Introduction
Temozolomide (TMZ) is the main chemotherapeutic drug
for the treatment of malignant gliomas (1-3)
and melanoma (4-6);
it is also proposed for colon cancer chemotherapy (7) and as a second line
gastroenteropancreatic neuroendocrine carcinoma treatment (8). Being used as a system therapy, TMZ
treatment is accompanied by a number of short- and long-term side
effects, including fatigue, nausea, vomiting, thrombocytopenia,
neutropenia (9), myelosupression
(10) and rarely myelodysplastic
syndrome or aplastic anaemia (11).
In addition, previous data have been published on the effects of
TMZ on quality of life, depression and increased anxiety; however,
published results on these topics are contradictory.
According to previous studies, TMZ chemotherapy does
not affect cognitive function and emotional functioning (12) in patients receiving standard
chemotherapy (13,14). Glioblastoma (GBM) treatment can
aggravate the wellbeing of patients (15) and causes depression, particularly in
the 3rd month of the treatment, which requires additional
specialized treatment (16).
By contrast, TMZ administration has been
demonstrated to cause behavioral impairments in animal models in
vivo. In adult mice, TMZ treatment leads to anxiety- and
depression-like behavior both with a short administration of 3 days
(17) and with a longer
administration of 5-15 weeks (18-20).
Several studies have shown that TMZ chemotherapy disrupts
hippocampus-dependent learning (21) and impairs social recognition
(17) and spatial and episodic
memory (22).
An important issue is that the majority of the
studies have been conducted on young animals, whereas GBM and other
malignant tumors occur mainly in adult and elderly patients. It was
proposed that elderly patients aged 70 years and older (who are
considered eligible for combined modality treatment) should receive
a short-course radiotherapy with concomitant and adjuvant TMZ
treatment up to 12 cycles to reduce the negative impact on
health-related quality of life (23,24).
However, the effects of TMZ on the behavior of adult and elderly
animals have not been sufficiently studied. In a previous conducted
by the authors, it was revealed that long-term treatment with TMZ
to adult Wistar rats resulted in a significant decrease in their
locomotor activity (25), whereas
its effects on the anxiety of the animals remained unknown.
Anti-GBM chemotherapy is accompanied with
dexamethasone (DXM) treatment in the majority of cases to prevent
brain edema (26). Such treatment
can potentially interfere with TMZ effects; therefore, in the
present study, a single TMZ treatment regimen was used with DXM
treatment (alone or in combination with TMZ). This concept was
supported by previously published data on the effect of DXM
influence on long-term behavioral characteristics of experimental
animals. It was shown that DXM causes affective changes in humans,
such as depression and anxiety (27,28)
and elevated levels of other glucocorticoid (GC) cortisol may
contribute to anxiety of patients (29). Prolonged use of GC during clinical
practice in adult patients with glioma results in negative
consequences, including psychiatric symptoms (30). In an animal model, adult
9-10-week-old C57BL/6J mice that were injected daily with DXM
demonstrated a variety of depression-like behaviors and activated
stress-related genes in the prefrontal cortex and hippocampus
(31). A different study indicated
a different effect of DXM on the behavior of animals depending on
the dose. Anxiolytic effects were noted in lower doses and
anxiogenic effects were observed at a high dose (32).
Various experimental models are used to assess the
molecular mechanisms of the effect of chemotherapeutic drugs on the
development of malignant gliomas (33). The majority of the studies
investigating TMZ have been focused on the role of extracellular
matrix (ECM) in the development of malignant gliomas (34-36)
and the development of TMZ resistance (37,38).
The main components of brain ECM are complex glycosylated
proteoglycan (PGs) molecules, which are composed of the various
core proteins with covalently attached polysaccharide chains of
glycosaminoglycans (GAGs) (36).
The effect of TMZ on the expression of PGs and GAGs in normal brain
tissues has been studied inadequately. It has been revealed that
TMZ exhibits no significant effect on PG expression in the
hippocampus and cerebral cortex of 2-month-old Wistar rats;
however, it demonstrates age- and brain zone-specific effects on
the content of such GAGs, such as heparan sulfate (HS) and
chondroitin sulfate (CS) (39). In
brain tissues derived from 10-week-old mice, TMZ did not affect the
expression of PG core proteins, whereas it reduced the CS content
in the subcortical brain structures of severe combined
immunodeficiency mice (40).
Concomitantly, no data have been reported on the potential
influence of long-term TMZ administration on glycosylated
macromolecules in adult brain tissues and this research area should
be investigated further.
The aim of the present study was to investigate the
effect of long-term administration of TMZ and/or DXM on the
development of anxiety of adult Wistar rats and on the content of
the PGs and GAGs in the brain tissue of these animals.
Materials and methods
Animals
Nine-month-old male Wistar rats (Crl:WI) weighing
400-500 g were used at the beginning of the experiment (40 animals
in total). The animals were obtained from the Institute of Cytology
and Genetics (Novosibirsk, Russia) and housed in polycarbonate
cages (36x50x28 cm) with free access to food and water; the
temperature was maintained at 25±1˚C and the humidity range was
50-60%. The animals were weighed once a day and maintained at a
light/dark 12/12 cycle. The number of animals per cage was 3 or 4.
The animals were sacrificed by decapitation using a guillotine
according to the American Veterinary Medical Association guidelines
for the euthanasia of animals (2013). All efforts were made to
minimize animal suffering and to reduce the number of animals used.
All procedures were conducted in accordance with the European
Communities Council Directive 2010/63/EU and in compliance with the
Federal Research Center for Fundamental and Translational Medicine
Ethical committee (approval no. N3/2017 from 23.06.2017;
Novosibirsk, Russia).
TMZ and DXM administration
Rats were randomly assigned to 4 groups (control,
TMX, DXM and TMZ + DXM; 10 animals/group). Simple randomization
using random number generation was used. TMZ-based drug (TMZ; Teva
Pharmaceutical Industries, Ltd.) was administered orally (150
mg/m2) per day as a water suspension. The synthetic GC
agonist (Dexamethasone; KrkA) was administered intraperitoneally at
a dose of 2.5 mg/kg twice a week. The animals received 5 cycles of
TMZ in the TMZ and TMZ/DXM groups for 5 consecutive days, with
intermissions of 16 days between cycles. The control group received
saline injections/peroral administration of the same volume as the
experimental groups. During the experiment, the weight of the
animals was monitored. The animals were sacrificed by decapitation
and the brain from each animal was collected; one hemisphere was
collected in RNALater solution (Invitrogen; ThermoFisher
Scientific, Inc.) for reverse transcription-quantitative PCR
(RT-qPCR) analysis. The other hemisphere was incubated in a 10%
neutral buffered formalin for 48 h at room temperature and used to
prepare paraffin blocks.
Elevated plus maze (EPM) test
EPM test was employed for studying mechanisms
underlying anxiety and anti-anxiety drugs (41,42).
The maze apparatus was composed of polyvinylchloride and comprised
two opposing closed arms (50x10x40 cm) and two opposing open arms
(50x10 cm). The maze was elevated at a height of 50 cm above the
floor. Each rat was placed in the center of the maze, facing an
open arm, and allowed to freely explore the maze for 5 min. The
percentages of time spent in the center, in open arms and during
head dipping in open arms, were used as an indication for
anxiolytic behaviour. The percentages of time spent in closed arms
and stretching in closed arms and the number of faecal boli were
used as indicators for anxiolytic behavior. The EPM test was
performed at the same time of the day at 13.00-15.00 pm. The first
behavioral test was performed prior to the initiation of the drug
administration (rats were naive) and the second test following the
end of drug administration (rats had already been trained).
RT-qPCR analysis
Сoronal sections of the mouse brain (~1.5 mm thick)
were obtained from the bregma (5±0.5 mm) and used for RT-qPCR
analysis. Total RNA was extracted from the brain samples using the
QIAzol reagent (Qiagen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. cDNA was synthesized from 1 µg
total RNA using a RevertAid H Minus First Strand cDNA Synthesis Kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The analysis was performed using the CFX96 Real-Time
PCR Detection System (Bio-Rad Laboratories, Inc.) and real-time
BioMaster HS-qPCR SYBR Blue Mastermix (2x) (Biolabmix) under the
following conditions: 95˚C for 2 min, followed by 40 cycles at 95˚C
for 10 sec and 60˚C for 30 sec. The total reaction volume was 25
µl. The relative amount of mRNA was normalized against GAPDH mRNA,
and the fold-change for each mRNA was calculated by the
2-ΔΔCq method (43). The
primer sequences used for the rat genes are presented in Table I.
 | Table ISequences of primers used in reverse
transcription-quantitative PCR analysis. |
Table I
Sequences of primers used in reverse
transcription-quantitative PCR analysis.
| Protein | Gene | Primer sequence
(5'→3') |
|---|
| Syndecan-1 | Sdc1 | F:
GAACCCACCAGCAGGGATAC |
| | | R:
CACACTTGGAGGCTGATGGT |
| Syndecan-3 | Sdc3 | F:
TGCTCGTAGCTGTGATCGTA |
| | | R:
TGTCGGGCTTCTGGTATGTG |
| Glypican-1 | Gpc1 | F:
GCCAGATCTACGGGGCTAAG |
| | | R:
AGACGCAGCTCAGCATACAG |
| Heparan sulfate
proteoglycan 2/perlecan | Hspg2 | F:
TGATGACGAGGACTTGCTGG |
| | | R:
ACACCACACTGACAACCTGG |
| Decorin | Dcn | F:
AATGCCATCTCCGAGTGGTG |
| | | R:
TTGTCGTGGAGTCGAAGCTC |
| Biglycan | Bgn | F:
GAACAGTGGCTTTGAACCCG |
| | | R:
CCTCCAACTCGATAGCCTGG |
| Lumican | Lum | F:
AATTTGACCGAGTCCGTGGG |
| | | R:
GCCTTTCAGAGAAGCCGAGA |
| Brevican | Bcan | F:
AGGGGACCTCACAAGTTCTTC |
| | | R:
ATTTGACTCGGGGAAAGCCC |
| Neurocan | Ncan | F:
AACCTGTGCGAGAAGGACAC |
| | | R:
GGGAGTGGACACTTGTCAGG |
| Aggrecan | Acan | F:
CAGATGGCACCCTCCGATAC |
| | | R:
GACACACCTCGGAAGCAGAA |
| Versican | Vcan | F:
ATGTGGATCATCTGGACGGC |
| | | R:
GTTTCGATGGTGGTTGCCTC |
| Chondroitin sulfate
proteoglycan 4/Ng2 |
Cspg4/Ng2 | F:
ATCTGGGAGGGGGCTATTGT |
| | | R:
GTACGCCATCAGAGAGGTCG |
| Chondroitin sulfate
proteoglycan 5 | Cspg5 | F:
CTCCCATCCAAATGACATGGA |
| | | R:
CTCGAGTTTGGGTGACATGGA |
| Proteintyrosine
phosphatase receptor type Z1/phosphacan | Ptprz1 | F:
TGTGTCATCGGAAGGATCGG |
| | | R:
GTCCGCATCGAAGCAGTAGA |
|
Glyceraldehyde-3-phosphate
dehydrogenase | Gapdh | F:
ATGGCCTTCCGTGTTCCTAC |
| | | R:
TCCAGGGTTTCTTACTCCTTGG |
Histological analysis
To study the morphology of the rat brain, one
hemisphere of the rat brain tissue was fixed in 10% neutral
formalin for 48 h at room temperature, dehydrated in a series
ethanol solution of increasing concentration and embedded in
paraffin. Serial 3-4 µm coronal sections of the mouse brain were
obtained from the bregma (5±0.5 mm), stained with hematoxylin and
eosin (H&E) for 2 min, and analysed using light microscope
LeicaDM 4000B (Leica Microsystems, Inc.) with the LeicaDFC 320
camera (Leica Microsystems, Inc.). The cerebral cortex was studied
within the primary and secondary motor cortex (M1, M2) and primary
somatosensory (S1HL, S1FL, S1DZ, S1BF) cortical zones.
Immunohistochemical (IHC)
analysis
For immunohistochemistry, 3-mm sections of
formalin-fixed, paraffin-embedded tissue samples were
deparaffinized in xylene and ethanol series. Tissue sections were
stained using Mouse and Rabbit Specific HRP/DAB (ABC) Detection IHC
kit (cat. no. ab64264; Abcam) according to the manufacturer's
instructions. Sections were incubated with Hydrogen Peroxide Block
(cat. no. ab64264; Abcam) for 10 min at room temperature, and then
exposed to Protein Block (cat. no. ab64264; Abcam) for 10 min at
room temperature. The antigen was retrieved following treatment
with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween-20)
at 95-98˚C for 20 min. The following primary antibodies were used
for immunostaining: mouse monoclonal anti-CS (1:100; cat. no.
C8035; Sigma-Aldrich; Merck KGaA), rabbit polyclonal anti-aggrecan
(1:100; cat. no. ab36861; Abcam), rabbit polyclonal anti-decorin
(1:100; cat. no. ab175404; Abcam), and rabbit polyclonal
anti-brevican (1:100; cat. no. ab111719; Abcam) for 1 h at room
temperature; mouse monoclonal anti-GFAP (ready-to-use; cat. no.
MS-1376-R7; Thermo Fisher Scientific) and mouse monoclonal
anti-Olig2 (ready-to-use; cat. no. 211F1.1; Cell Marque) for 30 min
at room temperature The staining patterns for CS, aggrecan, decorin
and brevican were visualized with Mouse and Rabbit Specific HRP/DAB
(ABC) Detection IHC kit (cat. no. ab64264; Abcam). The sections
were counterstained with hematoxylin and observed by light
microscopy using an AxioScopeA1 microscope (Zeiss AG). Quantitative
analysis was performed with ZENblue 2.3 software (Zeiss AG). A
total of ten fields for each section were analyzed.
Dot-blot analysis
Coronal brain tissue sections 1.5-mm thick were
obtained from the bregma (5±0.5 mm), lysed with RIPA-buffer (Thermo
Fisher Scientific, Inc.), containing complete protease inhibitor
cocktail (Roche Diagnostics), sonicated (20 kHz, 3 times for 10
sec) and centrifuged for 15 min at 14,000 x g. The protein
concentration was quantified using Pierce™ BCA Protein Assay Kit
(Thermo Fisher Scientific, Inc.). A total of 1 mg total protein was
dot-blotted onto polyvinylidene fluoride membranes at a volume of 2
ml. The membranes were blocked with 5% non-fat milk for 1 h at room
temperature and incubated with mouse anti-CS primary antibody
(1:500; cat. no. C-8035; MilliporeSigma), mouse anti-HS primary
antibody (1:500; cat. no. MAB2040; MilliporeSigma) overnight at 4˚C
followed by incubation with secondary peroxidase-conjugated
antibodies goat anti-mouse IgG (1:2,000; cat. no. ab6823; Abcam)
for 1 h at room temperature. GAGs were detected with an Optiblot
ECL Detection Kit (Abcam) according to the manufacturer's
instructions. The blot images were captured using ChemiDoc (Bio-Rad
Laboratories, Inc.) and analyzed semi-quantitatively using ImageJ
software (v.1.52; National Institutes of Health).
Statistical analysis
One-way ANOVA analysis with Fisher's Least
Significant Difference post hoc test and Kruskal-Wallis' test was
performed to determine the statistical significance between the
studied groups P<0.05 was considered to indicate a statistically
significant difference. The number of the repeats of the
experiments was three. The data are expressed as the mean ±
standard deviation. Statistical analysis was performed using Origin
Pro 8.5 software (OriginLab).
Results
Study design
To model anti-GBM chemotherapy in an experimental
animal model in vivo, adult Wistar rats were treated with
TMZ and/or DXM for 4 months. The effects of TMZ and/or DXM on
animal anxiety, morphology of the cerebral cortex, PG expression
and GAG content in the rat brain tissue were investigated.
TMZ treatment results in anxiety-like
behavior of the experimental animals
The effects of TMZ and/or DXM on anxiety were
investigated in adult rats using the EPM test. The main monitored
parameters included the following: Percentage of time spent in open
and closed arms as well as in the center of the arena, head dipping
in open-arms, stretching in closed arms and the number of fecal
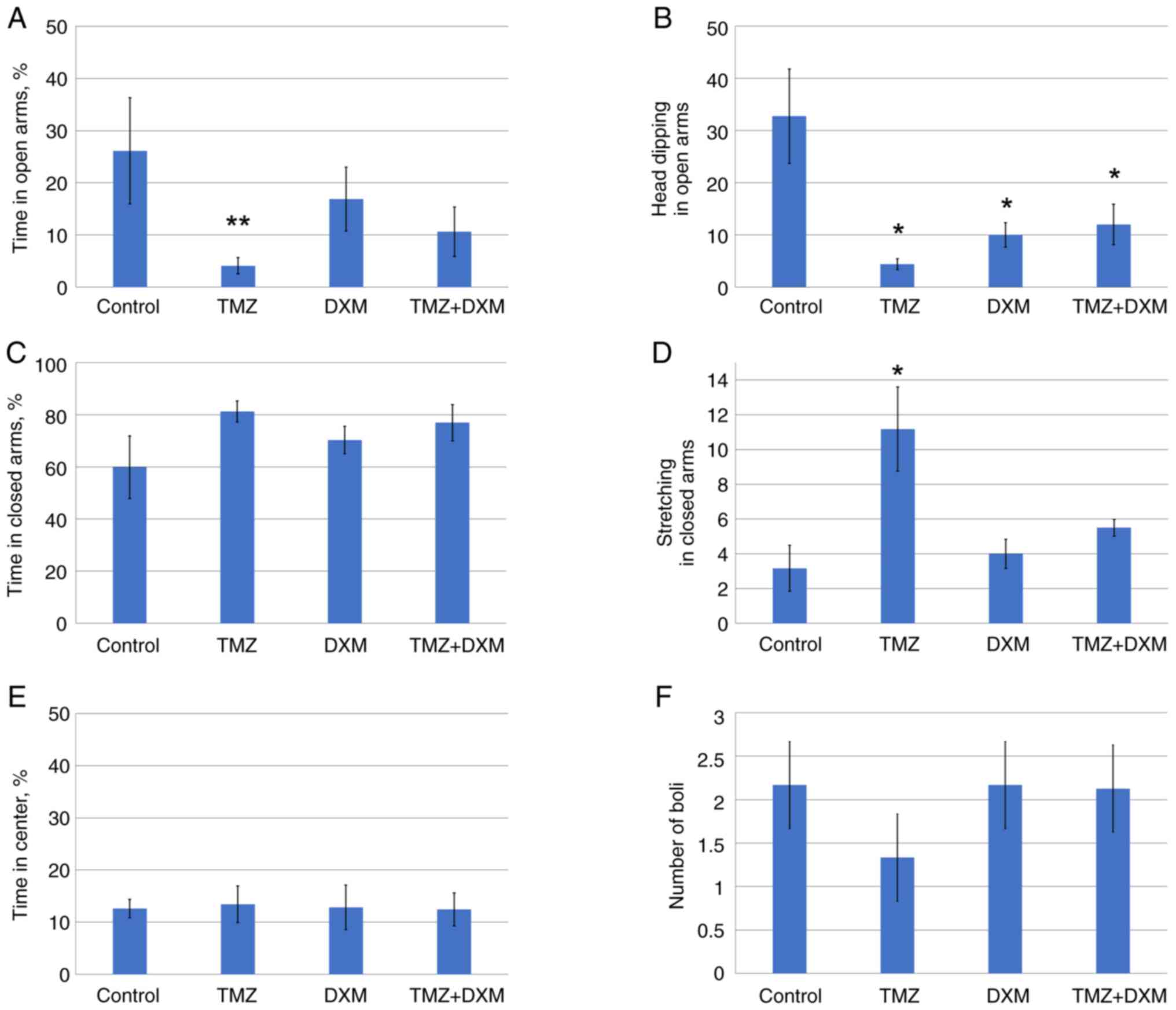
boli (Fig. 1A-F).
Long-term TMZ treatment of the adult rats resulted
in a decrease in the percentages of time and head dipping in
open-arms and an increase in stretching in closed arms in the EPM
test (Fig. 1A, B and D,
respectively), which indicated a significant increase in the
anxiety of the animals. The combination of TMZ with DXM did not
demonstrate significant differences with TMZ alone, suggesting a
negligible effect of DXM on the animal anxiety. The observation was
further supported by the DXM administration as a mono-regimen,
which did not affect the majority of the studied behavioral
parameters except for a reduction in head dipping in open-arms
(Fig. 1B).
These results demonstrated that the long-term use of
TMZ can affect anxiety of the adult Wistar rats in the experimental
system in vivo.
TMZ does not affect the morphology of
the cerebral cortex
To look for potential morphological basis of the
demonstrated behavioral changes, paraffin sections of the brain of
the rats were stained with H&E (Fig. 2A).
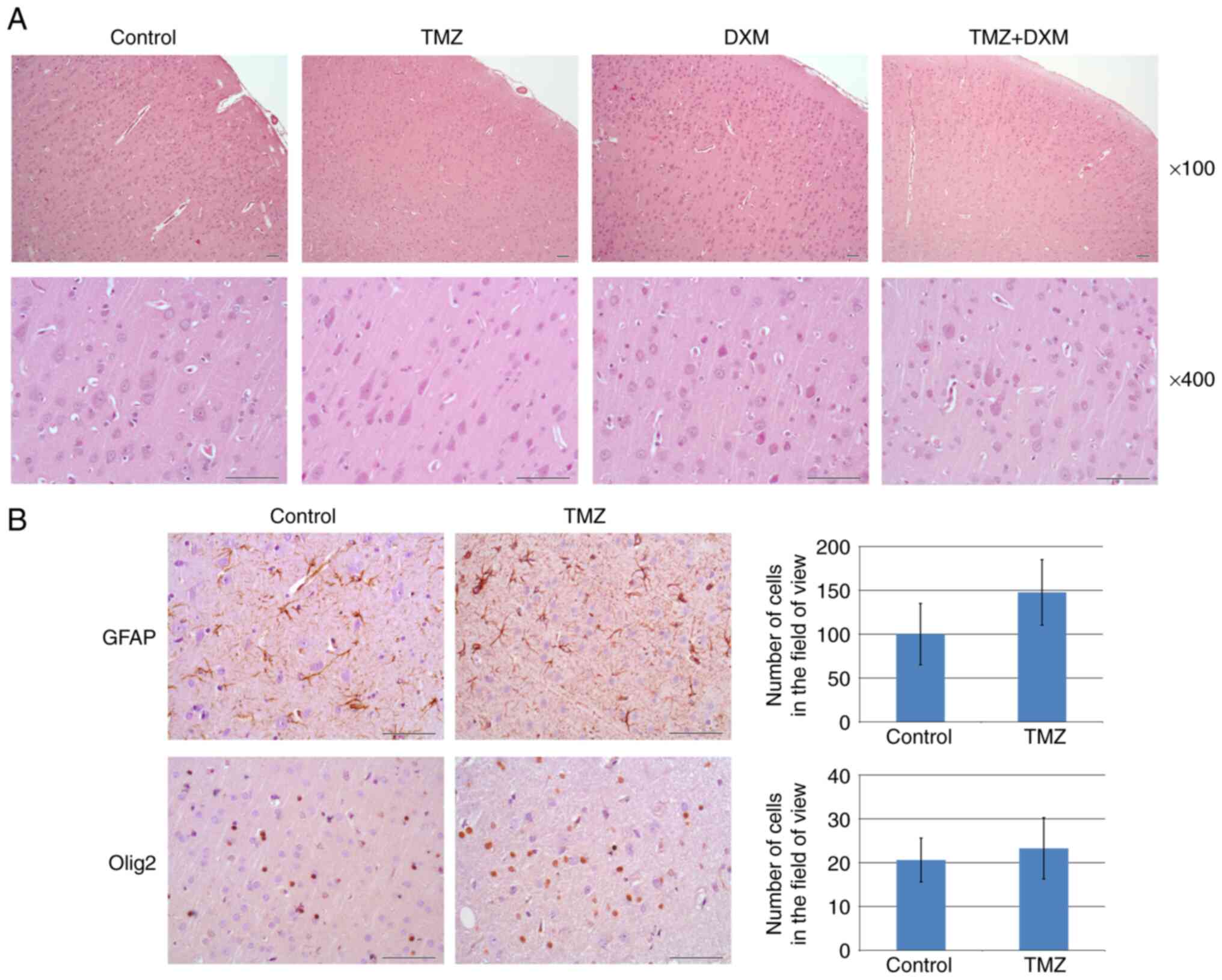 | Figure 2Histological analysis of rat brain
tissues in the control, TMZ, DXM and TMZ + DXM rat groups. (A)
Hematoxylin and eosin staining (magnification, x100; scale bar, 50
µm). (B) Immunohistochemical analysis of GFAP and OLIG2 expression
in the TMZ rat group. Quantitative analysis of the DAB signal was
performed with ZENblue software (magnification, x400; scale bar, 50
µm). The bars represent the mean ± standard deviation from
triplicate experiments (Origin 8.5; Kruskal-Wallis' test). Control,
non-treated rat brain tissues; TMZ, temozolomide, DXM;
dexamethasone; GFAP, glial fibrillary acidic protein; OLIG2,
oligodendrocyte transcription factor 2; DAB,
3,3'-diaminobenzidine. |
The cerebral cortex of the Wistar rat (neocortex)
has a typical six-layer structure in all groups (Fig. 2A). During the morphological analysis
of the tissue of the cerebral cortex, the architectonics of the
cerebral cortex were not disturbed; in addition, circulatory
disorders and other pathological changes, including necrobiosis and
necrosis of cellular elements and inflammatory infiltration, were
not detected. The H&E slides of the cortex tissue of the
animals (control and experimental groups) were characterized by
usual tinctorial properties of neurons and neuropil with minimal
visible variations. The most prominent in the cortex of all
experimental groups was the fifth layer, which contained
comparatively large neurons of pyramidal shape. It is important to
note that in all experimental groups in this layer (and to a lesser
degree in the third layer) the attention was attracted on the
moderately pronounced eosinophilia of the cytoplasm of pyramidal
neurons without signs of cyto-destruction or other structural
reactions, such as gliosis, peri-cellular infiltration, edema and
destruction of blood vessels (Fig.
2A).
The number of astrocytes and oligodendrocytes in the
cerebral cortex tissues of the Wistar rats was analyzed by IHC
staining of paraffin sections with antibodies to glial fibrillary
acidic protein (GFAP; marker of astrocytes) and nuclear protein
oligodendrocyte transcription factor 2 (OLIG2; marker of
oligodendrocytes and astrocytes). A tendency towards an increase in
the content of astrocytes derived from the brain tissues of
TMZ-treated animals was observed. However, the trend was not
statistically significant (Fig.
2B). The number of astrocytes and oligodendrocytes in the
TMZ-treated brain tissues did not demonstrate an evident difference
compared with that of the control cells (Fig. 2B).
Taken together, the obtained data did not reveal
significant deterioration of astroglia and oligodendroglia derived
from the cerebral cortex of the brain tissue of TMZ-treated adult
animals.
TMZ inhibits aggrecan expression in
rat brain tissues
Despite the unaltered morphological structure and
cellular composition of the brain tissue, certain molecular changes
may occur following TMZ/DXM treatment to the brain ECM. The main
components of the ECM are PGs. As the first step, transcriptional
profiling of the core proteins of the main PGs investigated was
performed by RT-qPCR (Fig. 3).
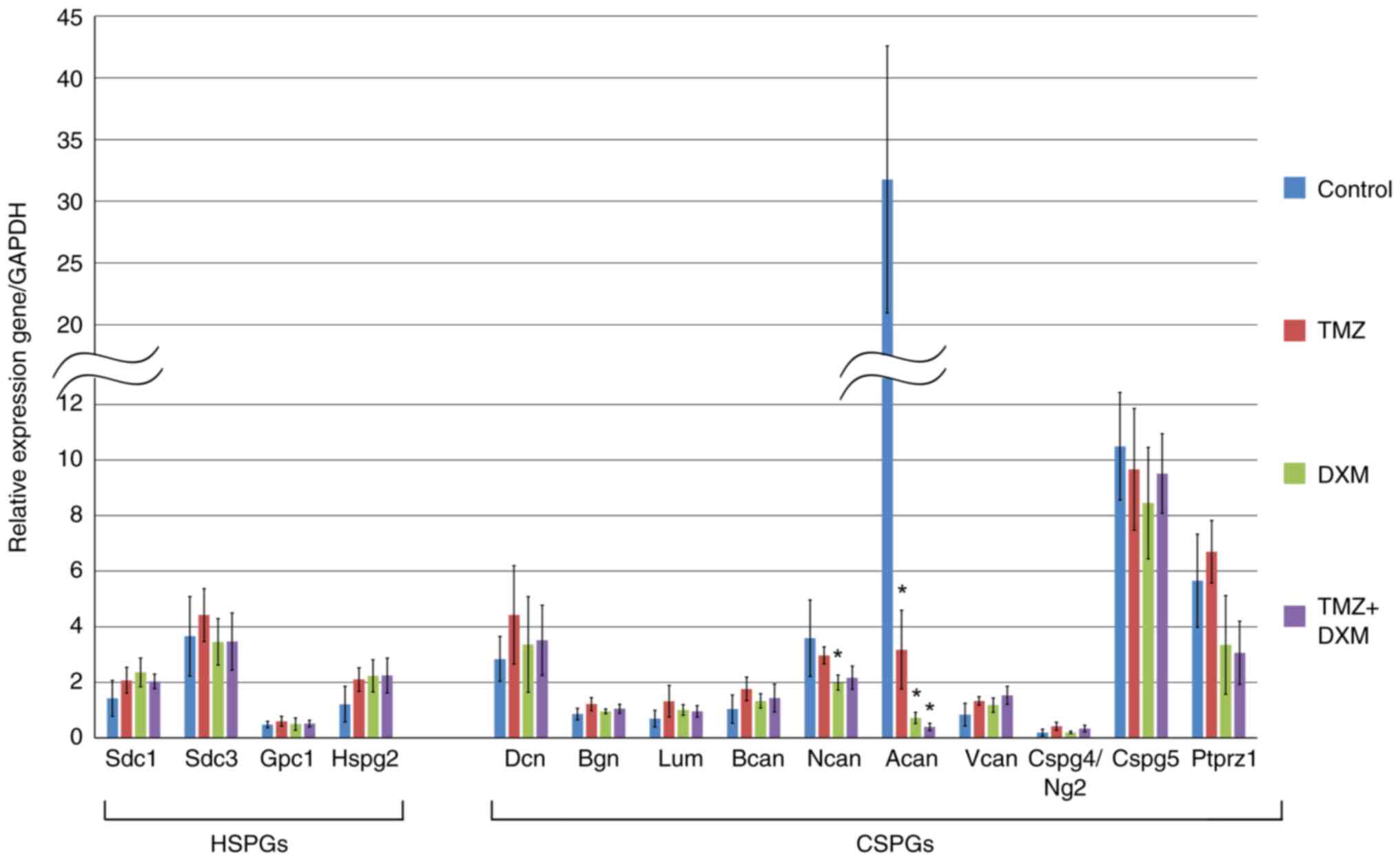 | Figure 3Expression of HSPGs and СSPGs core
proteins in rat brain tissues of the control, TMZ-treated,
DXM-treated and TMZ + DXM-treated experimental animals. Reverse
transcription-quantitative PCR, expression normalized to that of
GAPDH (OriginPro 8.5). The bars represent the mean ± standard
deviation from triplicate experiments (OriginPro 8.5). ANOVA +
Fisher's Least Significant Difference test; *P<0.05.
Control, non-treated rat brain tissue; TMZ, temozolomide; DXM,
dexamethasone; HSPG, heparan sulfate proteoglycan; CSPG,
chondroitin sulfate proteoglycan; Sdc1, syndecan-1; Sdc3,
syndecan-3; Gpc1, glypican-1; Hspg2, heparan sulfate proteoglycan
2/perlecan; Dcn, decorin; Bgn, biglycan; Lum, lumican; Bcan,
brevican; Ncan, neurocan; Acan, aggrecan; Vcan, versican;
Cspg4/Ng2, chondroitin sulfate proteoglycan 4; Cspg5, chondroitin
sulfate proteoglycan 5; Ptprz1, proteintyrosine phosphatase
receptor type Z1/phosphacan. |
The expression levels of all HSPGs (syndecan-1,
syndecan-3, glypican-1, and perlecan) and the majority of the
studied CSPGs (decorin, biglycan, lumican, brevican, versican,
Cspg4/Ng2, Cspg5 and phosphacan) were not significantly affected by
the long-term treatment with TMZ and/or DXM (except for neurocan
and aggrecan). Aggrecan was the most sensitive to TMZ/DXM treatment
and its expression was downregulated following TMZ (-10-fold), DXM
(-45-fold), and TMZ-DXM (-80-fold) treatment. Neurocan expression
demonstrated -1, and 8-fold decreases following single treatment
with DXM.
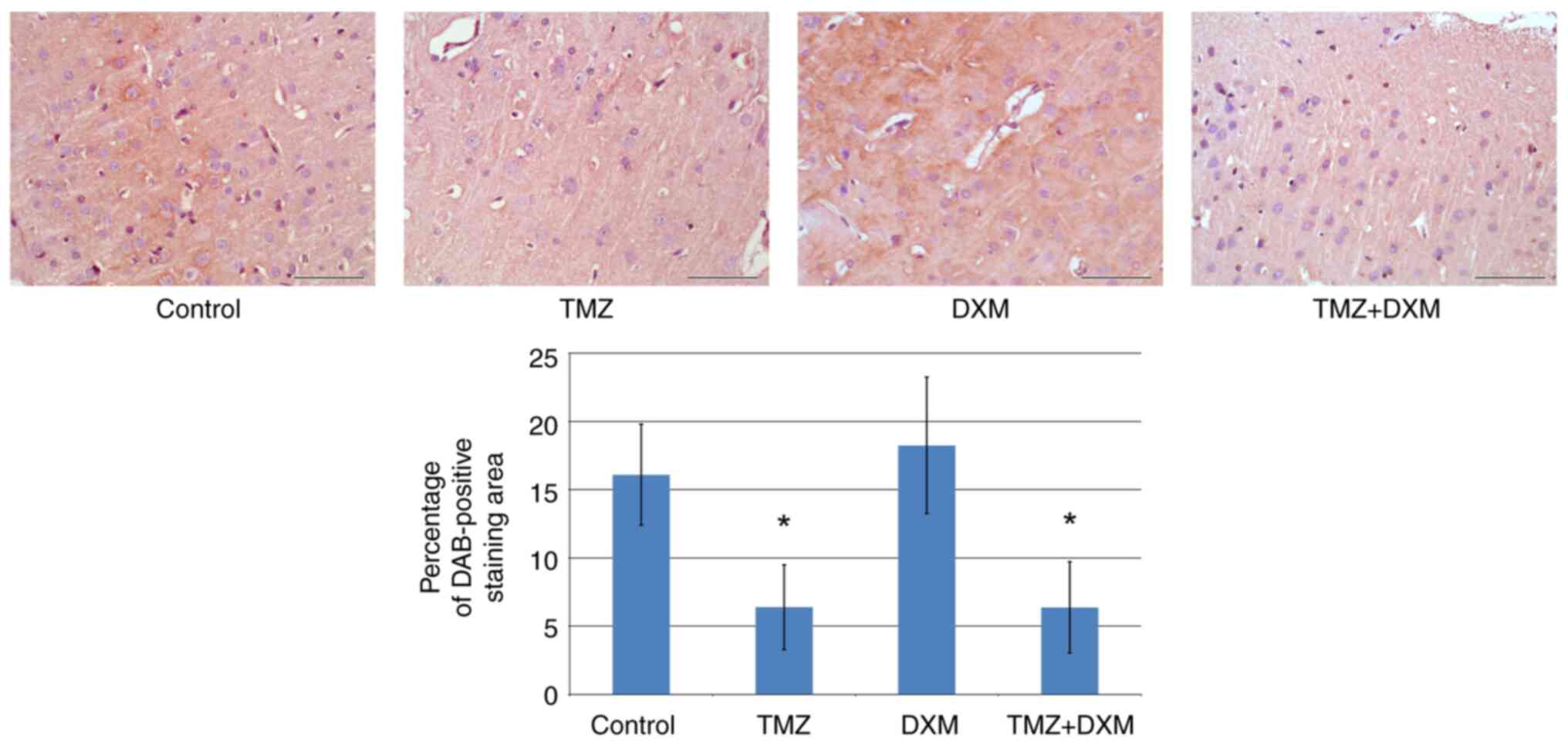
To confirm the validation of these results at the
protein level, IHC staining was performed on the brain sections
derived from these animals with antibodies to aggrecan, decorin and
brevican (Fig. 4).
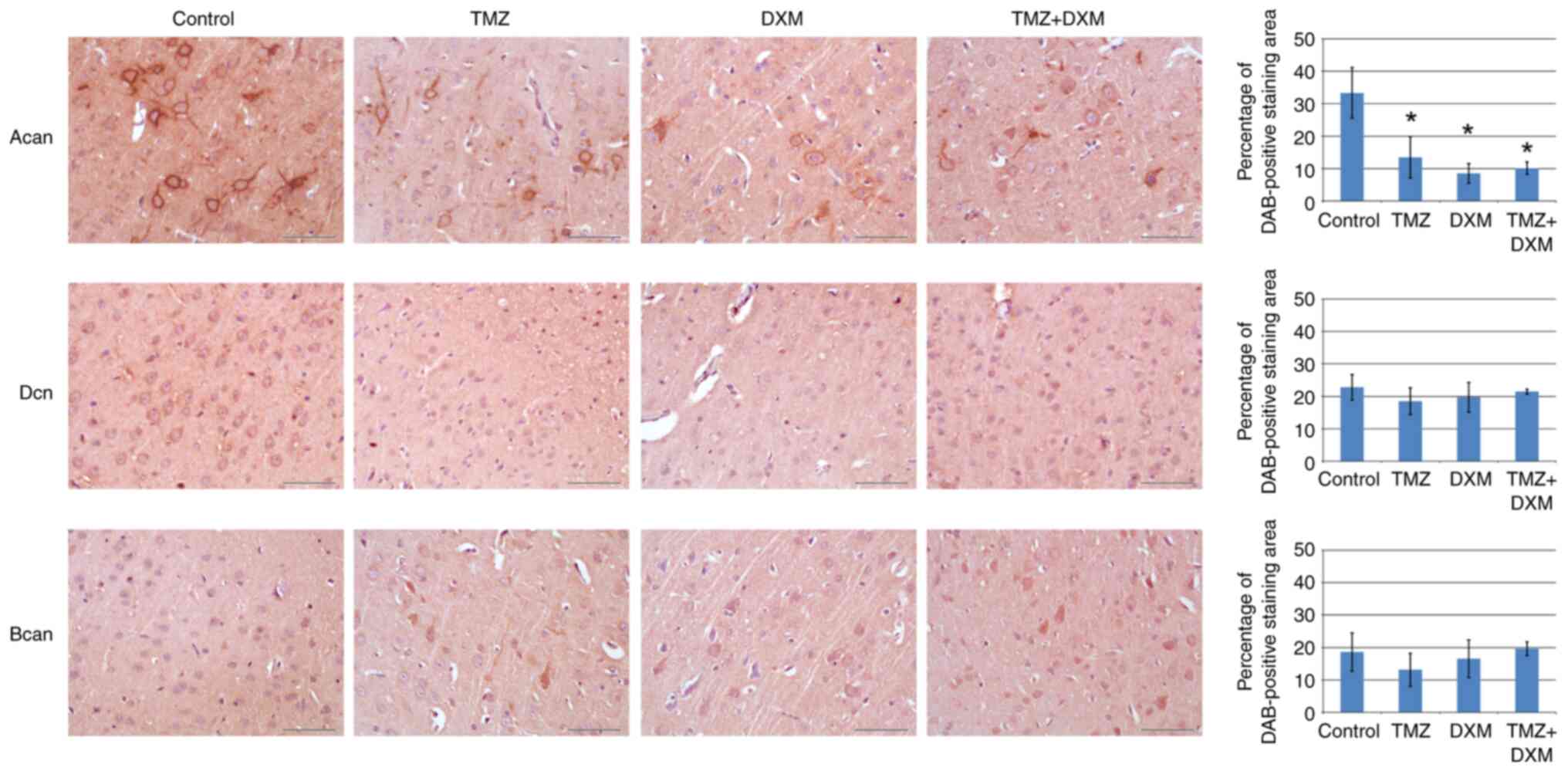 | Figure 4Immunohistochemical analysis of
aggrecan, decorin and brevican content in the control and
TMZ/DXM-treated experimental rats. Quantitative analysis of
aggrecan, decorin and brevican content was performed with ZENblue
software (magnification, x400; scale bar, 50 µm). The bars
represent the mean ± standard deviation from triplicate experiments
(OriginPro 8.5). ANOVA + Fisher's Least Significant Difference
test; *P<0.05. Control, non-treated rat brain tissue;
TMZ, temozolomide; DXM, dexamethasone; Acan, aggrecan; Dcn,
decorin; Bcan, brevican; DAB, 3,3'-diaminobenzidine. |
The protein content of aggrecan, decorin and
brevican corresponded to transcriptional data obtained by RT-qPCR
(Fig. 3), supporting the result
obtained on the significant decrease of aggrecan expression both at
the mRNA and protein levels.
TMZ reduces CS content in rat brain
tissues
Since PGs are complex protein-carbohydrate
molecules, it was important to assess whether changes occurred in
the content of carbohydrate chains of HSPGs and CSPGs. The total HS
and CS contents in the control and TMZ/DXM-treated brain tissues
were investigated by dot-blot analysis with anti-HS and anti-CS
primary antibodies specific for the polysaccharide HS and CS
epitopes, respectively (Fig.
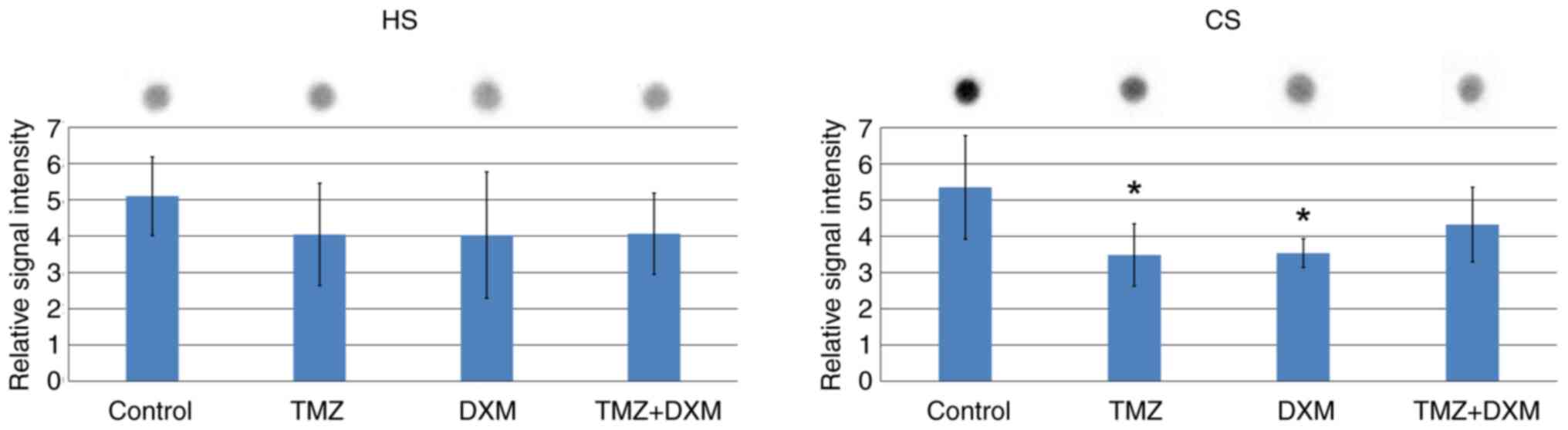
5).
It was revealed that the HS content was not affected
by TMZ and/or DXM treatments (Fig.
5A), whereas the CS content was decreased by 1.5-fold in the
brain tissues derived from the TMZ- and DXM-treated groups
(Fig. 5B). IHC staining supported
the results of dot-blot analysis, demonstrating a significant
(2-2.5-fold) decrease of the CS content in the brain cerebral
cortex following long-term TMZ exposure both as a mono-regimen and
as a combination treatment with DXM (Fig. 6).
Discussion
In the present study, the effects of long-term
administration of TMZ and/or DXM on the anxiety of adult Wistar
rats were assessed. Moreover, the content of the PGs and GAGs in
the brain tissues of these animals were studied. The dose and
regimen of TMZ and DXM treatments were selected to maximally mimic
a chemotherapeutic anti-GBM treatment.
Patients with GBM receive radiotherapy plus TMZ
daily (75 mg/m2), followed by six cycles of TMZ (150-200
mg/m2 for 5 days during each 28-day cycle) (1). For animals, the TMZ amount is
calculated based on the surface area of the animal's body, which is
further calculated by the following formula:
S=(K*W2/3)/10,000, where S is the surface area
(m2), W is the body weight obtained on the day of dosing
(g), and K ¼ 9.0 (constant for estimating surface area) (44,45).
The dose of DXM used to treat patients with GBM is
not clearly regulated. The maximum dose of DXM is 16 mg daily,
administered in 4 equal doses and recommended for symptomatic
patients (46). The dosage range
used in experimental models is considerably wide ranging from 0.1
to 50 mg/kg. A dosage of 2.5 mg/kg was selected in the present
study based on literature data (47-52)
and experimental conditions. Due to the fact that the animals
received the drug for a long time (4 months, 2 times/week), the
moderate DXM dose (2.5 mg/kg) was selected to reduce toxicity.
In the present study, it was shown that long-term
use of TMZ led to an increase in the anxiety of adult rats. The
data are consistent with the results of the study, where TMZ
administration to adult mice (once daily) for three days resulted
in the decrease of the time spent by the mice in open arms and
consequently increased anxiety (17). Treatment of adult two-month-old mice
with TMZ and radiotherapy three times per week for 6 weeks also led
to an increase in anxiety and neurogenesis deficit in the mice of
the EPM test (20). An additional
study indicated that following 5 and 15 weeks of radiochemotherapy,
anxiety-like behavior and anxiety- and depression-like behavior
were observed in 6-month-old mice, respectively (18).
The present study indicated that the long-term use
of TMZ and/or DXM therapy in a Wistar rat model leads to changes in
the brain ECM and increase of anxiety in elderly rats. These data
are in agreement with previous studies reporting that elderly
patients aged 70 years and older who are considered eligible for
combined modality treatment should receive a short-course of
radiotherapy with concomitant and adjuvant TMZ treatment up to 12
cycles (24); in addition, 6
courses of TMZ for elderly patients appear to be sufficient for an
optimal treatment response (53).
Taken together, these data may suggest the reduction in the number
of TMZ cycles that can in turn reduce the toxicity and side effects
of chemotherapy.
Previous studies have suggested the presence of
different neurophysiological mechanisms of behavioral disorders
during and following TMZ chemotherapy that are responsible for
reducing neurogenesis (17,19). Moreover, a decrease in theta
activity in the hippocampal tissues of adults (21) and changes in markers of oxidative
stress (catalase, superoxide dismutase, lipoxygenase and reduced
glutathione) in the hippocampal and frontal cortex regions of the
brain have been proposed as additional mechanisms (22). In the present study, it was
hypothesized that one of the mechanisms responsible for the
behavioral disturbance may be attributed to the changes in the
expression levels of PG core proteins and their CS carbohydrate
chains.
According to the findings of the present study,
long-term administration of TMZ resulted in the decrease of the
expression of aggrecan core proteins present in the cerebral cortex
of adult rats, which could cause impairments in neurogenesis and
plasticity (54). These results are
hard to compare with those reported from previous studies since the
information on this matter is very scarce. It has been revealed
that in 2-month-old Wistar rats, TMZ did not affect PG core protein
expression in the brain tissue at the mRNA level, although certain
changes were noted in the decorin and syndecan-1 protein contents
as well as in the HS/CS content (39). It can be hypothesized that aggrecan
becomes sensitive to long-term TMZ administration in elderly
patients suggesting that this PG can be a potential target for
brain protection at the elder age.
The effects of TMZ and DXM on the polysaccharide CS
chains were studied with dot-blot analysis and IHC. According to
the data of dot-blot analysis, the CS content is decreased after
TMZ and DXM treatment, according to IHC analysis CS content is
decreased after TMZ and TMZ/DXM treatments. As for DXM, there are
differences in the presented data on CS content coming from the
methodological basis of dot-blots and IHC. These methods were
performed with the use of different tissue samples-whole tissue
lysates for dot-blot and paraffin-embedded tissue for IHC.
Additionally, methodology includes different approaches to the
registration of the changes observed-quantification of the total CS
signal (dot-blot) and microphotography of the brain cortex (IHC).
The effects of TMZ on the polysaccharide CS chains have been
investigated in previous studies. In younger (2-month-old) Wistar
rats, TMZ differentially affected CS in different brain zones
increasing the CS content in the hippocampus but not in the brain
cortex (39). Concomitantly, TMZ
administration (by three cycles of 5 consecutive days) to
10-week-old mice decreased the CS-AC content in the cerebral cortex
(40). The results demonstrated in
the current study complement the previous data reported on the CS
content of the brain tissue following TMZ/DXM treatment and
contribute to the identification of a molecular mechanism of CS
sensitivity to systemic chemotherapy.
Taken together, the obtained results demonstrated
that long-term use of TMZ and/or DXM causes behavioral disorders in
adult rats, which are accompanied by changes in the expression of
CSPG aggrecan core protein and in the CS content. This may be a
novel molecular mechanism of long-term side-effects of TMZ.
Prevention of aggrecan loss may be a novel strategy for
neuroprotection and can potentially improve the quality of life of
elderly patients with cancer receiving long-term treatment with
TMZ.
Acknowledgements
The work was performed on the equipment of the
Multi-Access Center ‘Proteomic Analysis’ (https://frcftm.ru/cpk_proto/?lang=en), supported by
funding from the Ministry of Science and Higher Education of the
Russian Federation (agreement No. 075-15-2021-691).
Funding
Funding: The present study was supported by the Ministry of
Science and Higher Education of the Russian Federation (grant no.
122032200240-8), the Russian Science Foundation (grant no.
19-75-00051) and the Scholarship of Russian Federation President
for young scientists (grant no. SP-4000.2022.4).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
EVG and AVS conceptualized the study. EVK, VSU, NVM
and SVA developed methodology. AVS and OPM performed software
analysis. AVS, DKS and VSU validated data. DKS, OPM, EVK, NVM, GMK
and EEK conducted formal analysis. DKS, EVK, VSU, MOP, NVM, GMK,
EEK and SVA conducted investigation. EVG provided resources. AVS
performed data curation. AVS prepared the original draft. EVG
reviewed and edited the manuscript. AVS, GMK and SVA conducted data
visualization. AVS and EVG supervised the study. AVS conducted
project administration. AVS and EVG acquired funding. All authors
have read and approved the final version of the manuscript. VSU and
AVS confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and approved by the
Institutional Ethics Committee of the Institute of Molecular
Biology and Biophysics, Federal Research Center of Fundamental and
Translational Medicine (FRC FTM; approval no. N3/2017 from
23.06.2017; Novosibirsk, Russia).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N\ Engl J Med. 352:987–996. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
McAleavey PG, Walls GM and Chalmers AJ:
Radiotherapy-drug combinations in the treatment of glioblastoma: A
brief review. CNS Oncol. 11(CNS86)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yuen CA, Barbaro M and Haggiagi A: Newly
diagnosed glioblastoma in elderly patients. Curr Oncol Rep.
24:325–334. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wilson MA and Schuchter LM: Chemotherapy
for Melanoma. Cancer Treat Res. 167:209–229. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Harries M, Malvehy J, Lebbe C, Heron L,
Amelio J, Szabo Z and Schadendorf D: Treatment patterns of advanced
malignant melanoma (stage III-IV)-A review of current standards in
Europe. Eur J Cancer. 60:179–189. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Khunger A, Buchwald ZS, Lowe M, Khan MK,
Delman KA and Tarhini AA: Neoadjuvant therapy of locally/regionally
advanced melanoma. Ther Adv Med Oncol.
11(1758835919866959)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pietrantonio F, Randon G, Romagnoli D, Di
Donato S, Benelli M and de Braud F: Biomarker-guided implementation
of the old drug temozolomide as a novel treatment option for
patients with metastatic colorectal cancer. Cancer Treat Rev.
82(101935)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mollazadegan K, Welin S and Crona J:
Systemic treatment of gastroenteropancreatic neuroendocrine
carcinoma. Curr Treat Options Oncol. 22(68)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Scaringi C, De Sanctis V, Minniti G and
Enrici RM: Temozolomide-related hematologic toxicity. Onkologie.
36:444–449. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Le Rhun E, Oppong FB, Vanlancker M, Stupp
R, Nabors B, Chinot O, Wick W, Preusser M, Gorlia T and Weller M:
Prognostic significance of therapy-induced myelosuppression in
newly diagnosed glioblastoma. Neuro Oncol. 24:1533–1545.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gilbar PJ, Pokharel K and Mangos HM:
Temozolomide-induced aplastic anaemia: Case report and review of
the literature. J Oncol Pharm Pract. 27:1275–1280. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ahn GS, Hwang K, Kim TM, Park CK, Chang
JH, Jung TY, Kim JH, Nam DH, Kim SH, Yoo H, et al: Influence of
Concurrent and Adjuvant Temozolomide on Health-Related Quality of
Life of Patients with Grade III Gliomas: A Secondary Analysis of a
Randomized Clinical Trial (KNOG-1101 Study). Cancer Res Treat.
54:396–405. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hilverda K, Bosma I, Heimans JJ, Postma
TJ, Peter Vandertop W, Slotman BJ, Buter J, Reijneveld JC and Klein
M: Cognitive functioning in glioblastoma patients during
radiotherapy and temozolomide treatment: Initial findings. J
Neurooncol. 97:89–94. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lombardi G, Bergo E, Del Bianco P, Bellu
L, Pambuku A, Caccese M, Trentin L and Zagonel V: Quality of life
perception, cognitive function, and psychological status in a
real-world population of glioblastoma patients treated with
Radio-therapy and Temozolomide: A single-center prospective study.
Am J Clin Oncol. 41:1263–1271. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rooney AG, Brown PD, Reijneveld JC and
Grant R: Depression in glioma: A primer for clinicians and
researchers. J Neurol Neurosurg Psychiatry. 85:230–235.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Q, Qi F, Song X, Di J, Zhang L, Zhou
Y, Lu X, Chang J and Yu Y: A prospective longitudinal evaluation of
cognition and depression in postoperative patients with high-grade
glioma following radiotherapy and chemotherapy. J Cancer Res Ther.
14 (Supplement):S1048–S1051. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pereira-Caixeta AR, Guarnieri LO, Medeiros
DC, Mendes EMAM, Ladeira LCD, Pereira MT, Moraes MFD and Pereira
GS: Inhibiting constitutive neurogenesis compromises long-term
social recognition memory. Neurobiol Learn Mem. 155:92–103.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dey D, Parihar VK, Szabo GG, Klein PM,
Tran J, Moayyad J, Ahmed F, Nguyen QA, Murry A, Merriott D, et al:
Neurological impairments in mice subjected to irradiation and
chemotherapy. Radiat Res. 193:407–424. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Egeland M, Guinaudie C, Du Preez A,
Musaelyan K, Zunszain PA, Fernandes C, Pariante CM and Thuret S:
Depletion of adult neurogenesis using the chemotherapy drug
temozolomide in mice induces behavioural and biological changes
relevant to depression. Transl Psychiatry. 7(e1101)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gan H, Zhang Q, Zhu B, Wu S and Chai D:
Fluoxetine reverses brain radiation and temozolomide-induced
anxiety and spatial learning and memory defect in mice. J
Neurophysiol. 121:298–305. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nokia MS, Anderson ML and Shors TJ:
Chemotherapy disrupts learning, neurogenesis and theta activity in
the adult brain. Eur J Neurosci. 36:3521–3530. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pathak N, Cheruku SP, Rao V, Vibhavari
RJA, Sumalatha S, Gourishetti K, Rao CM and Kumar N:
Dehydrozingerone protects temozolomide-induced cognitive impairment
in normal and C6 glioma rats besides enhancing its anticancer
potential. 3 Biotech. 10(438)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Minniti G, Scaringi C, Baldoni A, Lanzetta
G, De Sanctis V, Esposito V and Enrici RM: Health-related quality
of life in elderly patients with newly diagnosed glioblastoma
treated with short-course radiation therapy plus concomitant and
adjuvant temozolomide. Int J Radiat Oncol Biol Phys. 86:285–291.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Minniti G, Lombardi G and Paolini S:
Glioblastoma in elderly patients: Current management and future
perspectives. Cancers (Basel). 11(336)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Suhovskih AV, Molodykh OP, Ushakov VS,
Politko MO, Sokolov DK, Koldysheva EV and Grigorieva EV: Long-Term
exposure to temozolomide affects locomotor activity and cartilage
structure of elderly experimental rats. Biomedicines.
8(541)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Afshari AR, Sanati M, Aminyavari S,
Shakeri F, Bibak B, Keshavarzi Z, Soukhtanloo M, Jalili-Nik M,
Sadeghi MM, Mollazadeh H, et al: Advantages and drawbacks of
dexamethasone in glioblastoma multiforme. Crit Rev Oncol Hematol.
172(103625)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nguyen ET, Streicher J, Berman S, Caldwell
JL, Ghisays V, Estrada CM, Wulsin AC and Solomon MB: A mixed
glucocorticoid/mineralocorticoid receptor modulator dampens
endocrine and hippocampal stress responsivity in male rats.
PhysiolBehav. 178:82–92. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nayana J, Shankaranarayana Rao BS and
Srikumar BN: Mifepristone's effects on depression- and anxiety-like
behavior in rodents. Steroids. 184(109058)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lenze EJ, Hershey T, Newcomer JW, Karp JF,
Blumberger D, Anger J, Doré P and Dixon D: Antiglucocorticoid
therapy for older adults with anxiety and co-occurring cognitive
dysfunction: Results from a pilot study with mifepristone. Int J
Geriatr Psychiatry. 29:962–969. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Mantilla EC Jr, Abramowitz J, Dan TU and
Pan E: Prolonged steroid dependence in adult patients with glioma.
Anticancer Res. 40:2059–2064. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Skupio U, Tertil M, Sikora M, Golda S,
Wawrzczak-Bargiela A and Przewlocki R: Behavioral and molecular
alterations in mice resulting from chronic treatment with
dexamethasone: Relevance to depression. Neuroscience. 286:141–150.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Vafaei AA, Rashidy-Pour A and Taherian AA:
Peripheral injection of dexamethasone modulates anxiety related
behaviors in mice: An interaction with opioidergic neurons. Pak J
Pharm Sci. 21:285–289. 2008.PubMed/NCBI
|
|
33
|
Gómez-Oliva R, Domínguez-García S,
Carrascal L, Abalos-Martínez J, Pardillo-Díaz R, Verástegui C,
Castro C, Nunez-Abades P and Geribaldi-Doldán N: Evolution of
experimental models in the study of glioblastoma: Toward finding
efficient treatments. Front Oncol. 10(614295)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Martin S, Janouskova H and Dontenwill M:
Integrins and p53 pathways in glioblastoma resistance to
temozolomide. Front Oncol. 2(157)2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dong F, Eibach M, Bartsch JW, Dolga AM,
Schlomann U, Conrad C, Schieber S, Schilling O, Biniossek ML,
Culmsee C, et al: The metalloprotease-disintegrin ADAM8 contributes
to temozolomide chemoresistance and enhanced invasiveness of human
glioblastoma cells. Neuro Oncol. 17:1474–1485. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Farace C, Oliver JA, Melguizo C, Alvarez
P, Bandiera P, Rama AR, Malaguarnera G, Ortiz R, Madeddu R and
Prados J: Microenvironmental modulation of decorin and lumican in
temozolomide-resistant glioblastoma and neuroblastoma cancer
stem-like cells. PLoS One. 10(e0134111)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sayegh ET, Kaur G, Bloch O and Parsa AT:
Systematic review of protein biomarkers of invasive behavior in
glioblastoma. Mol Neurobiol. 49:1212–1244. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pibuel MA, Poodts D, Díaz M, Hajos SE and
Lompardía SL: The scrambled story between hyaluronan and
glioblastoma. J Biol Chem. 296(100549)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tsidulko AY, Bezier C, de La Bourdonnaye
G, Suhovskih AV, Pankova TM, Kazanskaya GM, Aidagulova SV and
Grigorieva EV: Conventional Anti-glioblastoma chemotherapy affects
proteoglycan composition of brain extracellular matrix in rat
experimental model in vivo. Front Pharmacol. 9(1104)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Tsidulko AY, Shevelev OB, Khotskina AS,
Kolpakova MA, Suhovskih AV, Kazanskaya GM, Volkov AM, Aidagulova
SV, Zavyalov EL and Grigorieva EV: Chemotherapy-Induced degradation
of glycosylated components of the brain extracellular matrix
promotes glioblastoma relapse development in an animal model. Front
Oncol. 11(713139)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Walf AA and Frye CA: The use of the
elevated plus maze as an assay of anxiety-related behavior in
rodents. Nat Protoc. 2:322–328. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pádua Carobrez A, Kincheski GC and
Bertoglio LJ: Elevated Plus. Maze. In: Encyclopedia of
Psychopharmacology. Stolerman I.P. (ed). Springer, Berlin,
Heidelberg, 2010.
|
|
43
|
Livak KJ and Schmittgen TD: . Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Freireich EJ, Gehan EA, Rall DP, Schmidt
LH and Skipper HE: Quantitative comparison of toxicity of
anticancer agents in mouse, rat, hamster, dog, monkey and man.
Cancer Chemother Rep. 50:219–244. 1966.PubMed/NCBI
|
|
45
|
Reyderman L, Statkevich P, Thonoor CM,
Patrick J, Batra VK and Wirth M: Disposition and pharmacokinetics
of temozolomide in rat. Xenobiotica. 34:487–500. 2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kostaras X, Cusano F, Kline GA, Roa W and
Easaw J: Use of dexamethasone in patients with high-grade glioma: A
clinical practice guideline. Curr Oncol. 21:e493–e503.
2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen X, Lin YP, Wang D and Zhang JN:
Dexamethasone exacerbates spatial acquisition deficits after
traumatic brain injury in rats. Neurol Res. 32:1097–1102.
2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
From the American Association of
Neurological Surgeons (AANS), American Society of Neuroradiology
(ASNR), Cardiovascular and Interventional Radiology Society of
Europe (CIRSE), Canadian Interventional Radiology Association
(CIRA), Congress of Neurological Surgeons (CNS), European Society
of Minimally Invasive Neurological Therapy (ESMINT), European
Society of Neuroradiology (ESNR), European Stroke Organization
(ESO), Society for Cardiovascular Angiography and Interventions
(SCAI), Society of Interventional Radiology (SIR) et al.
Multisociety Consensus Quality Improvement Revised Consensus
Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J
Stroke. 13:612–632. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yao YY, Liu DM, Xu DF and Li WP: Memory
and learning impairment induced by dexamethasone in senescent but
not young mice. Eur J Pharmacol. 574:20–28. 2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Narang VS, Fraga C, Kumar N, Shen J, Throm
S, Stewart CF and Waters CM: Dexamethasone increases expression and
activity of multidrug resistance transporters at the rat
blood-brain barrier. Am J Physiol Cell Physiol. 295:C440–C450.
2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Drakulić D, Veličković N, Stanojlović M,
Grković I, Mitrović N, Lavrnja I and Horvat A: Low-dose
dexamethasone treatment promotes the pro-survival signalling
pathway in the adult rat prefrontal cortex. J Neuroendocrinol.
25:605–616. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Drakulić D, Stanojlović M, Nedeljković N,
Grković I, Veličković N, Guševac I, Mitrović N, Buzadžić I and
Horvat A: Upregulation of nucleoside triphosphate
diphosphohydrolase-1 and ecto-5'-nucleotidase in rat hippocampus
after re-peated low-dose dexamethasone administration. J Mol
Neurosci. 55:959–967. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Huang B, Yu Z and Liang R: Effect of
long-term adjuvant temozolomide chemotherapy on primary
glioblastoma patient survival. BMC Neurol. 21(424)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rowlands D, Lensjø KK, Dinh T, Yang S,
Andrews MR, Hafting T, Fyhn M, Fawcett JW and Dick G: Aggrecan
directs extracellular matrix-mediated neuronal plasticity. J
Neurosci. 38:10102–10113. 2018.PubMed/NCBI View Article : Google Scholar
|