1. Introduction
Sleep-disordered breathing is a notable health
concern. About one billion people aged 30-69 are estimated to
suffer from sleep-disordered breathing worldwide (1). Another study revealed that the
prevalence of sleep-disordered breathing was 24.0-83.8% in males
and 9.0-76.6% in females (2). As
sleep-wake disorders affect homeostasis and metabolic disturbances
underlie the onset of sleep disorder, the impact of these clinical
situations is notable. Differences in sleep duration and pattern
between sexes are already known. Sex hormones including
progesterone, androgens and estrogens are also related to
respiratory disorders. In addition, men are more likely to have
obstructive sleep apnea syndrome (OSAS), although women are more
likely to develop it after menopause. According with a study,
postmenopausal patients had a prevalence of sleep disturbed
breathing that was 3-6 times higher than it was in premenopausal
women (2). Women generally get
better sleep than men (3). They
have twice as many sleep spindles, deeper deep sleep and a slower
age-related decrease in delta activity, a sign of deep sleep
(4). Only 26% of women report
excellent or very good quality sleep (5). Women aged 40-65 years are more likely
to experience sleep problems (6).
Between the ages of 20 and 70 years, 50% of females develop OSAS. A
total of 20% of females exhibit moderate sleep apnea and 6% develop
severe cases (7). Male rats
undergoing intermittent and chronic hypoxia have altered testicular
morphology and lose spermatogenic cells throughout the
spermatogenic cycle (8).
Increasing evidence of clinically significant
differences between the sexes and genders correlates with the rise
in obesity and its associated comorbidities (1,3,9).
Although most research shows the impact of sleep disorder on the
cardiovascular system, the effect on sexual function and fertility
have received less attention (10,11).
Disturbed breathing during sleep leads to repeated hypoxemia,
activation of the sympathetic nervous system and excessive daytime
sleepiness. Of mechanisms that can underlie breathing-associated
sleep-disorders, OSAS is the most common (12,13).
OSAS is a heterogeneous disorder that includes anatomical
compromise of upper airway patency, loss of tone of the pharyngeal
dilator muscles and a reduced threshold for respiratory stimulus.
Due to repeated interruptions of breathing during sleep, repeated
episodes of hypoxemia and disturbances of normal sleep architecture
appear such as decreased time in the deeper stages of sleep, poor
sleep quality and microawakenings lasting up to several minutes
(14). Consequently, sympathetic
activation, cortical excitation, and circadian disturbance of
hormonal secretions occur, generating cardiovascular disease and
endocrine and reproductive disorder (15).
In patients with OSAS, a decrease in
minute-ventilation during sleep, an increase in upper airway
resistance and a decrease in the response of the respiratory
centres to stimuli induced by hypoxia and hypercapnia are present
(16,17). In response to respiratory changes
during sleep, microtremors, erectile dysfunction or fertility
disorders frequently emerge (18).
Large-scale epidemiological studies have estimated
that ~4% of males and 2% of women suffer from OSAS (12,19).
Additionally, it is estimated that 80-90% of OSAS cases remain
undiagnosed (20).
Infertility is a notable issue for public health.
According to the World Health Organization, infertility affects 48
million couples and 186 million individuals worldwide (21). To the best of our knowledge, the
association between OSAS and infertility has rarely been studied
(22,23). Infertility due to sleep-disordered
breathing has been proposed to occur via direct mechanisms such as
impaired spermatogenesis as well as indirect mechanisms, such as
altered gonadal hormone secretion and decreased libido (22,23).
The present review aimed to describe the main
mechanisms that alter the sex hormone homeostasis and fertility
status in patients with OSAS and whether continuous positive airway
pressure (CPAP) therapy can improve these.
2. OSAS pathogenesis
OSAS is characterized by repeated episodes of apnea
and hypopnea with a frequency of at least five episodes/h, caused
by partial or total obstruction of the pharynx. The pharynx is a
duct that is kept open due to pharyngeal dilator muscle activity.
Under normal conditions, when the tonus of the pharyngeal muscles
decreases (as during sleep), there are no notable airflow changes.
However, certain factors, including obesity and facial features
(retrognathia, acromegaly, dental malocclusion, longer distance
from the hyoid bone to the mandibular plane, relaxed pharyngeal
soft tissue and large tongue base), may result in airflow
disturbances due to increased upper airway resistance leading to
severe apnea (24).
Following episodes of apnoea and hypopnoea,
nocturnal hypoxemia is initially associated with impaired
respiratory flow; when recovery capacity is exceeded on resumption
of pulmonary ventilation, continuous hypoxia occurs due to carbon
dioxide retention (25). In
addition, at the end of apnea hypopnea episodes, due to increased
respiratory effort, microtremors appear, which may produce a
transition from sleep to wakefulness, with stage N1 increase and
stage N3 and rapid eye movement (REM) decrease (26). Chronic nocturnal hypoxemia
stimulates both hematopoiesis, causing polyglobulia and increased
blood vascularity, and the gonadal glands, increasing hormone
synthesis (27).
OSAS during infancy has been shown to influence
normal growth and development by impairing the secretion of growth
hormone (28). Data showing
increased cortisol and adrenocorticotropic hormone levels
associated with sleep-wake cycle disruption reflect the effort to
maintain wakefulness (29,30). It is unclear whether apnea control
via CPAP treatment normalizes testosterone levels. Testosterone
therapy in patients with OSAS may generate notable adverse effects
like increased cardiovascular morbidity, increase serum
prostate-specific antigen levels, erythrocytosis. Thus, it should
not be introduced without careful consideration (31). To the best of our knowledge, there
is limited analysis of the impact of OSAS on female gonadal
function (32-34).
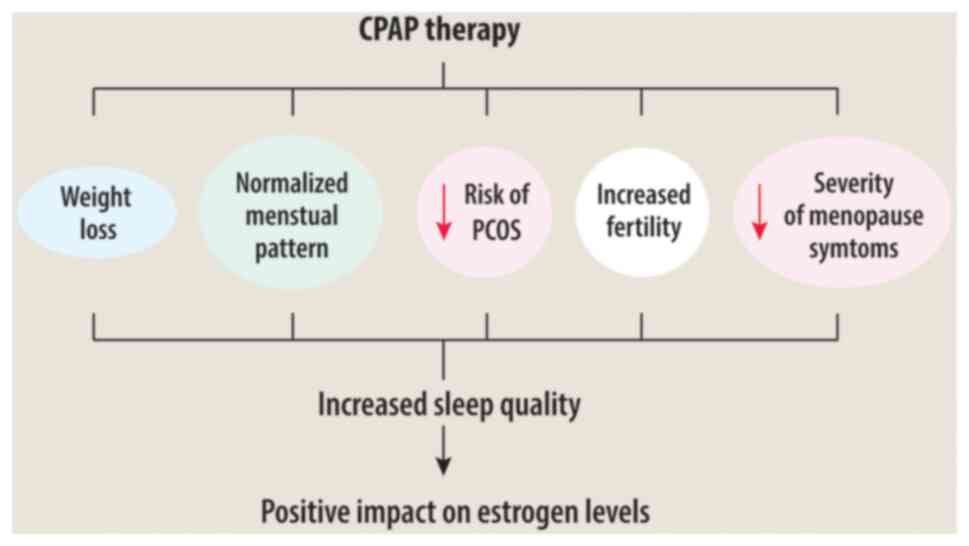
The association between CPAP therapy and estrogen
levels may depend on several factors (Fig. 1). Effective CPAP treatment can
reduce hormonal problems related to OSAS, menstrual irregularities
and PCOS risk, potentially increasing fertility and alleviating
menopausal symptoms by enhancing sleep quality and managing weight
problems (32).
3. Hypothalamic-pituitary-gonadal axis
Gonadal function is controlled by gonadotropins
secreted by the anterior pituitary, including follicle-stimulating
hormone (FSH) and luteinizing hormone (LH). In turn, the pituitary
gonadotropins controlled by gonadotropin-releasing hormone (GnRH)
released by the median eminence of the hypothalamus into
fenestrated capillaries of portal circulation and carried to the
anterior pituitary (35). The
ovarian follicle granulosa cells are stimulated by FSH to release
aromatase, which converts androgens produced by thecal cells into
estradiol (E2). FSH stimulates ovarian follicles to grow and
mature; in combination with intratesticular testosterone, it
supports spermatogenesis. LH stimulates ovulation and corpus luteum
development and controls the androgen synthesis by the Leydig
cells. The loss of negative estrogen feedback to the hypothalamus
and pituitary gland due to androgens leads to increased FSH and LH
levels (36,37). Depending on the serum values of
gonadotropin and sex steroids, it is possible to differentiate
between gonadal or hypothalamic/pituitary reproductive deficiency.
Gonadal dysfunction is characterized by elevated FSH levels and
reduced sex steroid levels, indicating primary dysfunction of the
ovaries or testes. Hypothalamic/pituitary dysfunction is marked by
low FSH and LH levels with decreased or normal sex steroid levels,
signaling a problem in the regulatory signals from the brain to the
gonads (38).
4. OSAS and gonads
There are strong interrelationships between sex
hormones and the respiratory function (39) however it is not clear which hormone
serves a crucial role. Androgens, progesterone and E2 act directly
through receptors in the nervous system. Sleep-disordered
breathing, such as OSAS, can alter the hormonal homeostasis. The
mechanisms may be different between sexes, as there are differences
in terms of prevalent hormones and breathing control due to
differences in lung size, hormonal fluctuations and body
composition (40).
Numerous studies have shown a negative association
between changes in sleep duration or architecture and the gonadal
axis (41,42). There is a complex association
between testosterone secretion, OSAS severity and obesity. Obesity
is the leading cause of OSAS and low testosterone levels have been
associated with increased body mass index (BMI) (43). Conversely, low testosterone levels
facilitate weight gain. Certain authors have attributed the
severity of OSAS to decreased testosterone concentration (44,45).
Although most studies have investigated male subjects, similar
effects have been observed in other sexes, with patients being more
affected after than before menopause (1,2).
A study found that testosterone levels decrease with
the increase in OSAS severity and are lower compared with those in
control subjects (45). In another
study that included obese men with associated metabolic syndrome
and OSAS, the control group comprising male patients with similar
clinical characteristics but without OSAS revealed that oxygen
desaturation index correlated negatively with total and free
testosterone levels (44).
Therefore, hypoxia exerts independent effects on the
pituitary-gonadal axis. A study comparing patients with OSAS with
severe oxygen desaturation and those with less severe desaturation
found a significant correlation between peak testosterone levels
and total desaturation time, suggesting that hypoxia affects the
circadian rhythm of testosterone (46). Similar findings were demonstrated in
a study that compared testosterone levels with oxygen saturation of
arterial blood (44). Loss of sleep
quality, as assessed by altered normal sleep stage architecture, is
also associated with reduced serum testosterone (47) and LH-testosterone profile (48). Although with advanced age there is a
notable risk of developing sleep disorders, Luboshitzky et
al (48) demonstrated that
hypogonadism in patients with OSAS is age-independent.
Subnormal morning LH levels have been observed in 16
men with OSAS in whom testosterone levels were normal (49). Decreased LH and testosterone levels
and their notable association with the Respiratory Disturbance
Index suggest that pituitary-gonadal dysfunction is a consequence
of OSAS rather than a primary independent disorder of the
hypothalamic-pituitary-gonadal axis (48).
Gonadal hormones receptors are present in various
types of tissues, including the lungs. The gonadal hormones have
been found in epithelial cells that line the airways and alveoli of
the lungs and can influence airway responsiveness and mucociliary
clearance (50-52).
Estrogen has been shown to affect the production of mucus in the
airways and contributes to symptoms in conditions such as asthma
and chronic obstructive pulmonary disease (53).
Activation of gonadal hormone receptors on smooth
muscle cells can affect airway tone and reactivity, potentially
contributing to bronchoconstriction or airway hyperresponsiveness.
Gonadal hormone receptors on endothelial cell influence vascular
function, including vasodilation, which may affect blood flow and
oxygen exchange in the lung. Some immune cells within lung tissue,
such as macrophages and lymphocytes, also express these receptors
and modulate immune response in the lungs and may affect
inflammation and immune cell function in respiratory disease.
The literature has revealed potential sites of
gonadal hormone receptors in the lungs (Table I) (50-58).
Research on gonadal hormones receptors in the lungs and their
specific functions is ongoing.
 | Table IPotential sites of gonadal hormone
receptors in the lung. |
Table I
Potential sites of gonadal hormone
receptors in the lung.
| Cell type | Gonadal hormone
receptor | First author,
year | (Refs.) |
|---|
| Epithelial | ERa, AR, PR | Tam et al,
2014; vom Steeg, 2020; Jain, 2012 | (50-52) |
| Smooth muscle | ER, AR, PR | Bhallamudi, 2020;
Kalidhindi, 2019; Matsui, 2000 | (54-56) |
| Endothelial | ER | Bhallamudi,
2020 | (54) |
| Immune | PR, ER, AR | vom Steeg, 2020;
Azeez, 2021; Kovats, 2015 | (51,57,58) |
Progesterone
Progesterone may help prevent premenopausal apnea
(59). The bronchodilator effect of
progesterone is caused by changes in smooth muscle tone of the
airways (60). Progesterones, such
as E2, prevent endothelial dysfunction and have a strong
vasodilatory effect on the pulmonary circulation (61,62).
Progesterone correlates with peak expiratory flow rate in humans
during the luteal phase of the menstrual cycle (63). In classic interstitial pneumonia,
progesterone receptors are expressed in the fibrotic regions
(64). In the lung, progesterone
results in upregulated IL-10, IL-1β, IL-5, IL-6, IL-22, tumor
necrosis factor (TNF)-α, IL-4, steroid receptor
coactivator/cyclin-dependent kinase inhibitor 1A, Erk, IL-9
and IL-13 as well downregulated or inhibited NF-κB, TGF-β1,
connective tissue growth factor, transgelin, plasminogen activator
inhibitor-1 and IFN-γ (65,66).
Progesterone is associated with hyperventilation
during pregnancy. Animal studies have shown that lack of
progesterone receptor correlates with loss of responsiveness to
hypoxia, suggesting a beneficial role of progesterone on the
airways (67,68,69).
Mouse responses to hypoxia and hypercapnia are
suppressed after receiving small interfering RNA (siRNA) against
membrane progesterone receptor-β but not receptor-αin the dorsal
brainstem. Furthermore, the number of apnea episodes increases. The
same study also revealed that membrane progesterone receptor β in
the dorsal brainstem establishes sex-specific chemoreflex responses
and reduces apnea frequency in adult mice, with females showing a
higher ventilatory response to hypoxia and hypercapnia than males.
Effects are eradicated by membrane progesterone receptor-β siRNA
but not α (70).
Sleep duration can influence human reproduction by
affecting hormonal regulation, menstrual regularity, fertility,
stress levels and sexual function, all of which serve a role in
reproductive health (32).
Some studies contend that the effect of progesterone
on breathing is mediated by cells in the hypothalamus that contain
progesterone receptors, either by modulating release of
neuromodulators such as serotonin or by binding to and altering the
function of γ-aminobutyric acid receptors (40,71,72).
Testosterone
Testosterone serves a protective role in the lung
because it relaxes the bronchial lumen and decreases histamine
response and airway inflammation (73-75).
Furthermore, testosterone causes up-[IL-2, IFN-γ, haemoglobin
subunit (Hbb)-β1, Hbb-γ and Hbb-θ1] and downregulation (IL-33,
thymic stromal lymphopoietin, IL-4, IL-5, IL-13, angiopoietin-like
4 and cytochrome P450, family 1, subfamily A, polypeptide 1) of
cytokines and inflammatory mediators in the lung (76-79).
A study suggested that the influence of testosterone
on respiratory function is mediated by the action of estrogens
(80). However, testosterone
secretion is dependent on sleep cycle; its serum concentration
increases during REM sleep. In addition, sleep of short duration (3
h) but with normal architecture is sufficient for normal levels of
testosterone secretion (81).
However, changes in serum testosterone levels have been
demonstrated in sleep disorders in both animal models and human
studies (82,83). A study of male rats found that sleep
deprivation is followed by a reduction in serum testosterone levels
and sperm quality compared with controls (82). Testosterone and prolactin levels are
significantly lower in sleep-deficient individuals (83).
Although shorter sleep duration does not appear to
alter testosterone levels, studies have shown that sleep duration
of 5 h/night is associated with a 10-15% decrease in blood
testosterone, decreased libido and vigour scores (assessed by
measuring vitality, energy and general physical and mental
well-being) (84,85).
In healthy young men, in the first part of sleep,
dominated by periods of REM sleep, there is an increase in
testosterone secretion (86). Sleep
disturbance leads to decreased daily testosterone secretion
(87). Following testosterone
therapy, ventilatory sensitivity to carbon dioxide during sleep and
wakefulness is enhanced in female patients (88).
Estrogen
The effect of estrogen on lung disease is not fully
known. In the lung, estrogens induce cytokine and inflammatory
mediator up-(IL-1β, IL-6, type I-IFN, TNF-α, NF-κB and toll-like
receptor 8) and downregulation (TGF-β1 and IL-10) (58,89,90).
The increased activity of thioredoxin, activation of the nuclear
factor erythroid 2-related factor 2 (Nrf-2) and p38
mitogen-activated protein (p38 MAP) kinases, inhibition of vagal
C-fibers and decrease expression of hypoxia-inducible factor
(HIF)-1 have been linked to the positive effects of estrogen and
phytoestrogens on obstructive sleep apnea and associated
co-morbidities by decreasing OSAS severity, improving sleep
quality, and mitigating hormonal and cardiovascular factors
(91). The activation of p38 MAP
kinase by estrogen may suppress HIF-1 to decrease lung
inflammation, which may inhibit activation of vagal C-fibers to
decrease bronchoconstriction to avoid obstruction when sleeping. In
addition, estrogen-mediated thioredoxin and Nrf-2 upregulation
enhances antioxidant defense and decreases inflammation (91). Another study reveals that the
estrogen-related receptor-α/slow myosin heavy chain transcriptional
regulatory cascade is a key factor in E2-mediated muscle
protection, suggesting a potential novel therapeutic target for the
treatment of postmenopausal OSAS (92).
OSAS is significantly more prevalent during and
after the menopause. Decreased E2 may be associated with increased
risk of OSAS in hormonally depressed patients during the peri- and
postmenopause, in addition to greater BMI and age, supporting the
hypothesis that decreased E2 associated with menopause affects
upper airway patency (93).
Patients are more likely to develop OSAS during pregnancy,
polycystic ovary syndrome, during late menopause and post-menopause
(94). There is an inverse link
between E2 and OSAS based on reports that postmenopausal patients
receiving hormone replacement therapy (HRT; estrogen or estrogen +
progesterone) have a lower prevalence of OSAS than those without
(32,91,92).
Consequently, estrogen may provide protection during OSAS
pathogenesis, but it is unclear if estrogen causes OSAS (93).
A previous study suggested that patients with an
apnea-hypopnea index ≥25 require CPAP because they do not respond
to HRT. HRT may be a good option for patients with less severe OSAS
(94,95).
5. Fertility and OSAS
HIF-dependent and -independent pathways are two
distinct mechanisms that can explain hypoxic effects on endocrine
cells (96). Variations in protein
phosphorylation and global transcription/translation efficiency are
the key regulators of the HIF-independent process. Long-term
control of the HIF-dependent pathway is triggered when HIF changes
the expression of target genes.
As an effect of hypoxia, protein phosphorylation may
change HIF activity (Fig. 2). Thus,
protein phosphorylation serves an important role in mediating the
activity of HIFs by regulating their stability, nuclear
translocation and interaction with coactivators. This allows cells
to adapt to hypoxic conditions by adjusting expression of specific
genes involved in oxygen homeostasis and cell survival (e.g.
erythropoietin, vascular endothelial growth factor, glucose
transporter 1) (97,98).
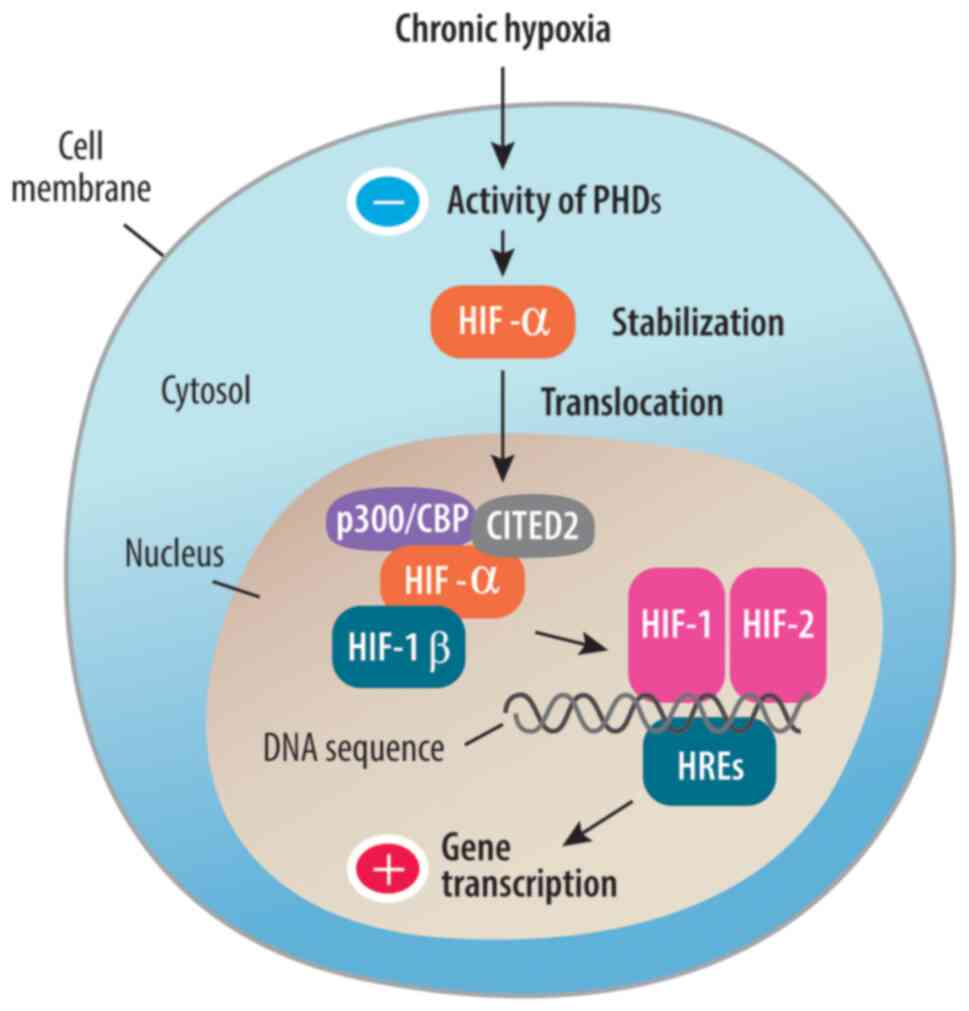 | Figure 2HIF pathway in chronic hypoxia. HIFs
are heterodimeric proteins composed of α (HIF-1α, HIF-2α or HIF-3α)
and β subunits. Under normoxic conditions, HIF-α subunits are
rapidly degraded by the proteasome. However, under hypoxic
conditions, PHDs that target HIF-α for degradation become less
active due to decreased oxygen availability. This leads to the
stabilization of HIF-α subunits. Once stabilized, HIF-α subunits
translocate into the cell nucleus, where they form a complex with
HIF-1β. This complex is required to form either transcription
factor HIF-1 or HIF-2. Phosphorylation of HIF-α subunits enhances
their interaction with coactivator proteins, such as p300/CBP and
CITED2. These coactivators recruit RNA polymerase and other
transcriptional machinery to the target genes, promoting gene
expression. Phosphorylation regulates the stability of HIF-α
subunits. Phosphorylation of HIF-α subunits occurs as a result of
signaling pathways activated by growth factors, cytokines or other
stimuli. Phosphorylation modulates HIF activity in response to
cellular signals. DNA sequences influence HREs by containing
specific binding sites that allow HIF proteins to attach to them,
regulating the expression of target genes under hypoxic conditions.
HIF, hypoxia-inducible factor; PHD, prolyl hydroxylases; p300-a
transcriptional coactivator; CBP CREB-binding protein; CITED2p300,
CBP-interacting transactivator with Glu/Asp-rich C-terminal domain
2; HRE, hypoxia response element. |
The association between hypoxia caused by lung
disease, such as chronic obstructive pulmonary disease, and
decreased serum testosterone in men has been described (99,100).
It has also been shown that erectile dysfunction is frequently
present in these patients (99).
Other factors that may contribute to infertility include increased
oxidative stress, insulin resistance, systemic inflammation and
aberrant secretion of reproductive hormones (100).
Sexual dysfunction has been reported in people with
from sleep disorders and is a pathology of interest (101).
Oxidative stress is considered the link between OSAS
and infertility. Increased oxidative stress may be an etiological
factor for decreased sperm fertility and studies on mice and human
male subjects have revealed that intermittent hypoxia correlates
with increased testicular oxidative stress and decreased sperm
motility (102,103). Chronic inflammation is associated
with impairments in spermatogenesis, sperm quality and,
consequently, fertility (104). In
chronic inflammation, IL-6, TNF and C-reactive protein are the
primary markers increased in patients with OSAS but are decreased
by CPAP (105).
The risk of infertility is significantly increased
with duration of OSAS. In addition, patients with untreated OSAS
have an increased risk of infertility (22).
6. CPAP therapy
CPAP therapy has not been unequivocally shown to
normalize the pituitary-gonadal axis in male patients (36,37,48).
Studies published so far show controversial effects, as is the case
with testosterone therapy (31,88).
Although studies have shown that CPAP therapy contributes to
increased testosterone levels, meta-analyses have not confirmed
significant changes in this hormone after initiation of therapy
(106-109).
Conflicting results may be generated by suboptimal studies or
therapeutic methodologies, such as insufficient duration of CPAP
therapy (Table II).
 | Table IIEffect of continuous positive airway
pressure therapy on plasma testosterone in patients with
obstructive sleep apnea syndrome. |
Table II
Effect of continuous positive airway
pressure therapy on plasma testosterone in patients with
obstructive sleep apnea syndrome.
| First author,
year | Number of
patients | Duration of study,
months | Timing of
measurement | Testosterone
levels | (Refs.) |
|---|
| Meston et
al, 2003 | 101 | 1 | Mid-morning | Decreased | (109) |
| Zhang et al,
2016 | 207 | 3 | Morning | No change | (107) |
| Celec et al,
2014 | 67 | 6 | Morning | No change | (33) |
The independent effect of OSAS on blood testosterone
concentration has been demonstrated in certain studies (44,110-114)
but not in all cross-sectional studies (33,47).
Following 3 months of CPAP treatment, serum
testosterone concentration is normalized (115,116). CPAP therapy (long- or short-term)
does not affect hormonal status in female patients (117). Compared with female patients,
higher long-term CPAP is required for male patients with minimally
symptomatic OSAS (118).
Serum levels of FSH, LH, progesterone and
testosterone are significantly lower in 153 patients with OSAS
patients than in controls (8). CPAP
therapy has no effect on prolactin, E2 and progesterone levels and
increases serum levels of FSH, LH and testosterone (119). The hypothalamic-pituitary-gonadal
axis is primarily influenced by nocturnal hypoxemia and sleep
disturbances in OSAS, which lowers blood levels of sex hormones.
The decrease in testosterone is likely caused by a decrease in
Leydig cell population, according to a study on brown Norway rats
(120). Long-term exposure to
hypoxia can lead to low sperm count and infertility as testosterone
is a key paracrine factor for spermatogenesis (121).
Weight loss may be a key factor in reversing gonadal
dysfunction; massive weight loss (20% weight loss) normalizes
testosterone levels (34,48,122).
7. Discussion
According to studies, sleep-disordered breathing is
underdiagnosed and may underlie several multisystem disorders
(6,12,13,19).
Hypoxia, frequent nighttime awakening and stress influence hormonal
secretion, which affects physical and cognitive development,
obesity and infertility, which pose public health problems
(92,100,102,103).
Studies have reported associations between sexual
dysfunction, hormonal disorder and severity of apnea-hypopnea index
(123,124). These studies have shown lower
levels of progesterone and estrogen in patients with OSAS
associated with development of sexual disorder. OSAS can have a
negative impact on sexual function, either through direct effects
or by causing hormonal imbalances (95,125).
Although most studies (43-46)
have reported an association between sleep disorders and plasma
testosterone levels, estrogens and progesterone impact on upper
airway stability and respiratory control during the menstrual
cycle. These hormones have been characterized as protective and are
hypothesized to results in lower rates of OSAS in female patients.
Levels of progesterone, E2 and 17-OH progesterone are lower in
female patients with OSAS (123).
In addition, previous polysomnographic investigations have shown
that, regardless of body weight, OSAS is more common and severe in
postmenopausal compared with premenopausal patients (2,124).
Breathing disorders during sleep affect secretion of
gonadal hormones through various mechanisms (32-34).
Sleep apnea often leads to disrupted sleep patterns characterized
by repeated awakening due to breathing interruptions (14,41,42).
These awakenings affect the normal circadian rhythm of gonadal
hormone secretion (123).
Prolactin secretion typically follows a diurnal pattern, with
higher levels during the night and lower levels during the day.
Sleep disruption can alter this pattern, leading to irregular
prolactin secretion (123). These
interruptions affect the normal circadian rhythm of hormone
secretion, including progesterone (9). Progesterone levels typically vary
throughout the menstrual cycle and are influenced by sleep quality
and duration. Sleep apnea can be a source of chronic stress for the
body, as it continually activates the stress response system,
including the release of stress hormones such as cortisol (29,30).
Elevated stress hormones can decrease the secretion of gonadal
hormones. Prolonged stress leads to changes in the balance of
gonadal hormones (32). Sleep apnea
is characterized by episodes of hypoxia during breathing
interruptions. Hypoxia can affect the function of the hypothalamus
and pituitary gland, which are involved in regulating gonadal
hormone secretion and changes in ovarian function (36,37).
Thus, hypoxia can lead to dysregulation of hormone secretion
pathways, potentially altering hormones levels. Sleep apnea is
often associated with obesity and metabolic changes, such as
insulin resistance (100). These
factors contribute to alterations in gonadal hormone regulation.
Sleep apnea leads to increased activity of the sympathetic nervous
system (12). This heightened
sympathetic activity influences hormone secretion, including
prolactin. The exact mechanisms by which sympathetic activation
affects prolactin are complex and not fully understood (12).
CPAP therapy is currently the gold standard of
treatment for patients with sleep apnoea syndrome (13). Although this therapy allows
effective control of breathing during sleep, a notable number of
patients stop using it or use it inconsistently 12 months after
initiation (118). Studies on the
effect of CPAP therapy suggest that conflicting results may also be
due to suboptimal studies or therapeutic methodologies, such as
insufficient duration of CPAP therapy (36,37,107,118). On the other hand, although
respiratory events are resolved by CPAP treatment, obesity may be
an important risk factor for hypogonadism (38,48).
To the best of our knowledge, there is relatively
limited data on the link between hypoxia induced by
sleep-disordered breathing and gonadal hormonal imbalance (99,100,121). Moreover, data are often
inconsistent, potentially due to the presence of factors such as
obesity, stress and the long time required for the onset of
sleep-disordered breathing disease. Social factors including
obesity, socio-economic disparities, lifestyle choices,
environmental conditions, urban noise pollution and sleep schedules
influenced by workplace demands may also contribute to
sleep-disordered breathing.
CPAP therapy does not have a clear positive impact
on restoring gonadal hormonal balance (107). Long-term therapy may be more
likely to normalize gonadal function, but, to the best of our
knowledge, long-term studies have not been performed.
Although there is an association between
sleep-disordered breathing and gonadal dysfunction via direct
effects of hypoxia and hypothalamic-pituitary-gonadal axis
dysfunction, CPAP therapy does not normalize this dysfunction
(36,37). Patients suffering from
sleep-disordered breathing diseases might experience a prolonged
period before their serum gonadal hormone levels return to normal
due to these dysfunctions (32).
Hormonal dysfunctions secondary to sleep apnea syndrome affect
quality of life and hormonal homeostasis.
Acknowledgements
The authors would like to thank Professor Florin
Pinzariu (George Enescu National University of Arts, Iasi, Romania)
for constructing figures.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
CC and EC conceptualized the study. CC, EC, LP, CP
and SDR designed the methodology and wrote the manuscript. CC, EC,
LP, CP and SDR performed the literature review. SDR constructed
tables. LP and CP edited the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjafield AV, Ayas NT, Eastwood PR,
Heinzer R, Ip MSM, Morrell MJ, Nunez CM, Patel SR, Penzel T, Pépin
JL, et al: Estimation of the global prevalence and burden of
obstructive sleep apnoea: a literature-based analysis. Lancet
Respir Med. 7:687–698. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Matsumoto T and Chin K: Prevalence of
sleep disturbances: Sleep disordered breathing, short sleep
duration, and non-restorative sleep. Respir Investig. 57:227–237.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bixler EO, Papaliaga MN, Vgontzas AN, Lin
HM, Pejovic S, Karataraki M, Vela-Bueno A and Chrousos GP: Women
sleep objectively better than men and the sleep of young women is
more resilient to external stressors: effects of age and menopause.
J Sleep Res. 18:221–228. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gaillard JM and Blois R: Spindle density
in sleep of normal subjects. Sleep. 4:385–391. 1981.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Casper-Gallup State of Sleep in America
2022 Report. https://www.gallup.com/analytics/390536/sleep-in-america-2022.aspx.
|
|
6
|
Song Z, Jiang R, Li C, Jin F and Tao M:
Menopausal Symptoms and sleep quality in women aged 40-65 years.
Biomed Res Int. 2022(2560053)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Franklin KA, Sahlin C, Stenlund H and
Lindberg E: Sleep apnoea is a common occurrence in females. Eur
Respir J. 41:610–615. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Farias JG, Bustos-Obregón E, Orellana R,
Bucarey JL, Quiroz E and Reyes JG: Effects of chronic hypobaric
hypoxia on testis histology and round spermatid oxidative
metabolism. Andrologia. 37:47–52. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Martins FO and Conde SV: Gender
differences in the context of obstructive sleep apnea and metabolic
diseases. Front Physiol. 12(792633)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yeghiazarians Y, Jneid H, Tietjens JR,
Redline S, Brown DL, El-Sherif N, Mehra R, Bozkurt B, Ndumele CE
and Somers VK: Obstructive sleep apnea and cardiovascular disease:
A scientific statement from the american heart association.
Circulation. 144:e56–e67. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mehra R, Chung MK, Olshansky B, Dobrev D,
Jackson CL, Kundel V, Linz D, Redeker NS, Redline S, Sanders P, et
al: Sleep-disordered breathing and cardiac arrhythmias in adults:
Mechanistic insights and clinical implications: A scientific
statement from the American heart association. Circulation.
146:e119–e136. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Young T, Palta M, Dempsey J, Skatrud J,
Weber S and Badr S: The occurrence of sleep-disordered breathing
among middle-aged adults. N Engl J Med. 328:1230–1235.
1993.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Franklin KA and Lindberg E: Obstructive
sleep apnea is a common disorder in the population-a review on the
epidemiology of sleep apnea. J Thorac Dis. 7:1311–1322.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Benkirane O, Delwiche B, Mairesse O and
Peigneux P: Impact of sleep fragmentation on cognition and fatigue.
Int J Environ Res Public Health. 19(15485)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cowie MR, Linz D, Redline S, Somers VK and
Simonds AK: Sleep disordered breathing and cardiovascular disease:
JACC state-of-the-art review. J Am Coll Cardiol. 78:608–624.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Douglas NJ, White DP, Pickett CK, Weil JV
and Zwillich CW: Respiration during sleep in normal man. Thorax.
37:840–844. 1982.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sunwoo BY and Owens RL: Sleep deficiency,
sleep apnea, and chronic lung disease. Clin Chest Med. 43:337–352.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Francis ME, Kusek JW, Nyberg LM and Eggers
PW: The contribution of common medical conditions and drug
exposures to erectile dysfunction in adult males. J Urol.
178:591–596. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jennum P and Sjøl A: Epidemiology of
snoring and obstructive sleep apnoea in a Danish population, age
30-60. J Sleep Res. 1:240–244. 1992.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen L, Pivetta B, Nagappa M, Saripella A,
Islam S, Englesakis M and Chung F: Validation of the STOP-Bang
questionnaire for screening of obstructive sleep apnea in the
general population and commercial drivers: A systematic review and
meta-analysis. Sleep Breath. 25:1741–1751. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
World Health Organisation: Infertility.
[(accessed on 10 August 2022)]. Available online: https://www.who.int/health-topics/infertility#tab=tab_1.
|
|
22
|
Jhuang YH, Chung CH, Wang ID, Peng CK,
Meng E, Chien WC and Chang PY: Association of obstructive sleep
apnea with the risk of male infertility in Taiwan. JAMA Netw Open.
4(e2031846)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lim ZW, Wang ID, Wang P, Chung CH, Huang
SS, Huang CC, Tsai PY, Wu GJ, Wu KH and Chien WC: Obstructive sleep
apnea increases risk of female infertility: A 14-year nationwide
population-based study. PLoS One. 16(e0260842)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Espinoza-Cuadros F, Fernández-Pozo R,
Toledano DT, Alcázar-Ramírez JD, López-Gonzalo E and
Hernández-Gómez LA: Speech signal and facial image processing for
obstructive sleep apnea assessment. Comput Math Methods Med.
2015(489761)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kumagai H, Sawatari H, Kiyohara Y, Kanoh
A, Asada K, Kawaguchi K, Arita A, Murase Y, Konishi N, Hoshino T,
et al: Nocturnal hypoxemia is related to morning negative
affectivity in untreated patients with severe obstructive sleep
apnea. Sci Rep. 12(21262)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bianchi MT, Cash SS, Mietus J, Peng CK and
Thomas R: Obstructive sleep apnea alters sleep stage transition
dynamics. PLoS One. 5(e11356)2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Alvarez-Martins I, Remédio L, Matias I,
Diogo LN, Monteiro EC and Dias S: The impact of chronic
intermittent hypoxia on hematopoiesis and the bone marrow
microenvironment. Pflugers Arch. 468:919–932. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nieminen P, Löppönen T, Tolonen U, Lanning
P, Knip M and Löppönen H: Growth and biochemical markers of growth
in children with snoring and obstructive sleep apnea. Pediatrics.
109(e55)2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chapotot F, Buguet A, Gronfier C and
Brandenberger G: Hypothalamo-pituitary-adrenal axis activity is
related to the level of central arousal: Effect of sleep
deprivation on the association of high-frequency waking
electroencephalogram with cortisol release. Neuroendocrinology.
73:312–321. 2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nicolaides NC, Vgontzas AN, Kritikou I and
Chrousos G: HPA axis and sleep. Endotext [Internet]. Feingold KR,
Anawalt B, Blackman MR, et al (eds). South Dartmouth (MA):
MDText.com, Inc., 2020. https://www.ncbi.nlm.nih.gov/books/NBK279071/.
Accessed May 2, 2023.
|
|
31
|
Grech A, Breck J and Heidelbaugh J:
Adverse effects of testosterone replacement therapy: An update on
the evidence and controversy. Ther Adv Drug Saf. 5:190–200.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kalmbach DA, Arnedt JT, Pillai V and
Ciesla JA: The impact of sleep on female sexual response and
behavior: A pilot study. J Sex Med. 12:1221–1232. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Celec P, Mucska I, Ostatníková D and
Hodosy J: Testosterone and estradiol are not affected in male and
female patients with obstructive sleep apnea treated with
continuous positive airway pressure. J Endocrinol Invest. 37:9–12.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Petersen M, Kristensen E, Berg S, Giraldi
A and Midgren B: Sexual function in female patients with
obstructive sleep apnea. J Sex Med. 8:2560–2568. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Clifton DK and Steiner RA:
Neuroendocrinology of reproduction. Yen & Jaffe's Reproductive
Endocrinology, pp3-33, 2009.
|
|
36
|
Hsueh AJW, Kawamura K, Cheng Y and Fauser
BCJM: Intraovarian control of early folliculogenesis. Endocr Rev.
36:1–24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Thibault C and Levasseur MC: Ovulation.
Hum Reprod. 3:513–523. 1988.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Richard-Eaglin A: Male and female
hypogonadism. Nurs Clin North Am. 53:395–405. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fuentes N and Silveyra P: Endocrine
regulation of lung disease and inflammation. Exp Biol Med
(Maywood). 243:1313–1322. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Behan M and Wenninger JM: Sex steroidal
hormones and respiratory control. Respir Physiol Neurobiol.
164:213–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Schmid SM, Hallschmid M, Jauch-Chara K,
Lehnert H and Schultes B: Sleep timing may modulate the effect of
sleep loss on testosterone. Clin Endocrinol (Oxf). 77:749–754.
2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Touzet S, Rabilloud M, Boehringer H,
Barranco E and Ecochard R: Relationship between sleep and secretion
of gonadotropin and ovarian hormones in women with normal cycles.
Fertil Steril. 77:738–744. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kim SD and Cho KS: Obstructive sleep apnea
and testosterone deficiency. World J Mens Health. 37:12–18.
2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gambineri A, Pelusi C and Pasquali R:
Testosterone levels in obese male patients with obstructive sleep
apnea syndrome: Relation to oxygen desaturation, body weight, fat
distribution and the metabolic parameters. J Endocrinol Investig.
26:493–498. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bercea RM, Patacchioli FR, Ghiciuc CM,
Cojocaru E and Mihaescu T: Serum testosterone and depressive
symptoms in severe OSA patients. Andrologia. 45:345–350.
2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kouchiyama S, Honda Y and Kuriyama T:
Influence of nocturnal oxygen desaturation on circadian rhythm of
testosterone secretion. Respiration. 57:359–363. 1990.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Barrett-Connor E, Dam TT, Stone K,
Harrison SL, Redline S and Orwoll E: Osteoporotic Fractures in Men
Study Group. The association of testosterone levels with overall
sleep quality, sleep architecture, and sleep-disordered breathing.
J Clin Endocrinol Metab. 93:2602–2609. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Luboshitzky R, Aviv A, Hefetz A, Herer P,
Shen-Orr Z, Lavie L and Lavie P: Decreased pituitary-gonadal
secretion in men with obstructive sleep apnea. J Clin Endocrinol
Metab. 87:3394–3398. 2002.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shahar E, Redline S, Young T, Boland LL,
Baldwin CM, Nieto FJ, O'Connor GT, Rapoport DM and Robbins JA:
Hormone replacement therapy and sleep-disordered breathing. Am J
Respir Crit Care Med. 167:1186–1192. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tam A, Wadsworth S, Dorscheid D, Man SFP
and Sin DD: Estradiol increases mucus synthesis in bronchial
epithelial cells. PLoS One. 9(e100633)2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
vom Steeg LG, Dhakal S, Woldetsadik YA,
Park HS, Mulka KR, Reilly EC, Topham DJ and Klein SL: Androgen
receptor signaling in the lungs mitigates inflammation and improves
the outcome of influenza in mice. PLoS Pathog.
16(e1008506)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Jain R, Ray JM, Pan JH and Brody SL: Sex
hormone-dependent regulation of cilia beat frequency in airway
epithelium. Am J Respir Cell Mol Biol. 46:446–453. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Siegfried JM: Sex and gender differences
in lung cancer and chronic obstructive lung disease. Endocrinology.
163(bqab254)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bhallamudi S, Connell J, Pabelick CM,
Prakash YS and Sathish V: Estrogen receptors differentially
regulate intracellular calcium handling in human nonasthmatic and
asthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol
Physiol. 318:L112–L124. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kalidhindi RSR, Katragadda R, Beauchamp
KL, Pabelick CM, Prakash YS and Sathish V: Androgen
receptor-mediated regulation of intracellular calcium in human
airway smooth muscle cells. Cell Physiol Biochem. 53:215–228.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Matsui K, Takeda K, Yu ZX, Valencia J,
Travis WD, Moss J and Ferrans VJ: Downregulation of estrogen and
progesterone receptors in the abnormal smooth muscle cells in
pulmonary lymphangioleiomyomatosis following therapy. An
immunohistochemical study. Am J Respir Crit Care Med.
161:1002–1009. 2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Azeez JM, Susmi TR, Remadevi V, Ravindran
V, Sasikumar Sujatha A, Ayswarya RNS and Sreeja S: New insights
into the functions of progesterone receptor (PR) isoforms and
progesterone signaling. Am J Cancer Res. 11:5214–5232.
2021.PubMed/NCBI
|
|
58
|
Kovats S: Estrogen receptors regulate
innate immune cells and signaling pathways. Cell Immunol.
294:63–69. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Martin SE, Mathur R, Marshall I and
Douglas NJ: The effect of age, sex, obesity and posture on upper
airway size. Eur Respir J. 10:2087–2090. 1997.PubMed/NCBI View Article : Google Scholar
|
|
60
|
LoMauro A and Aliverti A: Respiratory
physiology of pregnancy: Physiology masterclass. Breathe (Sheff).
11:297–301. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Artem'eva MM, Kovaleva YO, Medvedev OS and
Medvedeva NA: Effect of chronic administration of estradiol on
responsiveness of isolated systemic and pulmonary blood vessels
from ovariectomized wistar rats with hypoxic pulmonary
hypertension. Bull Exp Biol Med. 159:427–430. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
English KM, Jones RD, Jones TH, Morice AH
and Channer KS: Gender differences in the vasomotor effects of
different steroid hormones in rat pulmonary and coronary arteries.
Horm Metab Res. 33:645–652. 2001.PubMed/NCBI View Article : Google Scholar
|
|
63
|
de Zambotti M, Nicholas CL, Colrain IM,
Trinder JA and Baker FC: Autonomic regulation across phases of the
menstrual cycle and sleep stages in women with premenstrual
syndrome and healthy controls. Psychoneuroendocrinology.
38:2618–2627. 2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Vafashoar F, Mousavizadeh K, Poormoghim H,
Haghighi A, Pashangzadeh S and Mojtabavi N: Progesterone aggravates
lung fibrosis in a mouse model of systemic sclerosis. Front
Immunol. 12(742227)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Migliaccio A, Piccolo D, Castoria G, Di
Domenico M, Bilancio A, Lombardi M, Gong W, Beato M and Auricchio
F: Activation of the Src/p21ras/Erk pathway by progesterone
receptor via cross-talk with estrogen receptor. EMBO J.
17:2008–1018. 1998.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kunzmann S, Ottensmeier B, Speer CP and
Fehrholz M: Effect of progesterone on Smad signaling and
TGF-β/Smad-regulated genes in lung epithelial cells. PLoS One.
13(e0200661)2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Potvin C, Rossignol O, Uppari N,
Dallongeville A, Bairam A and Joseph V: Reduced hypoxic ventilatory
response in newborn mice knocked-out for the progesterone receptor.
Exp Physiol. 99:1523–1537. 2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Saaresranta T and Polo O: Hormones and
breathing. Chest. 122:2165–2182. 2002.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Chiarella SE, Cardet JC and Prakash YS:
Sex, cells, and asthma. Mayo Clin Proc. 96:1955–1969.
2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Boukari R, Rossignol O, Baldy C,
Marcouiller F, Bairam A and Joseph V: Membrane progesterone
receptor-β, but not-α, in dorsal brain stem establishes
sex-specific chemoreflex responses and reduces apnea frequency in
adult mice. J Appl Physiol (1985). 121:781–791. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Robinson JE and Kendrick KM: Inhibition of
luteinizing hormone secretion in the ewe by progesterone:
Associated changes in the release of gamma-aminobutyric Acid and
noradrenaline in the preoptic area as measured by intracranial
microdialysis. J Neuroendocrinol. 4:231–236. 1992.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rupprecht R: Neuroactive steroids:
Mechanisms of action and neuropsychopharmacological properties.
Psychoneuroendocrinology. 28:139–168. 2003.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Verma MK, Miki Y and Sasano H: Sex steroid
receptors in human lung diseases. J Steroid Biochem Mol Biol.
127:216–222. 2011.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Hoffman EA, Ahmed FS, Baumhauer H, Budoff
M, Carr JJ, Kronmal R, Reddy S and Barr RG: Variation in the
percent of emphysema-like lung in a healthy, nonsmoking multiethnic
sample. The MESA lung study. Ann Am Thorac Soc. 11:898–907.
2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kouloumenta V, Hatziefthimiou A, Paraskeva
E, Gourgoulianis K and Molyvdas PA: Non-genomic effect of
testosterone on airway smooth muscle. Br J Pharmacol.
149:1083–1091. 2006.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Laffont S, Blanquart E, Savignac M, Cénac
C, Laverny G, Metzger D, Girard JP, Belz GT, Pelletier L, Seillet C
and Guéry JC: Androgen signaling negatively controls group 2 innate
lymphoid cells. J Exp Med. 214:1581–1592. 2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lamont KR and Tindall DJ: Androgen
regulation of gene expression. Adv Cancer Res. 107:137–162.
2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Cephus JY, Stier MT, Fuseini H, Yung JA,
Toki S, Bloodworth MH, Zhou W, Goleniewska K, Zhang J, Garon SL, et
al: Testosterone attenuates group 2 innate lymphoid cell-mediated
airway inflammation. Cell Rep. 21:2487–2499. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP
and Jänne OA: Androgen receptor and androgen-dependent gene
expression in lung. Mol Cell Endocrinol. 317:14–24. 2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zabka AG, Mitchell GS and Behan M:
Conversion from testosterone to oestradiol is required to modulate
respiratory long-term facilitation in male rats. J Physiol.
576:903–912. 2006.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Wittert G: The relationship between sleep
disorders and testosterone in men. Asian J Androl. 16:262–265.
2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Alvarenga TA, Hirotsu C, Mazaro-Costa R,
Tufik S and Andersen ML: Impairment of male reproductive function
after sleep deprivation. Fertil Steril. 103:1355–1362.e1.
2015.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Jauch-Chara K, Schmid SM, Hallschmid M,
Oltmanns KM and Schultes B: Pituitary-gonadal and pituitary-thyroid
axis hormone concentrations before and during a hypoglycemic clamp
after sleep deprivation in healthy men. PLoS One.
8(e54209)2013.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Leproult R and Van Cauter E: Effect of 1
week of sleep restriction on testosterone levels in young healthy
men. JAMA. 305:2173–2174. 2011.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Su L, Zhang SZ, Zhu J, Wu J and Jiao YZ:
Effect of partial and total sleep deprivation on serum testosterone
in healthy males: A systematic review and meta-analysis. Sleep Med.
88:267–273. 2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Luboshitzky R, Herer P, Levi M, Shen-Orr Z
and Lavie P: Relationship between rapid eye movement sleep and
testosterone secretion in normal men. J Androl. 20:731–737.
1999.PubMed/NCBI
|
|
87
|
Luboshitzky R, Zabari Z, Shen-Orr Z, Herer
P and Lavie P: Disruption of the nocturnal testosterone rhythm by
sleep fragmentation in normal men. J Clin Endocrinol Metab.
86:1134–1139. 2001.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Ahuja D, Mateika JH, Diamond MP and Badr
MS: Ventilatory sensitivity to carbon dioxide before and after
episodic hypoxia in women treated with testosterone. J Appl Physiol
(1985). 102:1832–1838. 2007.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Smith LC, Moreno S, Robertson L, Robinson
S, Gant K, Bryant AJ and Sabo-Attwood T: Transforming growth factor
beta1 targets estrogen receptor signaling in bronchial epithelial
cells. Respir Res. 19(160)2018.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Shim B, Pacheco-Rodriguez G, Kato J,
Darling TN, Vaughan M and Moss J: Sex-specific lung diseases:
Effect of oestrogen on cultured cells and in animal models. Eur
Respir Rev. 22:302–311. 2013.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Zhang L, Ou X, Zhu T and Lv X: Beneficial
effects of estrogens in obstructive sleep apnea hypopnea syndrome.
Sleep Breath. 24:7–13. 2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Chen HH, Lu J, Guan YF, Li SJ, Hu TT, Xie
ZS, Wang F, Peng XH, Liu X, Xu X, et al: Estrogen/ERR-α signaling
axis is associated with fiber-type conversion of upper airway
muscles in patients with obstructive sleep apnea hypopnea syndrome.
Sci Rep. 6(27088)2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Galvan T, Camuso J, Sullivan K, Kim S,
White D, Redline S and Joffeet H: Association of estradiol with
sleep apnea in depressed perimenopausal and postmenopausal women: A
preliminary study. Menopause. 24:112–117. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Fogel RB, Malhotra A, Pillar G, Pittman
SD, Dunaif A and White DP: Increased prevalence of obstructive
sleep apnea syndrome in obese women with polycystic ovary syndrome.
J Clin Endocrinol Metab. 86:1175–1180. 2001.PubMed/NCBI View Article : Google Scholar
|
|
95
|
White DP: The hormone replacement dilemma
for the pulmonologist. Am J Respir Crit Care Med. 167:1165–1166.
2003.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Lee HC and Tsai SJ: Endocrine targets of
hypoxia-inducible factors. J Endocrinol. 234:R53–R65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Gleadle JM and Ratcliffe PJ: Induction of
hypoxia-inducible factor-1, erythropoietin, vascular endothelial
growth factor, and glucose transporter-1 by hypoxia: Evidence
against a regulatory role for Src kinase. Blood. 89:503–509.
1997.PubMed/NCBI
|
|
98
|
Déry MAC, Michaud MD and Richard DE:
Hypoxia-inducible factor 1: Regulation by hypoxic and non-hypoxic
activators. Int J Biochem Cell Biol. 37:535–540. 2005.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Cojocaru C, Turcanu A, Mihaescu T, Ciobica
A, Timofte D, Alexinschi O, Anton E and Cojocaru E: A biological
perspective for the management of chronic obstructive pulmonary
disease by testosterone. Arch Biol Sci. 67:257–259. 2015.
|
|
100
|
Palnitkar G, Phillips CL, Hoyos CM, Marren
AJ, Bowman MC and Yee BJ: Linking sleep disturbance to idiopathic
male infertility. Sleep Med Rev. 42:149–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Hammoud AO, Carrell DT, Gibson M, Peterson
CM and Meikle AW: Updates on the relation of weight excess and
reproductive function in men: Sleep apnea as a new area of
interest. Asian J Androl. 14:77–81. 2012.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Torres M, Laguna-Barraza R, Dalmases M,
Calle A, Pericuesta E, Montserrat JM, Navajas D, Gutierrez-Adan A
and Farréet R: Male fertility is reduced by chronic intermittent
hypoxia mimicking sleep apnea in mice. Sleep. 37:1757–1765.
2014.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Alahmar AT: Role of oxidative stress in
male infertility: An updated review. J Hum Reprod Sci. 12:4–18.
2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Seshadri S, Bates M, Vince G and Jones
DIL: The role of cytokine expression in different subgroups of
subfertile men. Am J Reprod Immunol. 62:275–282. 2009.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Yokoe T, Minoguchi K, Matsuo H, Oda N,
Minoguchi H, Yoshino G, Hirano T and Adachi M: Elevated levels of
C-reactive protein and interleukin-6 in patients with obstructive
sleep apnea syndrome are decreased by nasal continuous positive
airway pressure. Circulation. 107:1129–1134. 2003.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Zhang XB, Jiang XT, Du YP, Yuan YT and
Chen B: . Efficacy of continuous positive airway pressure on
testosterone in men with obstructive sleep apnea: A meta-analysis.
PLoS One. 9(e115033)2014.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Zhang XB, Lin QC, Zeng HQ, Jiang XT, Chen
B and Chen X: Erectile dysfunction and sexual hormone levels in men
with obstructive sleep apnea: Efficacy of continuous positive
airway pressure. Arch Sex Behav. 45:235–240. 2016.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Cignarelli A, Castellana M, Castellana G,
Perrini S, Brescia F, Natalicchio A, Garruti G, Laviola L, Resta O
and Giorgino F: Effects of CPAP on testosterone levels in patients
with obstructive sleep apnea: A meta-analysis study. Front
Endocrinol (Lausanne). 10(551)2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Meston N, Davies RJO, Mullins R, Jenkinson
C, Wass JAH and Stradling J: Endocrine effects of nasal continuous
positive airway pressure in male patients with obstructive sleep
apnoea. J Intern Med. 254:447–454. 2003.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Canguven O, Selepci B, Albayrak S,
Selimoglu A, Balaban M and Bulbul M: Is there a correlation between
testosterone levels and the severity of the disease in male
patients with obstructive sleep apnea? Arch Ital Urol Androl.
82:143–147. 2010.PubMed/NCBI
|
|
111
|
Kirbas G, Abakay A, Topcu F, Kaplan A,
Ünlü M and Peker Y: Obstructive sleep apnoea, cigarette smoking and
serum testosterone levels in a male sleep clinic cohort. J Int Med
Res. 35:38–45. 2007.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Hammoud AO, Walker JM, Gibson M, Cloward
TV, Hunt SC, Kolotkin RL, Adams TD and Meikle W: Sleep apnea,
reproductive hormones and quality of sexual life in severely obese
men. Obesity (Silver Spring). 19:1118–1123. 2011.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Grunstein RR, Handelsman DJ, Lawrence SJ,
Blackwell C, Caterson ID and Sullivan CE: Neuroendocrine
dysfunction in sleep apnea: Reversal by continuous positive airways
pressure therapy. J Clin Endocrinol Metab. 68:352–358.
1989.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Clarke BM, Vincent AD, Martin S, Adams R,
Appleton S, Vakulin A, Jesudason D and Wittert GA: Obstructive
sleep apnea is not an independent determinant of testosterone in
men. Eur J Endocrinol. 183:31–39. 2020.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Santamaria JD, Prior JC and Fleetham JA:
Reversible reproductive dysfunction in men with obstructive sleep
apnoea. Clin Endocrinol (Oxf). 28:461–470. 1988.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Jayaraman G, Majid H, Surani S, Kao C and
Subramanian S: Influence of gender on continuous positive airway
pressure requirements in patients with obstructive sleep apnea
syndrome. Sleep Breath. 15:781–784. 2011.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Li Z, Tang T, Wu W, Gu L, Du J, Zhao T,
Zhou X, Wu H and Qin G: Efficacy of nasal continuous positive
airway pressure on patients with OSA with erectile dysfunction and
low sex hormone levels. Respir Med. 119:130–134. 2016.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Turnbull CD, Bratton DJ, Craig SE, Kohler
M and Stradling JR: In patients with minimally symptomatic OSA can
baseline characteristics and early patterns of CPAP usage predict
those who are likely to be longer-term users of CPAP. J Thorac Dis.
8:276–281. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Lima N, Cavaliere H, Knobel M, Halpern A
and Medeiros-Neto G: Decreased androgen levels in massively obese
men may be associated with impaired function of the gonadostat. Int
J Obes Relat Metab Disord. 24:1433–1437. 2000.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Chen H, Hardy MP, Huhtaniemi I and Zirkin
BR: Age-related decreased Leydig cell testosterone production in
the brown Norway rat. J Androl. 15:551–557. 1994.PubMed/NCBI
|
|
121
|
Li Z, Wang S, Gong C, Hu Y, Liu J, Wang W,
Chen Y, Liao Q, He B, Huang Y, et al: Effects of environmental and
pathological hypoxia on male fertility. Front Cell Dev Biol.
9(725933)2021.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Saad F, Doros G, Haider KS and Haider A:
Differential effects of 11 years of long-term injectable
testosterone undecanoate therapy on anthropometric and metabolic
parameters in hypogonadal men with normal weight, overweight and
obesity in comparison with untreated controls: Real-world data from
a controlled registry study. Int J Obes (Lond). 44:1264–1278.
2020.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Netzer NC, Eliasson AH and Strohl KP:
Women with sleep apnea have lower levels of sex hormones. Sleep
Breath. 7:25–29. 2003.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Dancey DR, Hanly PJ, Soong C, Lee B and
Hoffstein V: Impact of menopause on the prevalence and severity of
sleep apnea. Chest. 120:151–155. 2001.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Köseoğlu N, Köseoğlu H, Itil O, Oztura I,
Baklan B, Ikiz AO and Esen AA: Sexual function status in women with
obstructive sleep apnea syndrome. J Sex Med. 4:1352–1357.
2007.PubMed/NCBI View Article : Google Scholar
|