Introduction
Laryngeal cancer is one of the most common head and
neck cancers. Based on the Global Burden of Disease study between
1990 and 2017, the incidence of laryngeal cancer increased by
58.67%, from 132,740 to 210,610 cases. The death- and
disability-adjusted life-years were also increased by 33.84 and
25%, respectively (1). The most
common diagnostic pathology is laryngeal squamous cell carcinoma
(LSCC) (2). Treatment modality
depends on various factors, including Tumor-Node-Metastasis (TNM)
classification, and health and financial status (3). In early stage LSCC, three main options
can be selected in single modality: Radiotherapy, transoral laser
microsurgery and open partial laryngectomy. In advanced stage LSCC,
combined modalities are applied; surgery and postoperative
radiotherapy have long been the most common options (4). At present, numerous options have
become available. Conservation laryngeal surgery and
chemoradiotherapy are useful options as part of individualized
treatment. The treatment modalities were considered based on host
factor, tumor factor, surgeon factor, institutional factor,
academic research center and financial factor. The outcome of every
treatment approach can impact functional impairments, long-term
morbidity and quality of life. Recurrent disease also reduces
overall survival (OS) and disease-specific survival (DSS) (5).
Tumorigenesis is universally investigated. Tumor
formation includes 10 major characteristics: Unlimited
multiplication, evasion from growth suppressors, promoting invasion
and metastasis, resisting apoptosis, stimulating angiogenesis,
maintaining proliferative signaling, elimination of cell energy
limitation, evading immune destruction, genome instability and
mutation, and tumor-enhanced inflammation (6,7).
However, some clinical characteristics of tumor recurrence remain
unknown. In clinical practice, the TNM classification does not
indicate the responsiveness of treatment and tumor recurrence. The
expression of a number of proteins can be used for prognosis.
However, a standardized prognostic protein expression is not yet
established to assist in decision-making (4,8,9) For
example, epidermal growth factor (EGFR) is a member of the ErbB
family of tyrosine kinase receptors. It is thought to play a major
role in enhancing tumor growth, invasion and metastasis. Studies in
LSCC show an association between high EGFR expression and poor
prognosis, including OS (10,11).
By contrast, other studies revealed that EGFR overexpression is
associated with longer OS (12,13).
Ki-67 is a nuclear protein which can be detected in all cell cycle
phases except G0. It is associated with proliferation of normal and
neoplastic cells. In immunohistochemistry, it is considered an
important prognostic marker in various cancers including lung,
brain, breast, prostate, esophagus and kidney cancer (14,15).
Ki-67 in LSCC shows association between higher expression and
advanced clinical stage, pathological characteristics, nodal
metastasis and also shorter survival (16-19).
By contrast, patients with LSCC and higher Ki-67 expression have
notably improved response to radiotherapy compared with those
patients with lower Ki-67 expression (20). The current study aimed to identify
prognostic factors based on protein expression to predict recurrent
LSCC.
Materials and methods
Ethical process
All protocols in the current study were approved by
the Institutional Review Board (IRB) of the Faculty of Medicine,
Chulalongkorn University (approval no. IRB 889/63; certificate of
approval, Faculty of Medicine, Chulalongkorn University no.
0158/2022; Bangkok, Thailand) which also covered any research
conducted at King Chulalongkorn Memorial Hospital with the 1964
Declaration of Helsinki. Written informed consent was obtained from
all individual participants included in the present study. Patients
diagnosed with LSCC through histological confirmation by
pathologists (K.R., N.K. and S.K.) were selected for inclusion in
the present study. The clinical data of the patients were obtained
from the Department of Otolaryngology, Head and Neck Surgery, King
Chulalongkorn Memorial Hospital between January 2009 and December
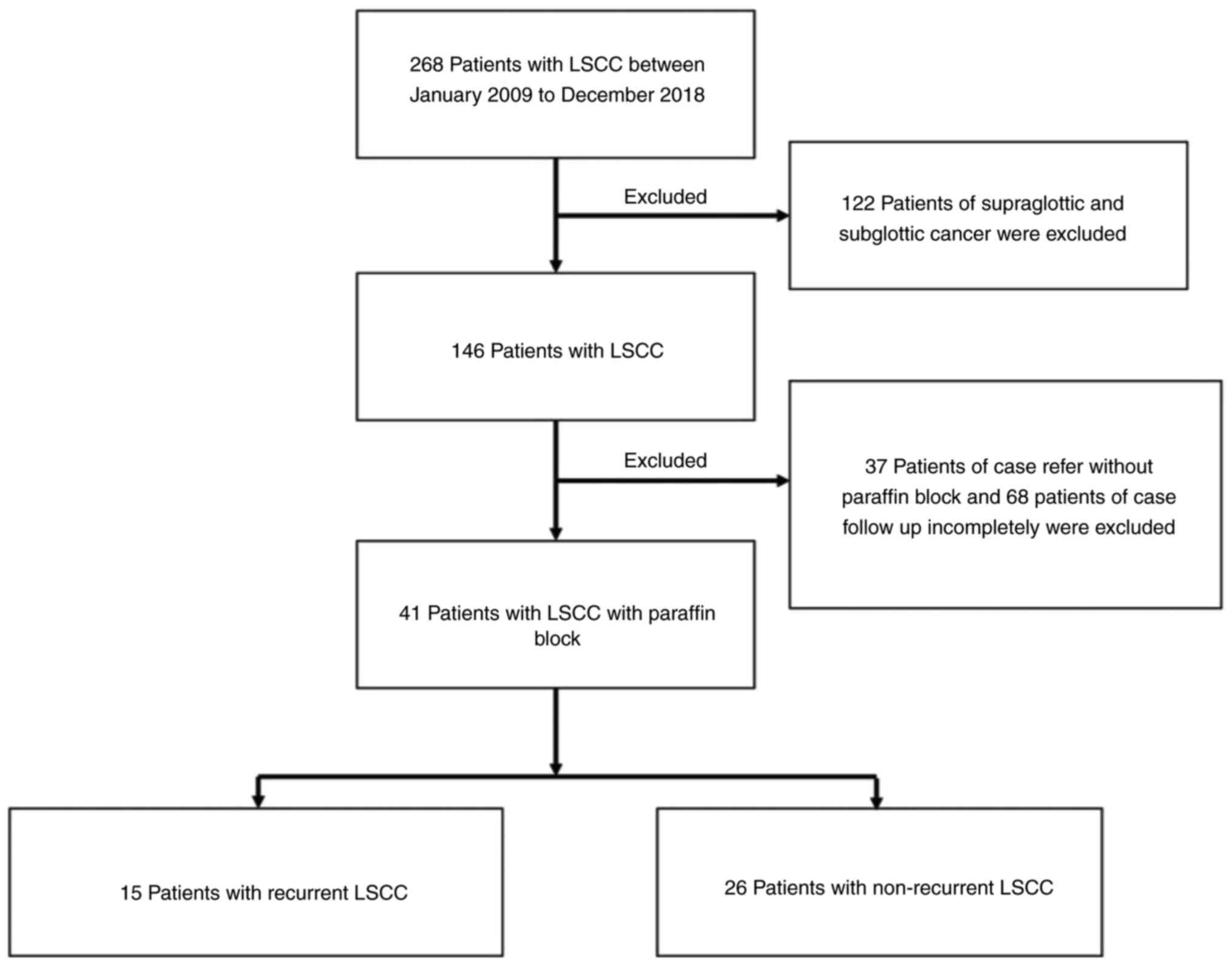
2018. The medical records of a total of 268 patients with LSCC were
assessed for inclusion in the present study. The exclusion criteria
included: i) Supraglottic in origin (122 cases); ii) referral
without paraffin block (37 cases); and iii) incomplete follow-up
(68 cases). Follow-up was done every 2 months during year 1 after
treatment, every 3 months in year 2 and every 6 months from year 3
to 5. The recurrence times were calculated from the last day of
treatment completion to the date of recurrence. Finally, 41
patients were included in the present study. There were 15 cases of
recurrent LSCC and 26 of non-recurrent LSCC (Fig. 1). The demographic data of the
patients, including age, sex, staging, histological grading, median
recurrence time and rate were retrieved from the clinical chart
records. TNM stage was categorized according to the 8th edition
American Joint Committee on Cancer TNM Staging System (3).
An immunohistochemical technique was used to
investigate the prognostic proteins between recurrent and
non-recurrent LSCC. Antibodies were selected based on literature
reviews and adapted from tumorigenesis (6). Cell cycle, proliferation signal
maintenance, cell replication and survival, and cell-resistant
apoptosis were considered in the experiments of the present study.
Furthermore, the mechanism of recurrence was considered to be
through angiogenesis stimulation, tumor invasion and metastasis,
and evasion from growth suppressors. Finally, antibodies of Ki-67,
pRb, cyclin D1, p16, c-Met, PI3K, HIF-1α, VEGF, β-catenin, p53,
RPA32, CD44, BAX, BAK, Bcl-xl and Bcl-2 were examined (Fig. S1).
Immunohistochemistry
Laryngeal tissue was fixed in 4% paraformaldehyde
for 8 h at room temperature, then embedded in paraffin and sliced
into 3-µm thick sections. The sections were stained first with
Mayer's hematoxylin (cat. no. C0303; Diapath S.p.A) for 5 min and
then stained with eosin (cat. no. C0353; Diapath S.p.A) for 5 min
at room temperature. For pRb, the sections were heated in a water
bath at 95˚C with Dako Target Retrieval Solution (Dako; Agilent
Technologies, Inc.) for 20 min. After washing with Tris Buffered
Saline, sections were incubated with mouse monoclonal pRb
antibodies (1:500, cat. no. 9307S; Santa Cruz Biotechnology, Inc.)
for 20 h at room temperature. Then, samples were incubated for 20
min at room temperature with EnVision reagent (Dako; Agilent
Technologies, Inc.) and horseradish peroxide. The visualization of
the reaction was completed with 3,3'-Diaminobenzidine solution.
After that the sections were stained with Hematoxylin II (cat. no.
790-2208; Roche Diagnostics, Ltd.) for 12 min at room temperature
and Bluing Reagent for 4 min (cat. no. 760-2037; Roche Diagnostics,
Ltd.) at room temperature. Antibody information can be found in
Table SI. Positive controls were
performed in each experiment. Negative controls were performed in
the same condition without primary antibodies. Full slide images
were reviewed and evaluated by three pathologists under a light
microscope (BX45; Olympus Corporation; magnification, x40).
Scoring
The slides were evaluated by three pathologists
(K.R., N.K. and S.K.) who were blinded from all clinical
information. All pathologists were trained and evaluated by a
Cohen's κ coefficient 0.97. All immunostaining slides were scanned
using Aperio ScanScope XT (Leica Microsystems, Inc.). Image
analysis was automatically scored using Aperio ImageScope (version
12.1.0.5029), and immunological expression was calculated by
Nuclear (https://tmalab.jhmi.edu/aperiou/userguides/IHC_Nuclear.pdf;
version 9) and Membranous algorithms (https://tmalab.jhmi.edu/aperiou/userguides/IHC_Membrane.pdf;
version 9). For H-score assessment, the staining intensity of
malignant cells was categorized as 0 (negative), 1+
(weak), 2+ (moderate) and 3+ (strong).
Thereafter, the total number of cells in each field and the number
of cells stained at each intensity were counted. The percentage of
positive cells was calculated following the formula of the H-score
and reported in range 0-300: H-score=(% of cells stained at
1+ x 1) + (% of cells stained at 2+ x 2) + (%
of cells stained at 3+ x 3).
Statistical analysis
All pathologists were trained and evaluated by a
Cohen's κ coefficient (κ index) 0.97. Data analysis was performed
using SPSS (version 23.0; IBM Corp.) and GraphPad Prism (version
9.0; Dotmatics). The age, sex, stage grouping, treatment
modalities, histological grading and lymphatic involvement of the
patients were reported. Significant factors to the number of
recurrent LSCC were analyzed using Pearson's χ2-test.
Differences in H-score between the recurrent and non-recurrent
groups were tested using the Mann-Whitney U test. Then, a receiver
operating characteristic (ROC) curve was analyzed to extract the
optimum cut-off point to distinguish high and low immunological
expression. OS was analyzed and disease-free survival (DFS) curves
were plotted using the Kaplan-Meier method and compared using the
log-rank test. Crude HR and adjusted HR were calculated using the
Cox regression test. The significance of tests was evaluated at 95%
confidence interval (CI). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients were divided into two groups based on
clinical information: The recurrent LSCC and the non-recurrent LSCC
group. Overall, the median OS of the patients in the current study
was 7.55 years. The median DFS was 5.95 years. Patients with
recurrent LSCC had a shorter OS than those with non-recurrent LSCC
(median, 4.73 vs. 9.14 years; P=0.003). The 5-year DFS after
treatment was 57.6%. Table I shows
the frequency of recurrent and non-recurrent LSCC in relation to
various parameters. A significant association was found between
histological grading (21) and
number of patients with recurrent LSCC (Pearson's χ2
test, 4.374; P=0.036).
 | Table IClinical and pathological parameters
of total LSCC (n=41), recurrent LSCC (n=15) and non-recurrent LSSC
(n=26). |
Table I
Clinical and pathological parameters
of total LSCC (n=41), recurrent LSCC (n=15) and non-recurrent LSSC
(n=26).
|
Characteristics | Total LSSC, n | Recurrent LSSC, n
(%) | Non-recurrent LSSC,
n (%) | P-value |
|---|
| Age, years | | | | 0.322 |
|
≤60 | 12 | 3 (20.00) | 9 (34.60) | |
|
>60 | 29 | 12 (80.00) | 17 (65.40) | |
| Sex | | | | N/A |
|
Male | 41 | 15 (100.00) | 26(100) | |
|
Female | 0 | 0 (0.00) | 0 (0.00) | |
| Smoking | | | | 0.827 |
|
Never | 7 | 2 (18.20) | 5 (21.70) | |
|
Current | 17 | 5 (45.50) | 12 (52.20) | |
|
Former | 10 | 4 (36.40) | 6 (26.10) | |
|
No data
available | 7 | | | |
| Alcohol | | | | 0.953 |
|
Never | 7 | 2 (18.20) | 5 (22.70) | |
|
Current | 20 | 7 (63.60) | 13 (59.10) | |
|
Former | 6 | 2 (18.20) | 4 (18.20) | |
|
No data
available | 8 | | | |
| Stage | | | | 0.215 |
|
Early | 7 | 4 (26.70) | 3 (11.50) | |
|
Advance | 34 | 11 (73.30) | 23 (88.50) | |
| Treatment | | | | 0.983 |
|
Definite
radiation | 7 | 3 (20.00) | 4 (15.40) | |
|
Concurrent
chemo radiotherapy | 6 | 2 (13.30) | 4 (15.40) | |
|
Radical
resection | 3 | 1 (6.70) | 2 (7.70) | |
|
Post
operation radiation | 25 | 9 (60.00) | 16 (61.50) | |
| Histological
grade | | | | 0.036a |
|
Well-differentiated | 25 | 6 (40.00) | 19 (73.10) | |
|
Moderately-differentiated | 16 | 9 (60.00) | 7 (26.90) | |
|
Poorly-differentiated | 0 | 0 (0.00) | 0 (0.00) | |
| Lymph node
status | | | | 0.548 |
|
Negative | 27 | 9 (60.00) | 18 (69.20) | |
|
Positive | 14 | 6 (40.00) | 8 (30.80) | |
| Anterior commissure
invasion | | | | 0.446 |
|
Negative | 16 | 7 (46.70) | 9 (34.60) | |
|
Positive | 25 | 8 (53.30) | 17 (65.40) | |
For immunohistochemistry, immunological expression
was detected in the nucleus of tumors: Ki67, pRb, RPA32, cyclin D1,
p53 and HIF-1α. Stained cytoplasm was detected in BAX, BAK, Bcl-xl,
c-Met, PI3K, β-catenin, p16 and CD44. Weakly stained cytoplasm was
also identified in VEGF and Bcl-2. Table SII shows the comparison of the
median H-scores of 16 proteins between the recurrent and
non-recurrent groups using the Mann-Whitney U test. A significantly
different H-score was found between the recurrent group and the
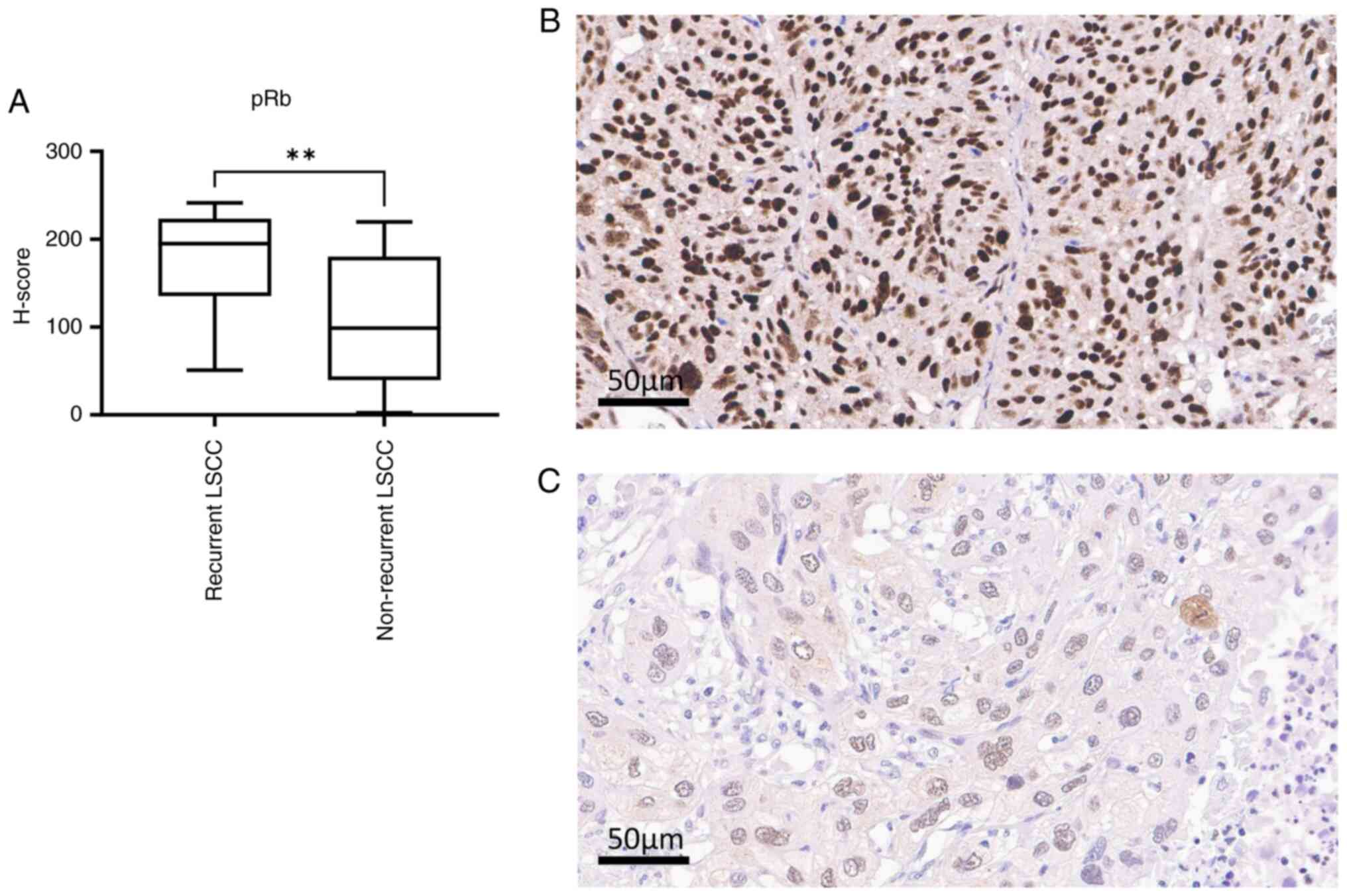
non-recurrent group in terms of pRb (P=0.0014) and c-Met expression
(P=0.0012). The median H-scores of pRb were 194.90 and 98.80 for
recurrent and non-recurrent LSCC, respectively (Mann-Whiteney U
test, P=0.014; Fig. 2A-C), and
there was a high expression in recurrent LSCC. The median H-scores
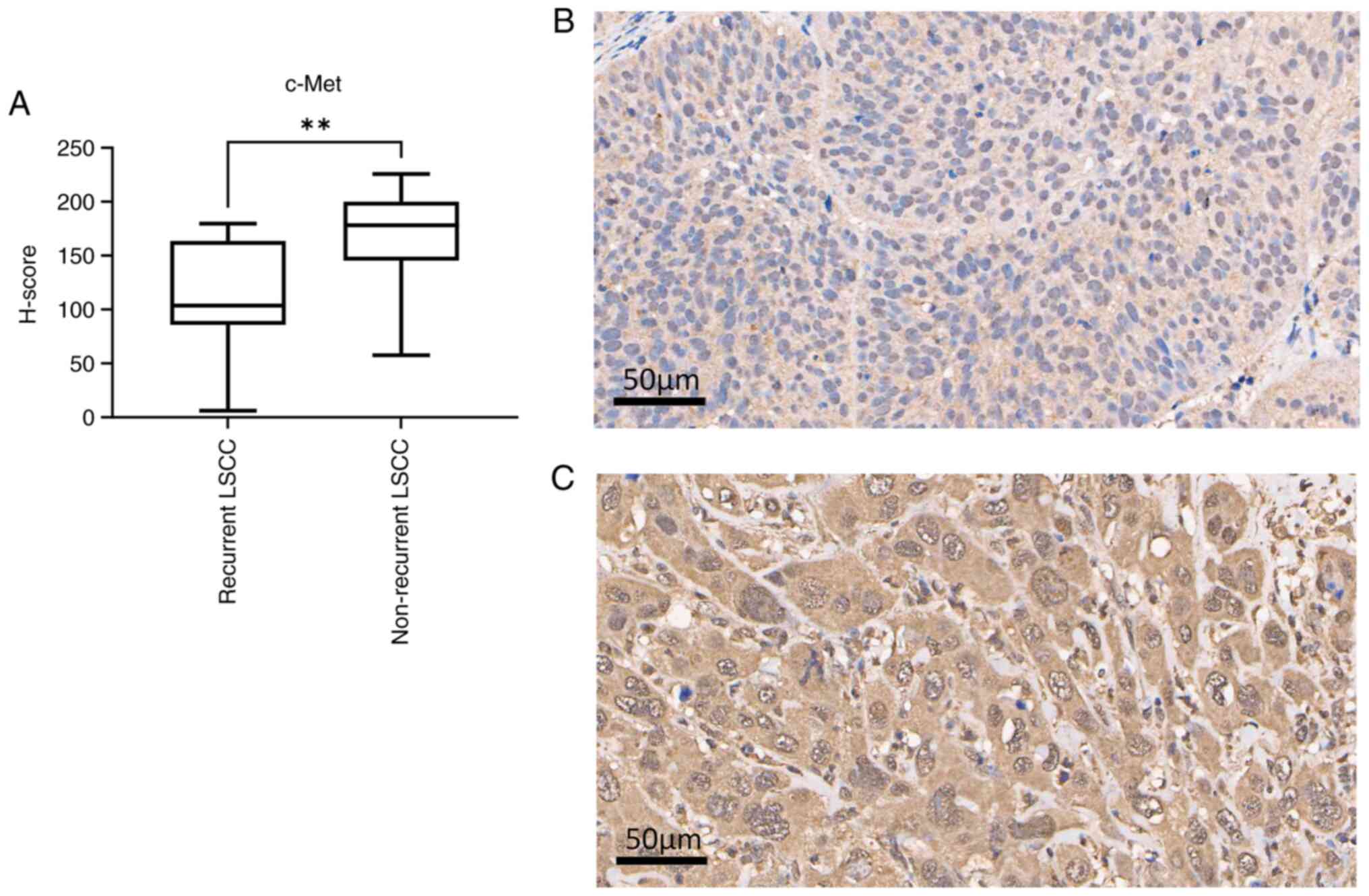
of c-Met were 103.60 and 178.20 for recurrent and non-recurrent
LSCC, respectively (Mann-Whitney U test; P=0.0012). The expression
levels of c-Met were lower in recurrent LSCC than in non-recurrent
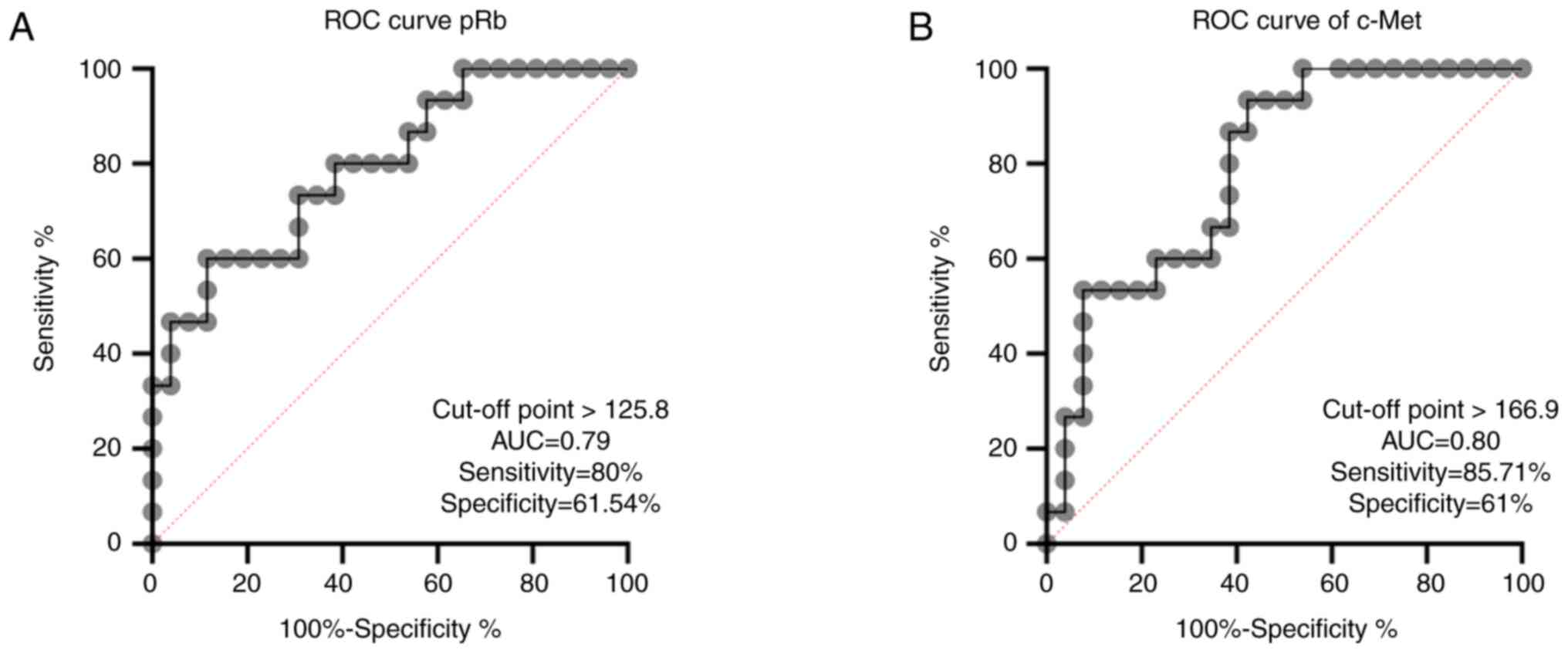
LSCC (Fig. 3A-C). A ROC curve was
plotted from the sensitivity and specificity of pRb and c-Met. The
optimum cut-off point of pRb to distinguish recurrent LSCC was
125.8, which demonstrated a sensitivity and specificity of 80.00
and 61.54%, respectively (Fig. 4A).
For c-Met, the optimum cut-off point was 166.9, which demonstrated
sensitivity and specificity of 85.71 and 61%, respectively
(Fig. 4B).
All patients were followed up until recurrence or
until December 2018. Kaplan-Meier survival analysis for DFS was
completed, and results showed that patients with
well-differentiated LSCC had a longer DFS time compared with that
of patients with moderate-differentiation, with DFS times of 6.95
and 4.32 years, respectively (log-rank χ2=5.268;
P=0.022). For the expression of all proteins, data showed that
patients with low pRb expression had a significantly longer DFS
time (7.83 years) compared with those with high pRb expression
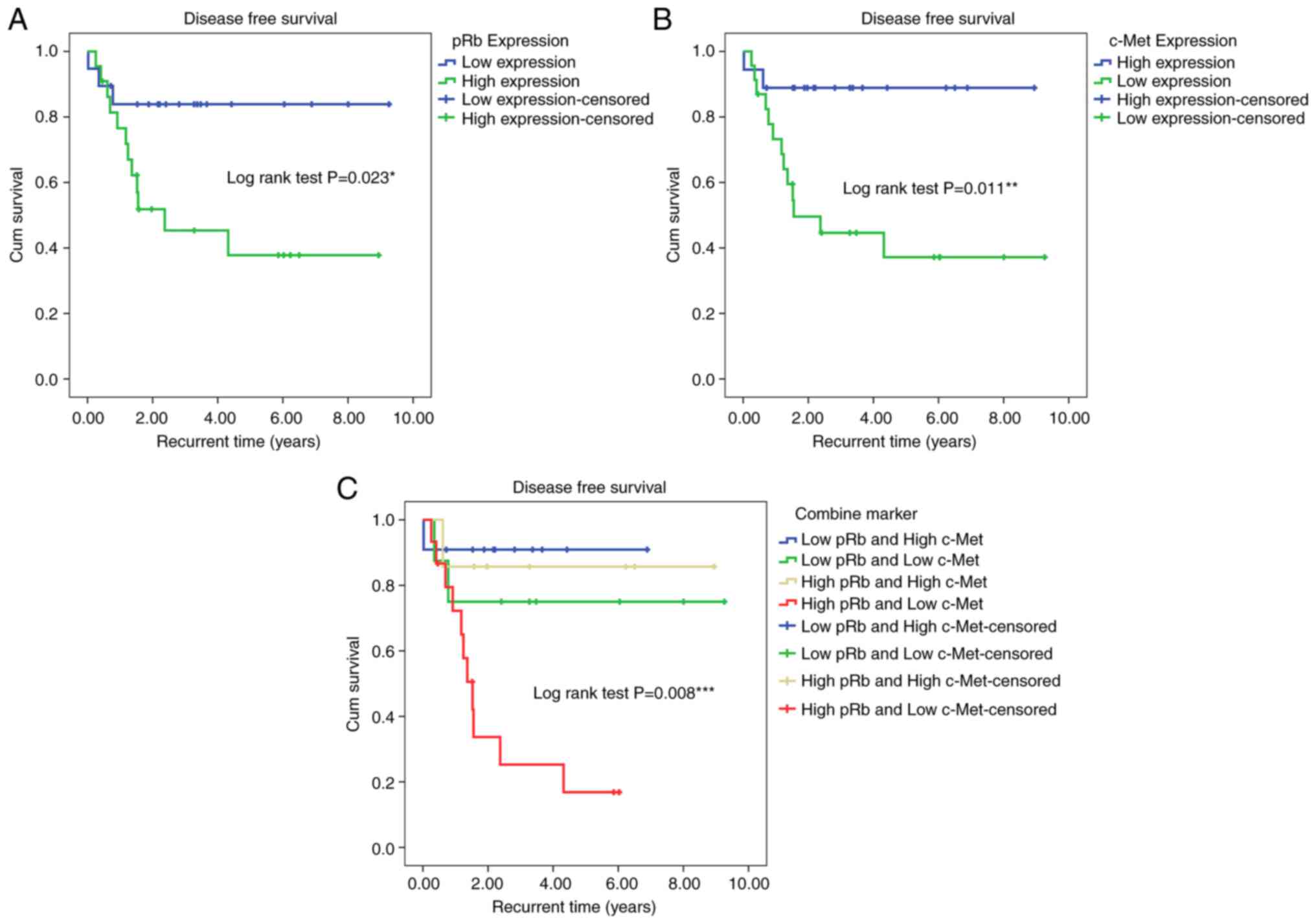
(4.34 years; log-rank χ2=5.161; P=0.023; Fig. 5A). By contrast, patients with high
c-Met expression had a longer DFS (7.98 years) compared with those
with low c-Met expression (4.54 years; log-rank
χ2=6.441; P=0.011; Fig.
5B). Moreover, patients with high pRb and low c-Met expression
had a short DFS compared with high pRb and high c-Met, low pRb and
high c-Met and low pRb and low c-Met with DFS 2.40 years compared
with patients with low pRb and high c-Met expression (log-rank
χ2=11.827; P=0.008; Fig.
5C). The incidence rate of recurrent LSCC in
well-differentiated LSCC was 6.92 cases per 100 person-years, while
that in moderately-differentiated LSCC was 25.42 cases per 100
person-years. For the combined high pRb expression and low c-Met
expression, the incidence rate of recurrent LSCC was 34.16 cases
per 100 person-years, more than that of other combined settings
(Table II).
 | Table IIAssociation between pathological
factors and median recurrence time of total LSCC (n=41), recurrent
LSCC (n=15) and non-recurrent LSSC (n=26). |
Table II
Association between pathological
factors and median recurrence time of total LSCC (n=41), recurrent
LSCC (n=15) and non-recurrent LSSC (n=26).
| Variables | Total patients with
LSCC, n | Patients with
recurrent LSCC, n | DFS (95% CI) | Person-years of
Observation | Incident rate/100
person years | P-value |
|---|
| Histological
grade | | | | | | 0.022a |
|
Well-differentiated | 25 | 6 | 6.95
(5.375-8.520) | 86.64 | 6.92 | |
|
Moderately-differented | 16 | 9 | 4.32
(2.319-6.325) | 35.4 | 25.42 | |
|
Poorly-differentiated | 0 | 0 | 0.00 | 0.00 | 0.00 | |
| pRb expression | | | | | | 0.023a |
|
Low | 19 | 3 | 7.83
(6.345-9.312) | 63.26 | 4.74 | |
|
High | 22 | 12 | 4.34
(2.655-6.017) | 58.79 | 20.41 | |
| c-Met
expression | | | | | | 0.011a |
|
Low | 23 | 13 | 4.54
(2.806-6.273) | 62.86 | 19.09 | |
|
High | 18 | 2 | 7.98
(6.729-9.234) | 58.78 | 3.40 | |
| Combined
markers | | | | | | 0.008a |
|
Low pRb and
high c-Met | 11 | 1 | 6.26
(5.095-7.427) | 29.67 | 3.37 | |
|
Low pRb and
low c-Met | 8 | 2 | 7.08
(4.474-9.694) | 33.59 | 5.95 | |
|
High pRb and
high c-Met | 7 | 1 | 7.75
(5.591-99.909) | 29.10 | 3.43 | |
|
High pRb and
low c-Met | 15 | 11 | 2.40
(1.291-3.519) | 29.27 | 34.16 | |
Several factors were significantly associated with
the risk of recurrence and differentiation, including the
expression of both pRb and c-Met. There was a significant increase
in patients with moderately-differentiated LSCC compared with those
with well-differentiated LSCC (Cox regression test; crude HR, 3.16;
95% CI, 1.12-8.92; P=0.030). In terms of protein expression, the
risk of recurrence increased in high pRb and low c-Met expression
compared with that in low pRb and high c-Met expression (Cox
regression test; crude HR, 9.53; 95% CI, 1.214-74.819;
P=0.032).
The adjusted HR model analysis revealed the same
results. Histological grading was an independent prognostic factor
for recurrent LSCC (Cox regression test; adjusted HR, 4.19; 95% CI,
1.321-13.268; P=0.015). High pRb and low c-Met expression were
independent prognostic factors for recurrent LSCC (Cox regression
test; adjusted HR, 8.73; 95% CI, 1.094-69.638; P=0.041; Table III).
 | Table IIICrude HR and adjusted HR analysis of
factors associated with recurrent LSCC. |
Table III
Crude HR and adjusted HR analysis of
factors associated with recurrent LSCC.
| Variables | Crude HR (95%
CI) | P-value | Adjusted HR | P-value |
|---|
| Histological
grade | | | | |
|
Well-differentiated | 1 | | 1 | |
|
Moderately-differentiated | 3.16
(1.213-3.980) | 0.030a | 4.19
(1.321-13.268) | 0.015a |
|
Poorly-differentiated | N/A | N/A | N/A | N/A |
| Combined
markers | | | | |
|
Low pRb and
high c-Met | 1 | | 1 | |
|
Low pRb and
low c-Met | 2.54
(0.230-28.112) | 0.447 | 1.41
(0.123-16.260) | 0.781 |
|
High pRb and
high c-Met | 1.38
(0.086-22.109) | 0.82 | 0.80
(0.048-13.153) | 0.875 |
|
High pRb and
low c-Met | 9.53
(1.214-74.819) | 0.032a | 8.73
(1.094-69.638) | 0.041a |
Discussion
In clinical practice, it is challenging to identify
a prognostic factor that can predict the treatment outcome of LSCC.
TNM staging is not a precise and accurate marker for prognostic
outcomes (15,22). Patients with identical TNM staging
may have a variable clinical course, response to treatment and
prognosis.
In the current study, a significant association
between histological grading and recurrent LSCC and DFS was found.
The present study is consistent with previous studies; Wang et
al (23) found an association
between well-differentiated LSCC and OS. A previous study on 998
patients with LSCC showed that patients with well- to
moderately-differentiated LSCC had notably improved survival
outcomes, including DSS, DFS and OS, than patients with
poorly-differentiated LSCC; that study included all supraglottic,
glottic and subglottic LSCC (24).
Meanwhile, the present study focused only on glottic cancer. It was
considered that supraglottic and subglottic LSCC might affect the
prognosis. Chen et al (25)
studied 110 patients with LSCC, and there were 55 patients with
well-differentiated and poorly-differentiated LSCC, including all
subsites. Patients with well-differentiated LSCC were found to have
significantly different OS (HR, 0.18; 95% CI, 0.07-0.46; P=0.001),
DSS (HR, 0.16; 95% CI, 0.05-0.45; P<0.001) and DFS (HR, 0.17;
95% CI, 0.07-0.41; P=0.003) than patients with
poorly-differentiated LSCC. Another study on all subsites of 250
patients with early glottic LSCC found that the risk of mortality
of patients with poor-differentiation was 1.45-fold more than that
of patients with a well-differentiated tumor (HR, 2.45; 95% CI,
1.19-5.40; P=0.01) (26). By
contrast, Piccirillo et al (27) studied 196 LSCC cases and found no
statistical significance between histological grading and symptom
duration, and survival rate.
The location of tumors at the anterior commissure
was frequently discussed as a prognostic factor. In the present
study, tumor location was not significantly associated with
recurrent LSCC. By contrast, numerous previous studies showed
significance in terms of local control and recurrence (28-35).
However, due to the small sample size of the present study, the
significance of local control and recurrence rate was not
determined.
In immunohistochemistry, the expression of 16
proteins was investigated in accordance with tumorigenesis. The
results showed a statistically significant expression only in pRb
and c-Met. pRb is a protein product of the RB tumor suppressor
gene. It controls the cell cycle, preserving genetic integrity and
mediating cell differentiation (36). It plays a role in the negative
control of the cell cycle and tumor progression. It works at the G1
checkpoint for block entrance of S-phase and inhibits cell
progression. The loss of pRb function may lead to cell cycle
dysregulation and malignant transformation. Similarly to a previous
study, a high pRb expression showed higher recurrent LSCC and lower
DFS (37). Moreover, Mizokami et
al (38) found that the loss of
pRb expression was associated with invasive tumor behavior, such as
high T classification or histological grade, which could predict
disease relapse. Similarly, Lee et al (39) found that a low pRb expression could
be notable in predicting recurrence and 3-year DFS.
c-Met, a mesenchymal-epithelial transition factor,
is a transmembrane receptor of tyrosine kinase found on the surface
of various epithelial cells. Hepatocyte growth factor/scatter
factor (HGF/SF) is a common ligand to c-Met receptors (40,41). A
disruption of HGF/c-Met signaling can cause uncontrolled
proliferation, motility, invasion and angiogenesis that could lead
to head and neck squamous cell carcinoma (HNSCC) (42). The present study obtained results
different from those of previous meta-analyses (43-45).
The present study was shown that a lower c-Met expression had
higher recurrence and shorter DFS. Crossing over with previous
studies, a higher c-Met expression had a predisposition for tumor
recurrence, and was associated with shorter OS and DFS (43-45).
Immunohistochemistry results were also considered. The small sample
size and the different clones of primary antibodies might affect
the results.
Results showed that pRb and c-Met are co-expressed
in HNSCC. They play a major role in the cell cycle and
tumorigenesis. They could lead to uncontrolled cell cycle, cell
proliferation, invasion, angiogenesis, affecting tumor recurrence
and survival. However, their precise associated functions are not
revealed. The small sample size and different clones of primary
antibodies used in the present study might affect the difference.
Moreover, the variant molecular and subsequent cellular alterations
might impact the results. Further large-scale studies including
multiple cohorts should be performed.
In clinical practice, it will be useful to define
the risk of recurrent LSCC based on pRb and c-Met expression from
routine pathological reports. Currently, there are small molecule
inhibitors and monoclonal antibodies of c-Met in various trials.
One of them is crizotinib which was examined in a phase I cohort
study of gastroesophageal cancer to check the responses to
Met-amplified metastatic disease (46). In the part of pRb, it works with
mechanism of the cyclin-dependent kinase 4 and 6 (CDK4/6)
inhibitors. The United States Food and Drug Administration has
approved three agents: Palbociclib, ribociclib and abemaciclib for
the treatment of advanced breast cancer in combination with
endocrine therapy (47). In locally
advanced HNSCC, there is currently no clinical use of CDK4/6
inhibitors concurrently with radiotherapy (48). Further research could provide the
potential for targeted therapeutic agents in the future.
In the current study, the degree of differentiation
of SCCA is an important clinicopathological factor in predicting
recurrent LSCC. The immunohistochemistry of the biological markers
pRb, and c-Met is the most useful prognostic factor for
distinguishing recurrent from non-recurrent disease. The present
study showed that the combined upregulated pRb expression and
downregulated c-Met expression is useful in predicting recurrent
LSCC. The assessment of pRb and c-Met expression should be
considered in clinical practice.
Supplementary Material
A total of 16 antibodies were used to
investigate the mechanism of tumorigenesis.
Types of 16 primary antibodies,
specification of the clones, lot number, incubated time, dilutions,
manufacturer and type of immunostainer.
Median histoscore of protein
expression in recurrent and non-recurrent LSCC, Mann-Whitney U
Test.
Acknowledgements
The authors would like to thank the
Immunohistochemistry Unit of the Department of Pathology, Faculty
of Medicine, Chulalongkorn University and King Chulalongkorn
Memorial Hospital for their assistance in the pathological
process.
Funding
Funding: The present study was funded by The Graduate School,
Chulalongkorn University to commemorate the 90th Anniversary of
Chulalongkorn University (Rachadapisek Sompote Fund, grant no.
GCUGR1125643036M). The funding body did not interfere with the
design of the study and collection, analysis, or interpretation of
data, and in writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST, KR, NK, SK and PM conceptualized the study. ST,
KR, NK, SK and PM carried out data curation. ST, NK and PM
completed formal analysis. ST and SK acquired funding. ST, KR, NK,
SK and PM completed the investigation. ST, NK and PM developed
methodology used. ST, NK and PM carried out project administration.
ST, KR, NK, SK and PM provided resources. ST, KR, NK and SK used
software. NK, SK and PM supervised the study. ST, NK and PM
validated the data. SK and PM visualized the data. ST and PM wrote
the original draft. ST, KR, NK, SK and PM reviewed and edited the
manuscript. ST, NK, SK and P.M. confirm the authenticity of all the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
All protocols used in the present study were
approved by the IRB of the Faculty of Medicine, Chulalongkorn
University (approval no. IRB 889/63; COA-MDCU no. 0158/2022,
Bangkok, Thailand) which also covered any study conducted in the
King Chulalongkorn Memorial Hospital in accordance with the
Declaration of Helsinki. All participants provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deng Y, Wang M, Zhou L, Zheng Y, Li N,
Tian T, Zhai Z, Yang S, Hao Q, Wu Y, et al: Global burden of larynx
cancer, 1990-2017: Estimates from the global burden of disease 2017
study. Aging (Albany NY). 12:2545–2583. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Morshed K, Skomra D, Korobowicz E,
Szymański M, Polz-Dacewicz M and Gołabek W: An immunohistochemical
study of cyclin D1 protein expression in laryngeal squamous cell
carcinoma. Acta Otolaryngol. 127:760–769. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
American Joint Committee on Cancer. Amin
MB, Edge SB, Schilsky RL and Gaspar LE: AJCC cancer staging manual.
Springer, New York, NY, 2017.
|
|
4
|
Bradford CR, Ferlito A, Devaney KO,
Mäkitie AA and Rinaldo A: Prognostic factors in laryngeal squamous
cell carcinoma. Laryngoscope Investig Otolaryngol. 5:74–81.
2020.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Brandstorp-Boesen J, Sørum Falk R, Boysen
M and Brøndbo K: Impact of stage, management and recurrence on
survival rates in laryngeal cancer. PLoS One.
12(e0179371)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu
Y, Gong Z, Zhang S, Zhou J, Cao K, et al: Role of tumor
microenvironment in tumorigenesis. J Cancer. 8:761–773.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leesutipornchai T, Ratchataswan T,
Vivatvakin S, Ruangritchankul K, Keelawat S, Kerekhanjanarong V,
Bongsebandhu-Phubhakdi S and Mahattanasakul P: EGFR cut-off point
for prognostic impact in laryngeal squamous cell carcinoma. Acta
Otolaryngol. 140:610–614. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vivatvakin S, Ratchataswan T,
Leesutipornchai T, Ruangritchankul K, Keelawat S, Mahattanasakul P
and Bongsebandhu-Phubhakdi S: MCM-2, Ki-67, and EGFR downregulated
expression levels in advanced stage laryngeal squamous cell
carcinoma. Sci Rep. 11(14607)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Almadori G, Cadoni G, Galli J, Ferrandina
G, Scambia G, Exarchakos G, Paludetti G and Ottaviani F: Epidermal
growth factor receptor expression in primary laryngeal cancer: An
independent prognostic factor of neck node relapse. Int J Cancer.
84:188–191. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maurizi M, Almadori G, Ferrandina G,
Distefano M, Romanini ME, Cadoni G, Benedetti-Panici P, Paludetti
G, Scambia G and Mancuso S: Prognostic significance of epidermal
growth factor receptor in laryngeal squamous cell carcinoma. Br J
Cancer. 74:1253–1257. 1996.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Furuta Y, Takasu T, Asai T, Yoshimura S,
Tokuchi F, Shinohara T, Nagashima K and Inuyama Y: Clinical
significance of the epidermal growth factor receptor gene in
squamous cell carcinomas of the nasal cavities and paranasal
sinuses. Cancer. 69:358–362. 1992.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marioni G, Staffieri A, Bertolin A,
Giacomelli L, D'Alessandro E, Ottaviano G, Accordi D, Stramare R,
de Filippis C and Blandamura S: Laryngeal carcinoma lymph node
metastasis and disease-free survival correlate with MASPIN nuclear
expression but not with EGFR expression: A series of 108 cases. Eur
Arch Otorhinolaryngol. 267:1103–1110. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cavaliere M, Bisogno A, Scarpa A, D'Urso
A, Marra P, Colacurcio V, De Luca P, Ralli M, Cassandro E and
Cassandro C: Biomarkers of laryngeal squamous cell carcinoma: A
review. Ann Diagn Pathol. 54(151787)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gioacchini FM, Alicandri-Ciufelli M,
Magliulo G, Rubini C, Presutti L and Re M: The clinical relevance
of Ki-67 expression in laryngeal squamous cell carcinoma. Eur Arch
Otorhinolaryngol. 272:1569–1576. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cai K, Luo Y, Li L and Liu Y: Expression
and significance of MCM2, Ki-67 and Rb protein in laryngeal
squamous cell carcinomas. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke
Za Zhi. 26:425–428. 2012.PubMed/NCBI(In Chinese).
|
|
17
|
Re M, Zizzi A, Ferrante L, Stramazzotti D,
Goteri G, Gioacchini FM, Olivieri F, Magliulo G and Rubini C: p63
and Ki-67 immunostainings in laryngeal squamous cell carcinoma are
related to survival. Eur Arch Otorhinolaryngol. 271:1641–1651.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nowinska K, Chmielewska M, Piotrowska A,
Pula B, Pastuszewski W, Krecicki T, Podhorska-Okołow M, Zabel M and
Dziegiel P: Correlation between levels of expression of
minichromosome maintenance proteins, Ki-67 proliferation antigen
and metallothionein I/II in laryngeal squamous cell cancer. Int J
Oncol. 48:635–645. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Krecicki T, Jeleń M, Zalesska-Krecicka M
and Szkudlarek T: Ki-67 immunostaining and prognosis in laryngeal
cancer. Clin Otolaryngol Allied Sci. 23:539–542. 1998.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kropveld A, Slootweg PJ, Blankenstein MA,
Terhaard CH and Hordijk GJ: Ki-67 and p53 in T2 laryngeal cancer.
Laryngoscope. 108:1548–1552. 1998.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Roland NJ, Caslin AW, Nash J and Stell PM:
Value of grading squamous cell carcinoma of the head and neck. Head
Neck. 14:224–229. 1992.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cordes C, Münzel AK, Rudolph P, Hoffmann
M, Leuschner I and Gottschlich S: Immunohistochemical staining of
Ki-67 using the monoclonal antibody Ki-s11 is a prognostic
indicator for laryngeal squamous cell carcinoma. Anticancer Res.
29:1459–1465. 2009.PubMed/NCBI
|
|
23
|
Wang N, Huang X and Cheng J: BIRC5
promotes cancer progression and predicts prognosis in laryngeal
squamous cell carcinoma. PeerJ. 10(e12871)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu Y, Shi X, Zhu X, Diao W and Chen X:
Association between pathological differentiation and survival
outcomes of patients with laryngeal squamous cell carcinoma. Eur
Arch Otorhinolaryngol. 279:4595–4604. 2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen P, Yu W, Huang J, Xu H, Li G, Chen X
and Huang Z: Matched-pair analysis of survival in patients with
poorly differentiated versus well-differentiated glottic squamous
cell carcinoma. Oncotarget. 8:14770–14776. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fararouei M, Daneshi N, Mohammadianpanah
Μ, Reza Tabatabaei H, Zare-Bandamiri M and Dianatinasab M: Factors
predicting survival in patients with early stage laryngeal cancer:
A cohort study between 2000 to 2015. J BUON. 22:996–1003.
2017.PubMed/NCBI
|
|
27
|
Piccirillo JF, Wells CK, Sasaki CT and
Feinstein AR: New clinical severity staging system for cancer of
the larynx. Five-year survival rates. Ann Otol Rhinol Laryngol.
103:83–92. 1994.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kitani Y, Kubota A, Furukawa M and Sato K:
Prognostic factors for local control in patients receiving
radiation therapy for early glottic cancer: Anterior commissure
involvement and effect of chemoradiotherapy. Eur Arch
Otorhinolaryngol. 273:1011–1017. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chone CT, Yonehara E, Martins JE, Altemani
A and Crespo AN: Importance of anterior commissure in recurrence of
early glottic cancer after laser endoscopic resection. Arch
Otolaryngol Head Neck Surg. 133:882–887. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hakeem AH, Tubachi J and Pradhan SA:
Significance of anterior commissure involvement in early glottic
squamous cell carcinoma treated with trans-oral CO2 laser
microsurgery. Laryngoscope. 123:1912–1917. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zouhair A, Azria D, Coucke P, Matzinger O,
Bron L, Moeckli R, Do HP, Mirimanoff RO and Ozsahin M: Decreased
local control following radiation therapy alone in early-stage
glottic carcinoma with anterior commissure extension. Strahlenther
Onkol. 180:84–90. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Le QT, Fu KK, Kroll S, Ryu JK, Quivey JM,
Meyler TS, Krieg RM and Phillips TL: Influence of fraction size,
total dose, and overall time on local control of T1-T2 glottic
carcinoma. Int J Radiat Oncol Biol Phys. 39:115–126.
1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bron LP, Soldati D, Zouhair A, Ozsahin M,
Brossard E, Monnier P and Pasche P: Treatment of early stage
squamous-cell carcinoma of the glottic larynx: Endoscopic surgery
or cricohyoidoepiglottopexy versus radiotherapy. Head Neck.
23:823–829. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Allegra E, Saita V, Azzolina A, De Natale
M, Bianco MR, Modica DM and Garozzo A: Impact of the anterior
commissure involvement on the survival of early glottic cancer
treated with cricohyoidoepiglottopexy: A retrospective study.
Cancer Manag Res. 10:5553–5558. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mannelli G, Comini LV, Santoro R, Bettiol
A, Vannacci A, Desideri I, Bonomo P and Piazza C: T1 glottic
cancer: Does anterior commissure involvement worsen prognosis?
Cancers (Basel). 12(1485)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dimaras H and Gallie BL: Retinoblastoma
protein, biological and clinical functions. In: Encyclopedia of
Cancer. Schwab M (ed). Springer, Berlin, pp3277-3280, 2011.
|
|
37
|
Morshed K, Korobowicz E, Skomra D,
Szymanski M, PolzDacewicz M, Gołabek W and Smolen A:
Immunohistochemical study of retinoblastoma protein expression in
laryngeal squamous cell carcinoma according to low and high
overexpression. Bulletin of the Veterinary Institute in Pulawy.
52:675–681. 2008.
|
|
38
|
Mizokami H, Sawatsubashi M, Tokunaga O and
Shin T: Loss of retinoblastoma protein expression in laryngeal
squamous cell carcinoma. Mod Pathol. 12:47–53. 1999.PubMed/NCBI
|
|
39
|
Lee LA, Fang TJ, Li HY, Huang CG, Chen TC,
Liao CT, Kang CJ, Chang KP and Yen TC: Low expression of pRB
predicts disease relapse in early glottic cancer treated with
transoral laser microsurgery. Laryngoscope. 129:E220–E226.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Raj S, Kesari KK, Kumar A, Rathi B, Sharma
A, Gupta PK, Jha SK, Jha NK, Slama P, Roychoudhury S and Kumar D:
Molecular mechanism(s) of regulation(s) of c-MET/HGF signaling in
head and neck cancer. Mol Cancer. 21(31)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan
C, Wu Y, Li X, Li X, Li G, et al: Function of the c-Met receptor
tyrosine kinase in carcinogenesis and associated therapeutic
opportunities. Mol Cancer. 17(45)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rothenberger NJ and Stabile LP: Hepatocyte
Growth Factor/c-Met signaling in head and neck cancer and
implications for treatment. Cancers (Basel). 9(39)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li L, Sun Z, Huang X, Li X, Sun L, Zhang
L, Zhang X, Ye L, Yuan J, Mao L and Li G: Role of c-Met expression
on prognosis of head and neck cancer: A literature review and
meta-analysis. Head Neck. 41:1999–2006. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jiang M, Zhang H, Xiao H, Zhang Z, Que D,
Luo J, Li J, Mao B, Chen Y, Lan M, et al: High expression of c-Met
and EGFR is associated with poor survival of patients with glottic
laryngeal squamous cell carcinoma. Oncol Lett. 15:931–939.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Szturz P, Budíková M, Vermorken JB, Horová
I, Gál B, Raymond E, de Gramont A and Faivre S: Prognostic value of
c-MET in head and neck cancer: A systematic review and
meta-analysis of aggregate data. Oral Oncol. 74:68–76.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mo HN and Liu P: Targeting MET in cancer
therapy. Chronic Dis Transl Med. 3:148–153. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shah M, Nunes MR and Stearns V: CDK4/6
inhibitors: Game changers in the management of hormone
receptor-positive advanced breast cancer? Oncology (Williston
Park). 32:216–222. 2018.PubMed/NCBI
|
|
48
|
Ngamphaiboon N, Chairoungdua A,
Dajsakdipon T and Jiarpinitnun C: Evolving role of novel
radiosensitizers and immune checkpoint inhibitors in
(chemo)radiotherapy of locally advanced head and neck squamous cell
carcinoma. Oral Oncol. 145(106520)2023.PubMed/NCBI View Article : Google Scholar
|