Introduction
Obesity is defined as an abnormal or excessive fat
accumulation that typically leads to health problems and currently
affects >2 billion people of the world population (1-3).
Its primary etiology is excessive energy intake combined with
physical inactivity, which leads to adipocytes with exacerbated
hypertrophy (1,4). Obesity is an important risk factor for
development of metabolic disorders, type 2 diabetes, cancer,
depression, dyslipidemia and cardiovascular diseases (2,4).
Therefore, it is important to seek strategies focused on its
prevention (3).
Strategies used for the prevention and treatment of
obesity include promoting regular physical activity at a moderate
intensity and following a diet with both adequate caloric content
and a correct distribution of macronutrients (1,2,4).
However, nutrigenomics may play an important role in the prevention
of obesity via the use of the bioactive compounds present in
certain foods with anti-obesogenic effects (5).
Ginger is a widely consumed plant around the world
with several beneficial health effects, such as improving blood
circulation, lowering blood lipids and glucose, and providing
anti-inflammatory, antioxidant and anti-obesogenic effects
(6-8).
The anti-obesogenic effect is associated with gingerols, the major
pungent compounds present in the rhizomes of ginger (9). Studies have confirmed the
antiadipogenic and/or lipolytic capacity of both 6-gingerol
(10-12)
and 6-shogaol (13), the most
abundant phenols in fresh and dried ginger root, respectively
(6). 6-gingerol inhibits
adipogenesis, decreases the accumulation of lipid droplets
(10) and promotes the browning of
3T3-L1 cells (14), while in
vivo approaches have demonstrated its role in decreasing weight
gain, body fat, inflammatory adipokines, adipocyte hypertrophy and
hyperplasia, as well as improving insulin sensitivity and glucose
tolerance in high-fat diet-induced obese mice (15,16).
In addition, the anti-obesogenic effects of 6-shogaol have been
demonstrated by the inhibition of 3T3-L1 preadipocyte proliferation
and differentiation (17), as well
as accumulation of lipids in mature adipocytes (13). In a clinical trial, the mean body
weight, body mass index and body fat levels were significantly
lower in patients with obesity receiving capsules of 6-shogaol
derived from an ethanolic extract of steamed ginger (18). A potential mechanism for its
anti-obesogenic effect is decreasing the expression of peroxisome
proliferator-activated receptor γ (PPARγ) and CCAAT
enhancer-binding protein α (C/EBPα), which in turn decreases the
levels of key regulators of adipogenesis and lipogenesis, such as
fatty acid synthase (FAS), acetyl CoA carboxylase (ACC) and fatty
acid binding protein 4 (FABP4) (6,10-13).
To the best of our knowledge, however, there are few studies
(10-13)
investigating the effects of other gingerols present in ginger,
particularly on adipose tissue.
Another abundant bioactive compound in ginger root
is 10-gingerol (10-G), which is notable for having high
bioavailability for humans (19).
Although it has been poorly studied, 10-G may be responsible for
the beneficial effects of ginger, especially its anti-obesogenic
effect (20). Therefore, the aim of
the present study was to evaluate anti-adipogenic activity of 10-G
on the 3T3-L1 cell line.
Materials and methods
3T3-L1 cell culture and
differentiation
Mouse 3T3-L1 cells were donated by Dr Trinidad
Garcia-Iglesias (Immunology Laboratory of the University Center of
Health Sciences, University of Guadalajara (Guadalajara, Mexico).
The incubation was performed at 37˚C and 5% CO2 in DMEM
(Sigma-Aldrich; Merck KGaA; cat. no. SIG-D6429) supplemented with
10% calf bovine serum (CBS; Cytiva; HyClone; cat. no. 12389812) and
1% antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin;
Gibco; Thermo Fisher Scientific, Inc.; cat. no. 15140122). 3T3-L1
preadipocytes were seeded in Petri dishes at a density of
1x105 cells/dish and the medium was replaced every 2-3
days until the cells reached 100% confluence. The day after cells
reached confluence (day 0), media were replaced with
differentiation medium composed of DMEM/F12 (Gibco; Thermo Fisher
Scientific, Inc.; cat. no. 21041025) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.; cat. no. 16000044), 1%
antibiotics and an adipogenic cocktail including 500 µM
3-Isobutyl-1-methylxanthine, 1 µM dexamethasone, 1.5 µg/ml insulin
and 1 µM rosiglitazone (3T3-L1 Differentiation kit; Sigma-Aldrich;
Merck KGaA; cat. no. SIG-DIF001-1KT) for 3 days at 37˚C. Media were
replaced with a maintenance medium composed of DMEM/F12
supplemented with 10% FBS, 1% antibiotics and 5 µg/ml insulin. The
maintenance medium was replaced every 2 days for a total of 8 days
of differentiation.
10-G preparation
10-G was acquired from Merck KGaA (Sigma-Aldrich;
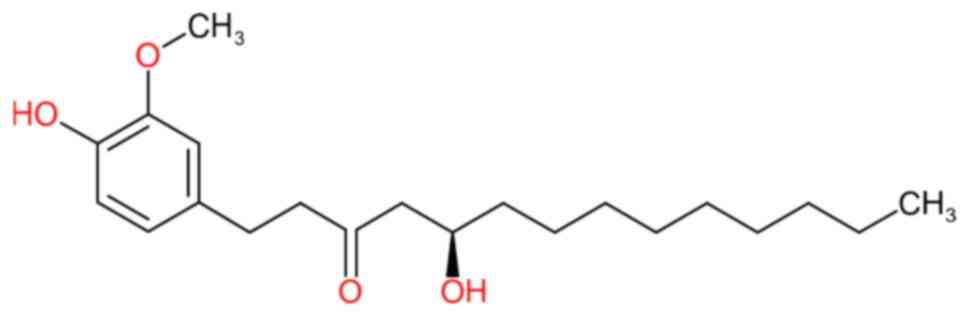
cat. no. G5798) at a standard concentration of 5 mg/ml (Fig. 1). A stock solution was prepared by
dissolving 10-G in methanol. Subsequently, dilutions were made to
50 µg/ml in DMEM supplemented with 10% CBS, 1%
antibiotic-antimycotic and 0.5% DMSO (Sigma-Aldrich; Merck KGaA;
cat. no. 276855-1L).
Dosage determination and
treatment
A dose-response curve assessment was performed with
1, 5, 10, 15, 25 and 35 µg/ml 10-G (Sigma Aldrich; Merck KGaA; cat.
no. SIG-G-027-1ML) during adipogenesis to select for further
experiments.
A total of three study groups was formed: Negative
control (NC; 3T3-L1 preadipocytes), positive control (PC; mature
3T3-L1 adipocytes) and 10-G (3T3-L1 preadipocytes stimulated with
10-G during adipogenic differentiation). In the 10-G group,
confluent preadipocytes were incubated at 37˚C and 5%
CO2 with 10-G until mature adipocytes were formed (8
days). Supernatant was collected in tubes and stored at -80˚C.
Finally, 3T3-L1 adipocytes were cryopreserved at -80˚C for further
experimental analysis.
MTT assay
3T3-L1 cells were seeded at a concentration of
5x103 cells/well in 96-well plates. Cells were
differentiated as aforementioned in the presence or absence of
10-G. Next, 1 mg/ml MTT (Invitrogen; Thermo Fisher Scientific,
Inc.; cat. No. M6494) solution was added to the wells and cells
were incubated for 1 h at 37˚C. The formazan crystals were
dissolved using an extraction buffer (20% SDS and 50%
dimethylformamide) followed by spectrophotometric measurement at
570 nm using a microplate reader (MultiScan GO; Thermo Fisher
Scientific, Inc.; cat. no. 51119300).
Oil red O staining
Fully differentiated 3T3-L1 cells were fixed with
10% (v/v) formaldehyde solution for 60 min at room temperature and
washed with distilled water. Then, lipid content in mature
adipocytes was stained with oil red O (Sigma-Aldrich; Merck KGaA;
cat. no. O0625) solution for 15 min and washed with distilled water
at room temperature. Briefly, the stained oil red O was dissolved
in 100% 2-propanol (Sigma-Aldrich; Merck KGaA; cat. no. I9516-1L)
followed by spectrophotometric measurement at 515 nm using a
microplate reader (MultiScan GO; Thermo Fisher Scientific, Inc.;
cat. no. 51119300).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from cells groups using the
RNeasy Mini kit according to the manufacturer's instructions
(Qiagen GmbH; cat. no. 74104). Then, cDNA was reverse-transcribed
from 1 µg total RNA using M-MLV Reverse Transcriptase (Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. 28025013) according to the
manufacturer's instructions. Gene expression was evaluated by qPCR
(LightCycler 96 Thermocycler; Roche Diagnostics) with the
OneTaq® Hot Start Master Mix (NEB-R; cat. no.
N01-M0484L) and TaqMan® probes (Thermo Fisher
Scientific, Inc.) as follows: 18S rRNA (Rn18s; cat. no.
Mm03928990_g1), C/ebpα (cat. no. Mm00514283_s1),
Pparγ (cat. no. Mm00440940_m1), Fabp4 (cat. no.
Mm00445878_m1), acetyl-coenzyme A carboxylase (Acaca; cat.
no. Mm01304257_m1), sterol regulatory element binding transcription
factor 1 (Srebf1; cat. no. Mm00482136_m1) and mechanistic
target of rapamycin complex (Mtor; cat. no. Mm00444968_m1).
The thermocycling conditions for qPCR were as follows: Initial
denaturation at 95˚C for 300 sec, followed by 30 cycles of
denaturation at 95˚C for 20 sec and amplification at 60˚C for 60
sec and final extension at 68˚C for 300 sec. Relative gene
expression was determined based on the 2-ΔΔCq method
(21) and normalized against the
mRNA expression of Rn18S. mRNA expression levels were
determined at days 4 and 8 of differentiation. All quantifications
were independently performed three times.
Western blot analysis
Preadipocytes and adipocytes were obtained by
washing cells with PBS buffer (Sigma-Aldrich; Merck KGaA; cat. no.
P3813) and lysed with standard buffer containing 100 mM HEPES, KCl
and ethylenediaminetetra-acetic acid (EDTA), 1% NP-40 cell lysis
buffer, 100 mM dithiothreitol, 1 M NaF, 10 mM
Na3VO4 and 100 mM phenylmethylsulfonyl
fluoride (PMSF). Total protein was obtained by centrifuging the
lysate at 15,000 x g for 20 min at 4˚C. Protein extract was
quantified by the Bradford method. 20 ug of protein/lane was
resuspended in SDS-containing Laemmli buffer, heated for 5 min at
85˚C and separated by 10% SDS-PAGE under reducing conditions
(2-mercaptoethanol). Proteins were then transferred to PVDF
membranes (Bio-Rad Laboratories, Inc.; cat. no. 1620177), blocked
for 2 h at room temperature with non-fat dry milk and incubated
overnight at 4˚C with specific antibodies against C/EBPα (1:1,000;
Santa Cruz Biotechnology, Inc.; cat. no. sc-166258). Afterward,
membranes were incubated with HRP-conjugated goat anti-mouse
secondary antibody (1:20,000; LI-COR Biosciences; cat. no.
926-80010) for 90 min at room temperature. Bands were detected
using Immobilon Western Chemiluminescent HRP substrate (EMD
Millipore; cat. no. WBKLS05000). Chemiluminescence was digitized
using the C-Digit Blot Scanner (LI-COR Biosciences; cat. no. 3600)
and analyzed using Image Studio Digits version 5.5.4 processing
software (licor.com/bio/image-studio/). All band density
quantifications were normalized against β-actin (1:1,000; Thermo
Fisher Scientific, Inc.; cat. no. 31464) as loading control.
Statistical analysis
Experiments were performed in triplicates. Data are
presented as the mean ± SEM. Data were analyzed by one-way ANOVA
followed by Tukey's post hoc test. Data were analyzed with GraphPad
Prism 9 (GraphPad Software, Inc.; Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell viability dose-response curve
with 10-G
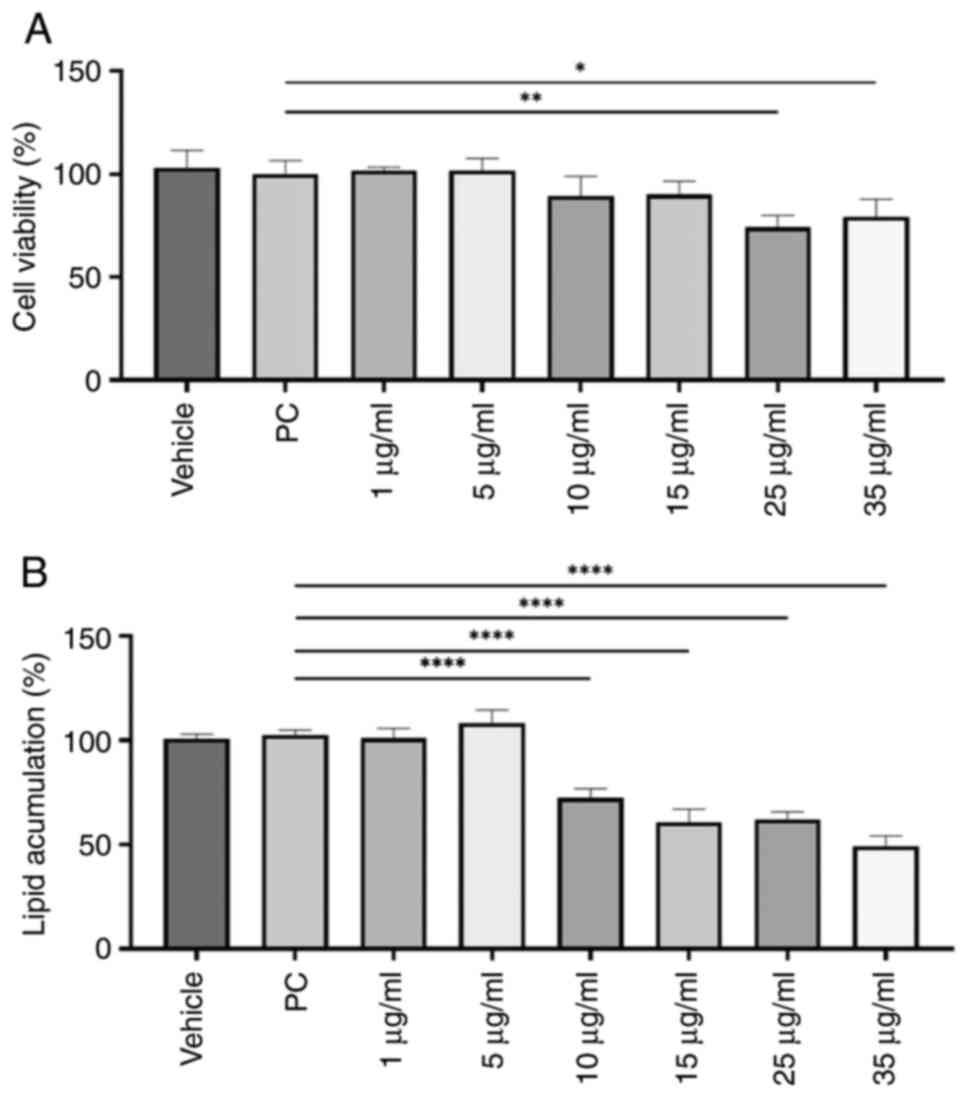
A dose-response curve with 10-G at 1, 5, 10, 15, 25
and 35 µg/ml was performed on the 3T3-L1 cell line to establish the
treatment dose. Subsequently, cell viability was evaluated by MTT
assay. Treatment of immature 3T3-L1 cells with ≥25 µg/ml 10-G
during the differentiation process significantly decreased the
percentage of living cells (Fig.
2A). Thus, doses of 25 and 35 µg/ml were excluded for
subsequent experiments.
Lipid content dose-response curve with
10-G
Amount of stored lipids was estimated by Oil Red O
staining. Except for 1 and 5 µg/ml, 10-G significantly reduced the
lipid content compared with the PC group (Fig. 2B). The dose of 35 µg/ml achieved the
most significant decrease in lipid content, by 50.73%. However,
this dose was not used for subsequent experiments due to low cell
viability.
Therefore, 15 µg/ml was chosen for treatment of
3T3-L1 preadipocytes during differentiation since it showed a
significant decrease in lipid content compared with PC without
significantly affecting the number of living cells.
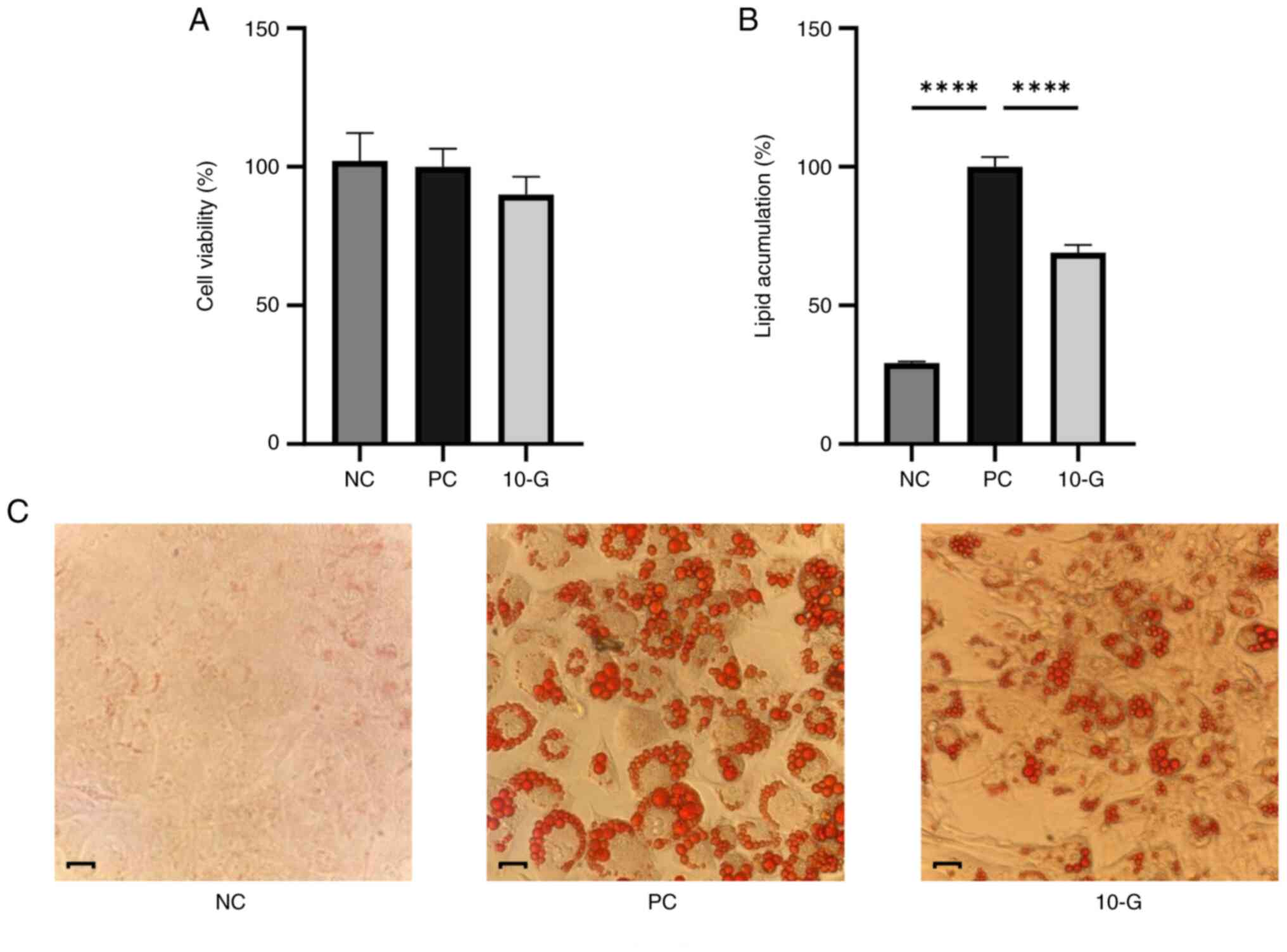
Effects of 10-G on cell viability and
adipocyte differentiation
Cell viability and adipocyte differentiation in the
presence or absence of 10-G were assessed. No significant
differences were in the percentage of viable cells between the
control and 10-G groups. Therefore, 10-G did not affect viability
of 3T3-L1 cells (Fig. 3A).
Regarding the adipocyte differentiation, Oil red O
staining revealed lipid content differences between groups. NC
cells showed no staining, while staining was observed in the PC and
10-G groups. The largest amount of stained lipid vacuoles was
observed in the PC, which indicated successful differentiation of
adipocytes (22). On the other
hand, the 10-G group showed less staining compared with the PC
group, which indicated lower levels of stored lipids (Fig. 3C). Lipid content of 10-G group was
71.17% and significantly decreased compared with the PC group
(Fig. 3B).
Effects of 10-G on pro-adipogenic and
lipogenic genes in 3T3-L1 adipocytes
Expression of genes related to the regulation of
adipogenesis and lipogenesis was assessed. The 10-G group showed
significantly decreased expression of C/ebpα, Pparγ,
Mtor, Acaca, and Fabp4 on day 4 compared with the PC
group (Fig. 4A). The 10-G group, on
day 8, showed higher mRNA expression of C/ebpα and
Pparγ, but the latter was not significant compared with the
PC group. On the other hand, the gene expression of Mtor and
Srebf1 showed a significant decrease in the 10-G compared
with the PC group. In addition, mRNA levels of the lipid
metabolism-associated genes Acaca and Fabp4 were
significantly decreased at day 8 of adipocyte differentiation in
the 10-G compared with the PC group (Fig. 4B).
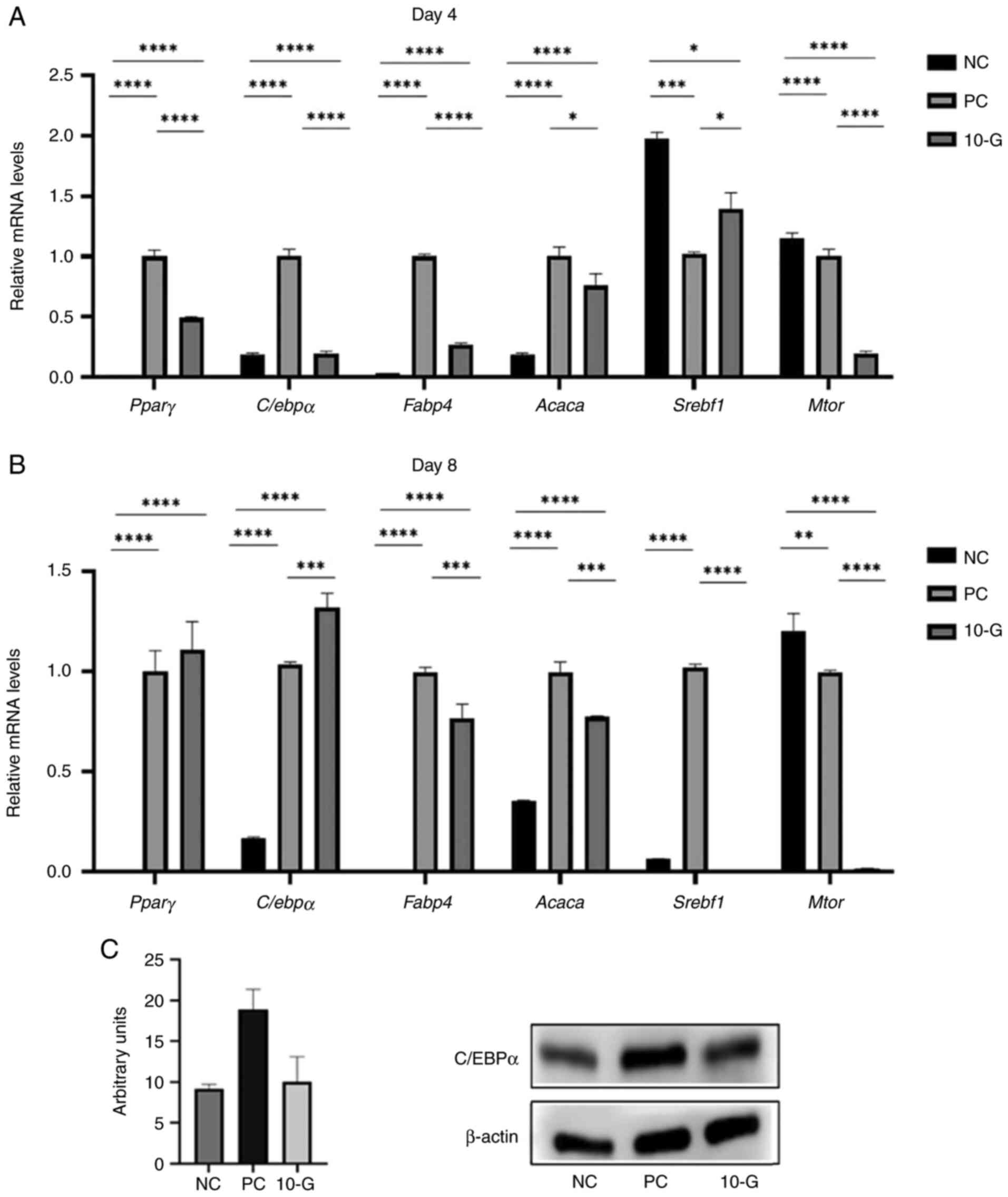 | Figure 4Effect of 10-G on pro-adipogenic and
lipogenic markers and C/EBPα protein expression. Pparγ,
C/ebpα, Fabp4, Acaca, Srebf1 and
Mtor mRNA levels in 3T3-L1 cells were examined using
quantitative PCR on day (A) 4 and (B) 8 of differentiation. The
relative gene expression of each sample was normalized to
Rn18S. (C) Protein levels of C/EBPα were determined by
western blot analysis after 8 days of differentiation. β-actin was
used as a loading control. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. Pparγ, peroxisome
proliferator-activated receptor γ; C/ebpα, CCAAT
enhancer-binding protein α; Fabp4, fatty acid binding
protein 4; Acaca, acetyl-coenzyme A carboxylase;
Srebf1, sterol regulatory element binding transcription
factor 1; Mtor, mechanistic target of rapamycin complex; NC,
negative control; PC, positive control; 10-G, 10-gingerol. |
Effect of 10-G on the protein
expression of adipocyte transcription factor C/EBPα
Treatment with 15 µg/ml 10-G notably decreased
C/EBPα protein levels in the 3T3-L1 adipocytes at day 8 of
differentiation compared with the PC group (Fig. 4C).
Discussion
The present study demonstrated that 10-G, one of the
most abundant phenols in ginger, decreased adipogenesis and
accumulation of cytoplasmic lipid droplets in 3T3-L1 cells via mRNA
downregulation of adipogenic transcriptional factors and lipid
metabolism-associated genes.
The proliferation of adipocytes and excessive fat
accumulation are associated with development of obesity and
metabolic complications (23).
Decreased adiposity is related to a decrease in number of
adipocytes and lipid content (10).
Ginger possesses abundant phenols, such as 6-gingerol and
6-shogaol, that exert beneficial health effects on obesity by
decreasing the intracellular lipid accumulation in 3T3-L1
preadipocyte cells (13,20,24).
Here, stimulation with 10-G during the
differentiation process significantly decreased lipid content in
adipocytes without affecting cell viability. Similar effects have
been described for other ginger phenols, primarily 6-gingerol
(10,14-16,20),
6-shogaol (13,17,18)
and galanolactone (25). Thus,
10-G, similar to other gingerols, serves a critical role in the
process of adipogenesis and accumulation of cytoplasmic lipid
droplets during 3T3-L1 cell differentiation (7).
Adipogenesis is a complex process that is regulated
by sequential activation of transcriptional factors. In the initial
stage, PPARγ and C/EBPα are required to trigger transcriptional
changes that lead to an adipose phenotype (26). Here, treatment with 10-G decreased
mRNA expression of Ppary and C/ebpa on day 4 of
differentiation, consistent with previous results for 6-gingerol
(10). Similarly, key fat cell
genes such as Acaca and Fabp4 were also decreased.
These results suggest that 10-G could act via inhibition of
adipogenic differentiation.
The expression levels of mechanistic target of
rapamycin complex (mTORC), a key factor that mediates increased
cell number and size (27), was
downregulated in cells treated with 10-G. Studies have shown that
mTORC is an important regulator for the formation of adipose tissue
and its function as lipid storage (24,28).
mTORC activation is implicated in the regulation of adipocyte
precursors, induction of preadipocyte differentiation into mature
adipocytes, as well as triglyceride synthesis (28,29).
Conversely, decreased expression and activation of mTORC is
associated with decreased expression of PPAR-γ and C/EBPα (24,30).
Consequently, there is decreased adiposity and impaired
adipogenesis (24). The present
results showed a higher expression of mTOR but lower
expression of Ppary and C/ebpa in NC cells that did
not receive hormonal stimuli to initiate the adipogenic
differentiation process (31).
However, after 8 days of differentiation, 10-G decreased the
expression of mTOR but did not decrease the expression of
Pparγ and C/ebpα in adipocytes. Although the present
gene expression results do not reflect those observed in other
studies reporting a decrease in Pparγ and C/ebpa
(11,13,20,25),
decreased C/EBPα protein expression was consistent with that
reported in other studies (10,13,20).
Therefore, protein expression of these pro-adipogenic markers may
be decreased.
mTOR is involved in a complex transcriptional
network. Ginger extract rich in 6-gingerol and 6-shogaol controls
mTOR expression through the activation of AMP-activated protein
kinase (AMPK) in a model of obesity (20,24).
AMPK expression was not evaluated in the present study and further
studies are needed to clarify the molecular mechanism by which 10-G
downregulates adipogenic differentiation of 3T3-L1 cells and
whether AMPK is also affected.
Moreover, mTOR has been implicated in the regulation
of triglyceride synthesis (28,29)
via phosphorylation of lipin 1, an inhibitor of sterol regulatory
element binding protein (SREBP). This enhances the nuclear
translocation of SREBP and promotes transcription of lipogenic
genes (27). Decreased mTOR
expression downregulates lipogenic pathways via decreased
activation of SREBP (27,29,30).
Here, following 8 days of differentiation, 10-G decreased the mRNA
expression of Srebf1, Acaca and Fabp4. Acaca
encodes ACC, a lipogenic enzyme that regulates endogenous fatty
acid synthesis and triglyceride storage (11,32).
The FABP4 gene is a marker of terminal adipocyte differentiation
and facilitates cellular uptake of long-chain fatty acids for their
metabolism (20). Downregulation of
Srebf1 decreases the expression of Acaca and
Fabp4, which decreases the synthesis and transport of fatty
acids and, consequently, lipid content in 3T3-L1 cells (32).
The present data suggested that 10-G could suppress
adipocyte differentiation via inhibition of mTOR expression,
which may lead to decreased expression of lipid
metabolism-associated genes, ultimately leading to decreased lipid
accumulation.
The present study did not assess expression and
phosphorylation status of the proteins involved in the mTOR
pathway. Therefore, further studies are necessary to confirm its
involvement in the anti-obesogenic effect of 10-G. Furthermore, it
is important to evaluate gene expression at multiple time points
since it can vary throughout differentiation process of
preadipocyte 3T3-L1 cells. The present study only performed western
blot analysis for C/EBPα at one time point. In addition, protein
expression of PPARγ, SREBP, mTORC, ACC and FABP4 should be
determined to confirm the mechanism of action of 10-G during
adipogenesis. Further studies are needed to address these
limitations.
A promising approach to test the therapeutic
properties of 10-G is to combine it with nanomedicine and novel
delivery systems designed for controlled and adjustable release of
the drug. This approach has the potential to improve the efficiency
of drug administration and targeting and to enable personalized
treatment in vivo (33,34).
In conclusion, the present study demonstrated the
antiadipogenic effects of 10-G during differentiation of 3T3-L1
cells to adipocytes. Further in vivo studies are necessary
to provide more complete data on its anti-obesity effect and
potential clinical use for the prevention and treatment of
obesity.
Acknowledgements
The authors would like to thank Dr Trinidad
Garcia-Iglesias (University of Guadalajara, Guadalajara, Mexico)
for generous donation of the 3T3-L1 cell line and the Biomedical
Sciences Research Institute of the University of Guadalajara for
providing the equipment used in the present study.
Funding
Funding: The present study was supported by Consejo Estatal de
Ciencia y Tecnología del Estado de Jalisco through the Jalisco
Scientific Development Fund (grant no. FODECIJAL-7944-2019).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
JRV and EML designed the experiments. GGO, MPO and
MPR performed the experiments. RRE and EML analyzed data. RRE, MPO
and JRV wrote the manuscript. EML and JRV confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
González-Muniesa P, Mártinez-González MA,
Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA and
Martinez JA: Obesity. Nat Rev Dis Prim. 3(17034)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Blüher M: Obesity: Global epidemiology and
pathogenesis. Nat Rev Endocrinol. 15:288–298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
World Health Organization (WHO): Obesity
and overweight. WHO, Geneva, 2021.
|
|
4
|
Heymsfield SB and Wadden TA: Mechanisms,
pathophysiology, and management of obesity. N Engl J Med.
376:254–266. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Doo M and Kim Y: . Obesity: Interactions
of genome and nutrients intake. Prev Nutr Food Sci. 20:1–7.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mao QQ, Xu XY, Cao SY, Gan RY, Corke H,
Beta T and Li HB: Bioactive compounds and bioactivities of ginger
(zingiber officinale roscoe). Foods. 8(185)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang J, Ke W, Bao R, Hu X and Chen F:
Beneficial effects of ginger Zingiber officinale Roscoe on obesity
and metabolic syndrome: A review. Ann N Y Acad Sci. 1398:83–98.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Saravanan G, Ponmurugan P, Deepa MA and
Senthilkumar B: Anti-obesity action of gingerol: Effect on lipid
profile, insulin, leptin, amylase and lipase in male obese rats
induced by a high-fat diet. J Sci Food Agric. 94:2972–2977.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Semwal RB, Semwal DK, Combrinck S and
Viljoen AM: Gingerols and shogaols: Important nutraceutical
principles from ginger. Phytochemistry. 117:554–568.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tzeng TF and Liu IM: 6-Gingerol prevents
adipogenesis and the accumulation of cytoplasmic lipid droplets in
3T3-L1 cells. Phytomedicine. 20:481–487. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li C and Zhou L: Inhibitory effect
6-gingerol on adipogenesis through activation of the Wnt/β-catenin
signaling pathway in 3T3-L1 adipocytes. Toxicol In Vitro. 30 (1 Pt
B):394–401. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rani MP, Krishna MS, Padmakumari KP, Raghu
KG and Sundaresan A: Zingiber officinale extract exhibits
antidiabetic potential via modulating glucose uptake, protein
glycation and inhibiting adipocyte differentiation: An in vitro
study. J Sci Food Agric. 92:1948–1955. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Suk S, Seo SG, Yu JG, Yang H, Jeong E,
Jang YJ, Yaghmoor SS, Ahmed Y, Yousef JM, Abualnaja KO, et al: A
bioactive constituent of ginger, 6-shogaol, prevents adipogenesis
and stimulates lipolysis in 3T3-L1 Adipocytes. J Food Biochem.
40:84–90. 2016.
|
|
14
|
Wang J, Zhang L, Dong L, Hu X, Feng F and
Chen F: 6-Gingerol, a functional polyphenol of ginger, promotes
browning through an AMPK-Dependent Pathway in 3T3-L1 Adipocytes. J
Agric Food Chem. 67:14056–14065. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng Z, Xiong X, Zhou Y, Wu F, Shao Q,
Dong R, Liu Q, Li L and Chen G: 6-gingerol ameliorates metabolic
disorders by inhibiting hypertrophy and hyperplasia of adipocytes
in high-fat-diet induced obese mice. Biomed Pharmacother.
146(112491)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hong KH, Um MY, Ahn J and Ha TY:
6-Gingerol ameliorates adiposity and inflammation in adipose tissue
in high fat diet-induced obese mice: Association with regulating of
adipokines. Nutrients. 15(3457)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jiao W, Mi S, Sang Y, Jin Q, Chitrakar B,
Wang X and Wang S: Integrated network pharmacology and cellular
assay for the investigation of an anti-obesity effect of 6-shogaol.
Food Chem. 374(131755)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Park SH, Jung SJ, Choi EK, Ha KC, Baek HI,
Park YK, Han KH, Jeong SY, Oh JH, Cha YS, et al: The effects of
steamed ginger ethanolic extract on weight and body fat loss: A
randomized, double-blind, placebo-controlled clinical trial. Food
Sci Biotechnol. 29:265–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zick SM, Djuric Z, Ruffin MT, Litzinger
AJ, Normolle DP, Alrawi S, Feng MR and Brenner DE: Pharmacokinetics
of 6-Gingerol, 8-Gingerol, 10-Gingerol, and 6-Shogaol and conjugate
metabolites in healthy human subjects. Cancer Epidemiol Biomarkers
Prev. 17:1930–1936. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Suk S, Kwon GT, Lee E, Jang WJ, Yang H,
Kim JH, Thimmegowda NR, Chung MY, Kwon JY, Yang S, et al:
Gingerenone A, a polyphenol present in ginger, suppresses obesity
and adipose tissue inflammation in high-fat diet-fed mice. Mol Nutr
Food Res. 61(10.1002/mnfr.201700139)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kraus NA, Ehebauer F, Zapp B, Rudolphi B,
Kraus BJ and Kraus D: Quantitative assessment of adipocyte
differentiation in cell culture. Adipocyte. 5:351–358.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Quintero-Fabián S, Ortuño-Sahagún D,
Vázquez-Carrera M and López-Roa RI: Alliin, a garlic (Allium
sativum) compound, prevents LPS-induced inflammation in 3T3-L1
adipocytes. Mediators Inflamm. 2013(381815)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee GH, Peng C, Jeong SY, Park SA, Lee HY,
Hoang TH, Kim J and Chae HJ: Ginger extract controls mTOR-SREBP1-ER
stress-mitochondria dysfunction through AMPK activation in obesity
model. J Funct Foods. 87(104628)2021.
|
|
25
|
Ahn EK and Oh JS: Inhibitory effect of
galanolactone isolated from Zingiber officinale Roscoe
extract on adipogenesis in 3T3-L1 cells. J Korean Soc Appl Biol
Chem. 55:63–68. 2012.
|
|
26
|
Madsen MS, Siersbæk R, Boergesen M,
Nielsen R and Mandrup S: Peroxisome proliferator-activated receptor
γ and C/EBPα synergistically activate key metabolic adipocyte genes
by assisted loading. Mol Cell Biol. 34:939–954. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu GY and Sabatini DM: mTOR at the nexus
of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol.
21:183–203. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee PL, Jung SM and Guertin DA: . The
complex roles of mechanistic target of rapamycin in adipocytes and
beyond. Trends Endocrinol Metab. 28:319–339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ricoult SJ and Manning BD: The
multifaceted role of mTORC1 in the control of lipid metabolism.
EMBO Rep. 14:242–251. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee PL, Jung SM and Guertin DA: The
complex roles of mechanistic target of rapamycin in adipocytes and
beyond. Trends Endocrinol Metab. 28:319–339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cave E and Crowther NJ: The Use of 3T3-L1
murine preadipocytes as a model of adipogenesis. Methods Mol Biol.
1916:263–272. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Okamoto M, Irii H, Tahara Y, Ishii H,
Hirao A, Udagawa H, Hiramoto M, Yasuda K, Takanishi A, Shibata S
and Shimizu I: Synthesis of a new [6]-gingerol analogue and its
protective effect with respect to the development of metabolic
syndrome in mice fed a high-fat diet. J Med Chem. 54:6295–6304.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liang T, Xing Z, Jiang L and Zhu JJ:
Tailoring nanoparticles for targeted drug delivery: From organ to
subcellular level. VIEW. 2(20200131)2021.
|
|
34
|
Geraili A, Xing M and Mequanint K: Design
and fabrication of drug-delivery systems toward adjustable release
profiles for personalized treatment. VIEW. 2(20200126)2021.
|