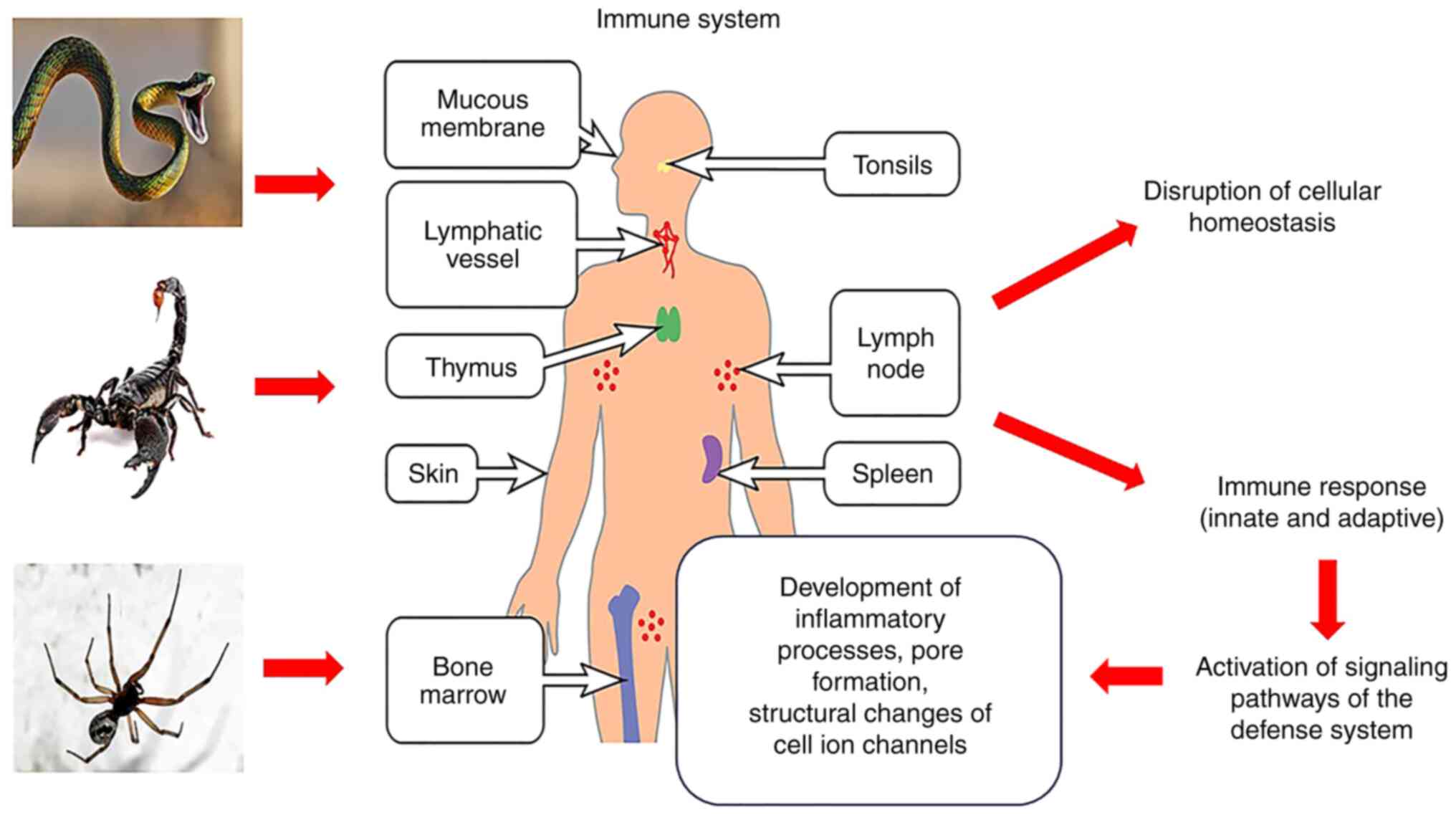
The human body is affected by environmental factors
(such as bacteria, viruses, climate, toxins). The dynamic balance
between the organism and its environment results from the influence
of natural, anthropogenic and social aspects. The action of any
elements of exogenous origin determines the development of adaptive
changes. Almost all organs and systems forming these adaptive
mechanisms, the coordinated activity of which maintains stability
in the internal environment, known as homeostasis (1). Homeostasis is provided by normal
functioning of the immune, nervous and endocrine systems.
Maintaining the stability of the internal environment must be
considered not only at the tissue, organ and system levels but also
at the molecular and cellular levels since this is where the
primary response to the action of external agents begins.
Maintaining dynamic balance depends on how long the damaging
factors act (2). The organism can
show self-regulation, reactivity and stability. However, damage to
the structural and functional components of the systems that ensure
the maintenance of homeostasis leads to disruption of coordinated
activity and pathological reactions (3,4).
Among the factors affecting the homeostasis system,
animal venom toxins play an important role. Venoms comprise
proteins, peptides, biogenic amines and salts produced by various
species of animal for protection or hunting prey. However, in the
case of bites of venomous animals, the body receives numerous
toxins that are distributed in the skin, blood vessels, skeletal
muscle fibres, and organs (5).
Typically, venoms containing substances of a protein nature also
include minor protein components and a number of organic and
inorganic substances, which together determine the physiological
activity and nature of the toxic effect (6). Animal venoms usually include enzymes
(hyaluronidase, phospholipase A, nucleotidase, phosphodiesterase,
deoxyribonuclease, L-amino acid oxidase, acid phosphatase and
acetylcholinesterase), proteins with specific properties (nerve
growth and anticomplementary factor), hemotoxins, neurotoxins,
biogenic amines (serotonin and histamine), monosaccharides and
polysaccharides (7).
Cells of the immune system are the first barrier to
pathogenic factors. Membrane receptors recognise changes in the
intercellular matrix and signal disruption of the homeostasis
system and the initiation of compensatory signalling cascades. The
mechanisms of the cellular response to the action of certain
stimuli depend mainly on the duration of disturbances in
homeostasis. There are four stages of the cellular response
(3,4). During the first, changes occur in
phosphorylation and dephosphorylation of key regulatory proteins to
restore impaired body functions or adapt to the changes. The second
stage involves activating the expression of fast-response genes. At
this stage, it is possible to recognise unfolded proteins due to
folding disorders in the endoplasmic reticulum (ER) and the
development of stress. Under stronger and prolonged exposure to
damaging agents, damage to the structure of organelles) occur due
to reprogramming of their genome and the concentration on
eliminating pathological changes (3-5).
These processes characterise the third stage of the cellular
response. At the fourth stage, activation of cell apoptosis
mechanisms mediated by ER stress or development of compensatory and
adaptive changes to a constantly acting stressor occurs. The
mechanisms of cell response are aimed at survival and preservation
of the organism (8,9).
Action of toxins of various origins, including the
components of animal venom, disrupt normal functioning and induce
structural rearrangements of organs (7). There are numerous venomous animals
with insufficiently studied proteome, peptidome and biological
activity that are the focus of an increasing number of experimental
studies (7,8).
The effects of venom range from mild clinical
symptoms to death. Symptoms of venom are divided into local and
systemic. Local symptoms include reddening of the skin at the site
of bite, swelling and enlargement and swelling of lymph nodes.
Systemic manifestations include nausea, vomiting, sweating,
bleeding, fever, difficulty breathing and anaphylaxis. Disturbances
of normal functioning of the lymphatic system, the development of
lymphadenitis and neuromuscular fasciculations are long-term
consequences of venomous animal bites (9,10).
Toxins leads to the activation of the immune system, which ensures
the formation of compensatory and adaptive changes (Fig. 1). Its dysregulation, mediated by the
action of the venom, can cause severe complications or even death
(11,12).
To protect against the components of animal venom, a
quick reaction is essential and is achieved by the coordinated work
of the innate immune system. The mechanisms include blood-tissue
barriers for an immediate but non-specific response to the action
of toxins. Physical barriers, such as skin and mucous membranes, as
well as a set of chemicals, including enzymes, that interact with
resident and migrating cells, are key in the host response. In
response to any stressor, the activation of pro-inflammatory
mediators such as cytokines and chemokines is observed (1-3).
In addition, numerous cells of the immune system are activated,
migration of leukocytes to the affected area is initiated and the
production of reactive oxygen species (ROS), reactive compounds of
nitrogen oxide (NO) and numerous proteases are generated, ensuring
maintenance of the regulation of innate effector functions and
homeostasis (13-16).
Key components of an innate immune response are
keratinocytes of the epidermis, which act as the first barrier when
bitten by venomous animals. Keratinocytes play a protective and
pro-inflammatory function and their cross-interactions with the
cells of the dermo-epidermal junctions underlie regulation of
immune cell maturation processes at the initial and late stages of
inflammation (17-19).
Similar to cells of the immune system, keratinocytes express
receptors for cytokines and pattern recognition receptor (PRR)
proteins that can recognise common molecular structures, such as
venoms. Their activation under the action of the components of
animal venom initiates synthesis and release of cytokines, NO and
alarmins (endogenous constitutive, chemotactic and
immune-activating peptides that are released in case of injury or
cell death or in response to induction of the immune defence
system) (20,21). These processes cause the formation
of foci of inflammation and involvement of numerous cells, both
resident and migratory (22).
Proteolytic enzymes induce the death of keratinocytes by apoptosis
or necrosis (23,24). The proteolytic degradation of
structures of the epidermis and dermis ensures the access of venom
components to the blood circulation, lymphatic system and target
organs. Apoptosis of keratinocytes may lead to excessive expression
of endogenous MMPs, which indirectly triggers the destruction of
tissues at the bite sites. Bites of the Loxosceles rufescens
spider cause key dermonecrotic consequences, systemic inflammatory
reaction and even fatal consequences, especially among children. In
this case, the pathophysiological mechanisms of action of toxins
are attractive, which consist in the stimulation of apoptosis of
epidermal cells by the enzyme sphingomyelinase D, which increases
the synthesis of membrane-bound MMP-2 and MMP-9 in cell culture
(25-28).
Endothelial cells of the walls of blood vessels also
play an essential role in recognising and protecting the body from
toxins. As the main point of contact with toxic components of
natural venoms that have entered the bloodstream, endotheliocytes
perform the function of recognising them by expressing numerous
PRRs, among which toll-like receptor (TLR), TNF receptors and IL-1
and cause activation of pro-inflammatory genes and pathological
changes in the microcirculation of blood vessels (26,27).
Endotheliocytes also express molecules of the major
histocompatibility complex (MHC) classes I and II and CD40 ligands,
ensuring intravascular presentation of foreign agents, including
animal venom toxins, to effector cells of the immune system
(28). Endothelial cells modulate
function of the immune system by affecting migration of leukocytes.
Adhesion and extravasation of leukocytes are characteristic
phenomena in response to highly selective expression of cell
adhesion molecules-1 and selectin on the apical surface of
endotheliocytes. Endothelial dysfunction as a result of venom
toxins, characterised by distortion of structure and functions of
endothelial cells leads to changes in the immune response. Rat
experiments have proven a violation of the permeability and
stability of vessel walls under endothelial dysfunction. In the
case of snake and spider venoms, an increase in secretion of IL-6
and 8 and monocyte chemoattractant protein-1 (MCP-1) is also
observed in vitro in culture of endothelial cells (29,30).
With increased production of these compounds, neutrophils exhibit
adhesive properties in relation to endotheliocytes through
selectin-mediated connections, and this notably increases
intracellular levels of Ca2+ and release of proteolytic
enzymes responsible for tissue degradation (30,31).
The monocyte-macrophage system (MMS) is a key
component of innate immunity. Most animal toxins disrupt the
structure and function of MMS cells (32). Toxins of Crotalus durissus
terrificus viper decreases migratory and phagocytic ability of
macrophages in the peritoneal cavity of rats. The crotoxin of their
venom has enzymatic properties and is capable of significantly
decreasing the expression of MHC type II molecules that presenting
foreign agents to T lymphocytes, as well as costimulatory molecules
such as CD40, CD80 and CD86. In addition, crotoxin inhibits the
production of IL-6, TNF-α and IL-12 and interferes with the
phosphorylation of NF-κB and MAPK p38. However, it stimulates
production of IL-10, TGFβ and prostaglandin E2 (PGE2) and
phagocytic activity of macrophages and causes toxin-mediated
changes in cytoskeleton proteins of these cells. The effect of
crotoxin is accompanied by production of NO in macrophages by
activation of inducible NO synthase (iNOS). Under these conditions,
glucose metabolism and the amino acid glutamine are disturbed in
the cells due to induction of hexokinase, glucose-6-phosphate
dehydrogenase and glutaminase. Such enzymatic hyperactivity in
macrophages is associated with an increase in levels of ATP and
numerous metabolites, as well as stimulation of the inflammatory
response, primarily via the production of NADPH (32,34).
NADPH serves as a substrate for NADPH+ oxidase and ROS
secretion, in particular, H2O2 (33-35).
Other studies show that the venom of Bothrops alternatus
snake increases the phagocytic activity of macrophages and their
production of superoxide radicals, which are involved in tissue
destruction at the sites of bites (35-37).
Experiments on mice using macrophage cell culture revealed powerful
pro-inflammatory properties (36,37).
In particular, Androctonus crassicauda scorpion toxin
induces expression of IL-12p40 mRNA, which is a chemoattractant of
macrophages that stimulates migration of dendritic cells and is
associated with activation of the inflammatory cascade (38-40).
Toxins Ts1 and Ts6 of Tityus
serrulatus scorpion contribute to the production of NO and
H2O2 in macrophages and modulates the
inflammatory response, characterised by an increase in the blood
levels of TNF-α, IL-6, IL-1α, IL-1β and IL-8 (41-43)
in rats. C-type lectin-like proteins obtained from the venom of
Bothrops jararacussu snake enhance production of TNF by
macrophages and the activity of CD14 without affecting the
proliferative capabilities of these cells (44). Sphingomyelinase D in Loxosceles
laeta spider venom promotes macrophage migration and cytokine
release by skin fibroblasts. Bothrops snake toxins stimulate
secretion of pro-inflammatory mediators, PGE2, macrophage
inflammatory protein-1 (MIR-1) and IL-1β and NF-κB activation in
cultured human MMS cells (45,46).
Therefore, the components of the venom of various species of
predatory animals exert an immunostimulating effect on the MMS and
contribute to development of a systemic inflammatory response
(47-49).
Neutrophils are involved in rapid response to the
inoculation of animal venom toxins. Like other cells of the immune
system, they are potent producers of cytokines and chemokines
capable of activating pro-inflammatory mechanisms. Following bites
of venomous animals, exocytosis of secretory granules of
neutrophils, containing ~700 different proteins, mainly enzymes,
that enter the extracellular matrix, is characteristic (50,51).
Defensins, serine proteases, neutrophil elastase, proteinase 3 and
cathepsins are among the main enzymes capable of inactivating the
toxic components of venom through their proteolytic degradation
(50). The role of proteolytic
enzymes in the degradation of necrotic tissues has also been
established. Studies have demonstrated the role of neutrophils in
stimulating the programmed death of cells affected by venom of
predatory animals (52-55).
Tissue basophils, stimulation of which is associated
with inflammasome activation and caspase-1 expression, serve an
essential role in the mechanisms of the immune response to venomous
animal toxin (60,61). In addition, they release histamine
and lipid mediators, and their degranulation can cause development
of an anaphylactic reaction (62).
Congenital anomalies of basophils, including mutations in
mastocytosis, are causes of severe allergic reactions of the body
to animal bites and fatal consequences. Thus, it has been
demonstrated in rats that the toxins of B. atrox snakes
induce formation of fractions of the complement system C3a and C5a,
which contribute to degranulation of tissue basophils, chemotaxis,
activation of neutrophils and the development of severe anaphylaxis
(63-70).
In Brazil, 26,000 snakebites were reported in 2016,
with 109 deaths. The annual snakebite mortalities in India are
46,000, in Bangladesh-2 6,000 and 400 in Sri Lanka. The number of
isolated and identified animal toxins is increasing every year
(25). Therefore, researching their
molecular structure and mechanism of action is urgent. Most act by
modulating the main signalling cascades (PI3kinase pathway,
arachidonic acid cascade) of cells or directly interfering with ion
balance, which is maintained by cell membranes (71). Certain types of toxin penetrate the
bilipid layer of the plasmalemma, forming pores. By contrast,
others act on ion pumps or channels responsible for maintaining the
concentration gradients of ions (72). The molecular mechanisms underlying
the effect of animal venom on signalling cascades are that their
toxic components are directed at individual target points of cell
membranes, where, under normal conditions, secondary messengers
initiate a physiological response to stimuli. When interacting with
toxins, complex pathways of biochemical reactions are suppressed,
which causes a pathological response in host cells. Plasmolemma
targets of venoms include ligand-gated ion channels, G
protein-coupled and tyrosine kinase receptors, integrins and
specific lipids (73,74). Certain targets (receptors, ion
channels) are located inside cells, particularly in organelles
capable of forming a specific response to the action of the toxin.
Currently, two mechanisms of target changes at the level of host
cell membranes are known. The first consists of conformational
changes of receptors, opening of ion channels for the flow of ions
and depolarisation of the cell. The second mechanism is
translocation, which involves stimulus-induced movement of
transporters of certain compounds from cells to domains of the
outer surface of the plasmalemma (75-79).
With interaction between the components of animal
venoms and the host, processes such as energy metabolism,
post-translational changes, cytoskeleton stability, gene
expression, motility, secretion, cell division and specific
functions are disturbed. Under normal conditions, communication
between cells and natural ligands leads to controlled changes in
intracellular levels of second messengers such as cAMP,
Ca2+, inositol triphosphate and 1,2-diacylglycerol.
Protein kinases activated by these messengers phosphorylate
numerous molecules of the cellular substrate, stimulating
signalling pathways (80). However,
when the triggering of these mechanisms is caused by
non-physiological factors, such as animal venom toxins, the cascade
of signalling pathways is disrupted, leading to the development of
pathological changes in cells that can ultimately lead to their
death. Toxins typically enhance or inhibit the activity of proteins
and enzymes, which disrupts cellular homeostasis. Cell homeostasis
is ensured mainly by the integrity and stability of the
plasmalemma. This process is dynamic and regulated by cells to
compartmentalize and protect organelles and genetic material in the
nucleus. The primary requirement for membrane integrity is the
preservation of ion concentration gradients. The penetration of
animal toxins into the double lipid layer and the formation of
pores lead to a change in the concentration of ions and the death
of cells (81-83).
Pore-forming toxins are polypeptides that contain
both a hydrophilic/polar domain and a hydrophobic/nonpolar domain
that vary in size from small peptides and oligomers to large
macromolecules. These animal venom toxins increase permeability
and/or destroy the plasma membrane. Pardaxin, produced by certain
marine fish, exerts its effects through hydrophobic/lipophilic
interaction with phospholipids of biological membranes of host
cells and the formation of pores. This is associated with
colloid-osmotic changes in the cell, particularly swelling. In
addition, pardaxin stimulates the increase of the intracellular
levels of Ca2+, activation of PLA2, the
production of eicosanoids and numerous endonucleases, the release
of cytokines, the initiation of inflammatory mechanisms and
apoptosis (84-88).
Toxins of venomous animals have a pathological
effect on the ion channels of cell membranes. Under normal
conditions, these ion channels regulate transport of cations and
anions, maintain resting membrane potential and control action
potential in cells. Given the importance of ion concentration
gradients supporting the functioning of nervous, cardiac, skeletal
and smooth muscle tissue, many toxins have been investigated that
are capable of modulating the conductance and/or kinetics of ion
channels, serving as channel-opening or blocking agents (101-104).
In non-excitable cells, ion channels regulate transport of
nutrients, release of certain compounds and activation of cells of
the immune system. Most ion channels (Na+,
K+, Ca2+ and some Cl-) are
voltage-dependent, while others are insensitive to voltage changes
and are controlled by second messengers or intracellular or
extracellular mediators. Voltage-dependent ion channels open or
close depending on the concentration gradient on both sides of the
plasma membrane. Ions pass through channels according to their
electrochemical gradient. Toxins of scorpion and snake venoms
selectively change the activity of such channels (105-112).
Experimental studies have demonstrated that dendrotoxins produced
by several species of African snake (Dendroaspis angusticeps,
viridis and polylepis) block potential-dependent
K+ channels in neurons (108-111).
The effect of dendrotoxins increases release of acetylcholine in
neuromuscular junctions. In the nervous system, potential-dependent
K+ channels are responsible for membrane repolarisation
and control duration of the action potential. Dendrotoxins bind to
K+ channels of Ranvier intercepts of motoneurons,
blocking their activity. This increases duration of the action
potential and release of acetylcholine in synapses, leading to
excessive overexcitation and convulsions (113). The molecular mechanisms underlying
the interaction between dendrotoxins and potential-dependent
K+ channels are that their communication is initiated by
electrostatic connections between positively charged amino acid
radicals in the cationic region of dendrotoxin and negatively
charged radicals in the pores of ion channels. K+
channels have areas of negative charges localised at the front of
the channel. Dendrotoxin molecule is also capable of mechanically
blocking the channel pore. However, certain data suggest that
dendrotoxin blocks the channel by conformational changes in its
structure (114,115).
Toxic compounds of animal venom penetrating disrupt
the stability of the internal environment. The mechanisms
underlying pathological changes are associated with changes in the
structure, function and biochemical reactions. The first line of
defence against the negative effects of toxins is cells that
contribute to the restoration of damaged links of homeostasis or
the formation of specific adaptations. Toxins of various species of
venomous animals can interfere with the morpho-functional
properties of cells, destroying their protective membranes, forming
pores or disrupting the activity of ion channels. Components of the
immune, nervous and endocrine systems are key in defense and
adaptation processes in response to venom by triggering signalling
pathways. Coordinated activity supports the vital functions and the
dysfunction causes serious or fatal consequences. Therefore,
studying changes in the homeostasis, primarily at the cellular
level, under these conditions is key.
Not applicable.
Funding: No funding was received.
Not applicable.
RM and IS performed the literature review. IS
designed the study and wrote the manuscript. OM edited the
manuscript. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Jakob MO, Murugan S and Klose CSN:
Neuro-immune circuits regulate immune responses in tissues and
organ homeostasis. Front Immunol. 11(308)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Meizlish ML, Franklin RA, Zhou X and
Medzhitov R: Tissue homeostasis and inflammation. Annu Rev Immunol.
39:557–581. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mowel WK, Kotzin JJ, McCright SJ, Neal VD
and Henao-Mejia J: Control of immune cell homeostasis and function
by lncRNAs. Trends Immunol. 39:55–69. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vincze J and Vincze-Tiszay G: The Human
organism is a biophysical-biopsychological system. Technium.
2:29–35. 2018.
|
|
5
|
Larréché S, Chippaux JP, Chevillard L,
Mathé S, Résière D, Siguret V and Mégarbane B: Bleeding and
thrombosis: Insights into pathophysiology of Bothrops venom-related
hemostasis disorders. Int J Mol Sci. 22(9643)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Walker AA, Robinson SD, Hamilton BF,
Undheim EAB and King GF: Deadly proteomes: A practical guide to
proteotranscriptomics of animal venoms. Proteomics.
20(e1900324)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Warrell DA: Venomous bites, stings, and
poisoning: An update. Infect Dis Clin North Am. 33:17–38.
2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Almanza A, Carlesso A, Chintha C,
Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N,
Montibeller L, More S, et al: Endoplasmic reticulum stress
signalling-from basic mechanisms to clinical applications. FEBS J.
286:241–278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Smith M and Wilkinson S: ER homeostasis
and autophagy. Essays Biochem. 61:625–635. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sanhajariya S, Duffull SB and Isbister GK:
Pharmacokinetics of snake venom. Toxins (Basel).
10(73)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Casella-Martins A, Ayres LR, Burin SM,
Morais FR, Pereira JC, Faccioli LH, Sampaio SV, Arantes EC, Castro
FA and Pereira-Crott LS: Immunomodulatory activity of Tityus
serrulatus scorpion venom on human T lymphocytes. J Venom Anim
Toxins Incl Trop Dis. 21(46)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pucca MB, Fry BG, Sartim MA, Peigneur S
and Monteiro WM: Editorial: Venoms and toxins: At the crossroads of
basic, applied and clinical immunology. Front Immunol.
12(716508)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Avalo Z, Barrera MC, Agudelo-Delgado M,
Tobón GJ and Cañas CA: Biological effects of animal venoms on the
human immune system. Toxins (Basel). 14(344)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Minutti-Zanella C, Gil-Leyva EJ and
Vergara I: Immunomodulatory properties of molecules from animal
venoms. Toxicon. 191:54–68. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Santhosh KN, Pavana D and Thippeswamy NB:
Impact of scorpion venom as an acute stressor on the
neuroendocrine-immunological network. Toxicon. 122:113–118.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Strbo N, Yin N and Stojadinovic O: Innate
and adaptive immune responses in wound epithelialization. Adv Wound
Care (New Rochelle). 3:492–501. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lien WC, Zhou XR, Liang YJ, Ching CT, Wang
CY, Lu FI, Chang HC, Lin FH and Wang HD: Therapeutic potential of
nanoceria pretreatment in preventing the development of urological
chronic pelvic pain syndrome: Immunomodulation via reactive oxygen
species scavenging and SerpinB2 downregulation. Bioeng Transl Med.
8(e10346)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou Z, Li K, Chu Y, Li C, Zhang T, Liu P,
Sun T and Jiang C: ROS-removing nano-medicine for navigating
inflammatory microenvironment to enhance Anti-Epileptic therapy.
Acta Pharm Sin B. 13:1246–1261. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mansfield K and Naik S: Unraveling
Immune-Epithelial interactions in skin homeostasis and injury. Yale
J Biol Med. 93:133–143. 2020.PubMed/NCBI
|
|
20
|
Piipponen M, Li D and Landén NX: The
immune functions of keratinocytes in skin wound healing. Int J Mol
Sci. 21(8790)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pondeljak N and Lugović-Mihić L:
Stress-Induced interaction of skin immune cells, hormones, and
neurotransmitters. Clin Ther. 42:757–770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Eyerich S, Eyerich K, Traidl-Hoffmann C
and Biedermann T: Cutaneous barriers and skin immunity:
Differentiating a connected network. Trends Immunol. 39:315–327.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Costal-Oliveira F, Stransky S,
Guerra-Duarte C, Naves de Souza DL, Vivas-Ruiz DE, Yarlequé A,
Sanchez EF, Chávez-Olórtegui C and Braga VMM: L-amino acid oxidase
from Bothrops atrox snake venom triggers autophagy, apoptosis and
necrosis in normal human keratinocytes. Sci Rep.
9(781)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Al-Asmari AK, Riyasdeen A and Islam M:
Scorpion venom causes apoptosis by increasing reactive oxygen
species and cell cycle arrest in MDA-MB-231 and HCT-8 cancer cell
lines. J Evid Based Integr Med. 23(2156587217751796)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gutiérrez JM, Escalante T, Rucavado A,
Herrera C and Fox JW: A comprehensive view of the structural and
functional alterations of extracellular matrix by snake venom
metalloproteinases (SVMPs): Novel perspectives on the
pathophysiology of envenoming. Toxins (Basel).
8(304)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ben Yekhlef R, Felicori L, Santos LH, F B
Oliveira C, Fadhloun R, Torabi E, Shahbazzadeh D, Pooshang Bagheri
K, Salgado Ferreira R and Borchani L: Antigenic and substrate
preference differences between scorpion and spider dermonecrotic
toxins, a comparative investigation. Toxins (Basel).
12(631)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dunbar JP, Sulpice R and Dugon MM: The
kiss of (cell) death: Can venom-induced immune response contribute
to dermal necrosis following arthropod envenomations? Clin Toxicol
(Phila). 57:677–685. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Morales-Moreno HJ, Carranza-Rodriguez C
and Borrego L: Cutaneous loxoscelism due to Loxosceles rufescens. J
Eur Acad Dermatol Venereol. 30:1431–1432. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nentwig W, Pantini P and Vetter RS:
Distribution and medical aspects of Loxosceles rufescens, one of
the most invasive spiders of the world (Araneae: Sicariidae).
Toxicon. 132:19–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pober JS, Merola J, Liu R and Manes TD:
Antigen presentation by vascular cells. Front Immunol.
8(1907)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dalal PJ, Muller WA and Sullivan DP:
Endothelial cell calcium signaling during barrier function and
inflammation. Am J Pathol. 190:535–542. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
De Andrade CM, Rey FM, Cintra ACO, Sampaio
SV and Torqueti MR: Effects of crotoxin, a neurotoxin from Crotalus
durissus terrificus snake venom, on human endothelial cells. Int J
Biol Macromol. 134:613–621. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Franken L, Schiwon M and Kurts C:
Macrophages: Sentinels and regulators of the immune system. Cell
Microbiol. 18:475–487. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Freitas AP, Favoretto BC, Clissa PB,
Sampaio SC and Faquim-Mauro EL: Crotoxin isolated from Crotalus
durissus terrificus venom modulates the functional activity of
dendritic cells via formyl peptide receptors. J Immunol Res.
2018(7873257)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Leiguez E, Giannotti KC, Moreira V,
Matsubara MH, Gutiérrez JM, Lomonte B, Rodríguez JP, Balsinde J and
Teixeira C: Critical role of TLR2 and MyD88 for
functional response of macrophages to a group IIA-secreted
phospholipase A2 from snake venom. PLoS One.
9(e93741)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sieber M, Bosch B, Hanke W and Fernandes
de Lima VM: Membrane-modifying properties of crotamine, a small
peptide-toxin from Crotalus durissus terifficus venom. Biochim
Biophys Acta. 1840:945–950. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Echeverría S, Leiguez E, Guijas C, do
Nascimento NG, Acosta O, Teixeira C, Leiva LC and Rodríguez JP:
Evaluation of pro-inflammatory events induced by Bothrops
alternatus snake venom. Chem Biol Interact. 281:24–31.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Setubal SS, Pontes AS, Furtado JL, Kayano
AM, Stábeli RG and Zuliani JP: Effect of Bothrops alternatus snake
venom on macrophage phagocytosis and superoxide production:
Participation of protein kinase C. J Venom Anim Toxins Incl Trop
Dis. 17:430–441. 2011.
|
|
39
|
Darkaoui B, Lafnoune A, Chgoury F, Daoudi
K, Chakir S, Mounaji K, Karkouri M, Cadi R and Naoual O: Induced
pathophysiological alterations by the venoms of the most dangerous
Moroccan scorpions Androctonus mauretanicus and Buthus occitanus: A
comparative pathophysiological and toxic-symptoms study. Hum Exp
Toxicol. 41(9603271211072872)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Saadi S, Assarehzadegan MA, Pipelzadeh MH
and Hadaddezfuli R: Induction of IL-12 from human monocytes after
stimulation with Androctonus crassicauda scorpion venom. Toxicon.
106:117–121. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Saidi H, Bérubé J, Laraba-Djebari F and
Hammoudi-Triki D: Involvement of alveolar macrophages and
neutrophils in acute lung injury after scorpion envenomation: New
pharmacological targets. Inflammation. 41:773–783. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ait-Lounis A and Laraba-Djebari F:
TNF-alpha modulates adipose macrophage polarization to
M1 phenotype in response to scorpion venom. Inflamm Res.
64:929–936. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Corzo G and Espino-Solis GP: Selected
scorpion toxin exposures induce cytokine release in human
peripheral blood mononuclear cells. Toxicon. 127:56–62.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Pucca MB, Peigneur S, Cologna CT, Cerni
FA, Zoccal KF, Bordon Kde C, Faccioli LH, Tytgat J and Arantes EC:
Electrophysiological characterization of the first Tityus
serrulatus alpha-like toxin, Ts5: Evidence of a pro-inflammatory
toxin on macrophages. Biochimie. 115:8–16. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pires WL, Kayano AM, de Castro OB,
Paloschi MV, Lopes JA, Boeno CN, Pereira SDS, Antunes MM, Rodrigues
MMS, Stábeli RG, et al: Lectin isolated from Bothrops jararacussu
venom induces IL-10 release by TCD4+cells and TNF-α
release by monocytes and natural killer cells. J Leukoc Biol.
106:595–605. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Júnior FAN, Jorge ARC, Marinho AD,
Silveira JAM, Alves NTQ, Costa PHS, E Silva PLB, Chaves-Filho AJM,
Lima DB, Sampaio TL, et al: Bothrops alternatus snake venom induces
cytokine expression and oxidative stress on renal function. Curr
Top Med Chem. 19:2058–2068. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rojas JM, Arán-Sekul T, Cortés E, Jaldín
R, Ordenes K, Orrego PR, González J, Araya JE and Catalán A:
Phospholipase D from Loxosceles laeta spider venom induces IL-6,
IL-8, CXCL1/GRO-α, and CCL2/MCP-1 production in human skin
fibroblasts and stimulates monocytes migration. Toxins (Basel).
9(125)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bahloul M, Regaieg K, Chabchoub I, Kammoun
M, Chtara K and Bouaziz M: Severe scorpion envenomation:
Pathophysiology and the role of inflammation in multiple organ
failure. Med Sante Trop. 27:214–221. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Khemili D, Valenzuela C, Laraba-Djebari F
and Hammoudi-Triki D: Differential effect of Androctonus australis
hector venom components on macrophage KV channels:
Electrophysiological characterization. Eur Biophys J. 48:1–13.
2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ryan RYM, Seymour J, Loukas A, Lopez JA,
Ikonomopoulou MP and Miles JJ: Immunological responses to
envenomation. Front Immunol. 12(661082)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rørvig S, Østergaard O, Heegaard NH and
Borregaard N: Proteome profiling of human neutrophil granule
subsets, secretory vesicles, and cell membrane: Correlation with
transcriptome profiling of neutrophil precursors. J Leukoc Biol.
94:711–721. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kruger P, Saffarzadeh M, Weber AN, Rieber
N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J and Hartl
D: Neutrophils: Between host defence, immune modulation, and tissue
injury. PLoS Pathog. 11(e1004651)2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Nourshargh S and Alon R: Leukocyte
migration into inflamed tissues. Immunity. 41:694–707.
2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Setubal Sda S, Pontes AS, Nery NM, Bastos
JS, Castro OB, Pires WL, Zaqueo KD, Calderon Lde A, Stábeli RG,
Soares AM and Zuliani JP: Effect of Bothrops bilineata snake venom
on neutrophil function. Toxicon. 76:143–149. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tecchio C, Micheletti A and Cassatella MA:
Neutrophil-derived cytokines: Facts beyond expression. Front
Immunol. 5(508)2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zuliani JP, Soares AM and Gutiérrez JM:
Polymorphonuclear neutrophil leukocytes in snakebite envenoming.
Toxicon. 187:188–197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Khemili D, Laraba-Djebari F and
Hammoudi-Triki D: Involvement of toll-like receptor 4 in
neutrophil-mediated inflammation, oxidative stress and tissue
damage induced by scorpion venom. Inflammation. 43:155–167.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zoccal KF, Bitencourt Cda S, Paula-Silva
FW, Sorgi CA, de Castro Figueiredo Bordon K, Arantes EC and
Faccioli LH: TLR2, TLR4 and CD14 recognize
venom-associated molecular patterns from Tityus serrulatus to
induce Macrophage-Derived inflammatory mediators. PLoS One.
9(e88174)2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Moreira V, Teixeira C, Borges da Silva H,
D'Império Lima MR and Dos-Santos MC: The role of TLR2 in
the acute inflammatory response induced by Bothrops atrox snake
venom. Toxicon. 118:121–128. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zoccal KF, Ferreira GZ, Prado MKB,
Gardinassi LG, Sampaio SV and Faccioli LH: LTB4 and
PGE2 modulate the release of MIP-1α and IL-1β by cells
stimulated with Bothrops snake venoms. Toxicon. 150:289–296.
2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Palm NW and Medzhitov R: Role of the
inflammasome in defense against venoms. Proc Natl Acad Sci USA.
110:1809–1814. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zoccal KF, Sorgi CA, Hori JI, Paula-Silva
FW, Arantes EC, Serezani CH, Zamboni DS and Faccioli LH: Opposing
roles of LTB4 and PGE2 in regulating the inflammasome-dependent
scorpion venom-induced mortality. Nat Commun.
7(10760)2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Thangam EB, Jemima EA, Singh H, Baig MS,
Khan M, Mathias CB, Church MK and Saluja R: The Role of histamine
and histamine receptors in mast cell-mediated allergy and
inflammation: The hunt for new therapeutic targets. Front Immunol.
9(1873)2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Galli SJ, Starkl P, Marichal T and Tsai M:
Mast cells and IgE in defense against venoms: Possible ‘good side’
of allergy? Allergol Int. 65:3–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kovacova-Hanuskova E, Buday T, Gavliakova
S and Plevkova J: Histamine, histamine intoxication and
intolerance. Allergol Immunopathol (Madr). 43:498–506.
2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Krystel-Whittemore M, Dileepan KN and Wood
JG: Mast cell: A multi-functional master cell. Front Immunol.
6(620)2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Menaldo DL, Bernardes CP, Pereira JC,
Silveira DS, Mamede CC, Stanziola L, Oliveira FD, Pereira-Crott LS,
Faccioli LH and Sampaio SV: Effects of two serine proteases from
Bothrops pirajai snake venom on the complement system and the
inflammatory response. Int Immunopharmacol. 15:764–771.
2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Moon TC, Befus AD and Kulka M: Mast cell
mediators: Their differential release and the secretory pathways
involved. Front Immunol. 5(569)2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Stitt J and Katial R: Venom allergy. J
Allergy Clin Immunol Pract. 4:184–185. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Stone SF, Isbister GK, Shahmy S, Mohamed
F, Abeysinghe C, Karunathilake H, Ariaratnam A, Jacoby-Alner TE,
Cotterell CL and Brown SG: Immune response to snake envenoming and
treatment with antivenom; complement activation, cytokine
production and mast cell degranulation. PLoS Negl Trop Dis.
7(e2326)2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Tambourgi DV and van den Berg CW: Animal
venoms/toxins and the complement system. Mol Immunol. 61:153–162.
2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kumar N and Sastry GN: Study of lipid
heterogeneity on bilayer membranes using molecular dynamics
simulations. J Mol Graph Model. 108(108000)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Sandvig K, Bergan J, Kavaliauskiene S and
Skotland T: Lipid requirements for entry of protein toxins into
cells. Prog Lipid Res. 54:1–13. 2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Herzig V, Cristofori-Armstrong B, Israel
MR, Nixon SA, Vetter I and King GF: Animal toxins-Nature's
evolutionary-refined toolkit for basic research and drug discovery.
Biochem Pharmacol. 181(114096)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Van Baelen AC, Robin P, Kessler P, Maïga
A, Gilles N and Servent D: Structural and functional diversity of
animal toxins interacting with GPCRs. Front Mol Biosci.
9(811365)2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bekbossynova A, Zharylgap A and Filchakova
O: Venom-derived neurotoxins targeting nicotinic acetylcholine
receptors. Molecules. 26(3373)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Hung A, Kuyucak S, Schroeder CI and Kaas
Q: Modelling the interactions between animal venom peptides and
membrane proteins. Neuropharmacology. 127:20–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kasheverov IE, Oparin PB, Zhmak MN,
Egorova NS, Ivanov IA, Gigolaev AM, Nekrasova OV, Serebryakova MV,
Kudryavtsev DS, Prokopev NA, et al: Scorpion toxins interact with
nicotinic acetylcholine receptors. FEBS Lett. 593:2779–2789.
2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Luiken JJ, Glatz JF and Neumann D: Cardiac
contraction-induced GLUT4 translocation requires dual signaling
input. Trends Endocrinol Metab. 26:404–410. 2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
O Collaço RC, Hyslop S, Dorce VAC, Antunes
E and Rowan EG: Scorpion venom increases acetylcholine release by
prolonging the duration of somatic nerve action potentials.
Neuropharmacology. 153:41–52. 2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Shrestha A, Kahraman O and Haselwandter
CA: Regulation of membrane proteins through local heterogeneity in
lipid bilayer thickness. Phys Rev E. 102(060401)2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ernst R, Ballweg S and Levental I:
Cellular mechanisms of physicochemical membrane homeostasis. Curr
Opin Cell Biol. 53:44–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Gilbert RJ, Dalla Serra M, Froelich CJ,
Wallace MI and Anderluh G: Membrane pore formation at protein-lipid
interfaces. Trends Biochem Sci. 39:510–516. 2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Rádis-Baptista G: Cell-penetrating
peptides derived from animal venoms and toxins. Toxins (Basel).
13(147)2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Copolovici DM, Langel K, Eriste E and
Langel Ü: Cell-penetrating peptides: Design, synthesis, and
applications. ACS Nano. 8:1972–1994. 2014.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Dal Peraro M and van der Goot FG:
Pore-forming toxins: Ancient, but never really out of fashion. Nat
Rev Microbiol. 14:77–92. 2016.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Kalafatovic D and Giralt E:
Cell-penetrating peptides: Design strategies beyond primary
structure and amphipathicity. Molecules. 22(1929)2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Kerkis I, Hayashi MA, Prieto da Silva AR,
Pereira A, De Sá Júnior PL, Zaharenko AJ, Rádis-Baptista G, Kerkis
A and Yamane T: State of the art in the studies on crotamine, a
cell penetrating peptide from South American rattlesnake. Biomed
Res Int. 2014(675985)2014.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Lin King JV, Emrick JJ, Kelly MJS, Herzig
V, King GF, Medzihradszky KF and Julius D: A cell-penetrating
scorpion toxin enables mode-specific modulation of TRPA1 and pain.
Cell. 178:1362–1374. 2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Burin SM, Menaldo DL, Sampaio SV, Frantz
FG and Castro FA: An overview of the immune modulating effects of
enzymatic toxins from snake venoms. Int J Biol Macromol.
109:664–671. 2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Chan YS, Cheung RCF, Xia L, Wong JH, Ng TB
and Chan WY: Snake venom toxins: Toxicity and medicinal
applications. Appl Microbiol Biotechnol. 100:6165–6181.
2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Xiong S and Huang C: Synergistic
strategies of predominant toxins in snake venoms. Toxicol Lett.
287:142–154. 2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Ferraz CR, Arrahman A, Xie C, Casewell NR,
Lewis RJ, Kool J and Cardoso FC: Multifunctional toxins in snake
venoms and therapeutic implications: From pain to hemorrhage and
necrosis. Front Ecol Evol. 7(218)2019.
|
|
94
|
Muller SP, Silva VAO, Silvestrini AVP, de
Macedo LH, Caetano GF, Reis RM and Mazzi MV: Crotoxin from Crotalus
durissus terrificus venom: In vitro cytotoxic activity of a
heterodimeric phospholipase A2 on human cancer-derived
cell lines. Toxicon. 156:13–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Hong J, Lu X, Deng Z, Xiao S, Yuan B and
Yang K: How melittin inserts into cell membrane: Conformational
changes, Inter-Peptide cooperation, and disturbance on the
membrane. Molecules. 24(1775)2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kachel HS, Buckingham SD and Sattelle DB:
Insect toxins-selective pharmacological tools and drug/chemical
leads. Curr Opin Insect Sci. 30:93–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Khalil A, Elesawy BH, Ali TM and Ahmed OM:
Bee venom: From venom to drug. Molecules. 26(4941)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Khan S: Advances in usage of venom
proteins as diagnostics and therapeutic mediators. Protein Pept
Lett. 25:610–611. 2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Kim W: Bee venom and its sub-components:
Characterization, pharmacology, and therapeutics. Toxins (Basel).
13(191)2021.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Rady I, Siddiqui IA, Rady M and Mukhtar H:
Melittin, a major peptide component of bee venom, and its
conjugates in cancer therapy. Cancer Lett. 402:16–31.
2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Wehbe R, Frangieh J, Rima M, El Obeid D,
Sabatier JM and Fajloun Z: Bee venom: Overview of main compounds
and bioactivities for therapeutic interests. Molecules.
24(2997)2019.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Ghosh A, Roy R, Nandi M and Mukhopadhyay
A: Scorpion venom-toxins that aid in drug development: A review.
Int J Pept Res Ther. 25:27–37. 2019.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Gilchrist J, Olivera BM and Bosmans F:
Animal toxins influence voltage-gated sodium channel function.
Handb Exp Pharmacol. 221:203–229. 2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Kuzmenkov AI and Vassilevski AA: Labelled
animal toxins as selective molecular markers of ion channels:
Applications in neurobiology and beyond. Neurosci Lett. 679:15–23.
2018.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Swartz KJ: Ion channels: The scorpion
toxin and the potassium channel. Elife. 2(e00873)2013.
|
|
106
|
Chen N, Xu S, Zhang Y and Wang F: Animal
protein toxins: Origins and therapeutic applications. Biophys Rep.
4:233–242. 2018.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Kalia J, Milescu M, Salvatierra J, Wagner
J, Klint JK, King GF, Olivera BM and Bosmans F: From foe to friend:
Using animal toxins to investigate ion channel function. J Mol
Biol. 427:158–175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lahiani A, Yavin E and Lazarovici P: The
Molecular basis of toxins' interactions with intracellular
signaling via discrete portals. Toxins (Basel).
9(107)2017.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Oliveira IS, Ferreira IG, Alexandre-Silva
GM, Cerni FA, Cremonez CM, Arantes EC, Zottich U and Pucca MB:
Scorpion toxins targeting Kv1.3 channels: Insights into
immunosuppression. J Venom Anim Toxins Incl Trop Dis.
25(e148118)2019.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Ortiz E and Possani LD: Scorpion toxins to
unravel the conundrum of ion channel structure and functioning.
Toxicon. 150:17–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Quintero-Hernández V, Jiménez-Vargas JM,
Gurrola GB, Valdivia HH and Possani LD: Scorpion venom components
that affect ion-channels function. Toxicon. 76:328–42.
2013.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Xu Y, Sun J, Liu H, Sun J, Yu Y, Su Y, Cui
Y, Zhao M and Zhang J: Scorpion toxins targeting Voltage-Gated
sodium channels associated with pain. Curr Pharm Biotechnol.
19:848–855. 2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Zhang JZ, Yarov-Yarovoy V, Scheuer T,
Karbat I, Cohen L, Gordon D, Gurevitz M and Catterall WA: Mapping
the interaction site for a β-scorpion toxin in the pore module of
domain III of voltage-gated Na(+) channels. J Biol Chem.
287:30719–30728. 2012.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Adams DJ and Lewis RJ: Neuropharmacology
of venom peptides. Neuropharmacology. 127:1–3. 2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Gordon D, Chen R and Chung SH:
Computational methods of studying the binding of toxins from
venomous animals to biological ion channels: Theory and
applications. Physiol Rev. 93:767–802. 2013.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Norton RS and Chandy KG: Venom-Derived
peptide inhibitors of Voltage-Gated potassium channels.
Neuropharmacology. 127:124–138. 2017.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Cologna CT, Peigneur S, Rustiguel JK,
Nonato MC, Tytgat J and Arantes EC: Investigation of the
relationship between the structure and function of Ts2, a
neurotoxin from Tityus serrulatus venom. FEBS J. 279:1495–504.
2012.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Díaz-García A and Varela D: Voltage-gated
K+/Na+ channels and scorpion venom toxins in
cancer. Front Pharmacol. 11(913)2020.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Shen H, Li Z, Jiang Y, Pan X, Wu J,
Cristofori-Armstrong B, Smith JJ, Chin YKY, Lei J, Zhou Q, et al:
Structural basis for the modulation of voltage-gated sodium
channels by animal toxins. Science. 362(eaau2596)2018.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Wu Y, Ma H, Zhang F, Zhang C, Zou X and
Cao Z: Selective Voltage-Gated sodium channel peptide toxins from
animal venom: Pharmacological probes and analgesic drug
development. ACS Chem Neurosci. 9:187–197. 2018.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Cohen G, Burks SR and Frank JA:
Chlorotoxin-a multimodal imaging platform for targeting glioma
tumors. Toxins (Basel). 10(496)2018.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Dardevet L, Rani D, Aziz TA, Bazin I,
Sabatier JM, Fadl M, Brambilla E and De Waard M: Chlorotoxin: A
helpful natural scorpion peptide to diagnose glioma and fight tumor
invasion. Toxins (Basel). 7:1079–1101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Wang D, Starr R, Chang WC, Aguilar B,
Alizadeh D, Wright SL, Yang X, Brito A, Sarkissian A, Ostberg JR,
et al: Chlorotoxin-directed CAR T cells for specific and effective
targeting of glioblastoma. Sci Transl Med.
12(eaaw2672)2020.PubMed/NCBI View Article : Google Scholar
|