1. Introduction
Skeletal muscle exhibits three distinct types of
contraction based on its functional characteristics: i) Concentric
contraction, also known as concentric exercise, involves the active
shortening of muscle length, which results in the displacement of
the limb; ii) isometric contraction (isometric exercise) occurs
when the muscle length remains constant, preventing limb movement;
and iii) eccentric contraction (eccentric exercise) involves the
lengthening of the muscle as it resists external force or
decelerates (1-3).
Among the three types of contractions, eccentric contraction stands
out by generating higher muscle force, albeit with the recruitment
of a relatively minor number of motor units. Consequently, this
type of contraction is associated with lower energy consumption and
oxygen uptake (1). Due to its
advantageous characteristics, eccentric contraction training has
gained widespread recognition and utilization in various domains.
One notable advantage is its remarkable ability to enhance muscle
force while minimizing metabolite production. As a result, the
eccentric contraction training mode has found extensive application
in physical training and injury rehabilitation within competitive
sports and in the realm of sports rehabilitation for metabolic and
musculoskeletal disorders (1,4-6).
However, eccentric contraction can lead to noteworthy
ultrastructural changes in skeletal muscles, including Z-disk
streaming, disruption and sarcomere destruction. Delayed skeletal
muscle ultrastructural changes are remarkable for their delayed
onset, with the peak occurring between 24-72 h (1,7). The
scientific community widely acknowledges that skeletal muscle
ultrastructural changes actively contribute to symptoms associated
with delayed onset muscle soreness (DOMS). In addition to DOMS,
these symptoms encompass reduced muscle strength and various
indicators of skeletal muscle damage (1,7,8),
resulting in changes in the activation sequence and recruitment
patterns of muscle motor units. This compensatory mechanism further
induces the occurrence of skeletal muscle damage (7,9-11).
However, the degree of ultrastructural changes in eccentric
exercise-induced skeletal muscle does not match the degree of DOMS
symptoms (7,12). Hence, it becomes imperative to
investigate the mechanism underlying ultrastructural changes in
skeletal muscle induced by eccentric exercise and elucidate its
precise association with symptoms of DOMS. Such research endeavors
offer practical insights for formulating evidence-based physical
training or sports rehabilitation programs. Currently, the
mechanism underlying eccentric exercise-induced skeletal muscle
damage remains unknown, and the lack of understanding about
eccentric contraction mechanisms is partly due to the limitations
of the sliding filament theory (1,4,5). The
mechanism of skeletal muscle contraction is derived from the
sliding filament theory, first published in Nature in 1954 by
Huxley and Niedergerke (13), and
Huxley and Hanson (14). This
theory utilizes a two-filament sarcomere model, consisting of thin
filaments composed of actin and thick filaments composed of myosin,
to elucidate the mechanism of muscle contraction through the
sliding action of these filaments facilitated by cross-bridges. The
two-filament sliding theory explains both concentric and isometric
contraction mechanisms (4);
however, based solely on the cross-bridge swing, the sliding
filament theory fails to account for phenomena such as the stable
descending limb observed in the force-length relationship curve
during eccentric contraction (4,15,16).
It was found that lateral force transmission
increased after acute eccentric contraction (17), and that collagen fiber deposition in
the endomysium and perimysium maintained the morphological
integrity of muscle fiber after chronic prolonged eccentric
contraction (9,18). It is hypothesized that some factors
must ensure the stability of the stable descending limb during an
eccentric contraction while increasing lateral force transmission
and protecting muscle fiber from damage. It is implied that
exploring the mechanism of the stable descending limb is key to
re-examining the mechanism of eccentric exercise-induced skeletal
muscle damage.
In fact, Hanson and Huxley (19), in confirming the sliding filament
theory of the two-filament sarcomere model, hypothesized the
existence of a third filament between the Z-disks. Nevertheless,
the lack of evidence and the challenge of integrating the third
filament into the two-filament sliding theory resulted in the
publication of the sliding filament theory based solely on the
two-filament sarcomere model in Nature the following year.
Consequently, from its inception, the sliding filament theory of
the two-filament sarcomere model has yet to be completed due to the
absence of the third filament. In the book ‘Reflections on Muscle’,
Huxley AF (20), the pioneer of the
cross-bridge theory highlighted the inadequacy of the sliding
filament theory, which is based on cross-bridge swinging, in
explaining the mechanism of eccentric contraction (20). Subsequent studies have provided
compelling evidence supporting the inclusion of titin, a spring
protein spanning half of the sarcomere, as the third filament in
the sarcomere (21). By integrating
the two-filament sarcomere model with titin, a more comprehensive
understanding of the eccentric contraction mechanism has emerged
(22). The three-filament sarcomere
model, comprising the thin filament, thick filament and titin as
the third filament, derived from the sliding filament theory,
presents a novel framework for investigating the mechanisms
underlying skeletal muscle damage induced by eccentric exercise
(23), thereby providing a more
insightful explanation of the eccentric contraction process.
2. Overview of the sliding filament
theory
Until the 1950s (24), researchers widely considered that
the force produced during muscle contraction was directly
associated with shortening the length of the thick filament
positioned at the center of the sarcomere. However, in 1953, using
high-resolution electron microscopy imaging technology, Huxley HE
(25) discovered that the thick
filament did not undergo shortening during muscle contraction.
Subsequently, in 1954, Huxley and Hanson (14), as well as Huxley and Niedergerke
(13), published two research
papers in Nature proposing the sliding filament theory. This theory
presents a two-filament sarcomere model comprising a thick filament
composed of myosin and a thin filament composed of actin. According
to this theory, the thin filament slides toward the center of the
thick filament through the swinging motion of the cross-bridge,
while the lengths of the thick and thin filaments remain unchanged.
The purpose of developing this model is to clarify how muscle
contraction works. In 1957, Huxley AF (26) revealed the first molecular model of
sarcomere structure and an energy calculation formula, providing a
detailed explanation of the sliding filament theory. The model
proposed that myosin pulls actin using the swinging motion of the
cross-bridge, causing the thin filament to slide towards the M-band
at the center of the sarcomere. The energy needed for the swinging
movement of the cross-bridge comes directly from adenosine
triphosphate. Huxley HE (27)
proposed the theory of cross-bridge swinging rotation, which Huxley
and Simmons (28) later revised to
account for kinetic changes in the cross-bridge during abrupt
muscle force or length alterations.
3. Deficiencies in the interpretation of
eccentric contraction mechanism by the sliding filament theory of
the two-filament sarcomere model
To date, the two-filament sliding theory has
effectively elucidated the mechanisms underlying concentric and
isometric contractions at the cellular and molecular levels
(4). However, this theory must
fully explain the mechanism behind eccentric contractions (4,15).
In the two-filament sarcomere model, the
half-sarcomere and the sarcomere exhibit instability (16,29).
The positioning of the thick filament within the sarcomere depends
solely on the balancing force generated by the cross-bridge in
conjunction with the thin filament. Maintaining a constant balance
of forces acting on the cross-bridge is necessary. Otherwise, even
a slight imbalance could cause the thick filament to be pulled
towards the ‘stronger’ half-sarcomere, leading to a more pronounced
imbalance and an unstable force within the half-sarcomere (30).
As a result, during eccentric exercise, weaker
sarcomeres are prone to overstretching due to non-uniform passive
elongation and the less favorable structural stability of
sarcomeres on the descending limb of the force-length relationship
curve (31,32). However, a number of studies have
demonstrated that the active stretching of the myofibril leads to
the formation of highly stable structures on the descending limb of
the force-length relationship curve, despite the non-uniform
characteristics of sarcomere length (33,34).
Besides the myosin and actin filaments, the stability of the
sarcomere force-length relationship curve on the descending limb is
reliant on other components.
4. Proposal of the three-filament sarcomere
model
The concept of the third filament and
its role
Based on the aforementioned studies, it becomes
evident that the sliding filament theory of the two-filament
sarcomere model cannot adequately account for the mechanism of
eccentric contraction. The eccentric contraction mechanism
hypothesizes the involvement of an additional sarcomere component
in collaboration with myosin and actin. In 1953, Hanson and Huxley
(19) introduced the concept of the
two-filament sarcomere model. It was hypothesized by the authors
that the existence of a third filament, known as the S filament,
exists as part of the thin filament between the Z-disks.
Subsequently, in 1965, the PhD thesis of Dos Remedios at the
University of Sydney rediscovered the presence of other filaments
within the sarcomere, reigniting interest in studying the third
filament (35). In 1976, Maruyama
(36) directly detected the third
filament using atomic force microscopy, and in 1979, it was
subsequently named titin by Wang et al (37). However, it was not until 1988 that
Fürst et al (38) utilized
titin antibodies to provide the initial evidence that this elastic
filament extends continuously from the Z-disk to the M-band,
spanning half of the sarcomere. Subsequent research revealed that
titin, with a molecular weight of ~3,000-4,000 kDa, is the most
substantial protein constituent of the sarcomere. It primarily
comprises the I-band region (which spans the titin region of the
thin filament) and the A-band region (which lies within the thick
filament). The I-band region of titin comprises
tandem-immunoglobulin domain (Ig) regions, including the N2A
element (consisting of multiple Ig domains inserted into a single
sequence) and the proline-glutamate-valine-lysine (PEVK) element
located between the differentially spliced and distal Ig domains
(39). The PEVK element and the Ig
domains collectively represent the most critical elastic region of
titin. On the other hand, the A-band region of titin primarily
consists of Ig domains and repetitive fibronectin sequences,
lacking any stretching functionality.
The influence factor of titin during
eccentric exercise
During low-force stretching (eccentric contraction)
of the sarcomere, the elongation of titin primarily occurs through
the straightening of interdomain linkers in the Ig domains
(39). However, during high-force
stretching (eccentric contraction), the PEVK element assumes a
central role in stretching, conferring spring-like properties to
titin and enabling it to buffer external forces (Fig. 1). Furthermore, the spring stiffness
of the PEVK element increases with higher concentrations of
cytoplasmic Ca2+, acting as a regulator of sarcomere
force (40). Researchers consider
the underlying regulatory mechanism involves titin winding around
the thin filament via cross-bridge rotation. This process leads to
the shortening of the elastic region and consequently, to an
increase in the spring stiffness of the PEVK element (41). However, a number of different
studies have suggested that the increase in titin stiffness is not
dependent on the winding of the thin filament but rather on the
proximity of the distal domain of titin to the central M-band of
the sarcomere (the precise mechanism remains unknown) (42). Notably, using a skinned fibers model
(mice soleus), Labeit et al (43) investigated recombinant PEVK
molecules containing 28-residue PEVK repeats and E-rich motifs, and
they observed that Ca2+ could bind to the E-rich motif
of the PEVK element at the distal end of titin, thereby increasing
the stiffness of titin.
To summarize the aforementioned studies, the
increase in titin stiffness, in the context of elevated cytoplasmic
Ca2+ levels, does not result from binding to the thin
filament. Instead, it is associated with Ca2+ binding to
the PEVK element, preventing titin elongation and promoting the
proximity of the distal end of titin to the central M-band of the
sarcomere. Regardless of whether the mechanism underlying the
increase in titin stiffness is fully understood or not, it is
evident that titin, as the third filament, dynamically contributes
to the regulation of passive force changes within the sarcomere by
interacting with the thick and thin filaments (21,41,44).
This role of titin complements the limitations of the two-filament
sarcomere model (40), providing a
more comprehensive explanation for the mechanism of an eccentric
contraction.
The three-filament sarcomere model in
the interpretation of the eccentric contraction mechanism
According to several relevant studies (21,40,42,45,46),
combined with the three-filament sarcomere model, the mechanism of
eccentric contraction can be explained as follows (3,23,47)
(Fig. 2): When the sarcomere
undergoes active stretching, troponin actively binds to
Ca2+, resulting in a conformational change in actin. In
turn, it initiates the binding of myosin heads to actin,
facilitating the formation of cross-bridges. Through the swinging
motion of the cross-bridges, the thin filaments actively slide
towards the center of the thick filaments, thereby completing the
contraction process of the sarcomere. Simultaneously, during
contraction, the sarcomere is stretched by an external force,
resulting in the elongation of the proximal Ig domain and PEVK
element in the I-band region of titin. This elongation increases
the compliance of the sarcomere, acting as a buffer against the
external force (Fig. 2A). Under
high force, the N2A element attaches to actin, reducing the free
length of titin.
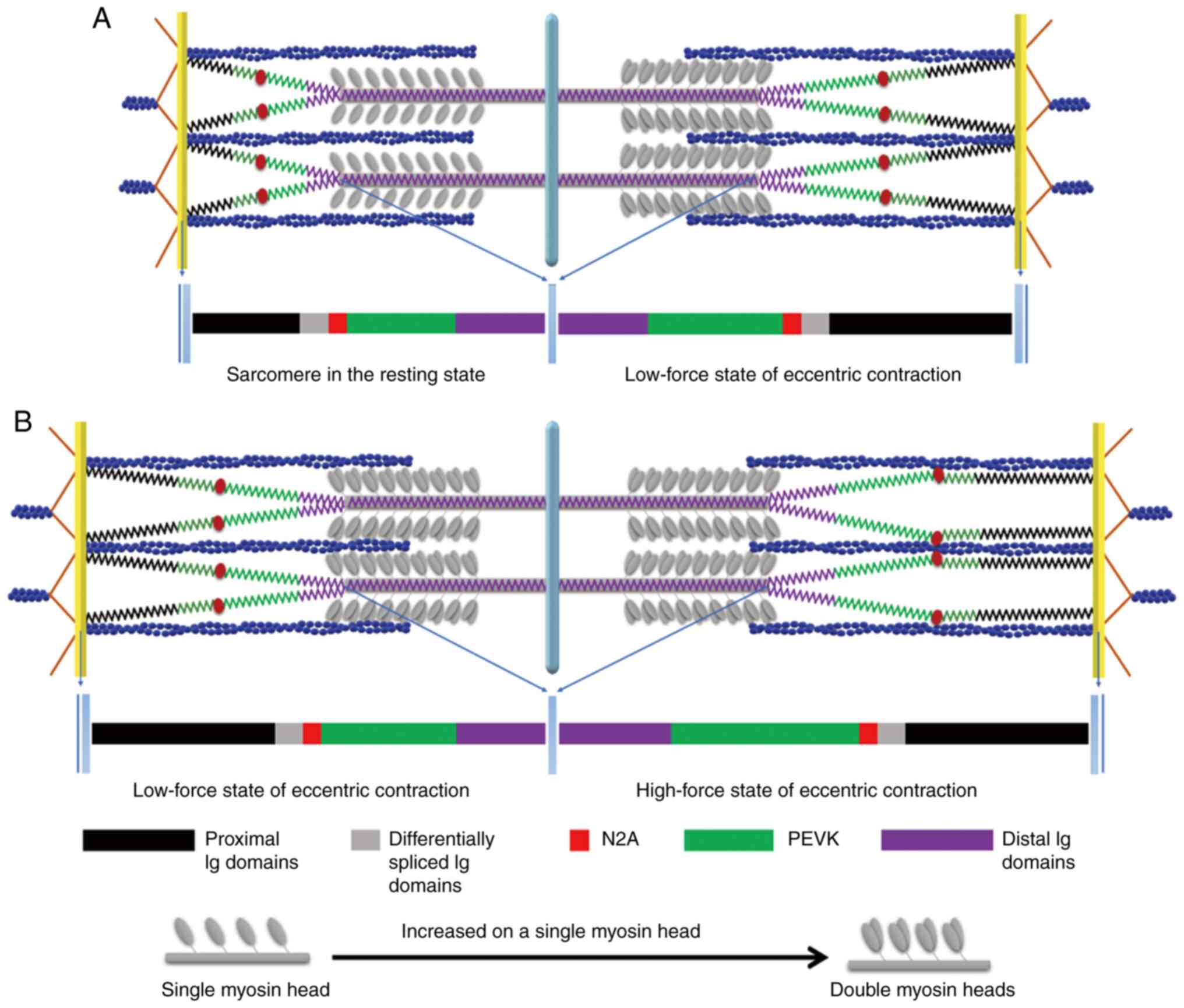 | Figure 2Illustration of the eccentric
contraction mechanism. In the resting state of the sarcomere (left
half-sarcomere), only one myosin head is activated to form the
cross-bridge during isometric and concentric contractions. However,
during an eccentric contraction, the increased strain on a single
myosin head may activate the second head, forming additional
cross-bridges and prolonged detachment time. During the low-force
stretch state of eccentric contraction [right half-sarcomere in (A)
and left half-sarcomere in (B)], the titin proximal Ig domain
undergoes stretching, causing the PEVK region to act as a spring
and increase in length. In the high-force stretch state of
eccentric contraction [right half-sarcomere in (B)], the increased
Ca2+ concentration results in the
Ca2+-dependent binding of titin N2A to actin, reducing
the titin-free length and increasing titin stiffness.
Simultaneously, Ca2+ binds to PEVK, preventing
over-stretching and increasing its stiffness, which plays a
protective role in the sarcomere. Data from a prior research of the
authors were used to create this visualization (23). Ig, immunoglobin; PEVK,
proline-glutamate-valine-lysine. |
On the other hand, binding the PEVK element to
Ca2+ prevents excessive elongation of PEVK. Furthermore,
the swing of the cross-bridge during its translation and rotation
brings the A-band and distal titin domains in closer proximity to
the central M-band of the sarcomere. This active process
effectively restrains the excessive elongation of the PEVK element
and enhances the stiffness of titin. Consequently, it prevents the
sarcomere from becoming overly compliant and safeguards the thick
and thin filaments against potential damage (Fig. 2B). Therefore, the sliding filament
theory of the titin-based three-filament sarcomere model provides
an improved understanding of the stability mechanism observed in
the descending limb of the sarcomere force-length relationship
curve during eccentric contraction. It indicates that the
spring-like properties and stiffness of titin help to maintain the
stability of the sarcomere during an eccentric contraction and the
functional characteristics of titin elucidate the mechanism of
eccentric exercise-induced skeletal muscle damage.
5. Remaining problems in the mechanism of
eccentric exercise-induced skeletal muscle damage
Compared with concentric and isometric exercises,
eccentric exercise actively increases muscle force while consuming
less energy, making it widely utilized in physical fitness training
and sports rehabilitation. However, unaccustomed exercise,
particularly eccentric exercise, can lead to skeletal muscle
damage, with the primary symptom being ultrastructural changes in
the muscle (mainly characterized by sarcomere structural changes,
such as Z-disk streaming and myofibril disruption or popping), as
well as symptoms of DOMS such as reduced muscle force, soreness,
swelling and increased concentration of creatine kinase (CK) in the
blood (1,7). Adverse effects such as pain, swelling
and impaired movement induce compensatory mechanisms in the
musculoskeletal system, further increasing the risk of sports
injury and potentially leading to chronic injury and pain, thus
exacerbating sports-related damage (7,9-11).
Consequently, some scholars have cautioned against using eccentric
exercise modes for chronic disease rehabilitation training
(1,6). Therefore, understanding the mechanism
of eccentric exercise-induced skeletal muscle damage has been a
crucial and challenging area of research in sports medicine, aiming
to address exercise practice issues. It has focused on
investigating the causes of ultrastructural changes in skeletal
muscle, which occur with a delay and parallel the symptoms of DOMS,
peaking within 24-72 h after exercise (7). Ultrastructural changes in skeletal
muscle can trigger an exercise-induced inflammatory response
closely associated with muscle soreness and swelling. Therefore,
the prevailing consensus attributes the symptoms of DOMS to the
ultrastructural changes in skeletal muscle, indicating that
eccentric exercise-induced muscle damage results from these
alterations (4,7).
Most scholars support the popping sarcomere
hypothesis as the mechanism of eccentric exercise-induced skeletal
muscle damage. The main arguments derive from the sliding filament
theory of the two-filament sarcomere model and the theory of
non-uniform sarcomere length. These theories propose that during
eccentric exercise, the passive elongation of the sarcomere is not
uniform and the sarcomere structure is most unstable on the
descending limb of the force-length relationship curve.
Consequently, weaker sarcomeres are prone to be excessively
stretched, leading to their disruption or destruction, often called
‘popping’. As the damage intensifies, the sarcolemma is breached,
resulting in uncontrolled entry of extracellular calcium ions into
the cytoplasm, activating the proteolytic enzyme calpains,
ultimately causing muscle damage (4,7,12,48).
However, a number of studies do not support the ‘popping’ theory,
as the integrity of the sarcolemma remains unaffected (49,50),
and the degree of ultrastructural changes in skeletal muscle does
not align with the symptoms of DOMS (7). The causal relationship between the
extent of ultrastructural changes in skeletal muscle and changes in
CK concentration and muscle force is also a subject of debate and
does not correspond to DOMS (4,7,12).
These findings indicated that the idea of attributing eccentric
exercise-induced skeletal muscle damage solely to sarcolemma damage
remains a subject of controversy.
6. The three-filament sarcomere model in the
interpretation of the mechanism of eccentric exercise-induced
skeletal muscle damage
The protective effect of titin
stiffness causes shear stress on the sarcolemma and extracellular
matrix (ECM)
Based on the mechanism of eccentric contraction
elucidated by the three-filament sarcomere model and recent
research, it is clear that the cause of skeletal muscle damage from
eccentric exercise is not only due to the overstretching of weaker
sarcomeres, which can lead to tearing of the sarcolemma. Instead,
researchers presume that the increased protection is a consequence
of the spring stiffness of the third filament titin (49,50).
Brynnel et al (45) conducted detailed studies using
skinned myofibers (diaphragm muscle and extensor digitorum longus)
to explore the protective effect of titin and the ECM on skeletal
muscle during eccentric contraction. Their findings revealed that
within the sarcomere's normal physiological working length range
(2.45-2.75 µm), titin primarily contributed to the increase in
passive component stiffness of skeletal muscle. However, beyond
this range, titin cooperates with ECM to further increase the
stiffness of the passive component. Furthermore, a previous study
revealed that the effect of increasing stiffness in titin is not
contingent upon the range of sarcomere length (51). This finding indicated that titin
works with the ECM to protect the structural integrity of the
sarcomere and sarcolemma during eccentric contraction.
Moreover, a number of studies have revealed that
sarcomere contraction actively transmits force to the tendons
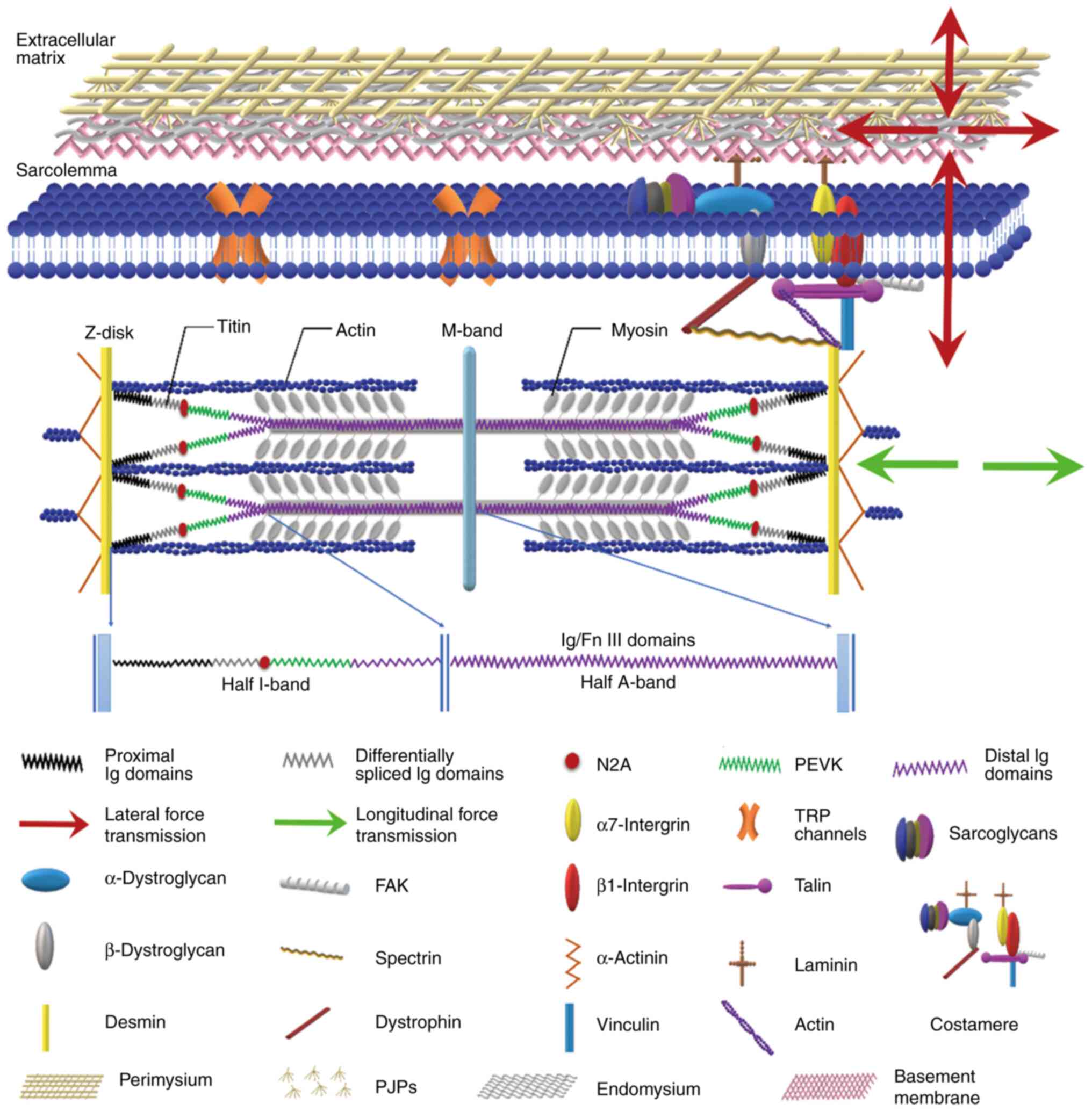
through two pathways (Fig. 1): i)
Longitudinal force transmission between the sarcomeres and ii) a
lateral force transmission pathway. The latter pathway involves the
transmission of force through costamere proteins (dystrophin and
α7β1 integrin) located near the Z-disk of the sarcolemma, followed
by lateral transmission to the endomysium, perimysium, epimysium
(also known as ECM) (52) and
eventually to the tendon (53,54).
After performing acute and chronic eccentric
contractions, a significant increase in the lateral force
transmission of skeletal muscle (17) and collagen fiber deposition in the
endomysium and perimysium (18) was
observed, respectively, suggesting a potential association between
the protective mechanism of titin stiffness and the enhancement of
lateral force transmission. Notably, the generation of shear stress
on the endomysium shared by adjacent myofibers is directly related
to the magnitude of lateral force transmission (17). During eccentric contractions, the
sarcolemma and ECM work together to produce shear stress, which is
essential in causing skeletal muscle damage.
The shear deformation induced by the
shear stress of the sarcolemma and ECM leads to skeletal muscle
damage
Hypothesis suggests that the endomysium experiences
heightened shear stress during an eccentric contraction,
facilitating enhanced lateral force transmission (17,18).
Experts consider this phenomenon helps to protect and change the
sarcolemma and ECM (17,18,23,45).
This repeated stretching induces increased permeability of the
sarcolemma and ECM damage, ultimately leading to skeletal muscle
damage. Recent studies support the notion that the sarcolemma
possesses structural characteristics capable of generating shear
stress at the cellular and molecular levels, thereby supporting the
plausibility of increased sarcolemma permeability due to shear
stress. The lateral force transmission mechanism connects the
sarcolemma with the ECM through costamere proteins, providing a
structural basis for ECM shear stress damage (17). Studies have revealed the involvement
of specific proteins in stabilizing the sarcolemma, such as
dystrophin and caveolae, which play a role in mitigating mechanical
stress and buffering the force on the sarcolemma during stretching
(55,56). Furthermore, the expression of the α7
integrin gene, a marker of mechanical stress, increases with
eccentric exercise, further highlighting the involvement of the
sarcolemma in shear stress (57).
Additionally, experiments utilizing electrical stimulation and
stretch-activated channel blockers have confirmed the increased
permeability of the sarcolemma and its role in eccentric
exercise-induced skeletal muscle damage (58).
In addition to sarcolemma damage, the shear
deformation of the ECM due to the sarcomere's lateral force
transmission also leads to ECM damage. Studies have revealed an
accumulation of ECM damage, characterized by collagen fiber
proliferation in the endomysium and perimysium, after eccentric
training, suggesting the involvement of ECM in DOMS (18). This finding supports the view that
DOMS originates from ECM damage, independent of ultrastructural
changes in skeletal muscle (23,49,50).
The understanding of these mechanisms has important practical
implications. Firstly, incorporating eccentric training into
rehabilitation programs enables the utilization of a
non-destructive approach to target chronic diseases, minimizing
harm to myofibers. Secondly, this approach aids in elucidating the
contentious association between skeletal muscle ultrastructural
changes and the manifestation of symptoms related to DOMS. These
mechanisms can operate autonomously, with DOMS symptoms sharing a
common mechanism involving ECM participation.
Mechanism of skeletal muscle damage:
Shear deformation theory
To sum up the aforementioned statements, the
mechanism of skeletal muscle damage is intricately linked to the
shear deformation experienced by the sarcolemma and ECM as forces
transmit laterally within the sarcomere. The authors refined and
renamed this concept the ‘shear deformation theory’ based on the
‘popping sarcomere hypothesis (32,48)’.
The critical argument posits that passive stretching leads to
non-uniformity in length by the sliding filament theory of the
three-filament sarcomere model and the theory of non-uniform
sarcomere length. Moreover, the structural stability of the
sarcomere is at its weakest on the descending limb of the
force-length relationship curve during eccentric exercise. The
stiffness of titin and ECM increases to safeguard the sarcomere and
sarcolemma from damage.
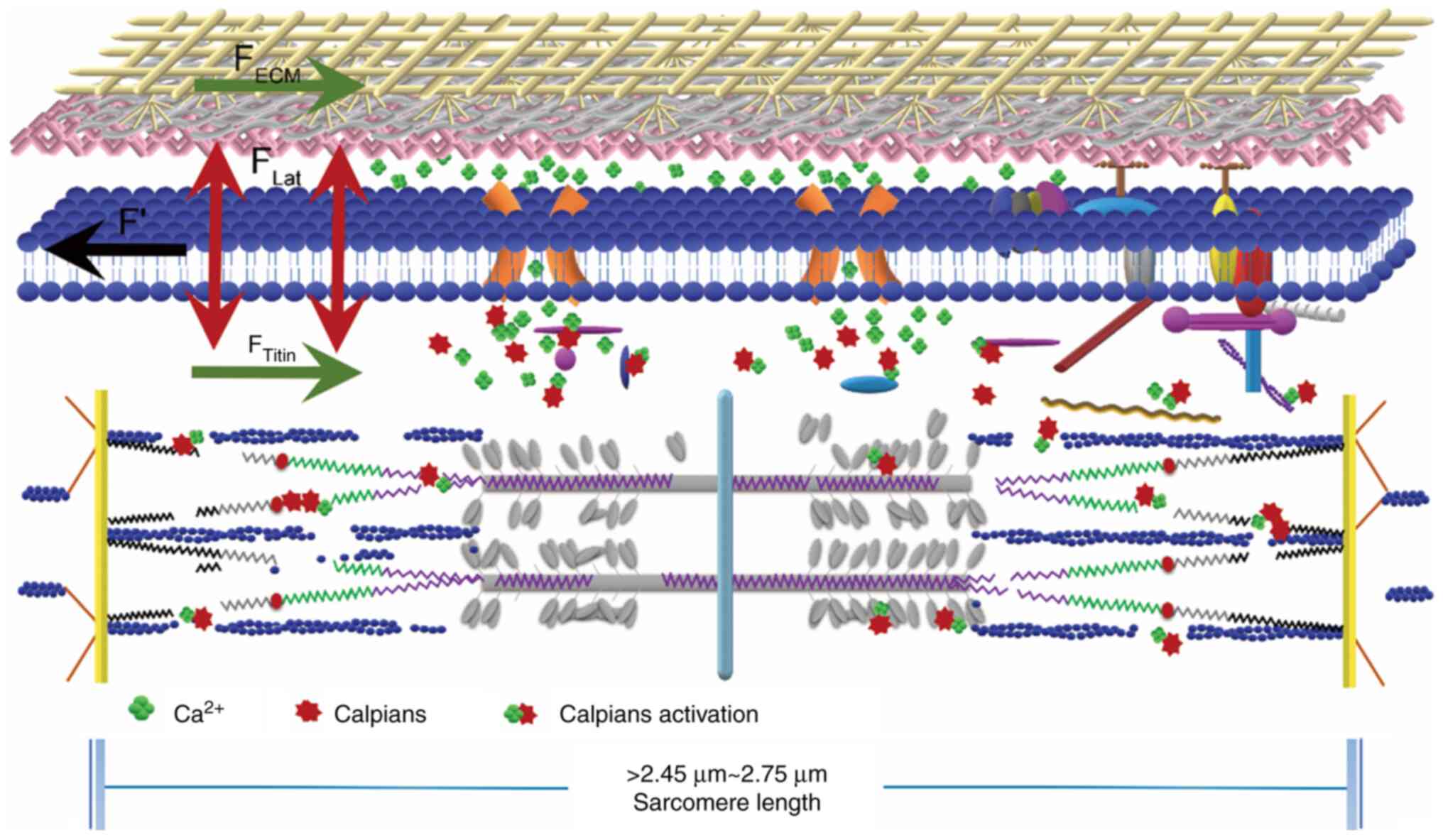
Consequently, the lateral transmission of forces
within the sarcomere triggers shear deformation of the sarcolemma
and ECM. This shear deformation prompts heightened permeability of
the sarcolemma, resulting in an uncontrolled influx of
extracellular calcium ions into the cytoplasm. Subsequently, the
activation of calpains ensues, leading to ultrastructural damage in
skeletal muscle. Simultaneously, the shear deformation of the ECM
induces shear damage, culminating in DOMS via an inflammatory
response (Fig. 3): When the
sarcomere length exceeds the physiological range of 2.45-2.75 µm,
the increased stiffness of titin and ECM functions to safeguard the
integrity of the sarcolemma. Simultaneously, the lateral force
transmission causes shear deformation, resulting in heightened
permeability of the sarcolemma and ECM, leading to shear damage.
These processes induce skeletal muscle ultrastructural changes and
contribute to DOMS.
7. Conclusions
The two-filament sarcomere model, which forms the
basis of the sliding filament theory, needs to be adequately
explained the mechanism of eccentric contraction. However, by
incorporating the third filament, titin, into the sliding filament
theory, the eccentric contraction mechanism through the
three-filament sarcomere model can be further elucidated. Per the
revised sliding filament theory based on the three-filament
sarcomere model, skeletal muscle exhibited a dual-layer protection
mechanism during an eccentric contraction involving increased titin
and ECM stiffness. Subsequently, the shear stress generated by
lateral force enhances the permeability of the sarcolemma and leads
to ECM damage, ultimately resulting in skeletal muscle damage.
Acknowledgements
Not applicable.
Funding
Funding: The present review was supported by the Natural Science
Foundation of Shandong (grant no. ZR2020MC080).
Availability of data and materials
Not applicable.
Authors' contributions
ZX conceived and designed the review. ZQ and LP were
major contributors to writing the manuscript. ZQ and LP wrote parts
of the manuscript and ZQ prepared the figures. All authors read and
approved the final version of the manuscript. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hody S, Croisier JL, Bury T, Rogister B
and Leprince P: Eccentric muscle contractions: Risks and benefits.
Front Physiol. 10(536)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu AS and Zhang XL: Research progress of
eccentric training interventions on type 2 diabetes based on matrix
metalloproteinases 2, 9 regulating extracellular matrix
homeostasis. Chin J Sports Med. 36:1004–1011. 2017.
|
|
3
|
Tomalka A: Eccentric muscle contractions:
From single muscle fibre to whole muscle mechanics. Pflugers Arch.
475:421–435. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Douglas J, Pearson S, Ross A and McGuigan
M: Eccentric exercise: Physiological characteristics and acute
responses. Sports Med. 47:663–675. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Annibalini G, Contarelli S, Lucertini F,
Guescini M, Maggio S, Ceccaroli P, Gervasi M, Ferri Marini C,
Fardetti F, Grassi E, et al: Muscle and systemic molecular
responses to a single flywheel based iso-inertial training session
in resistance-trained men. Front Physiol. 10(554)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lovering RM and Brooks SV: Eccentric
exercise in aging and diseased skeletal muscle: Good or bad? J Appl
Physiol (1985). 116:1439–1445. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang X, Zhang XL and Xu SS: Exercise and
skeletal muscle ultrastructural changes. Chin J Sports Med.
29:109–113. 2010.
|
|
8
|
Keriven H, Sánchez-Sierra A,
Miñambres-Martín D, González de la Flor Á, García-Pérez-de-Sevilla
G and Domínguez-Balmaseda D: Effects of peripheral electromagnetic
stimulation after an eccentric exercise-induced delayed-onset
muscle soreness protocol in professional soccer players: A
randomized controlled trial. Front Physiol.
14(1206293)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang XL, Gao XJ, Shi JP, Li JP, Zhou Y
and Wang R: The mechanism of eccentric exercise-induced skeletal
muscle overuse injuries. Chin J Sports Med. 31:1064–1074. 2012.
|
|
10
|
Thirupathi A, Freitas S, Sorato HR,
Pedroso GS, Effting PS, Damiani AP, Andrade VM, Nesi RT, Gupta RC,
Muller AP and Pinho RA: Modulatory effects of taurine on metabolic
and oxidative stress parameters in a mice model of muscle overuse.
Nutrition. 54:158–164. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang XL and Wang RY: The mechanism of
overuse injuries: skeletal muscle tensegrity complex imbalance
theory. Chin J Sports Med. 32:646–653. 2013.
|
|
12
|
Wang RY: Skeletal muscle and exercise.
People's Sports Publishing House, Beijing, 2013.
|
|
13
|
Huxley AF and Niedergerke R: Structural
changes in muscle during contraction; interference microscopy of
living muscle fibres. Nature. 173:971–973. 1954.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Huxley H and Hanson J: Changes in the
cross-striations of muscle during contraction and stretch and their
structural interpretation. Nature. 173:973–976. 1954.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Herzog W: Mechanisms of enhanced force
production in lengthening (eccentric) muscle contractions. J Appl
Physiol (1985). 116:1407–1417. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Novak I and Truskinovsky L: Nonaffine
response of skeletal muscles on the ‘descending limb’. Math Mech
Solids. 20:697–720. 2015.
|
|
17
|
Zhang X, Zhang XL, Kong M and Ye ML:
Changes in lateral force transmission of skeletal muscle induced by
acute eccentric exercise and acupuncture intervention effect. Chin
J Sport Sci Technol. 54:94–108. 2018.
|
|
18
|
Kong M, Zhang X, Ye ML and Zhang XL:
Changes of perimysial junctional plates induced by excessive
eccentric training and the effects of acupuncture intervention.
Sheng Li Xue Bao. 69:17–32. 2017.PubMed/NCBI(In Chinese).
|
|
19
|
Hanson J and Huxley HE: Structural basis
of the cross-striations in muscle. Nature. 172:530–532.
1953.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Huxley AF: Reflections on muscle.
Liverpool UK: Liverpool University Press, 1980.
|
|
21
|
Herzog W: The role of titin in eccentric
muscle contraction. J Exp Biol. 217:2825–2833. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Horowits R and Podolsky RJ: The positional
stability of thick filaments in activated skeletal muscle depends
on sarcomere length: Evidence for the role of titin filaments. J
Cell Biol. 105:2217–2223. 1987.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao Q and Zhang XL: Discussion on
revision of the sliding filament theory to resolve problems of
eccentric contraction mechanism. Acta Physiol Sinica. 73:143–147.
2021.
|
|
24
|
Herzog W, Schappacher G, Duvall M, Leonard
TR and Herzog JA: Residual force enhancement following eccentric
contractions: A new mechanism involving titin. Physiology
(Bethesda). 31:300–312. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huxley HE: Electron microscope studies of
the organisation of the filaments in striated muscle. Biochim
Biophys Acta. 12:387–394. 1953.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huxley AF: Muscle structure and theories
of contraction. Prog Biophys Biophys Chem. 7:255–318.
1957.PubMed/NCBI
|
|
27
|
Huxley HE: The mechanism of muscular
contraction. Science. 164:1356–1365. 1969.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Huxley AF and Simmons RM: Proposed
mechanism of force generation in striated muscle. Nature.
233:533–538. 1971.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Morgan DL, Whitehead NP, Wise AK, Gregory
JE and Proske U: Tension changes in the cat soleus muscle following
slow stretch or shortening of the contracting muscle. J Physiol.
522:503–513. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Iwazumi T: Molecular mechanism of muscle
contraction. Physiol Chem Phys Med NMR. 21:187–219. 1989.
|
|
31
|
Morgan DL: New insights into the behavior
of muscle during active lengthening. Biophys J. 57:209–221.
1990.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Morgan DL and Proske U: Popping sarcomere
hypothesis explains stretch-induced muscle damage. Clin Exp
Pharmacol Physiol. 31:541–545. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rassier DE, Herzog W and Pollack GH:
Dynamics of individual sarcomeres during and after stretch in
activated single myofibrils. Proc Biol Sci. 270:1735–1740.
2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rassier DE, Herzog W and Pollack GH:
Stretch-induced force enhancement and stability of skeletal muscle
myofibrils. Adv Exp Med Biol. 538:501–515. 2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dos Remedios CG: Comparative studies on
striated muscle. PhD thesis, University of Sydney, 1965.
|
|
36
|
Maruyama K: Connectin, an elastic protein
from myofibrils. J Biochem. 80:405–407. 1976.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang K, McClure J and Tu A: Titin: Major
myofibrillar components of striated muscle. Proc Natl Acad Sci USA.
76:3698–3702. 1979.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fürst DO, Osborn M, Nave R and Weber K:
The organization of titin filaments in the half-sarcomere revealed
by monoclonal antibodies in immunoelectron microscopy: A map of ten
nonrepetitive epitopes starting at the Z line extends close to the
M line. J Cell Biol. 106:1563–1572. 1988.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Linke WA and Krüger M: The giant protein
titin as an integrator of myocyte signaling pathways. Physiology
(Bethesda). 25:186–198. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Holt NC: Beyond bouncy gaits: The role of
multiscale compliance in skeletal muscle performance. J Exp Zool A
Ecol Integr Physiol. 333:50–59. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nishikawa KC, Monroy JA, Uyeno TE, Yeo SH,
Pai DK and Lindstedt SL: Is titin a ‘winding filament’? A new twist
on muscle contraction. Proc Biol Sci. 279:981–990. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
DuVall MM, Jinha A, Schappacher-Tilp G,
Leonard TR and Herzog W: Differences in titin segmental elongation
between passive and active stretch in skeletal muscle. J Exp Biol.
220:4418–4425. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Labeit D, Watanabe K, Witt C, Fujita H, Wu
Y, Lahmers S, Funck T, Labeit S and Granzier H: Calcium-dependent
molecular spring elements in the giant protein titin. Proc Natl
Acad Sci USA. 100:13716–13721. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Leonard TR and Herzog W: Regulation of
muscle force in the absence of actin-myosin-based cross-bridge
interaction. Am J Physiol Cell Physiol. 299:C14–C20.
2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Brynnel A, Hernandez Y, Kiss B, Lindqvist
J, Adler M, Kolb J, van der Pijl R, Gohlke J, Strom J, Smith J, et
al: Downsizing the molecular spring of the giant protein titin
reveals that skeletal muscle titin determines passive stiffness and
drives longitudinal hypertrophy. Elife. 7(e40532)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Weidner S, Tomalka A, Rode C and Siebert
T: How velocity impacts eccentric force generation of fully
activated skinned skeletal muscle fibers in long stretches. J Appl
Physiol (1985). 133:223–233. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shi J, Watanabe D and Wada M: Eccentric
muscle contraction potentiates titin stiffness-related contractile
properties in rat fast-twitch muscles. J Appl Physiol (1985).
133:710–720. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Owens DJ, Twist C, Cobley JN, Howatson G
and Close GL: Exercise-induced muscle damage: What is it, what
causes it and what are the nutritional solutions? Eur J Sport Sci.
19:71–85. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jørgensen A, Foster PP, Eftedal I, Wisløff
U, Paulsen G, Havnes MB and Brubakk AO: Exercise-induced
myofibrillar disruption with sarcolemmal integrity prior to
simulated diving has no effect on vascular bubble formation in
rats. Eur J Appl Physiol. 113:1189–1198. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yu JG, Liu JX, Carlsson L, Thornell LE and
Stål PS: Re-evaluation of sarcolemma injury and muscle swelling in
human skeletal muscles after eccentric exercise. PLoS One.
8(e62056)2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Powers JD, Bianco P, Pertici I, Reconditi
M, Lombardi V and Piazzesi G: Contracting striated muscle has a
dynamic I-band spring with an undamped stiffness 100 times larger
than the passive stiffness. J Physiol. 598:331–345. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lieber RL and Binder-Markey BI: .
Biochemical and structural basis of the passive mechanical
properties of whole skeletal muscle. J Physiol. 599:3809–3823.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ramaswamy KS, Palmer ML, van der Meulen
JH, Renoux A, Kostrominova TY, Michele DE and Faulkner JA: Lateral
transmission of force is impaired in skeletal muscles of dystrophic
mice and very old rats. J Physiol. 589:1195–1208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Minato K, Yoshimoto Y, Kurosawa T,
Watanabe K, Kawashima H, Ikemoto-Uezumi M and Uezumi A: Measurement
of lateral transmission of force in the extensor digitorum longus
muscle of young and old mice. Int J Mol Sci.
22(12356)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Le S, Yu M, Hovan L, Zhao Z, Ervasti J and
Yan J: Dystrophin as a molecular shock absorber. ACS Nano.
12:12140–12148. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lo HP, Hall TE and Parton RG: .
Mechanoprotection by skeletal muscle caveolae. Bioarchitecture.
6:22–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Boppart MD, Volker SE, Alexander N, Burkin
DJ and Kaufman SJ: . Exercise promotes alpha7 integrin gene
transcription and protection of skeletal muscle. Am J Physiol Regul
Integr Comp Physiol. 295:R1623–R1630. 2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhang BT, Whitehead NP, Gervasio OL,
Reardon TF, Vale M, Fatkin D, Dietrich A, Yeung EW and Allen DG:
Pathways of Ca²+ entry and cytoskeletal damage following
eccentric contractions in mouse skeletal muscle. J Appl Physiol
(1985). 112:2077–2086. 2012.PubMed/NCBI View Article : Google Scholar
|