 562692), CD45
(PeCy7; cat. no. 557748), CD161 (BV510; cat. no. 563212), CD4
(BV605 cat. no. 562658), CD8 (APC-Cy7; cat. no. 557834), γδTCR
(FITC; cat. no. 347903) and Vα7.2 (PE; cat. no. 566739) or isotype
control antibodies (BD Pharmingen; BD Biosciences) for 20 min at
8˚C.
To perform intracellular labeling, tubes containing
PBMCs were centrifuged at 400 x g for 5 min at 21˚C, the
supernatant was discarded, and 1 µg each antibody was added to the
cells, with the exception of the unlabelled tubes. Then, 100 µl PBS
containing 0.05% saponin permeabilization reagent was added, and
the tubes were stored at 4˚C for 30 min while protected from light.
After centrifugation at 400 x g for 5 min at 21˚C, the supernatant
was discarded by inverting each tube, and the cells were
resuspended in 300 µl PBS solution. The PBMCs were then incubated
with mouse anti-human IFN-γ (APC; cat. no. 551385) and IL-17 (Alexa
700; cat. no. 560613) or isotype control conjugated with the
corresponding fluorochromes for 20 min at 8˚C (BD Pharmingen; BD
Biosciences).
Using an LSRII Fortessa™ flow cytometer
(BD Biosciences), 500,000 events per PBMC sample were acquired in
the lymphocyte quadrant, as determined by their relative
size/granularity. Compensation was performed using adsorbed
microspheres (CompBeads; BD Biosciences) treated with the
antibodies used for extra- and intracellular staining. Cell gating
was based on the specific isotype control values as well as the
fluorochrome minus 1 setting.
CD45highCD14-CD19-CD3+CD161+Vα7.2+
live lymphocytes were considered MAIT cells (Fig. S1). The frequencies of
γδTCR+CD3+, CD4+CD8-,
CD8+CD4- and
CD19+CD8-CD4- live lymphocytes
were also determined to evaluate the frequency of γδT,
CD4+ T, CD8+ T and B cells, respectively.
Data analysis was performed using FlowJo software (Version 10.8;
Tree Star, Inc.), and only the extra- and intracellular staining of
viable cells was analyzed.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 (GraphPad Software; Dotmatics). In vitro data were
obtained from 6 separate experiments with 1 or 2 samples. P≤0.05
was considered to indicate a statistically difference, as assessed
by one-way ANOVA using Tukey's post hoc test for multiple
comparisons among all groups.
Results
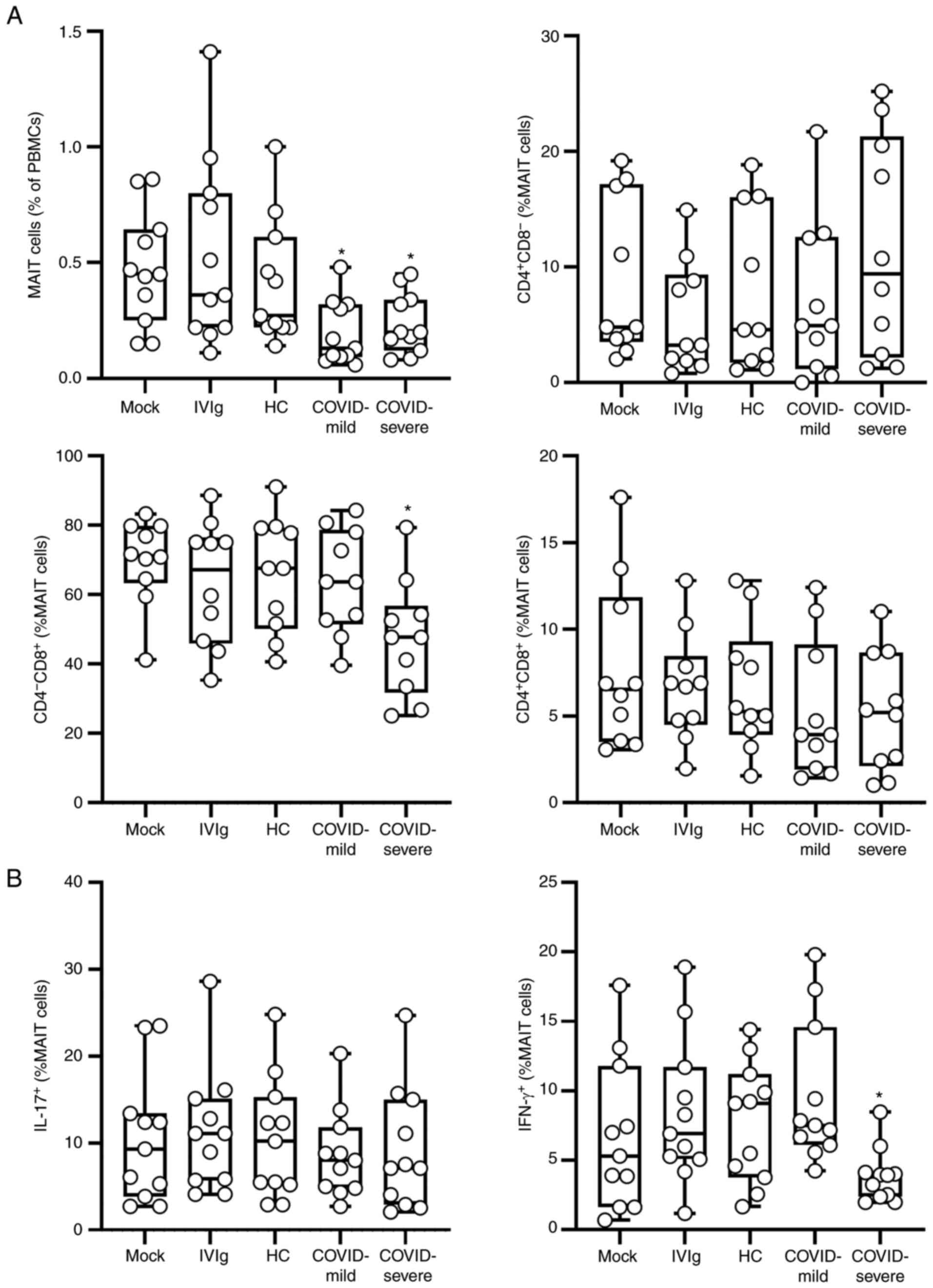
The frequency of MAIT cells (Fig. S1) was first identified and
evaluation of the results revealed that purified IgG obtained from
mild and severe cases of COVID-19 reduced the frequency of these
cells compared with the frequency under control conditions
(Fig. 1A). When evaluating the
expression of co-receptors, it was observed that purified IgG
obtained from severe COVID-19 cases reduced the frequency of
CD4-CD8+ MAIT cells compared with that of the
controls. No difference in the frequency of the phenotypes
CD4+CD8- or CD4+CD8+
was observed when all culture conditions were compared. When the
intracellular production of cytokines was evaluated, it was
observed that IgG from patients with mild or severe COVID-19 did
not influence the intracellular production of IL-17 in MAIT cells.
It was also observed that IgG from severe cases of COVID-19 reduced
the frequency of IFN-γ-producing MAIT cells compared with that of
the controls, while mild COVID-19 IgG had no effect (Figs. 1B and S2). Finally, whether the experimental
conditions influenced the frequency of important peripheral human
lymphocyte populations was evaluated, but none of the evaluated
conditions influenced the frequency of γδT, CD4+ T,
CD8+ T and B cells (Fig.
S3, Fig. S4 and Fig. S5).
Discussion
It was recently reported that downregulated levels
of peripheral MAIT cells were observed in the peripheral blood of
patients who had recovered from severe COVID-193,
corroborating previous and recent observations in the literature
(1,2). The results of the present study
indicate that the IgG repertoire induced during the development of
mild and severe COVID-19 has, per se, the in vitro
potential to reduce the frequency of the MAIT cell population in
healthy individuals. It has also been demonstrated that peripheral
lymphopenia could be related to COVID-19 disease severity and
mortality (14,15). However, this phenomenon is
potentially associated with several aspects of COVID-19
development, including intense cytokine production at the primary
site of infection, and may differentially encompass the peripheral
populations of lymphocytes, an aspect that continues to be
investigated and in which MAIT cells may have a role. Furthermore,
lymphopenia has been described in patients infected with various
viruses from different families, indicating that the possible
mechanisms engaged in this process are not specific to individual
viral families and may include the host antibody response (16), a mechanism that remains vague.
Combining those pieces of evidence, it may be hypothesized that the
reduced frequency of peripheral MAIT cells is due to the high
levels of circulating COVID-19-induced IgG, a major characteristic
of the convalescent period, corroborating the results of other
researchers (3). However, the
results obtained in the present study did not indicate differences
in the MAIT cell reduction intensity between mild or severe IgG,
possibly because the experimental protocols did not fully reproduce
in vivo conditions. These differences may include aspects
such as the concentration of IgG, which may differ between
conditions, and the inclusion criteria for each COVID-19 group,
which also differ between studies.
The ability of MAIT cells to produce IL-17 and
modulate inflammatory responses has been known for over a decade
(17). In the present study,
whether this major functional parameter was influenced by IgG
obtained from patients with different COVID-19 severity was
evaluated, but no influence was observed.
When evaluating the intracellular production of
cytokines, it was observed that the production of IFN-γ by MAIT
cells was reduced by the IgG of patients with severe COVID-19 and
not by that of patients with mild COVID-19. IFN-γ-producing MAIT
cells have been associated with the control of bacterial infections
(18), and their production is
related to the CD8+ MAIT cell phenotype and cytotoxic
activity (19). The IgG from
patients with severe COVID-19 reduced the frequency of
CD4-CD8+ and IFN-γ-producing MAIT cells,
indicating a reduction in the antiviral activity mediated by these
cells. This observation substantiates the development of more
severe disease development. It also corroborates previous evidence
about MAIT cells in COVID-19 by indicating that the total frequency
of MAIT cells is reduced and may specifically affect certain MAIT
cell subpopulations. However, the role of CD8+ MAIT
cells in COVID-19 severity is under investigation, and its precise
role requires elucidation.
Unfortunately, the approaches used in the present
study were limited and did not elucidate the possible mechanisms
that may mediate the IgG-induced reduction of peripheral MAIT
cells; however, from some similar approaches used in other studies,
some possibilities may be suggested. It was recently demonstrated
in a similar protocol using purified IgG in vitro that IgG
directly interacts with the membrane of another unconventional
T-cell population with a limited diversity of clonal receptors,
namely γδT cells (12). The
induction of apoptosis was not evaluated in the present study, but
it may be considered as a possible mechanism for the reduction in
MAIT cell frequency since single-cell transcriptomic profiling has
already indicated that cell death is a main cause of the reduction
in MAIT cell frequency during severe COVID-19(20). The biological relevance of a reduced
frequency of peripheral MAIT cells in the development of protective
immunity against SARS-Cov-2 requires elucidation. However, it was
recently demonstrated that MAIT cells might contribute to T-cell
responses, including the priming of T follicular helper cells, and
the induction of humoral immunity (21).
In conclusion, the findings of the present study
indicate the potential of a severe COVID-19-induced IgG response to
mediate a reduction in the frequency of peripheral MAIT cells,
particularly that of IFN-γ-producing MAIT cells. This unprecedented
observation contributes to elucidation of the mechanism by which
this peripheral MAIT cell reduction may occur in patients with
severe COVID-19. Finally, it is suggested that future
investigations should focus on understanding the COVID-19-induced
IgG repertoire as a mediator of immune alterations in severe and
critical cases.
Supplementary Material
Identification and evaluation of
peripheral MAIT cells in cultured PBMCs. A representative set of
flow cytometry plots from a PBMC sample in each group is presented.
Each line of plots illustrates the sequence of gatings to identify
MAIT cells in the same sample. MAIT cells were determined as
singlets, lymphocyte-compatible size/complexity and live, and cells
expressing the phenotype
CD45highCD14-CD19-CD3+CD161+Vα7.2+
cells were considered MAIT cells. MAIT, mucosal-associated
invariant T; PBMCs, peripheral blood mononuclear cells; Mock,
without IgG; IVIg, intravenous immunoglobulin IgG for therapeutic
use; HC, healthy control IgG; COVID-mild, purified IgG from
patients with mild COVID-19; COVID-severe, purified IgG from
patients with mild COVID-19; IgG, immunoglobulin G; COVID,
coronavirus disease 2019.
Representative flow cytometry plots
showing CD4/CD8 expression and the intracellular production of
IL-17 and IFN-γ by mucosal-associated invariant T cells in cultured
peripheral blood mononuclear cells. Mock, without IgG; IVIg,
intravenous immunoglobulin IgG for therapeutic use; HC, healthy
control IgG; COVID-mild, purified IgG from patients with mild
COVID-19; COVID-severe, purified IgG from patients with mild
COVID-19; IgG, immunoglobulin G; COVID, coronavirus disease
2019.
Frequency of γδT, CD4+ T,
CD8+ T and B cells in cultured PBMCs. PBMCs from healthy
individuals (n=10) were cultured with 100 μg/ml of purified
IgG from patients with mild COVID-19 (n=39) or severe COVID-19
(n=40) for 3 days. The controls were mock treated in the absence of
IgG, treated with IVIg or treated with IgG from HC individuals
(n=40) obtained prior to the COVID-19 pandemic. After culture,
PBMCs were evaluated for the frequency of (A) γδTCR+ T,
(B) CD4+ T, (C) CD8+ T and (D) B cells.
PBMCs, peripheral blood mononuclear cells; COVID, coronavirus
disease 2019; IgG, immunoglobulin G; IVIg, intravenous
immunoglobulin IgG for therapeutic use; HC, healthy control.
Representative flow cytometry plots
showing the frequency of γδT cells in peripheral blood mononuclear
cells cultured under various conditions. Mock, without IgG; IVIg,
intravenous immunoglobulin IgG for therapeutic use; HC, healthy
control IgG; COVID-mild, purified IgG from patients with mild
COVID-19; COVID-severe, purified IgG from patients with mild
COVID-19; IgG, immunoglobulin G; COVID, coronavirus disease
2019.
Representative flow cytometry plots
showing the frequency of CD4+/CD8+ T cells
and B cells in peripheral blood mononuclear cells cultured under
various conditions. B cells were identified as CD19+ DN
cells. DN, CD4/CD8 double negative; Mock, without IgG; IVIg,
intravenous immunoglobulin IgG for therapeutic use; HC, healthy
control IgG; COVID-mild, purified IgG from patients with mild
COVID-19; COVID-severe, purified IgG from patients with mild
COVID-19; IgG, immunoglobulin G; COVID, coronavirus disease
2019.
Information about the patients with
COVID-19.
Information on patients who donated
peripheral blood mononuclear cells.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Laboratory of
Medical Investigation-56, Medical School, University of São Paulo,
São Paulo, Brazil (LIM-56 HC-FMUSP), the National Council for
Scientific and Technological Development (CNPq; grant no.
302937/2021-8) and São Paulo Research Foundation (FAPESP; grant no.
2021/08225-8).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NRM and BOF performed in vitro experiments.
IGF and DTAR selected the patients and collected blood samples. MNS
collaborated with the design of the study and writing the
manuscript. JRV designed the study, wrote the manuscript, and
coordinated the activities of the other authors. JRV, NRM and BOF
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the School of Medicine at the University of São Paulo
(CAAE: 63361622.7.0000.0068 and 70823623.0.0000.0068).
Patient consent for publication
Not applicable.
Authors' information
Dr Jefferson Russo Victor, ORCID ID:
0000-0001-6092-8394
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parrot T, Gorin JB, Ponzetta A, Maleki KT,
Kammann T, Emgård J, Perez-Potti A, Sekine T and Rivera-Ballesteros
O: Karolinska COVID-19 Study Group et al. MAIT cell
activation and dynamics associated with COVID-19 disease severity.
Sci Immunol. 5(eabe1670)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Flament H, Rouland M, Beaudoin L, Toubal
A, Bertrand L, Lebourgeois S, Rousseau C, Soulard P, Gouda Z,
Cagninacci L, et al: Outcome of SARS-CoV-2 infection is linked to
MAIT cell activation and cytotoxicity. Nat Immunol. 22:322–235.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liechti T, Iftikhar Y, Mangino M, Beddall
M, Goss CW, O'Halloran JA, Mudd PA and Roederer M: Immune
phenotypes that are associated with subsequent COVID-19 severity
inferred from post-recovery samples. Nat Commun.
13(7255)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Victor JR: Do different IgG repertoires
play a role in B- and T-cell functional modulation during ontogeny?
The ‘hooks without bait’ theory. Immunol Cell Biol. 98:540–548.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
de-Oliveira MG, Lira AAL, Sgnotto FR,
Inoue AHS, Santos LS, Nakamatsu BY, Duarte AJS, Leite-de-Moraes M
and Victor JR: Maternal IgG impairs the maturation of offspring
intrathymic IL-17-producing γδT cells: Implications for murine and
human allergies. Clin Exp Allergy. 49:1000–1012. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sgnotto FDR, de Oliveira MG, Lira AAL,
Inoue AHS, Titz TO, Orfali RL, Bento-de-Souza L, Sato MN, Aoki V,
Duarte AJS and Victor JR: IgG from atopic dermatitis patients
induces IL-17 and IL-10 production in infant intrathymic TCD4 and
TCD8 cells. Int J Dermatol. 57:434–440. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sgnotto FDR, Oliveira MG, Lira AAL,
Bento-de-Souza L, Duarte AJDS and Victor JR: Low doses of IgG from
atopic individuals can modulate in vitro IFN-γ production by human
intra-thymic TCD4 and TCD8 cells: An IVIg comparative approach. Hum
Vaccin Immunother. 13:1563–1572. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Santos LS, Sgnotto FDR, Sousa TR, Orfali
RL, Aoki V, Duarte AJDS and Victor JR: IgG from atopic dermatitis
patients induces non-atopic infant thymic invariant natural killer
T (iNKT) cells to produce IL-4, IL-17, and IL-10. Int J Dermatol.
59:359–364. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Inoue AHS, Lira AAL, de-Oliveira MG, de
Sousa TR, Sgnotto FDR, Duarte AJDS and Victor JR: The potential of
IgG to induce murine and human thymic maturation of IL-10+ B Cells
(B10) revealed in a pilot study. Cells. 9(2239)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
de Sousa TR, Sgnotto FDR, Fagundes BO,
Duarte AJDS and Victor JR: Non-atopic neonatal thymic innate
lymphoid cell subsets (ILC1, ILC2, and ILC3) identification and the
modulatory effect of IgG from dermatophagoides pteronyssinus
(Derp)-atopic individuals. Front Allergy. 2(650235)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Sousa TR, Fagundes BO, Nascimento A,
Fernandes LA, Sgnotto FDR, Orfali RL, Aoki V, Duarte AJDS, Sanabani
SS and Victor JR: IgG from adult atopic dermatitis (AD) patients
induces thymic IL-22 production and CLA expression on CD4+ T cells:
Possible epigenetic implications mediated by miRNA. Int J Mol Sci.
23(6867)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fagundes BO, de Sousa TR, Nascimento A,
Fernandes LA, Sgnotto FDR, Orfali RL, Aoki V, Duarte AJDS, Sanabani
SS and Victor JR: IgG from adult atopic dermatitis (AD) patients
induces nonatopic neonatal thymic gamma-delta T cells (γδT) to
acquire IL-22/IL-17 secretion profile with skin-homing properties
and epigenetic implications mediated by miRNA. Int J Mol Sci.
23(6872)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
da Ressureição Sgnotto F, Souza Santos L,
Rodrigues de Sousa T, Feitosa de Lima J, Mara da Silva Oliveira L,
Saeed Sanabani S, José da Silva Duarte A and Russo Victor J: IgG
from HIV-1-exposed seronegative and HIV-1-infected subjects
differently modulates IFN-γ production by thymic T and B cells. J
Acquir Immune Defic Syndr. 82:e56–e60. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Toori KU, Qureshi MA and Chaudhry A:
Lymphopenia: A useful predictor of COVID-19 disease severity and
mortality. Pak J Med Sci. 37:1984–1988. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tavakolpour S, Rakhshandehroo T, Wei EX
and Rashidian M: Lymphopenia during the COVID-19 infection: What it
shows and what can be learned. Immunol Lett. 225:31–32.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Guo Z, Zhang Z, Prajapati M and Li Y:
Lymphopenia caused by virus infections and the mechanisms beyond.
Viruses. 13(1876)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dusseaux M, Martin E, Serriari N,
Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C,
Treiner E and Lantz O: Human MAIT cells are xenobiotic-resistant,
tissue-targeted, CD161hi IL-17-secreting T cells. Blood.
117:1250–1259. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Le Bourhis L, Martin E, Péguillet I,
Guihot A, Froux N, Coré M, Lévy E, Dusseaux M, Meyssonnier V,
Premel V, et al: Antimicrobial activity of mucosal-associated
invariant T cells. Nat Immunol. 11:701–708. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Dias J, Boulouis C, Gorin JB, van den
Biggelaar RHGA, Lal KG, Gibbs A, Loh L, Gulam MY, Sia WR, Bari S,
et al: The CD4-CD8-MAIT cell subpopulation is a functionally
distinct subset developmentally related to the main CD8+ MAIT cell
pool. Proc Natl Acad Sci USA. 115:E11513–E11522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shi J, Zhou J, Zhang X, Hu W, Zhao JF,
Wang S, Wang FS and Zhang JY: Single-cell transcriptomic profiling
of MAIT cells in patients with COVID-19. Front Immunol.
12(700152)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pankhurst TE, Buick KH, Lange JL, Marshall
AJ, Button KR, Palmer OR, Farrand KJ, Montgomerie I, Bird TW, Mason
NC, et al: MAIT cells activate dendritic cells to promote
TFH cell differentiation and induce humoral immunity.
Cell Rep. 42(112310)2023.PubMed/NCBI View Article : Google Scholar
|