Introduction
Cutaneous melanoma is the most common type of
melanoma and is a malignant tumor. Several subtypes that are
classified according to histological criteria, include superficial
spreading, nodular, lentigo maligna and acral lentiginous melanomas
(1). Although the histological
criteria are widely accepted, histological subtyping plays a very
small role in predicting the behavior and prognosis of the disease
(2). Cutaneous melanomas arise from
melanocytes, which are melanin-producing cells found in the basal
layer of the epidermis. Melanin prevents injury due to ultraviolet
exposure, which is the most important risk factor for cutaneous
melanoma (3). Besides ultraviolet
exposure, genetic mutations are another factor involved in
cutaneous melanoma-genesis (4).
Although cutaneous melanoma accounts for <5% of all skin
cancers, it can develop in individuals of all ages and colors and
is responsible for >70% of skin cancer-related deaths (5). Countries with a high incidence of
cutaneous melanoma are those with Caucasian populations and are
mostly located near the equator, where UV levels are the highest.
Despite its location, Thailand has lesser instances of melanoma,
but a high mortality rate (6).
P53 is a transcription factor that suppresses tumor
growth through cell cycle arrest and apoptosis (7). Mutations in BRAF, CDKN2A and MDMX are
frequently observed in cutaneous melanoma (5,8). BRAF
causes melanoma by dysregulating the activation of downstream
MEK/ERK effectors (9). CDKN2A, a
tumor suppressor gene, encodes p14ARF that inhibits MDM2 mediated
degradation of P53 (4,10). Accordingly, the loss of CDKN2A can
inactivate the P53 pathway. In addition, missense mutations are
common in TP53. Because of their predominant occurrence in the
DNA-binding domain, P53 cannot bind to DNA and regulate
transcription (11). Consequently,
mutations in P53 can disable tumor suppressor activity and confer
oncogenic potential, thereby enhancing cancer cell
proliferation.
Since P53 mutations are commonly found in human
cancers, researchers are attempting to identify chemotherapeutics
to target this mutation precisely. For example, there are
strategies that involve the development of small molecular
compounds and short peptides to restore normal functionality of the
gene (12).
Currently, there is scant data on the frequency of
P53 mutations in cutaneous melanoma, particularly in the Thai
population. A cohort of Thai patients with cutaneous melanoma was
examined. Owing to its simplicity of implementation and widespread
adoption by pathologists, immunohistochemistry (IHC) technique was
utilized as it serves as a widely accepted and reliable surrogate
marker for P53 mutational analysis (13,14).
The clinicopathological features and prognostic variables were
assessed in association with the prevalence of P53 mutations.
Materials and methods
Patient recruitment
The present study was approved (approval no.
1643/2564) by the Ethics Committee of the Faculty of Medicine of
Chulalongkorn University (Med Chula IRB; Bangkok, Thailand).
Written informed consent was signed by all participants. The
exclusion criteria are low amounts of pathologic tis-sue, a lack of
clinical data and poor follow-up. The population of the present
study consisted of 50 patients admitted to the Plastic and
Reconstructive Surgery Unit, Department of Surgery, King
Chulalongkorn Memorial Hospital (Bangkok, Thailand) between 2012
and 2018 (Table I). The median age
was 65.5 years (range, 28-95 years) and 26 patients (52%) were
women. According to the AJCC Cancer Staging Manual, Eighth Edition
(15), 28 of the 50 patients (56%)
were in the advanced stage (III-IV) at initial diagnosis, and 22
(44%) were in the early stage (I-II). Among them, 31 (62%)
presented with ulcers and 19 (38%) did not. Pathological records
were manually screened to record the histological characteristics
of the primary tumor and to identify recurrences and deaths.
Histological subtype analysis revealed that superficial spreading
accounted for 10% (five cases) of the diagnoses, nodular subtype
accounted for 48% (24 cases) and acral lentiginous subtype
accounted for 42% (21 cases). The majority were at Breslow level 4,
followed by Breslow levels 3, 2 and 1 (56, 20, 16 and 8%,
respectively). For survival analysis, all patients were followed-up
from the day of surgery to the day of death, recurrence, or final
follow-up. The median follow-up period for disease-free survival
(DFS) was 14 months (range: 2-53 months), despite the median
follow-up period for overall survival (OS) being 24.5 months
(range: 4-96 months). Owing to the authors' concern about the
relationship between the variables of interest, the possible
variable factors of interest were examined and used in the crude
and adjusted hazard ratio analyses.
 | Table IClinicopathological characteristics of
patients with cutaneous melanoma and P53 status. |
Table I
Clinicopathological characteristics of
patients with cutaneous melanoma and P53 status.
| Clinicopathological
characteristics | n | P53 wild-type | Mutated P53 | P-value |
|---|
| Sex | | | | 0.048a |
|
Male | 24 | 20 | 4 | |
|
Female | 26 | 15 | 11 | |
| Age | | | | 0.355 |
|
≤65.5 | 25 | 19 | 6 | |
|
>65.5 | 25 | 16 | 9 | |
| Histological
subtype | | | | 0.001b |
|
Superficial
spreading | 5 | 4 | 1 | |
|
Nodular | 24 | 11 | 13 | |
|
Acral
lentiginous | 21 | 20 | 1 | |
| Breslow level | | | | 0.006a |
|
1 | 4 | 4 | 0 | |
|
2 | 8 | 8 | 0 | |
|
3 | 10 | 9 | 1 | |
|
4 | 28 | 14 | 14 | |
| Tumor stage | | | | 0.804 |
|
Early stage
(I-II) | 22 | 15 | 7 | |
|
Advanced
stage (III-IV) | 28 | 20 | 8 | |
| Ulcer | | | | 0.086 |
|
Presence | 31 | 19 | 12 | |
|
Absence | 19 | 16 | 3 | |
| Staging node | | | | 0.308 |
|
0 | 24 | 16 | 8 | |
|
1 | 13 | 8 | 5 | |
|
2 | 6 | 4 | 2 | |
|
3 | 7 | 7 | 0 | |
|
Recurrencec | | | | 0.217 |
|
Recurrent | 29 | 18 | 11 | |
|
Not
Recurrent | 19 | 15 | 4 | |
| Deathc | | | | 0.869 |
|
Dead | 27 | 19 | 8 | |
|
Alive | 22 | 15 | 7 | |
| Total | 50 | 35 | 15 | |
IHC staining
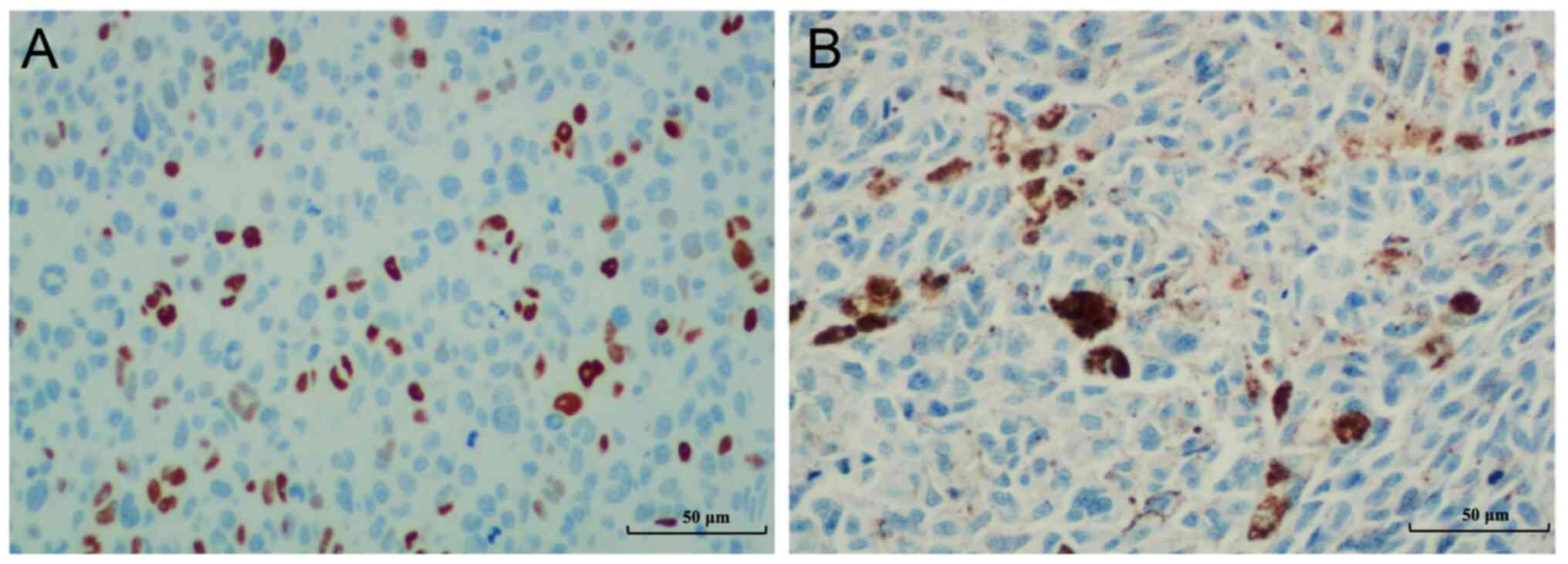
The anti-P53 monoclonal DO7 mouse antihuman antibody
(cat. no. GA61661-2; Dako; Agilent Technologies, Inc.) was applied
to 3-µm-thick paraffin-embedded tissue slides. IHC was performed
using an automated immunostainer (BenchMark XT, Ventana Medical
Systems; Roche Tissue Diagnostics; Roche Diagnostics, Ltd.),
following the manufacturer's instructions. Based on the previous
studies, positive IHC staining was considered to be the mutated
form of P53, whereas negative IHC staining for P53 was determined
to be the wild-type (13,14). A total of >10% of the strong and
homogeneous nucleus-stained tumor cell samples were considered
positive, whereas the samples with absent nuclear staining of tumor
cells were classified as negative. Representative images from each
category are shown in Fig. 1. Tumor
cells with scattered melanin pigments were identified and not
counted for P53 staining.
Statistical analysis
Chi-square or Fisher's exact tests were used (as
appropriate) to assess significant differences in the distribution
of mutated P53 staining. For comparisons between more than two
groups, Two way analysis of variance (ANOVA) was used. Kaplan-Meier
analysis was used to investigate the DFS and OS. Crude and adjusted
hazard ratios were calculated using a Cox regression model. The
significance level was set at a two-tailed P-value of less than
0.05. SPSS Statistics ver. 23.0 (IBM Corp.) was used for all
statistical analyses.
Results
Association of the mutated P53 with
clinicopathological characteristics
IHC staining for P53 was positive in 15 cases (30%),
which were considered to be mutant P53 (Fig. 1A). Negative P53 IHC staining was
determined to be the wild-type (Fig.
1B). The association between clinicopathological
characteristics and P53 IHC status is summarized in Table I. Among the 9 variables, only 3
showed statistical significance for association with the P53
mutation: sex (P=0.048), Breslow level (P=0.006), and histological
subtype (P=0.001). Cases with mutated P53 were considerably more
frequent in female patients (11 of 26 cases, 42.31%) than in male
patients (4 of 24 cases, 16.67%). Furthermore, Breslow level 4
cases had a higher prevalence of mutated P53 compared with Breslow
level 1-3 cases. Of the samples from 28 Breslow level 4 cases, 14
(50%) exhibited positive P53 IHC results, whereas only 1 of 22
cases (4.55%) with Breslow level 1-3 was positive for P53 staining.
As for histological subtype, positive P53 IHC was mostly observed
in the nodular subtype (13 of 24 cases, 54.2%), followed by
superficial spreading (1 of 5 cases, 20%) and acral lentiginous (1
of 21 cases, 4.76%). Other clinicopathological variables, including
age, tumor stage and clinical presence of ulcers, were not
significantly associated with mutated P53. Normal tissues adjacent
to melanoma were negative for P53 using IHC staining (Fig. S1).
DFS
During the follow-up period, 60.4% patients with
cutaneous melanoma experienced disease recurrence. Moreover,
mutated P53 and advanced tumor stage demonstrated higher crude
hazard ratios (HR) of 1.140 [95% Confidence Interval (CI):
0.527-2.469] and 1.249 (95% CI: 0.548-2.851), respectively
(Table II). Although the
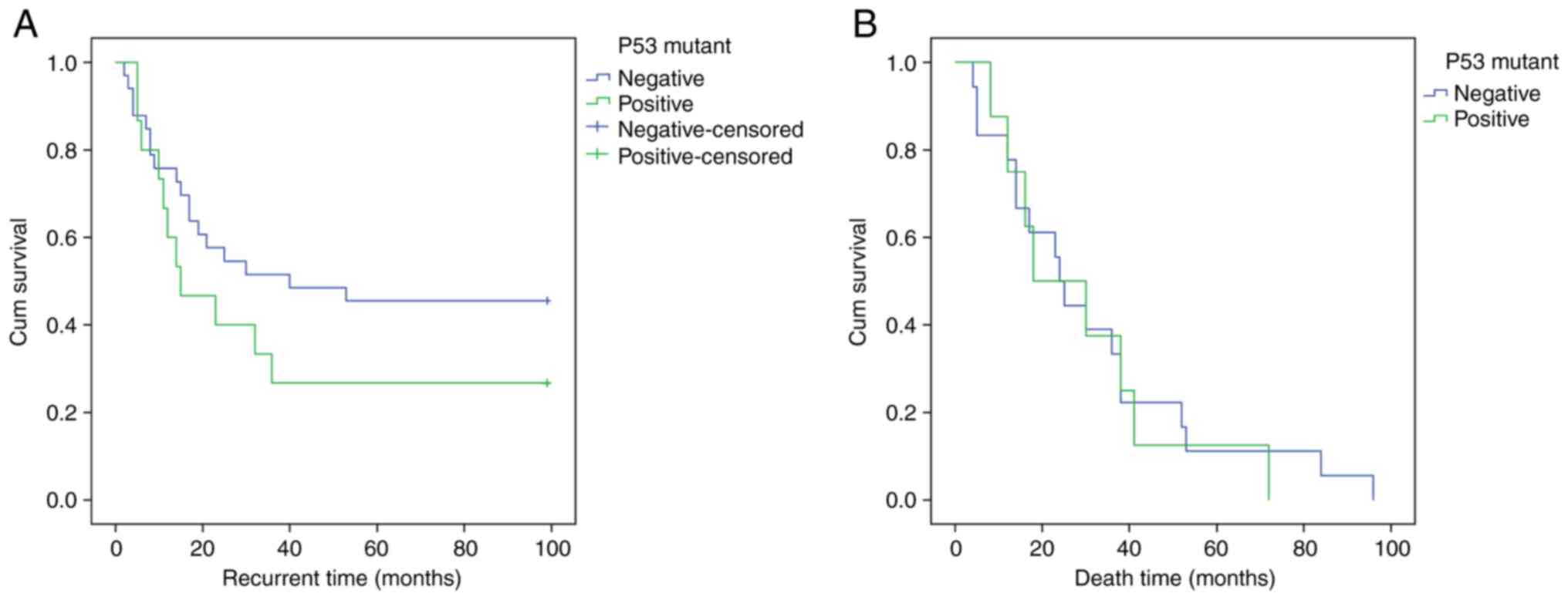
Kaplan-Meier curve revealed that patients with mutated P53 had a
shorter DFS time, the difference was not statistically significant
(P=0.739, Fig. 2A).
 | Table IIUnivariate and multivariate analysis
of DFS and P53 status in cutaneous melanoma patients. |
Table II
Univariate and multivariate analysis
of DFS and P53 status in cutaneous melanoma patients.
| | Crude hazard
ratio |
|---|
|
Characteristics | Recurrence, N
(%) | Hazard ratio | 95% Confidence
interval | P-value |
|---|
| Sex | | | | |
|
Male | 11 (22.4) | 1 | | |
|
Female | 18 (37.5) | 1.080 | 0.503-2.316 | 0.844 |
| Age | | | | |
|
≤65.5 | 12(25) | 1 | | |
|
>65.5 | 17 (35.4) | 0.696 | 0.326-1.489 | 0.351 |
| Histological
subtype | | | | |
|
Superficial
spreading | 4 (8.3) | 1 | | |
|
Nodular | 16 (33.3) | 0.961 | 0.315-2.931 | 0.944 |
|
Acral
lentiginous | 9 (18.8) | 0.431 | 0.123-1.513 | 0.189 |
| P53 | | | | |
|
P53 wild
type | 18 (37.5) | 1 | | |
|
Mutated
P53 | 11 (22.9) | 1.140 | 0.527-2.469 | 0.739 |
| Breslow level | | | | |
|
1 | 1 (2.1) | 1 | | |
|
2 | 3 (6.3) | 1.000 |
0.000-49486.161 | 1.000 |
|
3 | 5 (10.4) | 1.000 |
0.000-47365.258 | 1.000 |
|
4 | 20 (41.7) | 1.000 |
0.000-46358.619 | 1.000 |
| Tumor stage | | | | |
|
Early stage
(I-II) | 9 (18.8) | 1 | | |
|
Advanced
stage (III-IV) | 20 (41.7) | 1.249 | 0.548-2.851 | 0.597 |
| Ulcer | | | | |
|
Absence | 8 (16.7) | 1 | | |
|
Presence | 21 (43.75) | 1.342 | 0.565-3.185 | 0.505 |
| Staging node | | | | |
|
0 | 11 (22.9) | 1 | | |
|
1 | 8 (16.7) | 1.388 | 0.545-3.539 | 0.492 |
|
2 | 5 (10.4) | 0.916 | 0.307-2.734 | 0.875 |
|
3 | 5 (10.4) | 2.669 | 0.870-8.195 | 0.086 |
| Total | 29 (60.4) | | | |
OS
The crude and adjusted HR analyses for mortality in
patients with cutaneous melanoma are listed in Table III. The overall mortality rate in
the present study was 55.1%. The two significant variables were age
(P=0.023) and Breslow level (P=0.035). Patients over the age of
65.5 years had significantly longer survival times than younger
patients, with a crude HR of patient mortality of 0.368 (95% CI:
0.156-0.869). Remarkably, this result was very close to the
adjusted HR of 0.383 (95% CI, 0.156-0.937). Another significant
discovery in patients with Breslow level 4 was that their primary
tumor had a significantly lower HR for mortality of 0.073 (95% CI:
0.006-0.827) with a P-value of 0.035. After two years of follow-up,
the Kaplan-Meier curve revealed no difference between the OS time
associated with mutated P53 and wild type P53 (P=0.796, Fig. 2B).
 | Table IIIUnivariate analysis of overall
survival and P53 status in cutaneous melanoma patients. |
Table III
Univariate analysis of overall
survival and P53 status in cutaneous melanoma patients.
| | Crude hazard
ratio | Adjusted hazard
ratio |
|---|
|
Characteristics | Death (N, %) | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex | | | | | | | |
|
Male | 9 (18.4) | 1 | | | | | |
|
Female | 18 (36.7) | 1.003 | 0.441-2.277 | 0.995 | - | - | - |
| Age | | | | | | | |
|
≤65.5 | 12 (24.5) | 1 | | | 1 | | |
|
>65.5 | 15 (30.6) | 0.368 | 0.156-0.869 | 0.023a | 0.383 | 0.156-0.937 | 0.036a |
| Histological
subtype | | | | | | | |
|
Superficial
spreading | 4 (8.2) | 1 | | | | | |
|
Nodular | 15 (30.6) | 1.141 | 0.342-3.807 | 0.830 | - | - | - |
|
Acral
lentiginous | 8 (16.3) | 0.625 | 0.173-2.260 | 0.474 | - | - | - |
| P53 | | | | | | | |
|
P53 wild
type | 19 (38.8) | 1 | | | | | |
|
Mutated
P53 | 8 (16.3) | 1.119 | 0.478-2.620 | 0.796 | - | - | - |
| Breslow level | | | | | | | |
|
1 | 1 (2.0) | 1 | | | 1 | | |
|
2 | 4 (8.2) | 0.097 | 0.008-1.254 | 0.074 | 0.161 | 0.012-2.121 | 0.165 |
|
3 | 5 (10.2) | 0.104 | 0.008-1.343 | 0.083 | 0.130 | 0.010-1.672 | 0.117 |
|
4 | 17 (34.7) | 0.073 | 0.006-0.827 | 0.035a | 0.112 | 0.010-1.299 | 0.080 |
| Tumor stage | | | | | | | |
|
Early stage
(I-II) | 9 (18.4) | 1 | | | | | |
|
Advanced
stage (III-IV) | 18 (36.7) | 1.910 | 0.775-4.709 | 0.160 | - | - | - |
| Ulcer | | | | | | | |
|
Absence | 9 (18.4) | 1 | | | | | |
|
Presence | 18 (36.7) | 1.592 | 0.673-3.766 | 0.290 | - | - | - |
| Staging node | | | | | | | |
|
0 | 11 (22.5) | 1 | | | | | |
|
1 | 5 (10.2) | 1.960 | 0.587-6.544 | 0.274 | - | - | - |
|
2 | 6 (12.2) | 1.585 | 0.557-4.513 | 0.388 | - | - | - |
|
3 | 5 (10.2) | 1.814 | 0.587-5.608 | 0.301 | - | - | - |
| Total | 27 (55.1) | | | | | | |
Discussion
Wiriyakulsit et al (6), reported the incidence and mortality
rates of melanoma to be 0.52 and 0.25 per 100,000 individuals,
respectively (6). Although there
were few melanoma cases in Thailand, the death rate was significant
at 48.08%. This is in concurrence with the findings of the present
study, indicating the overall death rate as 55.1%.
The prevalence of P53 mutation in cutaneous melanoma
has received little attention, and there has been no prior research
on Thai patients. A study from Europe found that 17 out of 81 (21%)
cutaneous melanoma specimens harbored a P53 mutation (16). On similar lines, a systematic review
of reported cases in the United States found 68 of the 575 cases
(11.9%) analyzed were with the P53 mutation in cutaneous melanoma
(17). The present study revealed
that, P53 mutations were discovered in 30% of the participants,
which is higher than that previously reported. This higher
occurrence of cutaneous melanoma P53 mutation in Thailand
demonstrated the relationship between the two and could potentially
lead to the development of effective and optimum targeted
treatment.
Since melanoma originates from melanocytes, the
present study did not involve a direct comparison of melanoma cells
with normal melanocytes. This decision was primarily due to the
small proportion of melanocytes present in normal skin and the
difficulty in identifying melanocytes in H&E sections, which
typically require special staining techniques. However, during the
present's study inspection of the normal epithelium next to the
melanoma, no cells were identified that tested positive for the
anti-P53 antibody, as illustrated in Fig. S1. This particular finding confirms
the absence of P53 mutations in normal skin.
Furthermore, it was revealed that the P53 mutation
had a significant association with some clinicopathological
characteristics including female sex, nodular subtype and Breslow
level 4. However, it was not associated with age, tumor stage,
ulcer presentation and node stage. These clinicopathological
results have not been widely reported and varied across individual
studies. To the best of the authors' knowledge, a study in the
United States reported that P53 mutation is associated with older
age (18), which contrasts with the
present study. However, in line with the findings of the present
study, another study suggested no association between P53 mutation
and ulceration (18). These
controversies could be resolved in an improved way with larger
sample sizes. Further molecular testing of other methods, such as
next-generation sequencing could also be useful, as a report of
inter-method discrepancy exists (19).
Although the prevalence of mutated P53 in cutaneous
melanoma was moderate (30%), the rates of recurrence and death in
patients with P53-mutated cutaneous melanoma in the present study
were relatively high (60.5 and 55.1%, respectively). Furthermore,
the duration for recurrence in cases with the P53 mutation was
lower than that for wild-type P53. This poor clinical outcome may
stem from the dysfunction of mutated P53 itself and its interaction
with other oncogenic genes, especially BRAF, which was shown
in a study by Celesia et al (20) to be preferentially interacting with
mutated P53 than the wild-type. In the present study, it was also
revealed that the mortality rate associated with mutated P53
significantly increased with advanced age and Breslow level 4. This
is consistent with previous studies that found that older age was
strongly associated with higher mortality rates (21,22).
This association between mutated P53 and the
prognosis of cutaneous melanoma needs to be studied further to
elucidate the underlying mechanisms and develop an effective and
ideal targeted therapy to counter the resistance of melanoma to
current regimens. Accumulating evidence supports the idea that P53
plays an important role in the tumor suppression in multiple types
of cancer, including skin cancer (5). Stabilization and activation of P53,
known as the guardian of the genome, results in cell cycle arrest,
DNA repair, and/or apoptosis in severely and persistently damaged
tumor cells (5,11). Understanding the relationship
between P53 and other proteins such as MDMX, MDM2, BRAF, and
melanoma, is important. Thus, targeted therapy aimed at P53 is
currently promising, but also challenging, for the treatment of
cutaneous melanoma and other skin cancers in future (23).
In addition to BRAF, P53-targeted therapy is novel
and promising in this new era, and a number of researchers are
attempting to uncover its secrets. P53 is a key target of numerous
novel chemotherapeutics but is generally ineffective as a
stand-alone agent (24).
Therapeutic outcomes can be achieved by combining a drug with P53
activation and MDMX-MDM2, BRAF, or MEK inhibitors (5,11). The
drug currently under study, known as PRIMA-1MET,
activates P53, thereby leading to tumor suppression, and helps
sensitize melanoma cells to other targeted therapies. Furthermore,
the combination of PRIMA-1MET and pimasertib noticeably
promoted apoptosis in melanoma cells (5).
The limited sample size of the present study is a
key limitation because it restricts the extent to which the results
can be applied, including in terms of ethnicity. Thailand is a
multiethnic society; however, due to a strong homogenizing culture,
most Thai nationals identify as ethnic Tai, including in the
patient registry, regardless of their actual ethnic background.
This predicament obscures the genetic diversity in the research of
the present study. Therefore, further research with a larger sample
size is crucial to validate and reinforce these results. It is also
suggested to collect additional information on more lifestyle
factors related to melanoma for analysis, such as sunlight exposure
and the use of sun protective products.
In conclusion, the findings of the present study
revealed that cutaneous melanomas corresponded to P53 mutation,
recurrence rates and mortality rates. The addition of cancer cells
to mutated P53 still makes it an attractive target for melanoma
therapy. It is considered that these findings will be one of the
initial steps towards understanding disease incidence and
prognostic factors and promoting the innovation of future curative
therapies for melanoma. Consequently, additional studies with
larger populations should be conducted to investigate novel
targeted therapies.
Supplementary Material
An example of normal skin above the
melanoma. The normal skin exhibits negative staining for the P53
antibody.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the
Ratchadapiseksompotch Fund (grant no. RA67/022; Faculty of
Medicine, Chulalongkorn University).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors conceptualized the present study and
performed formal analysis. JM, KR and NK acquired funding. WS, SK,
TS, KR and NK developed methodology. JM and NK supervised the
study, performed project administration, provided resources and
conducted software analysis. JM, TS, KR and NK validated data. JM,
CM, PT, WS and SK performed data visualization. JM, CM, PT, WS, KR
and NK conducted investigation. JM, KR and NK confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved approval no.
1643/2564 by the Ethics Committee of the Faculty of Medicine of
Chulalongkorn University (Med Chula IRB; Bangkok, Thailand).
Written informed consent was signed by all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hassel JC and Enk AH: Melanoma. In: Kang
S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ and
Orringer JS (eds). Fitzpatrick's Dermatology. 9th edition. McGraw
Hill, New York, NY, pp1982-2011, 2019.
|
|
2
|
Smoller BR: Histologic criteria for
diagnosing primary cutaneous malignant melanoma. Mod Pathol. 19
(Suppl 2):S34–S40. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bandarchi B, Ma L, Navab R, Seth A and
Rasty G: From melanocyte to metastatic malignant melanoma. Dermatol
Res Pract. 2010(583748)2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu Y and Sheikh MS: Melanoma: Molecular
pathogenesis and therapeutic management. Mol Cell Pharmaco.
6(228)2014.PubMed/NCBI
|
|
5
|
Loureiro JB, Abrantes M, Oliveira PA and
Saraiva L: P53 in skin cancer: From a master player to a privileged
target for prevention and therapy. Biochim Biophys Acta Rev Cancer.
1874(188438)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wiriyakulsit N, Klomkleang P, Sornda T and
Kaewkong W: Melanoma: Incidence among the thai population and the
use of a molecular understanding of this cancer to improve the
strategy of targeted therapy. Thai Cancer J. 41:134–150. 2021.
|
|
7
|
Sullivan KD, Galbraith MD, Andrysik Z and
Espinosa JM: Mechanisms of transcriptional regulation by p53. Cell
Death Differ. 25:133–143. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Meevassana J, Anothaisatapon K, Subbalekha
S, Kamolratanakul S, Siritientong T, Ruangritchankul K, Pungrasami
P, Hamill KJ, Angsapatt A and Kitkumthorn N: BRAF V600E
immunohistochemistry predicts prognosis of patients with cutaneous
melanoma in Thai population. Plast Reconstr Surg Glob Open.
10(e4605)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ascierto PA, Kirkwood JM, Grob JJ, Simeone
E, Grimaldi AM, Maio M, Palmieri G, Testori A, Marincola FM and
Mozzillo N: The role of BRAF V600 mutation in melanoma. J Transl
Med. 10(85)2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shadfan M, Lopez-Pajares V and Yuan ZM:
MDM2 and MDMX: Alone and together in regulation of p53. Transl
Cancer Res. 1:88–89. 2012.PubMed/NCBI
|
|
11
|
Rivlin N, Brosh R, Oren M and Rotter V:
Mutations in the p53 tumor suppressor gene: Important milestones at
the various steps of tumorigenesis. Genes Cancer. 2:466–474.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu G, Pan C, Bei JX, Li B, Liang C, Xu Y
and Fu X: Mutant p53 in cancer progression and targeted therapies.
Front Oncol. 10(595187)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yemelyanova A, Vang R, Kshirsagar M, Lu D,
Marks MA, Shih IeM and Kurman RJ: Immunohistochemical staining
patterns of p53 can serve as a surrogate marker for TP53 mutations
in ovarian carcinoma: An immunohistochemical and nucleotide
sequencing analysis. Mod Pathol. 24:1248–1253. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Singh N, Piskorz AM, Bosse T,
Jimenez-Linan M, Rous B, Brenton JD, Gilks CB and Köbel M: p53
immunohistochemistry is an accurate surrogate for TP53 mutational
analysis in endometrial carcinoma biopsies. J Pathol. 250:336–345.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Keung EZ and Gershenwald JE: The eighth
edition American joint committee on cancer (AJCC) melanoma staging
system: implications for melanoma treatment and care. Expert Rev
Anticancer Ther. 18:775–784. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zerp SF, van Elsas A, Peltenburg LT and
Schrier PI: p53 mutations in human cutaneous melanoma correlate
with sun exposure but are not always involved in melanomagenesis.
Br J Cancer. 79:921–926. 1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hocker T and Tsao H: Ultraviolet radiation
and melanoma: A systematic review and analysis of reported sequence
variants. Hum Mutat. 28:578–588. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim DW, Haydu LE, Joon AY, Bassett RL Jr,
Siroy AE, Tetzlaff MT, Routbort MJ, Amaria RN, Wargo JA, McQuade
JL, et al: Clinicopathological features and clinical outcomes
associated with TP53 and BRAFNon-V600 mutations in
cutaneous melanoma patients. Cancer. 123:1372–1381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rusu S, Verocq C, Trepant AL, Maris C, De
Nève N, Blanchard O, Van Campenhout C, De Clercq S, Rorive S, Cotoi
OS, et al: Immunohistochemistry as an accurate tool for the
assessment of BRAF V600E and TP53 mutations in primary and
metastatic melanoma. Mol Clin Oncol. 15(270)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Celesia A, Franzò M, Di Liberto D,
Lauricella M, Carlisi D, D'Anneo A, Notaro A, Allegra M, Giuliano M
and Emanuele S: Oncogenic BRAF and p53 interplay in melanoma cells
and the effects of the HDAC inhibitor ITF2357 (Givinostat). Int J
Mol Sci. 24(9148)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tsai S, Balch C and Lange J: Epidemiology
and treatment of melanoma in elderly patients. Nat Rev Clin Oncol.
7:148–152. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iglesias-Pena N, Paradela S,
Tejera-Vaquerizo A, Boada A and Fonseca E: Cutaneous melanoma in
the elderly: Review of a growing problem. Actas Dermosifiliogr
(Engl Ed). 110:434–447. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jenkins RW and Fisher DE: Treatment of
advanced melanoma in 2020 and beyond. J Invest Dermatol. 141:23–31.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Box NF, Vukmer TO and Terzian T: Targeting
p53 in melanoma. Pigment Cell Melanoma Res. 27:8–10.
2014.PubMed/NCBI View Article : Google Scholar
|