Introduction
Zinc oxide nanoparticles (ZnO NPs) are widely used
in various industries, including biomedicine, due to their
versatility in optical and chemical properties and rating as
generally recognized as safe metal oxide by the US Food and Drug
Administration (1). Altering the
properties and morphology of ZnO NPs is simple and it can be used
to enhance their photocatalytic and photo-oxidizing potential
(1). Traditional chemical (such as
chemical vapor deposition and the use of toxic chemical reduction
reagents such as sodium borohydride) and physical (e.g. sol-gel
process and chemical co-precipitation) methods for NP synthesis are
known to increase the toxicity and subsequently decrease their
biocompatibility (2). To overcome
this, green synthesis uses eco-friendly sources and methods. In
green synthesis of NPs, substituting toxic reagents with
plant-based natural capping agents decreases toxicity and increases
biocompatibility. In this regard, papaya is used as a reducing
agent to produce ZnO NPs (3).
Papaya is widely available and easily accessible throughout the
world (4). The fruit is rich in
phytochemicals (phenolic and oleic acids and tannins) and
biomolecular compounds. In addition to being a good source of
nutrition, it is known for anti-inflammatory, antioxidant,
antibacterial, antiviral and anti-parasitic properties, which are
beneficial to health (5,6).
Despite the promising features of ZnO NPs and green
synthesis methods, NP toxicity assessment is crucial. Comprehensive
evaluation of the toxicology and hazardous properties of
nanomaterials is key to ensuring human and environmental safety
(6). The zebrafish (Danio
rerio) is a widely used model for assessing diverse biological
and toxicological responses (6-12).
The small size of zebrafish and rapid embryonic development allows
for cost-effective replication (8,9).
Furthermore, notable homologous genes and physiological response
similarities (such as immune response) between humans and zebrafish
make this model useful for understanding toxicity mechanisms
(8). Additionally, the zebrafish
model enables real-time and non-invasive tracking of NP
biodistribution (13).
Several studies regarding the toxicity of zebrafish
in metal oxide NP, specifically in ZnO NP, have been conducted
(9-12).
However, only a few studies assessed the toxicology evaluation of
ZnO NP synthesized via green synthesis methods (3,10,14).
The present study prepared ZnO NPs using papaya extract from two
solvents, aqueous and methanol, for toxicity evaluation using a
zebrafish model. Characterization of ZnO NPs obtained through green
synthesis was performed by scanning electron microscopy (SEM),
Ultraviolet Visible (UV-Vis) spectrophotometer, X-ray diffraction
(XRD) and Fourier transform infrared (FTIR) measurement. Assessment
of toxicology was performed in zebrafish by identifying embryonic
mortality, hatching rate and malformations. Subsequently, the
immune response were assessed by gene expression analysis using
quantitative (q)PCR targeting TNF-α and IL-1 as
pro-inflammatory gene, IL-10 as an anti-inflammatory gene
and the elongation factor 1 α promotor (EF1α) used as
reference gene. Therefore, the aim of the present study is to
investigate the toxicity of green synthesized ZnO NPs using papaya
fruit extract on zebrafish embryonic development and immune
response.
Materials and methods
Preparation of plant extract
Plant extracts were prepared with two solvents,
distilled water and methanol ACS grade (cat. no. 6501-04, CAS no.
67-56-1; Anhui Fulltime Specialized Solvent & Reagent Co.,
Ltd.). Whole papayas with medium ripeness were used. All fruits
were washed with distilled water, cut into small pieces, and dried
until excess water was evaporated. A total of 75 g fruits were
boiled with distilled water (1:2) for 30 min at 80˚C. The mixture
was cooled and filtered with a Buchner funnel using Whatmann filter
paper no. 1 three times to remove solid residue. The papaya extract
was kept in a fridge at 4˚C until further experiments. For the
methanol extraction, the fruits were separated and dried at 70˚C
overnight. The dried fruit was ground to a soft powder using a
blending machine. A total of 10 g fruits was weighed into a sterile
Erlenmeyer flask, then 100 ml 70% methanol was added and left for
72 h at room temperature on the shaker. Filtered extract was
evaporated at 40˚C using a rotary evaporator. The crude extract was
stored at 4˚C for further processing (15). Bioactive compound analysis was
conducted on both extracts using qualitative phytochemical tests,
including phenolic, tannin, flavonoid, saponin, triterpenoid,
steroid and alkaloid tests described by Ehiowemwenguan et al
(16).
Green synthesis of ZnO NPs from plant
extract
NP synthesis was conducted as described by Bayrami
et al (17) and Dmochowska
et al (18) with
modifications. A total of ~20 ml papaya extract was diluted in 80
ml distilled water. Then, 6.42 mg zinc nitrate
[Zn(NO3)2.6H2O, Sigma Aldrich; Merck KGaA;
cat. no. 228737-100G] was added and stirred using a magnetic
stirrer for 10 min. A total of 5 M NaOH was added until the pH
reached 12. The solution was oven-dried at 60˚C for 1 h or until
white precipitate was formed. The precipitate was rinsed with
distilled water and ethanol (3:1) after supernatant was decanted.
The pellet was centrifuged at 4,025 x g for 20 min at room
temperature and incubated in an oven at 60˚C for 24 h. Then, using
a crucible cup, the pellet was furnaced at 400˚C for 2 h, producing
a white powder of NPs. The product was stored in a hermetic tube
for testing and characterization.
Characterization of ZnO NPs
The structure of synthesized ZnO NPs was determined
by SEM analysis (JSM 6510 LA, JEOL Ltd.) using gold (99.9%) coating
with sputtering for 90 sec at room temperature, and the size of
particles was analyzed using ImageJ software version 1.53t
(ImageJ.org). The optical absorption spectra of ZnO NPs
were recorded using a UV-visible spectrophotometer (Shimadzu
Corporation; cat. no. UV-1900) at a range of 200-600 nm.
Diffraction patterns were determined by XRD at 1˚/min with two
angles from 20 to 80˚ (Bruker D8 Advance). Cu-K radiation (1.54060
Å) was operated at 40 kV and 40 mA. The results of XRD were
analyzed using OriginLab version 2023b (originlab.com) to identify the type, morphology and
crystal size of the measured particles. The diffraction peak
maximum was observed at the 101 plane and the crystallite size was
determined using Scherrer's physical formula as follows: D=0.94
λ/βcosθ where D is crystallite size, is the X-ray wavelength, and
is the full width at half the maximum of the peak (19). Functional groups and compound
classes of papaya extract and synthesized ZnO NPs were identified
using FTIR (Shimadzu Corporation; Prestige 21) at room temperature
with frequencies of 400-4,000 cm-1 (14).
Toxicity evaluation in zebrafish
Toxicity evaluations were conducted to identify the
lethality of extract and ZnO NPs in fish embryos for 96 h. The
mortality, abnormality, and hatching rate were assessed according
to standard procedure by OECD Fish Embryo Acute Toxicity Test (FET)
no. 236(20).
Adult wild-type zebrafish (n=30, 4-5 months, 0.4-0.6
g) purchased from a local breeder from Bogor, Indonesia were
maintained under standard laboratory conditions. Zebrafish were
maintained in a temperature-controlled room at 28̊C with a 14 h
light/10 h dark cycle in 12 L tanks with aerator. The zebrafish
were fed three times/day with commercial pellets. The eggs of
zebrafish were obtained 4-5 h post-fertilization (hpf) from
breeding adult fish in a 1:2 female: male ratio and analyzed under
stereo microscope (magnification 1.575x) to separate the viable
eggs. A total of ~20 viable embryos were transferred to each well
of a 24-well plate with 2 ml zebrafish culture medium (5 mM NaCl;
0,17 mM KCl; 0,33 mM CaCl2; 0,33 mM MgSO4, Sigma Aldrich). ZnO NPs
synthesized from distilled water [papaya aqueous extract (PAE);
0.01, 0.10, 1.00, 10.00 and 100.00 mg/l] and methanol extract
[papaya methanolic extract (PME); 1.25, 2.50, 5.00, 10.00 and 20.00
mg/l] were dispersed in distilled water before being added to the
wells. Negative controls (zebrafish culture medium) were used to
compare with positive controls (3,4-dichloroaniline) and treated
groups, while internal plate controls (also in zebrafish culture
medium) were used for checking the quality of the embryosaccording
to standard procedure (20). The
embryos were incubated for 96 h at 27±1˚C and observed every 24 h
for toxicity evaluation. Toxicity evaluation comprised mortality,
malformation, and hatching rate. The mortality and hatching rate
were expressed as the number of dead embryos or eggs hatched
compared with the control group. Abnormalities were analyzed by
observing coagulation of embryos, lack of somite formation,
pericardial/cardial edema and non-detachment of the tail. The
probit analysis were used to calculate the LC50 dosage which is a
method to analyze the relationship between the test
compound/treatment and the response (mortality) in a binominal
manner (21), The zebrafish embryos
used for toxicity evaluation were euthanized using excess clove oil
>100 ppm (22). All research
procedures were approved by Research Ethics Commission, Padjadjaran
University (approval no. 1026/UN6.KEP/EC/2022).
RNA isolation and cDNA synthesis
A total of ~40 zebrafish larvae 96 hpf from each
treatment (lethal concentration 50 (LC50) and untreated control
group were used for total RNA isolation using Quick-RNA™
MiniPrep Plus (Zymo Research Corp.), according to the
manufacturer's instructions. The RNA was quantified using NanoDrop
ND-1000 spectrophotometer (Nanodrop Technologies Inc.). cDNA strand
was then synthesized from total RNA templates using RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The synthesized cDNA was
stored at -20˚C for further experiments.
Gene expression analysis
cDNA amplification was performed using SYBR Green
Mastermix (GoTaq® qPCR Master Mix; cat. no. A6001;
Promega Corporation). Targeted genes associated with the immune
response in zebrafish were IL-10, IL-1, and TNF-α.
The housekeeping gene EF1α was used to normalize results. Primer
sequences are listed in Table I.
DNA amplification was performed according to the manufacturer's
instructions using 5 µl 2X PCR premix, 1 µl primer mix, 2 µl
nuclease-free water and 2 µl DNA template (nuclease-free water was
used as negative control). Thermocycling conditions were as
follows: Initial denaturation at 95˚C for 1 min, followed by 40
cycles (30 sec denaturation at 95˚C, 30 sec annealing at 58˚C and
45 sec extension at 72˚C ) and 1 min final extension at 60˚C.
Amplification and quantification were performed with CFX96 Biorad
system (Biorad) and Quantstudio™ 1 RT-PCR, for analysis
using QuantStudio Design and Analysis Software version 1.5.2
(Applied Biosystem, Thermo Fisher Scientific). Expression was
calculated by normalizing Cq values of the target gene to the Cq
value of the housekeeping gene (ΔCq) and normalized to untreated
control (ΔCq untreated-ΔCq treated) (23).
 | Table IPrimer sequences for gene expression
analysis. |
Table I
Primer sequences for gene expression
analysis.
| Primer | Sequence,
5'→3' | Accession no.
(NCBI) |
|---|
| EF1α
forward |
CTGGAGGCCAGCTCAAACAT | AI330352 |
| EF1α
reverse |
ATCAAGAAGAGTAGTAGTACC | |
| IL-10
forward |
AGCACTCCACAACCCCAATC | AY887900 |
| IL-10
reverse |
GACCCCCTTTTCCTTCATCT | |
| TNF-a
forward |
CGTCTGCTTCACGCTCCAT | BC124141 |
| TNF-a
reverse |
CTGGTCCTGGTCATCTCTCC | |
| IL-1
forward |
CGCAGCACAAAATGAAGCAG | NM 212844.2 |
| IL-1
reverse |
TGTAAGACGGCACTGAATCC | |
Statistical analysis
Data are presented as the mean ± standard error of
the mean and statistical significance of differences between groups
was analyzed by performing one-way ANOVA followed by Tukey's post
hoc test. For assessing mortality and hatching rate, a two-way
ANOVA followed by Tukey's post hoc test with dosage was utilized.
Data were obtained from three independent experiments. All the
statistical analysis was performed using IBM Corp. SPSS Statistics
29.1 for Windows. P<0.05 was considered to indicate a
statistically significant difference.
Results
Synthesis and characterization of ZnO
NPs
PAE and PME underwent preliminary phytochemical
screening test to identify the bioactive compounds involved in
synthesis of nanomaterials. Key phytochemical components of PME
included phenolic compounds, tannins, saponins and triterpenoids,
whereas PAE only contained triterpenoid (Table II).
 | Table IIPreliminary qualitative screening
analysis. |
Table II
Preliminary qualitative screening
analysis.
| Phytochemical | Papaya aqueous
extract | Papaya methanolic
extract |
|---|
| Phenol | - | + |
| Tanin | - | + |
| Flavonoid (HCl +
Mg) | - | - |
| Flavonoid
(H2SO4) | - | - |
| Flavonoid (NaOH
10%) | - | - |
| Saponin | - | ++ |
| Triterpenoid | + | + |
| Steroid | - | - |
| Alkaloid | - | - |
ZnO NPs were synthesized using both PAE and PME, as
corroborated by absorption bands at 387.4 and 364.0 nm using UV-Vis
spectroscopy, respectively (Fig.
1A). Both bands are characteristic of ZnO NP (1,2).
Furthermore, particles were also characterized using XRD, revealing
typical hexagonal wurtzite structure of ZnO NPs based on the
diffraction angles at 31.641, 34.291, 36.271, 47.481, 56.471,
62.801, 66.351, 67.711, 69.101 and 76.831, corresponding to the
reflection planes of 110, 002, 101, 102, 110, 103, 200, 112, 201
and 202, respectively (Fig. 1B).
The sharp and narrow diffraction peaks were in accordance with
Joint Committee on Powder Diffraction Standards card no. 36-1451,
which is a compiled database of diffraction patterns of various
high quality powder, confirming the pure crystallite form of the
ZnO hexagonal phase (wurtzite structure) (24). The diffraction peak maximum was
observed at the 101 plane and. Mean crystallite size of ZnO NP PAE
and PME was 13 and 12 nm, respectively. To visualize the structure
of ZnO NPs, particles were also subjected to SEM imaging. ZnO NP
synthesized had mean particle sizes of 198 and 152 nm for PAE and
PME, respectively (Fig. 1C). The
particles exhibited nanoflower morphology and surface structure,
with only slight variations in thickness.
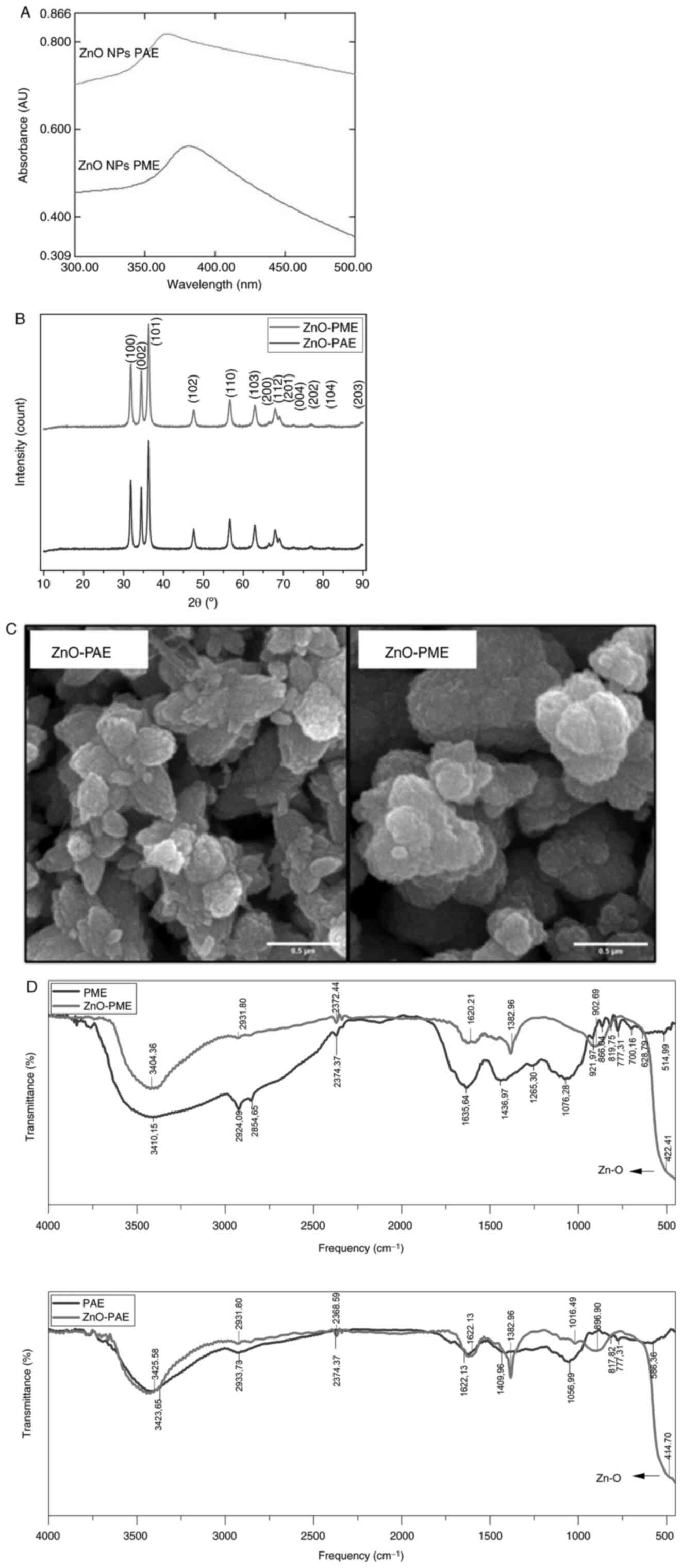 | Figure 1Characterization of ZnO NPs
synthesized from PAE and PME. (A) UV-Vis spectrum, (B) XRD pattern,
(C) morphology under scanning electron microscopy (magnification,
x40,000) and (D) FTIR spectrum analysis of papaya extract. ZnO NP,
zinc oxide nanoparticle; PAE, papaya aqueous extract; PME, papaya
methanolic extract; UV-Vis, Ultraviolet Visible Spectroscopy; XRD,
X-ray Diffraction; FTIR, Fourier Transform Infrared. |
To identify the chemistry of the compounds from PAE
and PME involved in the formation of ZnO NPs, analyses based on
FTIR spectra were conducted (Fig.
1D). For PAE, the vibration bands were observed at 586.36,
777.31 and 817.82 (C-H), 1056.99 (C-O stretch of alcohols), 1409.96
(OH bend of phenol), 1622.13 (C=C stretching alkene), 2933.73 (C-H
stretching of methylene) and 3423.65 cm-1 (O-H
stretching of alcohols). For PME, the bands were recorded at
514.99, 628.79, 777.31, 819.75 and 866.04 (C-H vibrations), 700.16
(C-C vibrations), 921.97 (-CH=CH2 vinyl terminal),
1,076.28 and 1,265.30 (C-O stretch of alcohols and phenol),
1,436.97 (C-H bend of methylene), 1,635.64 (C=C stretching alkene),
2,854.65 and 2,924.09 (C-H stretching of methylene) and 3,410.15
cm-1 (O-H stretching of alcohols). The functional groups
identified by FTIR analysis were consistent with the phytochemical
screening which indicated the presence of phenolic compounds,
tannins, saponins and triterpenoids.
FTIR analysis of ZnO-PME revealed vibration bands at
422.41, 902.69, 1,382.96 and 1,620.21 (C=C stretching alkene),
2,372.44 (N-H component), 2,931.8 (C-H stretching of methylene) and
3,404.36 cm-1 (O-H stretching of alcohols). For ZnO-PAE,
bands were observed at 414.7, 896.9, 1,016.49, 1,382.96 and
1,622.13 (C=C stretching alkene), 2,368.59 (N-H component), 2,931.8
(C-H stretching of methylene) and 3,425.58 cm-1 (O-H
stretching of alcohols). The vibration bands between 400 and 600
cm-1 were attributed to the Zn-O group due to vibration
of Zn and O atoms in ZnO (25,26).
Toxicity evaluation
Healthy zebrafish embryos (6 hpf) were used to
assess the toxicity in terms of mortality, hatching rate and
malformation. Mortality is defined as the number of zebrafish
embryos that died during observation, while hatching rate is
defined as the number of zebrafish embryos that hatched from their
chorion. Finally, malformation was considered to be a common
abnormality that occurs in pericardial edema and yolk sac
edema.
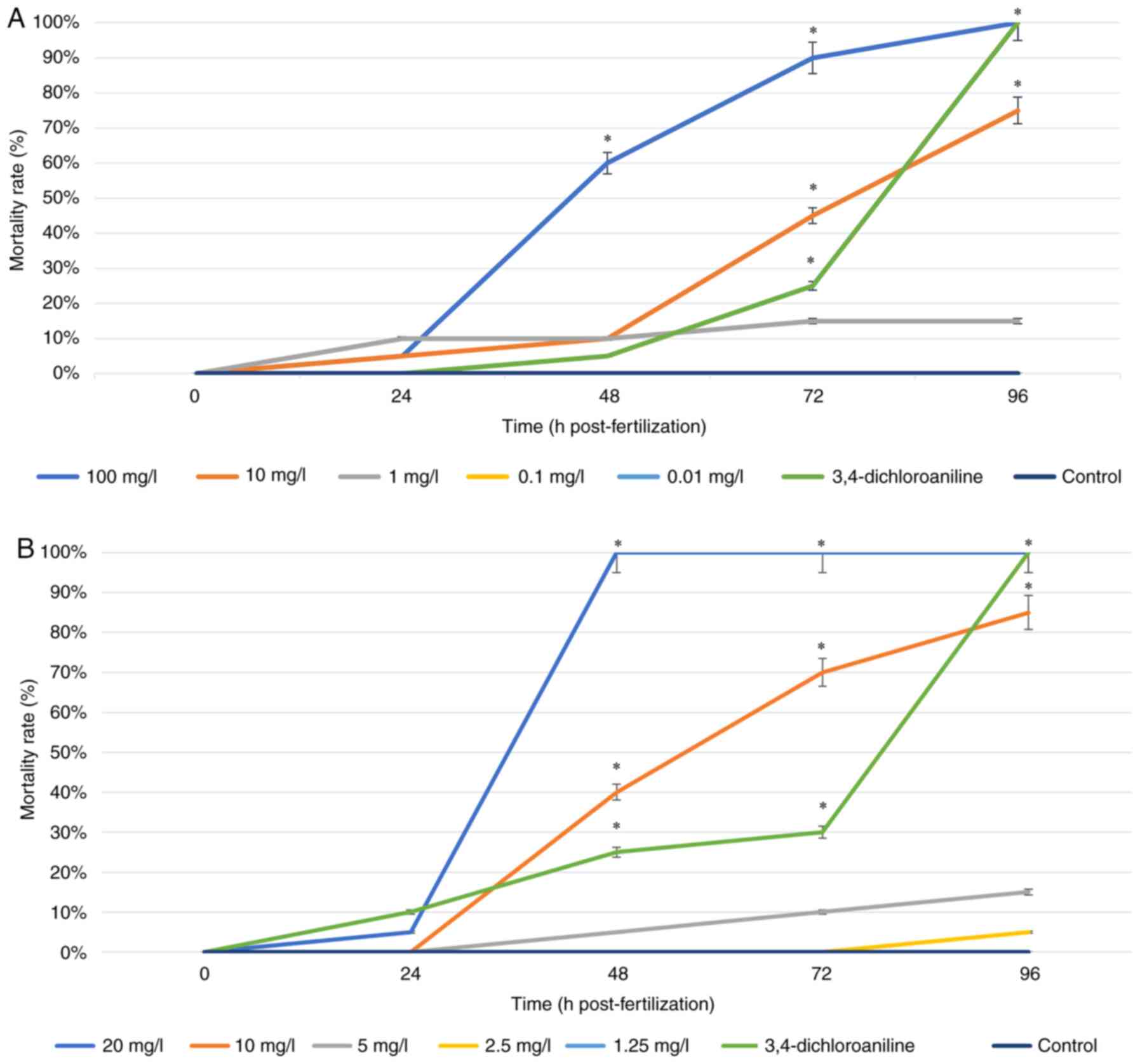
Mortality rate. The mortality rate of
zebrafish exposed to ZnO NPs synthesized from PAE and PME was
observed for 96 h (Fig. 2). Both
types of ZnO NP showed a tendency for higher concentrations to
cause significant mortality in zebrafish, with similar results for
the positive control (3,4-dicholoroaniline) after 96 hpf. The
highest concentration of ZnO NP PAE, 100 mg/l (Fig. 2A), led to the death of all zebrafish
embryos at 96 h of observation. ZnO NP PME (Fig. 2B) at 20 mg/l showed mortality after
48 hpf. The concentration of 10 mg/l in both ZnO NPs showed a
fairly high mortality, in which half of zebrafish embryos died. On
the other hand, lower concentrations displayed similar results to
the control group. For ZnO NP PAE, concentrations of 0.1 and 0.01
mg/l showed similar results at all time points. Similarly, the
results obtained from the lowest concentration of ZnO NP PME which
is 1.25 mg/l indicated similar results with that of the control
group. The 96-h LC50 values estimated by probit analysis for ZnO NP
PAE and PME were 8.246 and 6.568 mg/l, respectively.
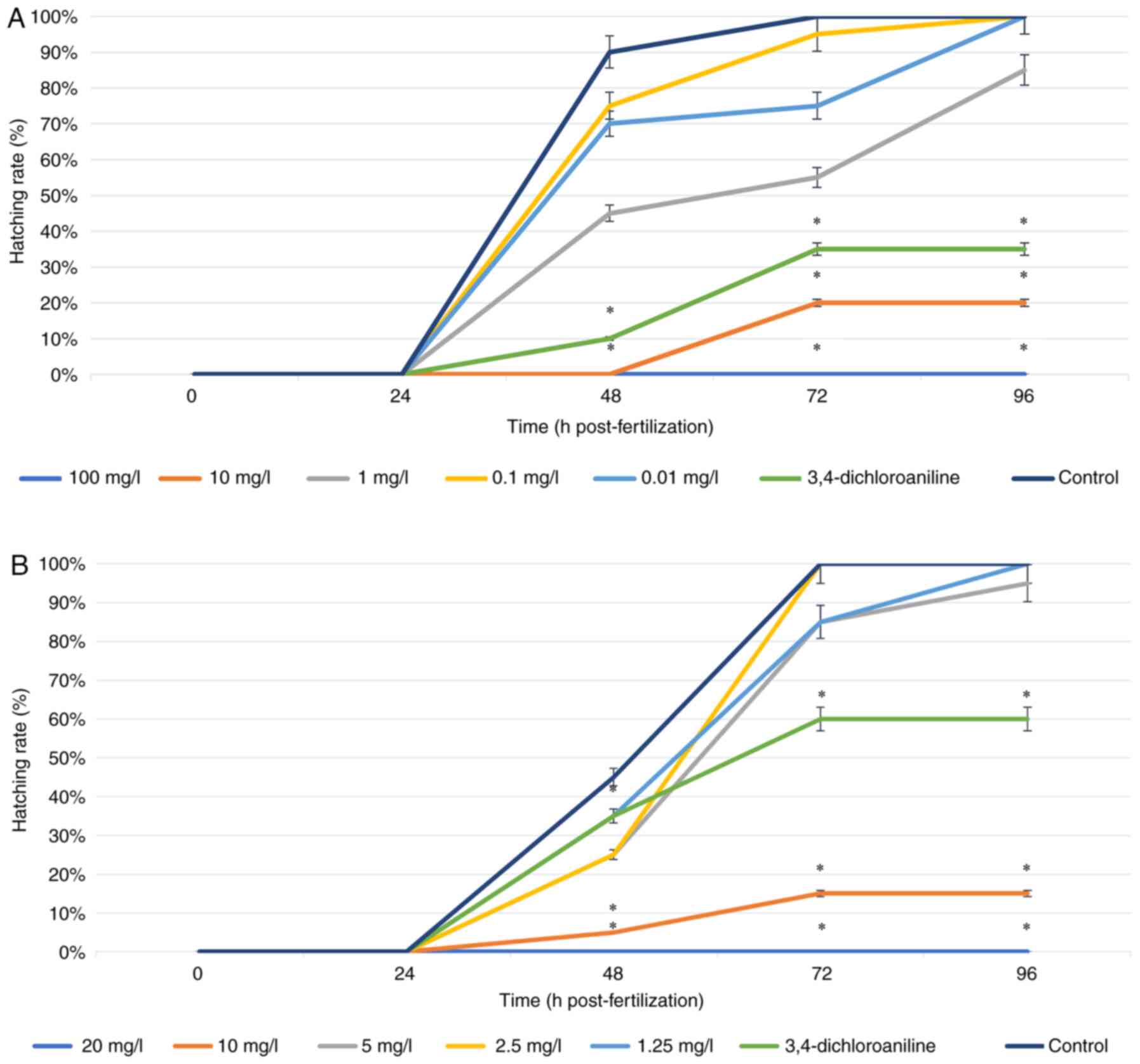
Hatching rate. The hatching rate of zebrafish
embryos was also investigated for 96 h. At 24 h, no embryos had
hatched and the hatching rate significantly increased at 48 h
(Fig. 3). In the control group,
normal embryos hatched at 48-72 h. The hatching rate in all
treatments using both ZnO NPs at low concentrations had the same
results as the control group at 96 h of observation. (ZnO NP PAE,
0.10 and 0.01; PME: 1.25 and 2.50 mg/l; Fig. 3A and B, respectivey). The higher concentrations
showed a significantly decreased hatching rate, with 100 PAE and 20
mg/l PME preventing all hatching. The concentration at 10 mg/l for
both types of ZnO NP yielded a lower hatching rate compared with
the positive control, showing that exposure to ZnO NP >10 mg/l
led to inhibition of the development of the zebrafish embryo. ZnO
NP exerted embryonic toxicity in a dose- and time-dependent
manner.
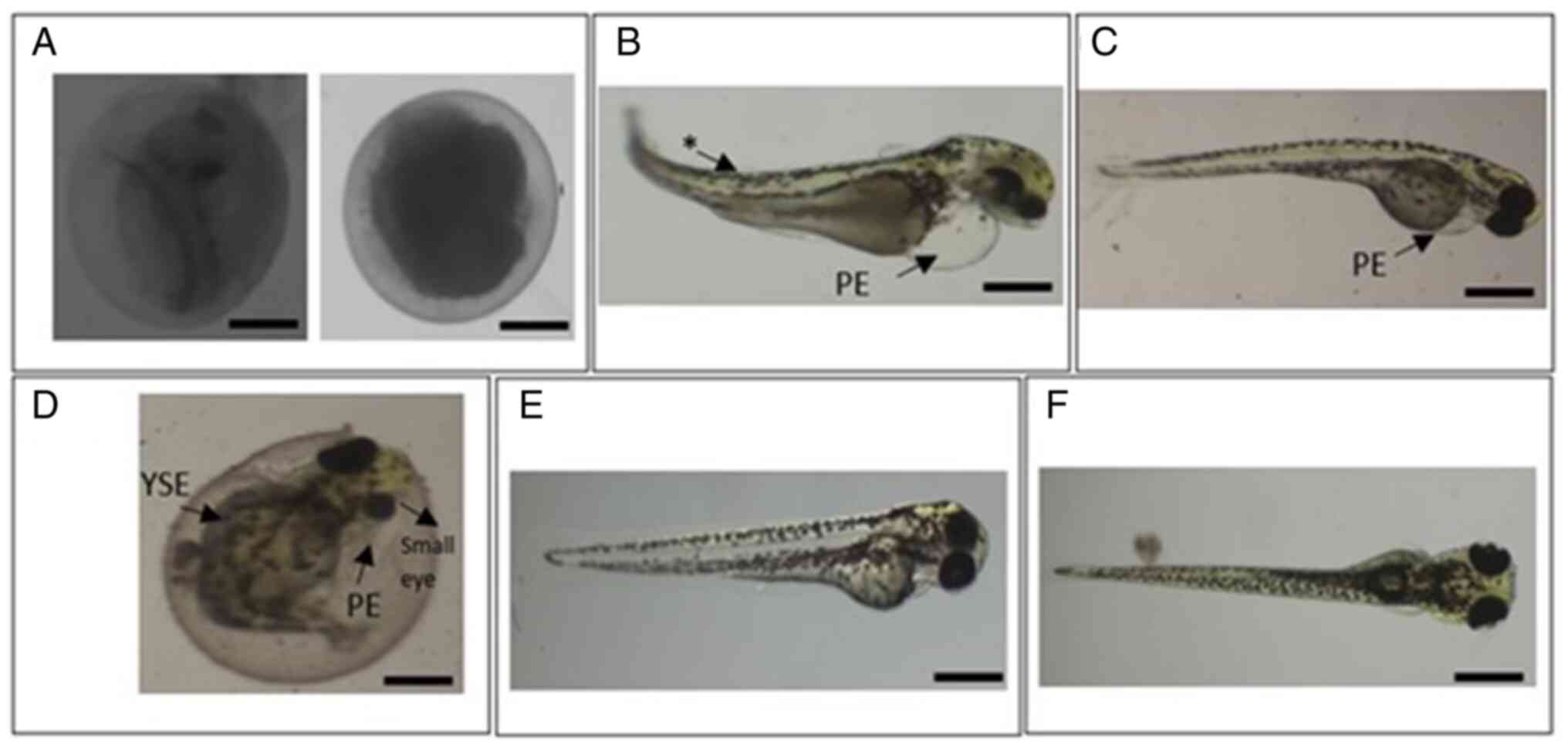
Malformation. After exposure to ZnO NP PAE
and PME, larvae showed several abnormalities or morphological
alterations typical of metal oxide NP-induced toxicity, such as
coagulation of the embryo and pericardial edema (20). Fish embryo acute toxicity tests have
four core endpoints: i) Coagulation of fertilized eggs; ii) lack of
somite formation; iii) non-detachment of the tail bud and iv) lack
of heartbeat (20). Zebrafish
embryos exposed to 20 mg/l ZnO NP PAE and PME showed coagulation
after 48 hpf (Fig. 4A). Coagulation
occurred at 24 hpf (Fig. 4A),
indicating early death, and in later developmental stages, where
the general development was delayed and the body typically started
coagulating from the tail and the yolk sac.
In addition to the endpoints, other observations
were recorded as lethal or sublethal endpoints (27). Malformations were identified
(arrows) at the spine and sac yolk compared with the control group
(Fig. 4B, C and F)
following treatment with 5 mg/l ZnO NP PME and 10 mg/l ZnO NP PME
and PAE, indicating a toxic effect on embryonic development.
Zebrafish embryos exhibited decreased eye size and the formation of
pericardial edema at 72 hpf following exposure to 10 mg/l ZnO NP
PME (Fig. 4D). However, lower
concentrations of ZnO NP PAE and 1.25 and 2.5 mg/l ZnO NP PME
showed no significant difference compared with the control
(Fig. 4E).
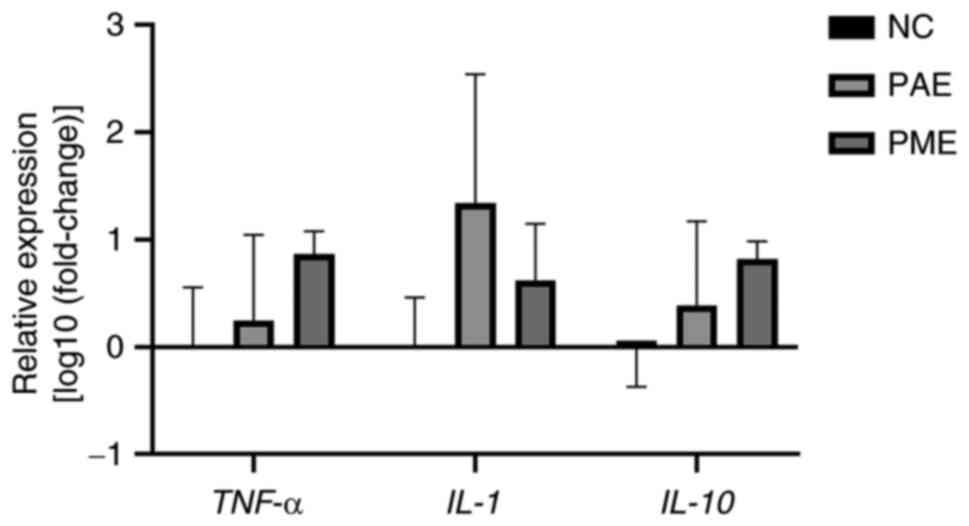
Expression levels of inflammatory
genes
LC50 of synthesized ZnO NPs upregulated transcripts
of IL-1 and -10 and TNF-α (Fig. 5). TNF-α and IL-1 and
-10 mRNA expression levels in whole zebrafish larvae were
stable in controls but changed following exposure to 8.246 mg/l ZnO
NP PAE and 6.568 mg/l PME for 96 h. The proinflammatory cytokine
TNF-α mRNA expression levels following ZnO NP PME exposure
were higher than following ZnO NP PAE exposure, while the highest
IL-1 mRNA expression was observed following exposure to
ZnO-PAE. IL-10 mRNA expression, an anti-inflammatory
cytokine, was observed to be upregulated after exposure to both ZnO
NP PAE and PME. This may indicate an immunomodulatory response
after exposure to ZnO NPs (28).
Discussion
ZnO NP have unique features associated with
biomedical applications, such as relatively high catalytic
reactivity and also have a good non-linear optical performance and
biochemical stability (2).
Synthesis of ZnO NP yields different size, morphology and structure
depending on methodology. Green synthesis is one of the most common
metal oxide NP synthesis methods using biological substances
(3). In the present study, ZnO NP
was synthesized from papaya extract. The present study synthesized
ZnO NP from papaya extract. Papaya contains abundant phytochemicals
(i.e. phenolic, terpenoid, tannin, and alkaloid compounds) and has
been shown to be beneficial in the treatment of several diseases,
such as inflammation, hyperglycemia, and hypertension. It also
possesses anticarcinogenic, antiparasitic and antimicrobial
activities (29). Here, papaya
extract was obtained using distilled water and methanol to explore
the effects of ZnO NP.
The phytochemical screening was carried out for both
extracts to determine the bioactive compound involved in ZnO NP
synthesis. Phytochemicals such as terpenoids, alkaloids, phenolics,
tannins, amino acids and saponins extracted from plants are
potential substitutes for stabilizing and reducing agents (5,30,31).
PME produced more phytochemicals such as phenolic compounds,
tannins, saponins and triterpenoids compared with PAE, which only
produced triterpenoid. These results may be due to differences in
the polarity of distilled water and methanol, which dissolve
different bioactive compounds during extraction. Papaya might
contain high levels of biocompounds that are soluble in water
(29). Phenolic and other
phytochemical compounds in the papaya fruit extract serve as
capping agents for NPs. Phenol, triterpenoid, and saponin are also
known for their antioxidant, anticarcinogenic and anti-inflammatory
activities (14,30). Coordination with -OH and COOH groups
stabilizes and caps synthesized ZnO NPs (31). Terpenoid and phenolic group
molecules are responsible for the reduction process (18).
Based on XRD and SEM analyses, there was a slight
difference in size between ZnO NP PAE and PME. ZnO NP PAE showed a
larger size (SEM, 207 nm; crystallite size from XRD, 13 nm)
compared with the ZnO NP PME (SEM, 188 nm; crystallite size from
XRD, 12 nm). The difference in size of ZnO NP might be due to the
number of phytochemicals involved in the reduction of ZnO NP as PME
contained more biocompounds extracted than PAE (29,30).
Despite that, surface morphology and crystallite structure analyzed
showed a similar nanoflower shape with wurtzite structure, which is
typical morphology for ZnO NP (5,26,32,33).
Furthermore, the FTIR analysis indicated that PME showed more
vibration bands than PAE, indicating that more phytochemicals were
contained in PME. Peaks observed from ZnO NP PAE and PME showed
that both synthesized ZnO NPs have organic functional groups from
phytochemical components of papaya fruit extract, which are
strongly attached to the surface of Zn precursor and act as both
capping and reducing agents (5,31). As
functional groups were similar for ZnO-PME and ZnO-PAE, similar
compounds may have been involved in the reductive synthesis using
both extracts and remained as the capping agents surrounding NPs,
such as phenol and triterpenoid (26). Biomolecules are bifunctional in the
formation and stabilization of ZnO NPs in aqueous medium; the
phenolic group prevents agglomeration, allowing metal NPs to form
and stabilize (34).
Numerous in vivo toxicity assessments of ZnO
NP have been conducted in various animal models to determine the
effect of ZnO NP in organisms (3,9-11,28,35,36),
Toxicity assessment has been performed in several systems (such as
pulmonary, renal and reproductive) in mammalian models (28,36).
However, assessment in mammalian models is time-consuming,
expensive and laborious due to invasive distribution of ZnO NP. For
initial screening of nanomaterial toxicity, zebrafish models
provide a quick and easy assessment (6). The zebrafish model is typically used
in developmental toxicology as embryos are optically transparent
and developmental observation is simple using microscopy. Moreover,
zebrafish share close homology with the human genome and similar
physiological responses to mammalian models (8,29).
Earlier developmental embryos are more susceptible
to external substances than adult or larval zebrafish (8,29).
Hence, administration of ZnO NPs synthesized from PAE and PME to
examine their potential toxicity was performed during the embryonic
period (4-96 hpf). The present study indicated that ZnO NPs
synthesized from PAE and PME led to a rise in mortality and a
decline in hatching rate in a concentration- and time-dependent
manner. Similar outcomes have been reported in previous studies of
nanomaterials, where the survival and hatching rates of zebrafish
embryos decreased (9-11,36-38).
Furthermore, the reduced hatching rate results from delays in the
development of the embryos and some of the embryos developed
malformation in some organs (37,38).
Common examples observed in malformed embryos were decreased
heartbeat or blood flow, lack pigmentation, delayed or altered
development, modified movement, distortion of the spine and
formation of various types of oedemata (6,7).
Oedemata in zebrafish appears to be of little mechanistic value and
is categorized as an unspecific side effect of both acute and
sublethal toxicity as changes typical of cardiotoxicity have also
been described following exposure to nanomaterials (18,29).
The toxic effect of ZnO NPs may be due to the dissolved ZnO NPs in
Zn2+ ions, which are free in intracellular cells. The
disruption of cellular Zn homeostasis in the cell is associated
with mitochondrial dysfunction and oxidative stress (27). Zn is known as an element of many
transcription factors, like zinc finger protein transcription
factors and enzymes (35,39).
The primary molecular mechanism underlying the
toxicity of NPs is the formation of reactive oxygen species (ROS),
which leads to the induction of inflammation (40). Inflammatory cytokines, such as ILs
and TNF are proteins secreted mainly by activated immune cells such
as macrophages and neutrophils (41). These key proinflammatory cytokines
produced by activated immune cells in zebrafish and the first
cytokines produced in the initial stages of inflammation (41,42).
TNF-α and IL-1 mediate the innate immune response,
which is an important activator of inflammation (43,44).
IL-10 is a potent anti-inflammatory cytokine that suppress
the transcription of proinflammatory cytokines (45).
The present study showed that expression of genes
associated with the immune response might be stimulated by ZnO NP
exposure. Similarly, in whole zebrafish embryos, copper (Cu) NPs
(25 nm; 1 mg/l), soluble Cu, and polystyrene NPs (25 nm; 10 mg/l)
were able to upregulate more mRNA expression of innate immune genes
[IL-1β and immunoresponsive gene 1 (irg1l)] on
the skin cells (epithelium) than in the intestine. Exposure to
CuNPs induces neutrophil migration in the tail as a result of
altered transcriptional changes in pro-inflammatory genes,
indicating NP-specific inflammatory response (45). ZnO NP exposure induces
immunotoxicity in BALB/c mice in an age-dependent manner, as aged
mice exhibited altered CD4 and CD8 cells, IL-6, TNF-α and
IFN-γ (28).
Exposure to ZnO NPs promotes production of ROS,
which leads to oxidative and endoplasmic reticulum stress, then
excessive uptake and formation of fatty acids, which are deposited
in the liver and cause non-alcoholic fatty liver disease (46). The cytotoxic activity of ZnO NPs has
been shown to be associated with the physicochemical properties of
their surface, which may be exert an indirect (influencing kinetics
and release of zinc ions, which are the main factor causing
toxicity) or direct (interacting with cell membranes) influence
(47-49).
Toxicity of ZnO NP in the aqueous environment depends on the
hydrodynamic diameter and concentration, with larger hydrodynamic
diameters and high concentrations associated with higher toxicity
(36). Nevertheless, the
quantitative association (amount of Zn2+ release from
ZnO NPs that leads ROS production in cells) of the dose-response
relationship for both ZnO NP synthesized should be determined
(48). In addition, expression of
genes associated with antioxidant, antibacterial, antiviral and
anti-parasitic properties need to be assessed in ZnO NPs from PAE
and PME.
Here, ZnO NPs synthesized from PME and PAE gave
similar outcomes in toxicity, with the mean safe dosage of ZnO NPs
<10 mg/l. This is also consistent with previous studies with ZnO
NPs synthesized from Amaranthus caudatus causing deformities
and a lower survival rate at ≥10 mg/ml (50). Although the present study did not
conduct experiments with different concentrations of papaya extract
to synthesize ZnO NPs or change the shape of ZnO NPs (spheres), to
the best of our knowledge, the present study is the first to screen
toxicity of ZnO NPs synthesized from plant extracts. One way to
reduce the toxicity of ZnO NPs is surface modification with silica
coating to prevent dissolution of ZnO NPs (51).
To summarize, the present study indicated that
exposure to ZnO NPs synthesized from plant extract leads to
developmental toxicity in embryos based on mortality, hatching rate
and malformation. ZnO NPs may pose a potential environmental
hazard, highlighting the need for further investigation into the
association between ZnO NP exposure, adverse effects and the
underlying biological mechanisms to evaluate safety of ZnO NPs.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Research, Community
Service, and Innovation Program for Physiology, Animal Development
and Biomedical Science Research Group, School Of Life Sciences and
Technology, Institut Teknologi Bandung (grant no.
52.A/IT1.C11.SK-PL/2022).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
IW, RMP, NGZ and APS contributed to the
conceptualization, study design, formal analysis, and data
interpretation. NGZ and APS performed all experiments and wrote the
manuscript. RKP performed toxicological experiments and data
analysis. NGZ and APS confirm the authenticity of all the raw data.
IW and RMP contributed to supervision and writing of original
draft. SD contributed to formal analysis, validation, and editing
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
Ethics Committee of the Faculty of Medicine, Universitas
Padjadjaran, Bandung, West Java, Indonesia (approval no.
1026/UN6.KEP/EC/2022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yadi M, Mostafavi E, Saleh B, Davaran S,
Aliyeva I, Khalilov R, Nikzamir M, Nikzamir N, Akbarzadeh A, Panahi
Y and Milani M: Current developments in green synthesis of metallic
nanoparticles using plant extracts: A review. Artif Cells Nanomed
Biotechnol. 46 (Suppl 3):S336–S343. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sadhasivam S, Shanmugam M, Umamaheswaran
PD, Venkattappan A and Shanmugam A: Zinc oxide nanoparticles: Green
synthesis and biomedical applications. J Clust Sci. 32:1441–1455.
2021.
|
|
3
|
Kalpana VN and Devi Rajeswari V: A review
on green synthesis, biomedical applications, and toxicity studies
of ZnO NPs. Bioinorg Chem Appl. 2018(3569758)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Asghar N, Naqvi SAR, Hussain Z, Rasool N,
Khan ZA, Shahzad SA, Sherazi TA, Janjua MR, Nagra SA, Zia-Ul-Haq M
and Jaafar HZ: Compositional difference in antioxidant and
antibacterial activity of all parts of the Carica papaya using
different solvents. Chem Cent J. 10(5)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rathnasamy R, Thangasamy P, Thangamuthu R,
Sampath S and Alagan V: Green synthesis of ZnO nanoparticles using
Carica papaya leaf extracts for photocatalytic and photovoltaic
applications. J Mater Sci Mater Electron. 28:10374–10381. 2017.
|
|
6
|
Jia HR, Zhu YX, Duan QY, Chen Z and Wu FG:
Nanomaterials meet zebrafish: Toxicity evaluation and drug delivery
applications. J Control Release. 311-312:301–318. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chahardehi AM, Arsad H and Lim V:
Zebrafish as a successful animal model for screening toxicity of
medicinal plants. Plants (Basel). 9(1345)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee KY, Jang GH, Byun CH, Jeun M, Searson
PC and Lee KH: Zebrafish models for functional and toxicological
screening of nanoscale drug delivery systems: Promoting preclinical
applications. Biosci Rep. 37(BSR20170199)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bai W, Zhang Z, Tian W, He X, Ma Y, Zhao Y
and Chai Z: Toxicity of zinc oxide nanoparticles to zebrafish
embryo: A physicochemical study of toxicity mechanism. J Nanopart
Res. 12:1645–1654. 2010.
|
|
10
|
Machado S, González-Ballesteros N,
Gonçalves A, Magalhães L, Sárria Pereira de Passos M,
Rodríguez-Argüelles MC and Castro Gomes A: Toxicity in vitro and in
zebrafish embryonic development of gold nanoparticles
biosynthesized using cystoseira macroalgae extracts. Int J
Nanomedicine. 16:5017–5036. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lacave JM, Retuerto A, Vicario-Parés U,
Gilliland D, Oron M, Cajaraville MP and Orbea A: Effects of
metal-bearing nanoparticles (Ag, Au, CdS, ZnO, SiO2) on developing
zebrafish embryos. Nanotechnology. 27(325102)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zakaria ZZ, Mahmoud NN, Benslimane FM,
Yalcin HC, Al Moustafa AE and Al-Asmakh M: Developmental toxicity
of surface-modified gold nanorods in the zebrafish model. ACS
Omega. 7:29598–29611. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Johnston HJ, Verdon R, Gillies S, Brown
DM, Fernandes TF, Henry TB, Rossi AG, Tran L, Tucker C, Tyler CR
and Stone V: Adoption of in vitro systems and zebrafish embryos as
alternative models for reducing rodent use in assessments of
immunological and oxidative stress responses to nanomaterials. Crit
Rev Toxicol. 48:252–271. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Singh C, Kumar J, Kumar P, Chauhan BS,
Nath G and Singh J: Green synthesis of silver nanoparticles using
aqueous leaf extract of Premna integrifolia (L.) rich in
polyphenols and evaluation of their antioxidant, antibacterial and
cytotoxic activity. Biotechnol Biotechnol Equip. 33:359–371.
2019.
|
|
15
|
Bere AW, Mulati O, Kimotho J and Ng'ong'a
F: Carica papaya leaf extract silver synthesized nanoparticles
inhibit dengue type 2 viral replication in vitro. Pharmaceuticals
(Basel). 14(718)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ehiowemwenguan G, Emoghene AO and
Inetianbor JE: Antibacterial and phytochemical analysis of Banana
fruit peel. IOSR J Pharm. 4:18–25. 2014.
|
|
17
|
Bayrami A, Parvinroo S, Habibi-Yangjeh A
and Rahim Pouran S: Bio-extract-mediated ZnO nanoparticles:
Microwave-assisted synthesis, characterization and antidiabetic
activity evaluation. Artif Cells Nanomed Biotechnol. 46:730–739.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dmochowska A, Czajkowska J, Jędrzejewski
R, Stawiński W, Migdał P and Fiedot-Toboła M: Pectin based banana
peel extract as a stabilizing agent in zinc oxide nanoparticles
synthesis. Int J Biol Macromol. 165:1581–1592. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sharma D, Sabela MI, Kanchi S, Bisetty K,
Skelton AA and Honarparvar B: Green synthesis, characterization and
electrochemical sensing of silymarin by ZnO nanoparticles:
Experimental and DFT studies. J Electroanal Chem. 808:160–172.
2018.
|
|
20
|
Organisation for Economic Co-operation and
Development: Test No. 236: Fish embryo acute toxicity (FET) test.
Guidelines for the Testing of Chemicals. Paris, France, 2013.
|
|
21
|
Halmi MIE, Rahim MBHA and Othman AR:
Estimation of LC50 and its confidence interval for the effect of
ferrous sulphate on Catla catla. J Environ Microbiol Toxicol.
6:21–23. 2018.
|
|
22
|
Fernandes IM, Bastos YF, Barreto DS,
Lourenço LS and Penha JM: The efficacy of clove oil as an
anaesthetic and in euthanasia procedure for small-sized tropical
fishes. Braz J Biol. 77:444–450. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Etape EP, Foba-Tendo J, Ngolui LJ, Namondo
BV, Yollande FC and Nguimezong MBN: Structural characterization and
magnetic properties of undoped and Ti-Doped ZnO nanoparticles
prepared by modified oxalate route. J Nanomater.
2018(9072325)2018.
|
|
25
|
Awasthi A, Bansal S, Jangir LK, Awasthi G,
Awasthi KK and Awasthi K: Effect of ZnO nanoparticles on
germination of Triticum aestivum seeds. Macromol Symp.
376(1700043)2017.
|
|
26
|
Awwad AM, Albiss B and Ahmad AL: Green
synthesis, characterization and optical properties of zinc oxide
nanosheets using Olea europea leaf extract. Adv Mater Lett.
5:520–524. 2014.
|
|
27
|
von Hellfeld R, Brotzmann K, Baumann L,
Strecker R and Braunbeck T: Adverse effects in the fish embryo
acute toxicity (FET) test: A catalogue of unspecific morphological
changes versus more specific effects in zebrafish (Danio
rerio) embryos. Environ Sci Eur. 32(122)2020.
|
|
28
|
Senapati VA, Gupta GS, Pandey AK, Shanker
R, Dhawan A and Kumar A: Zinc oxide nanoparticle induced age
dependent immunotoxicity in BALB/c mice. Toxicol Res (Camb).
6:342–352. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dotto JM and Abihudi SA: Nutraceutical
value of Carica papaya: A review. Sci Afr. 13(e00933)2021.
|
|
30
|
Truong DH, Nguyen DH, Ta NTA, Bui AV, Do
TH and Nguyen HC: Evaluation of the use of different solvents for
phytochemical constituents, antioxidants, and in vitro
anti-inflammatory activities of Severinia buxifolia. J Food Qual.
2019(8178294)2019.
|
|
31
|
Senthilkumar N, Nandhakumar E, Priya P,
Soni D, Vimalan M and Vetha Potheher I: Synthesis of ZnO
nanoparticles using leaf extract of Tectona grandis (L.) and their
anti-bacterial, anti-arthritic, anti-oxidant and in vitro
cytotoxicity activities. New J Chem. 41:10347–10356. 2017.
|
|
32
|
Dulta K, Koşarsoy Ağçeli G, Chauhan P,
Jasrotia R and Chauhan PK: Ecofriendly synthesis of zinc oxide
nanoparticles by Carica papaya leaf extract and their applications.
J Clust Sci. 33:603–617. 2022.
|
|
33
|
López-Miranda JL, España Sánchez BL,
Esparza R and Estévez M: Self-assembly of ZnO nanoflowers
synthesized by a green approach with enhanced catalytic, and
antibacterial properties. Mater Chem Phys. 289(126453)2022.
|
|
34
|
Hu YL, Qi W, Han F, Shao JZ and Gao JQ:
Toxicity evaluation of biodegradable chitosan nanoparticles using a
zebrafish embryo model. Int J Nanomedicine. 6:3351–3359.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vandebriel RJ and De Jong WH: A review of
mammalian toxicity of ZnO nanoparticles. Nanotechnol Sci Appl.
5:61–71. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Amin N, Zulkifli SZ, Azmai MNA and Ismail
A: Toxicity of zinc oxide nanoparticles on the embryo of javanese
medaka (Oryzias javanicus Bleeker, 1854): A comparative study.
Animals (Basel). 11(2170)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Duan J, Yu Y, Shi H, Tian L, Guo C, Huang
P, Zhou X, Peng S and Sun Z: Toxic effects of silica nanoparticles
on zebrafish embryos and larvae. PLoS One. 8(e74606)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhu X, Zhu L, Duan Z, Qi R, Li Y and Lang
Y: Comparative toxicity of several metal oxide nanoparticle aqueous
suspensions to zebrafish (Danio rerio) early developmental
stage. J Environ Sci Health A Tox Hazard Subst Environ Eng.
43:278–284. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Haque E and Ward AC: Zebrafish as a model
to evaluate nanoparticle toxicity. Nanomaterials (Basel).
8(561)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pandey A and Mishra AK: Immunomodulation,
toxicity, and therapeutic potential of nanoparticles. BioTech
(Basel). 11(42)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rosowski EE: Determining macrophage versus
neutrophil contributions to innate immunity using larval zebrafish.
Dis Model Mech. 13(dmm041889)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ott LW, Resing KA, Sizemore AW, Heyen JW,
Cocklin RR, Pedrick NM, Woods HC, Chen JY, Goebl MG, Witzmann FA
and Harrington MA: Tumor necrosis factor-alpha- and
interleukin-1-induced cellular responses: Coupling proteomic and
genomic information. J Proteome Res. 6:2176–2185. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kaneko N, Kurata M, Yamamoto T, Morikawa S
and Masumoto J: The role of interleukin-1 in general pathology.
Inflamm Regen. 39(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Iyer SS and Cheng G: Role of interleukin
10 transcriptional regulation in inflammation and autoimmune
disease. Crit Rev Immunol. 32:23–63. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Brun NR, Koch BEV, Varela M, Peijnenburg
WJGM, Spainkc HP and Vijver MG: Nanoparticles induce dermal and
intestinal innate immune system responses in zebrafish embryos†.
Environ Sci Nano. 5:904–916. 2018.
|
|
46
|
He M, Li X, Yu L, Deng S, Gu N, Li L, Jia
J and Li B: Double-sided nano-ZnO: Superior antibacterial
properties and induced hepatotoxicity in zebrafish embryos. Toxics.
10(144)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Czyżowska A and Barbasz A: A review: Zinc
oxide nanoparticlesi-friends or enemies? Int J Environ Health Res.
32:885–901. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Choi JS, Kim RO, Yoon S and Kim WK:
Developmental toxicity of zinc oxide nanoparticles to zebrafish
(Danio rerio): A transcriptomic analysis. PLoS One.
11(e0160763)2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu F, Harper BJ and Harper SL: Comparative
dissolution, uptake, and toxicity of zinc oxide particles in
individual aquatic species and mixed populations. Environ Toxicol
Chem. 38:591–602. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jeyabharathi S, Kalishwaralal K, Sundar K
and Muthukumaran A: Synthesis of zinc oxide nanoparticles (ZnONPs)
by aqueous extract of Amaranthus caudatus and evaluation of
their toxicity and antimicrobial activity. Mater Lett. 209:295–298.
2017.
|
|
51
|
Chia SL and Leong DT: Reducing ZnO
nanoparticles toxicity through silica coating. Heliyon.
2(e00177)2016.PubMed/NCBI View Article : Google Scholar
|