1. Introduction
Glioblastoma (GB) is a primary glial tumor of
astrocytic type, grade IV of malignancy according to the World
Health Organization (WHO) classification (1). Compared with other cancer types, the
incidence of GB is relatively low, varying from 3 to 5 cases per
100,000 individuals in North America, Europe and Central Asia
(2). The large majority of GB cases
occur sporadically, and the tumor is found in all age groups, but
is more frequently detected during the second half of life in men
of the so-called ‘non-Hispanic type’ (3).
Current treatment protocols for GB include
neurosurgery, high-dose radiation and chemotherapy (CT), most
frequently using such cytostatics as temozolomide (TMZ) and
lomustine, as well as the targeted antitumor agent, bevacizumab
(4). The effect of treatment is
insufficient, with a median relapse-free survival of 4-8 months,
and an overall survival of 15 months (5). A total of ~25% of patients are able to
survive for 24 months following diagnosis, which makes GB one of
the most dismal diseases in oncology.
Resistance to treatment is associated with the
heterogeneous nature of the cancer stem cell (CSC) population that
dominates in the GB cell hierarchy (6). High β-catenin content is the most
important characteristic of functionally active CSCs (7), due to their interaction with the local
immunosuppressive microenvironment, thus activating the
intracellular Wnt signaling pathway (8), PI3K/AKT/mTOR (9) and other mechanisms (10) that are responsible for plasticity
and tumor progression.
Cytostatic drugs fail to effectively destroy CSCs in
the body of a patient (11), and
attempts to regulate CSCs by combining TMZ, immune checkpoint
inhibitors (12) and
molecular-targeted drugs (13) have
not been unequivocally successful; thus, there is an urgent need to
supplement the existing GB treatment protocols with more effective
methods of regulating the functional activity of this cell
type.
It could be hypothesized that a combination of drugs
that inhibit the synthesis and biological activity of β-catenin
with immunotherapy may destabilize the interaction between CSCs and
the tumor microenvironment; block the mechanisms of CSC phenotypic
heterogeneity and plasticity; enhance the antiglioma effect of
cytostatics; hinder tumor progression; and prolong the life of a
patient.
The present study aimed to investigate the
possibility of regulating GB CSCs by combining antagonists of the
Wnt/β-catenin signaling pathway with immunotherapy.
2. Principles of GB treatment
Eliminating as many cancer cells (CCs) as possible
is the key principle of GB treatment. Surgery is the method of
choice for treating patients with GB (14), since it provides one-stage
elimination of numerous CCs, reduces intracranial pressure,
relieves brain compression, and improves the overall condition of
the patient (15). However, the
applicability of surgical treatment is limited. The severity of the
condition of the patient, a functional status <70 on the
Karnofsky scale, or subtentorial, thalamic, multifocal or
bihemispheric localization of the tumor often render it impossible
to treat the condition surgically without a direct risk to the life
of the patient, and make a strong case for the refusal of surgery
in patients with newly diagnosed GB (16,17).
Moreover, brain tissue infiltration by CCs, even with the use of
the most modern surgical technologies, does not allow the radical
removal of the tumor without causing severe and irreparable
neurological damage to the patient (18,19).
Cytoreduction effectiveness increases significantly
when surgery is combined with radiation (20). Radiation therapy damages DNA,
induces ageing processes and destroys GB cells (21). Intraoperative irradiation (22) can be used, as well as remote
γ-therapy (23), which can be
supplemented with brachytherapy (24), proton therapy (25) and neutron-capture therapy (26). The life expectancy of patients with
GB is associated with the dose of radiation received, which may
reach ≥60 Gy (27), while dose
escalation can result in radiation-induced brain necrosis, impaired
cerebral circulation, reactive gliosis, sclerosis, cyst formation
and development of psychoorganic syndrome (28).
The majority of cases exhibit tumor relapse in 4-8
months after its removal, with ≤30% of patients undergoing
reoperation (29), which improves
their overall condition and quality of life, reduces their
dependence on corticosteroids, and improves the effects of adjuvant
CT.
Reoperation requires special skill, as fluid-filled
cystic cavities that appear after radiotherapy can be misleading in
terms of correctly identifying the necessary extent of resection;
thus, careful planning of the surgery is necessary regarding clear
anatomical landmarks (such as sulci, ventricles and dura mater
boundaries), which is not always possible (30). It is partly for this reason that
<10% of patients who are reoperated due to GB progression or
recurrence undergo a third surgery, and only 2% of patients undergo
≥3 reoperations (17).
CT is recommended for all patients with GB. CT is
the main method of destroying CCs, and it is used in combination
with radiotherapy for treating a newly diagnosed GB to prolong
remission after standard chemoradiation therapy and to prolong the
life of patients with tumor recurrence (6). The drug of choice in CT is a
cytostatic alkylating antineoplastic agent called TMZ, which is a
tetrazine derivative.
Based on the potent cytotoxic activity of TMZ
against glioma and carcinoma cells, the current stage of
neuro-oncology development is termed the ‘temozolomide era’
(31). TMZ passes through the
blood-brain barrier (BBB), easily penetrates into the cerebrospinal
fluid, and, once in the bloodstream, undergoes a chemical
transformation into monomethyltriazenoimidazolcarboxamide, the
effect of which is attributed to the alkylation of guanine at O6
and N7 positions, with the subsequent triggering of aberrant
reduction of methyl residues.
Patients are recommended a TMZ dosage of 75
mg/m2 in combination with radiotherapy, followed by 6-8
cycles of TMZ with an increased dose of ≤150-200 and ≤400
mg/m2. The maximum tolerated dose per treatment cycle is
1,000 mg/m2. TMZ is usually employed in combination with
lomustine (32) and bevacizumab, an
inhibitor of endothelial growth factor in blood vessels.
Lomustine is an alkylating antineoplastic agent that
is a nitrosourea derivative and a second-line CT drug. In addition
to DNA alkylation, its antineoplastic effect is achieved via
inhibition of genome repair enzymes, DNA matrix damage and
suppression of some key enzymatic processes in CCs in the late
G1 and early S phases of the cell cycle (32). The recommended dose of lomustine in
adults is 130 mg/m2 when administered once orally every
6 weeks, and the total dose for all treatment regimens should not
exceed 1,000 mg/m2. Similar to TMZ, further doses of
lomustine should be determined based on the therapy effectiveness
and hematological response of the patient to the previous dose.
Previous attempts to enhance the antineoplastic
treatment effect by combining TMZ or lomustine with the
poly(ADP-ribose) polymerase inhibitor, olaparib (33), the histone deacetylase inhibitor,
vorinostat (34) and other blockers
of DNA repair enzymes (35) have
not yet been successful. Procarbazine, vincristine, paclitaxel and
platinum-based agents and other cytostatics have no significant
advantages over TMZ and lomustine, and their administration has a
detrimental effect on hematological status, increases toxicity and
does not prolong the life of patients with GB (36). Electromagnetic therapy, which has
been widely used in the last decade, expands the possibilities of
cytostatics (28), but does not
have any strategic advantages.
Thus, cytoreduction, cytotoxic and cytostatic
principles define the current paradigm of GB treatment, which is
able to ensure an average survival of patients of 15 months, with
an average cost of treatment of 62,602$ (37). The main reason for such a cost is
CT, the effectiveness of which is rather low despite the large
number of antineoplastic agents available. The effect of CT is
limited by the BBB permeability (28), but attempts to administer drugs into
the removed GB bed, intrathecal/intraventricular administration of
CT, or encasing cytostatics into nanocapsules for their targeted
delivery to the brain by using monoclonal antibodies have not yet
been successful (11), which is
usually explained by the phenotypic plasticity (6) and particular properties of CSCs
(38).
3. CC plasticity and resistance to
treatment
Traditionally, the plasticity of CCs has been
defined as their ability to transition into an undifferentiated
state and resist the effects of CT (39). A decisive step in understanding GB
plasticity was made by Verhaak et al (40), who described proneural, neural,
classical and mesenchymal tumor subtypes, showing the possibility
of their transformation during treatment. It is noteworthy that the
proneural subtype, identified in this study, was the most
proliferative, while the mesenchymal subtype of the tumor was
poorly proliferative and highly resistant to CT. Subsequently, high
genome heterogeneity in the structure of these tumor subtypes
(41) was revealed, while the
critically low degree of DNA methylation, which was inherent to the
mesenchymal GB subtype, predetermined the main trend of the problem
development.
Brennan et al (42) further described six classes of DNA
methylation in GB cells, with the highest methylation status
assigned to isocitrate dehydrogenase (IDH) mutant tumors as
the most differentiated and least plastic ones (42). The IDH mutation resulted in
an excess of 2-hydroxyglutarate in CCs, which was accompanied by
hypermethylation of the promoter regions of the
O6-methylguanine-DNA methyltransferase
(MGMT) gene, which provided direct DNA repair (43). Reducing the degree of genome
methylation made CCs more plastic (44). In light of this, in 2016, the WHO
selected IDH mutations as the main criterion for
systematization of gliomas, distinguishing IDH-wild-type and
mutant types of GB (45).
Theoretically, IDH-wild-type GB cells have
great replicative freedom and can use a wide arsenal of mechanisms
for repairing single- and double-stranded DNA breaks, including
homologous recombination and nonhomologous end joining (43-45).
IDH-wild-type GB was divided into seven additional classes
on the basis of genome methylation (46), which allowed Neftel et al
(47) to describe four types of
phenotypic states inherent in CCs: i) Neural progenitor-like cells
with amplification of the CDK4 gene; ii) oligodendroglial
progenitor-like cells with amplification of the PDGFRA gene;
iii) astrocyte-like cells with amplification of the EGFR
gene; and iv) mesenchymal-like cells with mutation of the
NF1 gene.
Such taxonomy makes the phenotypic plasticity of CCs
directly dependent on their local microenvironment (48-50),
which not only predetermines the C phenotype, but also appears to
be able to switch reproduction programs on and off depending on
external conditions (51-53).
In light of this, it can be presumed that the majority of
phenotypic plasticity is exhibited by CCs with a hypomethylated
genome, which are almost insusceptible to CT.
It is commonly considered that cells of this type,
which are called CSCs, were described in 1997 by Bonnet and Dick
(54) in their study on the
hierarchical structure of the cell population in acute myeloid
leukemia. However, as early as in 1877, Julius Friedrich Conheim, a
student of Rudolf Virchow, indicated the presence of neoplastic
elements with embryonic characteristics among the cells of gastric,
breast and other aggressive tumors (55).
It is highly probable that CSCs are transformed
descendants of normal neural stem cells (NSCs) that inhabit the
subventricular zone and other germinative centers of the human
brain. This is indicated by: i) The identity of >60% of proteins
in the NSC proteome and CSCs of GB (56); ii) complex attractive-permissive
interactions between cells of these types (57); iii) the CSC microenvironment,
including clones of astrocytic and oligodendroglial progenitor
cells that are transcriptomically similar to NSCs of the brain of a
patient (58); and iv) the presence
of NSC-like elements carrying the same mutations as differentiated
CCs at all stages of gliomagenesis (59).
Normal NSCs and CSCs have a number of similar
immunocytochemical markers on the cell surface, among which the
CD133 antigen (prominin-1) is considered to be the main marker of
GB stem cells (13). However, in
addition to NSCs, this glycoprotein is present in hematopoietic
stem cells (HSCs), endothelial progenitor cells, as well as in
kidneys, trachea, salivary and mammary glands, placenta, digestive
tract, testes, and other normal cells and tissues (60).
The role of CD133 antigen in the neoplastic process
is not fully understood, but its direct association with cancer is
clear. This marker is present in CSCs of lung cancer (61), colorectal carcinoma (62) and breast cancer (63). CSCs of the CD133+
immunophenotype rank the highest in the hierarchy of GB cells
(64) and are characterized by
their tumorigenicity and high proliferative activity. However, the
presence of differentiated non-tumorigenic CD133+ cells,
progenitor-like CD133+ cells with limited proliferative
potential, and CSCs that are tumorigenic and negative to this
marker (65) but immunopositive to
CD56, SRY-box transcription factor (SOX)2, SOX9, CD15, A2B5 and
other antigens (64), allows to
assert that the CSC phenomenon is not directly associated with
cells of one certain immunophenotype.
This interpretation explains the failure of previous
attempts to increase the effect of treating invasive gliomas by
combining TMZ or lomustine with monoclonal antibodies against
different CSC antigens (64,65).
Probably, at the initial stage of a neoplastic process, mutations
forming the primary stem lineage occur specifically in NSCs, which
have the highest proliferation rate among all the cells of the
nervous system. The proliferation of mutant cells leads to an
increase in cell mass and competition for oxygen, thus triggering
mechanisms that produce new generations of NSCs capable of
arbitrarily switching between anaerobic and aerobic types of
metabolism by regulating the level of lipid and glutamine
utilization, which proliferate or remain in a quiescent state
(66).
The functional activity of such cells is determined
by the local microenvironment, which activates a number of
molecular mechanisms, leading to the proliferation of CCs that have
adapted to certain local microconditions. Perivascular localization
is a characteristic of proneural CSCs, which are mesenchymal CSCs
that are extracted from hypoxic zones and areas of necrosis
(67), where local microconditions
are unfavorable. The proneural type of GB is characterized by
proliferating CSCs with a glycolytic type of metabolism and a low
level of lipid utilization, whereas the mesenchymal type of GB is
characterized by CSCs that are predominantly in the state of
proliferative quiescence and are able to switch between glycolysis
and aerobic respiration as well as exhibit a high level of lipid
metabolism.
In fact, proneural and mesenchymal tumor subtypes
reflect two possible states of CSCs, namely proliferation and
survival. The transition to the survival state occurs under the
influence of hypoxia (68),
cytostatics (69), irradiation
(70,71) and anti-angiogenic therapy (72). It can be assumed that the reverse
transition, triggering tumor relapse, is also precipitated by the
influence of the local microenvironment, which activates numerous
molecular mechanisms in CSCs (73),
with the canonical Wnt signaling pathway playing a particular role
among those mechanisms.
4. Wnt signaling pathway and the
microenvironment of CSCs
The canonical Wnt signaling pathway is the most
important intracellular signaling pathway, regulating embryogenesis
and differentiation of normal stem cells (74). In GB pathogenesis, this pathway
controls the balance between symmetric and asymmetric CSC
divisions, which predetermines the aggressiveness and fatality of
the tumor (75). The central link
of this mechanism is β-catenin, which even in the absence of
signaling is bound by a multiprotein ‘destructive complex’
represented by the adenomatous polyposis coli (APC), AXIN1/2, CK1
and glycogen synthase kinase (GSK)-3β proteins. GSK-3β protein
kinase, which is activated by the ‘destructive complex’,
phosphorylates β-catenin, which loses its functionality and
undergoes degradation in the proteasome (74).
The Wnt signaling pathway is activated by one of 19
secreted Wnt-proteins interacting with Frizzled and low-density
lipoprotein receptor-related protein (LPR)4/5 family receptors of
the CSC surface, which activates the intracellular Disheveled
protein and triggers a cascade of intermolecular interactions,
leading to the blockade of the ‘destructive complex’ and
accumulation of β-catenin in the CSC cytoplasm, which enters the
nucleus, activates T-cell factor (TCF), and triggers the expression
of pluripotency genes (76-78),
inluding MYC, CCND1, cellular communication network factor 4
(CCN4 also known as WISP1) and PPARG.
Mutations of the proline, glutamate and leucine rich
protein 1 (PELP-1) gene, that is a potential co-activator of
the canonical Wnt signaling pathway, are found in the majority of
patients with GB, while mutations of the APC gene are
identified in 14.5% of cases, and mutations of the FAT1
gene, a negative regulator of Wnt signaling, are detected in 57% of
patients (77-79).
Mutations of other components are rare (80). A high content of β-catenin is one of
the main characteristics of CD133+ CSCs (81,82),
which indicates the involvement of other mechanisms that cause its
accumulation in this type of cells.
One of the mechanisms previously described (83) is the activation of the PI3K/AKT/mTOR
signaling pathway, which enables both the stimulation of PI3K
(13) and the immediate activation
of intracellular AKT, which directly phosphorylates GSK-3β and
increases β-catenin content (12).
In turn, mTOR creates two multiprotein complexes, namely mTORC1 and
mTORC2, which antagonistically regulate each other's activity: The
first one decreases the β-catenin content in CSCs (84), while the second one increases it
(85).
The increase in β-catenin content activates
telomerases, thus leading to telomere lengthening, immortalization,
stabilization of the CSC genome, and survival of CSCs under
chemoradiotherapy (CRT) (86-88).
The β-catenin content in CSCs increases due to semaphorins. a
particular class of secreted and membrane proteins produced by
neuroblasts and neurons (89). The
synthesis of β-catenin in CSCs is induced by TGF-β secreted by CCs
and immunosuppressive microglia cells (90). Through the SMAD and death-associated
protein 6 (DAXX) signaling pathways, this cytokine activates the
PI3K/AKT/mTOR axis (91), leading
to β-catenin accumulation in CSCs, which indicates the strategic
role of the microenvironment in CSC regulation.
Thus, β-catenin is one of the most significant
regulators of CSC proliferation, with the local microenvironment
being the main regulator of the intracellular β-catenin content in
CSCs. The microenvironment regulates CSCs by triggering two
divergent processes, namely autophagy and epithelial-mesenchymal
transition (EMT) (73). The
inflammatory microenvironment triggers micro- and macro-autophagy,
which degrades intracellular proteins (92), thus providing energy-independent
utilization of β-catenin, critically reducing CSC activity, and
destabilizing the interaction with the extracellular matrix, which
allows CSCs to migrate, and survive hypoxia, metabolic acidosis and
CRT.
On the contrary, an immunosuppressive
microenvironment (93) produces
Wnt3a and Wnt5 ligands that activate the Wnt signaling pathway,
with WISP1 as one of the target genes (94), the protein product of which
increases the synthesis of other Wnt ligands, as well as that of
IL-10 and IL-35, thus suppressing autophagy, and leading to an
increase in β-catenin content and enhanced interaction with the
extracellular matrix. In turn, the activation of AKT, which is
mediated by α6β1 and other components of the integrin signaling
pathway (95), activates the
production of Wnt ligands, immunosuppressive cytokines, programmed
cell death (PD)-1 and cytotoxic T-lymphocyte-associated protein
4(94), which creates an ‘autocrine
loop’, leading to increased immunosuppression, niche development
and proliferation of CSCs (96).
Thus, the plasticity of CCs is caused by the
influence of an immunosuppressive microenvironment, contributing to
the increase of β-catenin content, which provides the transition
from survival to proliferation in CSCs. Numerous attempts have been
made to regulate these processes using targeted therapy.
5. Targeted therapy and CSC plasticity
Differentially activated signaling pathways differ
for proneural and mesenchymal types of CSCs (97). In the former, the main role belongs
to the signaling pathways of tyrosine kinase (TRK) β-receptor of
platelet-derived growth factor (PDGF) and Notch; in the latter, the
TGF-β, NF-κB and glycolysis signaling pathways are dominant.
Pharmacologists have paid close attention to these molecular
mechanisms; however, important breakthroughs have yet to
emerge.
PDGF has become one of the priority targets for the
inhibition of CSC proliferation. However, imatinib, an inhibitor of
the TRK activity of PDGF from a new class of targeted cytostatics,
failed trials in patients with GB (98). The multikinase inhibitor, sorafenib,
was marginally effective when delivered directly to the tumor and
only together with alternating electromagnetic field therapy
(99), while sunitinib showed no
effect at all in patients with GB (100).
Proliferation suppression of CSCs via the
TRK-signaling domain of epidermal growth factor receptor (EGFR) in
patients with GB has been attempted and failed. The peptide
vaccine, rindopepimut, against the EGFRvIII antigen failed to meet
expectations in combination with TMZ and bevacizumab (101). Depatuxizumab, a monoclonal
antibody against EGFR, loaded with the monomethyl auristatin F and
displaying antimitotic effects, has demonstrated modest results
both in combination with TMZ (102) and without it (103).
Onartuzumab, a monoclonal antibody directed against
TRK-receptor of hepatocyte growth factor (HGF), was not successful
in treating patients with GB in combination with TMZ and
bevacizumab (104). The
multikinase inhibitor cabozantinib, by blocking HGF signaling among
other mechanisms, was ineffective in treating patients with GB
(105). Only bevacizumab, by
inhibiting TRK-receptor of vascular endothelial growth factor
(VEGF) (106), in combination with
TMZ, increased the life expectancy of patients with GB by 4-6
months, which is a remarkable achievement for targeted therapy.
Poor efficiency of TRK receptor inhibitors has
spawned numerous unsuccessful attempts to target downstream
components of the PI3K/AKT/mTOR signaling pathway. Buparlisib, a
PI3K-kinase inhibitor, proved to be no more advantageous than
monotherapy with TMZ (107),
carboplatin and lomustine (108).
The inhibitors of AKT kinase (perifosine) (109) and mTOR kinase (temsirolimus)
failed to meet expectations both in combination with TMZ and
without it (110).
The BRAF-V600E mutation is found in 20% of tumors,
and it involves the substitution of valine-600 for glutamic acid,
permanently activating the RAS/RAF/MEK/ERK signaling pathway.
However, the inhibitors of the mutant RAF protein kinase, sorafenib
(99) and vemurafenib (111), have not exhibited any effect in
patients with GB.
The Notch signaling pathway is the oldest reported
mechanism regulating the processes of stem cell differentiation
(112). Activation of this pathway
occurs when one of four Delta-like ligands or Jag1-2 ligands, binds
to the membrane of one cell, directly interacts with one of four
Notch receptors on the membrane of the cell, receiving the signal
that triggers a cascade of intermolecular interactions, thus
leading to the activation of target genes. Suppression of these
mechanisms can block the proliferative functions of CSCs, but none
of the Notch-signaling inhibitors has shown any effect in GB
(113).
Attempts to affect CSCs via pharmacological
suppression of the EMT-related mechanisms were also ineffective.
The inhibitors of TGF-β, trabedersen (114) and galunisertib (115), did not produce a significant
increase in survival rates when combined with TMZ, procarbazine,
lomustine and vincristine.
Theories on a possible pharmacological regulation of
CSCs via the PI3K/AKT/NF-κB axis are inconsistent (116). There have been some reports on the
apoptosis of GB cells under the influence of this target (117) and the ability of cannabidiol to
inhibit NF-κB activity (118), but
this method has not been used for the regulation of CSCs in the
complex treatment of GB.
Therefore, attempts of regulating CSC with the help
of targeted therapy have been shown to be practically ineffective,
but certain prospects are associated with the suppression of the
Wnt/β-catenin signaling pathway.
6. β-catenin inhibitors and CT
β-catenin is a central factor that ensures the
transition of CSCs from survival to proliferation mode. The
strategy of counteracting this transition is based on (78) inhibiting the Wnt-ligand bind to the
Frizzled receptor complex, suppressing the antagonists of the
β-catenin destruction complex, and blocking the interaction between
β-catenin and TCF/LEF. Most inhibitors of the Wnt signaling pathway
have not yet undergone clinical trials; in this regard, repurposed
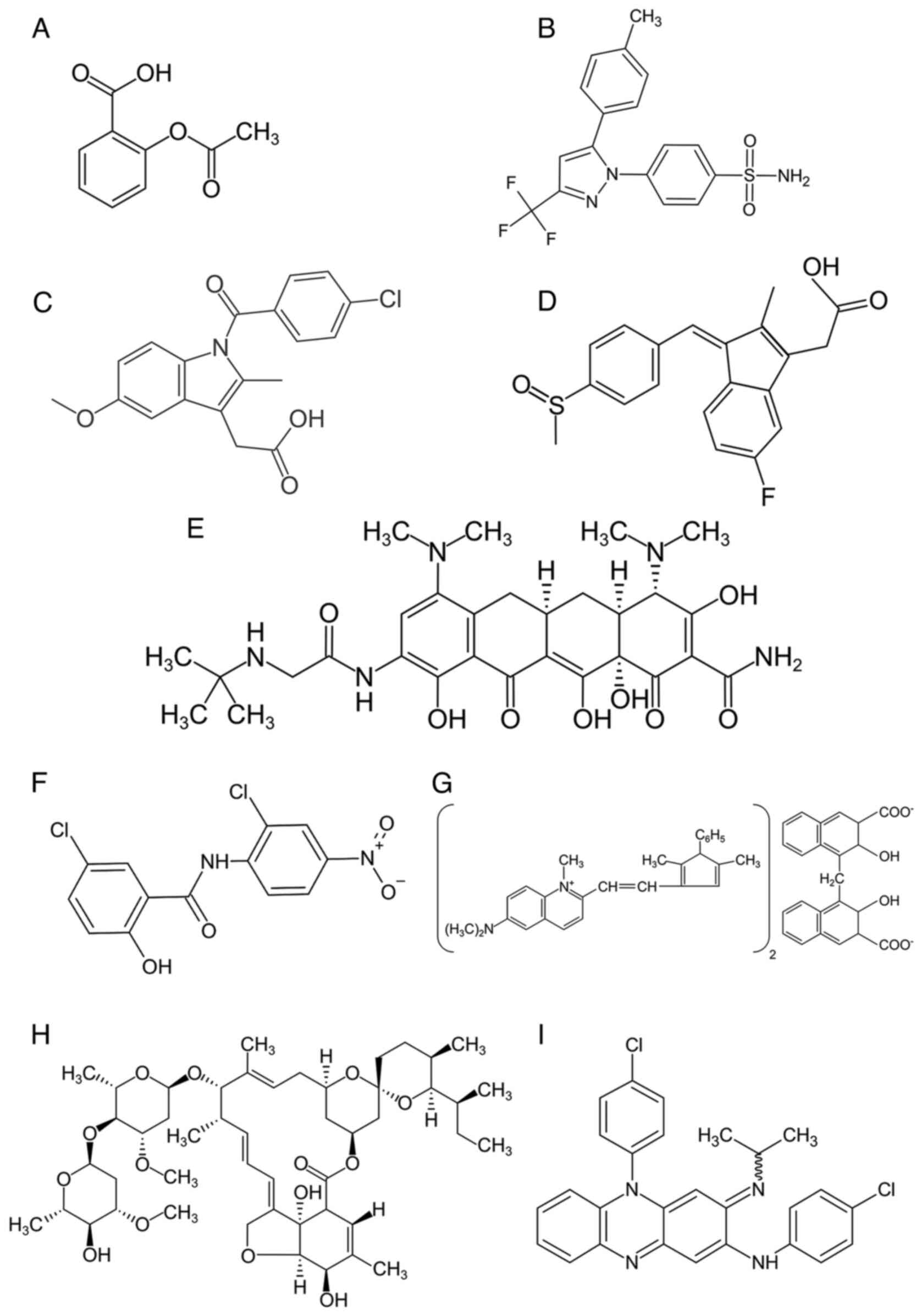
drugs are of particular interest (Fig.
1 and Table I).
 | Table IRepurposed drugs targeting the
Wnt/β-catenin signaling pathway. |
Table I
Repurposed drugs targeting the
Wnt/β-catenin signaling pathway.
| Name of the
drug | Mechanism of Wnt
signaling inhibition | Results of
preclinical/clinical studies | (Refs.) |
|---|
| Aspirin | -Prostaglandin
E2/cyclooxygenase-dependent | -Suppression of
proliferation in almost any Wnt-dependent cancer | (119) |
| | -Inactivation of
PP2A and phosphorylation of β-catenin | -Reduction of tumor
formation in the FAP mouse model, reduction of β-catenin levels in
the tumor | |
| | -Cross-talk between
other pathways (such as NF-κB) | -Retrospective
studies, especially for the prevention of colon cancer | |
| | | -Recommended for
the prevention of CRC in individuals 50 to 69 years of age | |
| Celecoxib | -Prostaglandin
E2/cyclooxygenase-dependent | -Violation of
proliferation in CRC, hepatoma, osteosarcoma and GB Decreased
CD133+ colon cancer stem cells | (120,121) |
| | -Promotes
proteasomal degradation of TCF1 and TCF4 | -Inhibition of
β-catenin-positive precancerous lesions in the colon of mice and in
a model of colon cancer in rats | |
| | -Cross-linking of
the c-Met/AKT pathway promoting GSK-3β phosphorylation | -Reduction of
polyps in patients with FAP after 6 months of treatment | |
| Indomethacin |
-PGE2/COX-dependent | -Growth suppression
of CRC cell lines | (122) |
| | -Degradation of
β-catenin through transcription inhibition | -Decreased tumor
burden in chemically-induced colon cancer | |
| | -Impaired formation
of the β-catenin/TCF4 complex | | |
| Sulindac |
-PGE2/COX-dependent | Growth suppression
of colorectal cancer cell lines | (122) |
| | -Inactivation of
PP2A and β-catenin phosphorylation | | |
| Tigecycline | -Decrease in the
content of β-catenin in the cytoplasm of CCs | -Suppression of the
growth of cervical cancer cells | (125) |
| | -Increased
synthesis of AXIN1 | -Inhibition of
growth of cervical cancer xenografts | |
| Niclosamide | -Promotion of FZD 1
endocytosis | -Antiproliferative
activity against osteosarcoma, CRC, breast cancer, lung cancer,
hepatoma and GB. | (126) |
| | -Inhibition of
DVL2 | -Reduces the levels
of β-catenin in mouse models of colorectal and basal breast
cancer | |
| | -LPR6
degradation | | |
| Pyrvinium
pamoate | -Activation of the
isoform of casein kinase 1α, part of the Wnt pathway destruction
complex | -Suppresses tumor
growth in a colon cancer model | (127) |
| Ivermectin | -Deactivation of
β-catenin by reducing C-terminal phosphory-lation due to
overactivation of phosphatases PP2A and PP1 | -Antiproliferative
against colon cancer (including stem cells) and lung cancer | (128) |
| | | -Reduced tumor
growth in colon cancer xenograft models | |
| Clofazimine | -Participation in
the inhibition of the TCF transcription complex | -Suppression of the
growth of squamous hepatocellular cancer and lung cancer | (129) |
| | -Cross-talk between
other pathways | -Suppression of the
growth of glioblastoma, lung and breast cancer | |
| | | -Combination and
monotherapy for hepatocellular carcinoma with moderate positive
results | |
Wnt-inhibitory activity has been described for a
number of agents from the group of non-steroidal anti-inflammatory
drugs. Aspirin is one of the most popular types of medication from
this group; its pharmacological effect is based on the inhibition
of cyclooxygenase enzymes that reduce the levels of β-catenin in
CCs, inhibit the expression of Wnt-target genes, enhance the
cytotoxic effect of TMZ and bevacizumab (119), and prevent the development of
colorectal cancer and a number of Wnt-dependent neoplasms (Fig. 1A).
Celecoxib, another non-steroidal anti-inflammatory
agent, reactivates GSK-3β, eliminates the effects of the activation
of the ‘β-catenin degradation complex’, inhibits TCF at a dose of
1,200-1,600 mg/day, and suppresses cyclooxygenase 2 and
carboanhydrase, which impairs the adaptation of CCs to hypoxia
(120) and enhances the cytotoxic
effect of TMZ. In light of this, the administration of celecoxib
preoperatively and before the end of CRT (121) has been described as a low-risk and
justifiable procedure, but the ability of celecoxib to inhibit
platelet aggregation combined with the hematological toxicity of
TMZ raises some doubts concerning such claims (Fig. 1B).
The ability to inhibit the intracellular
Wnt/β-catenin signaling pathway has also been observed in other
drugs of this group. Indomethacin (Fig.
1C) is an indoleacetic acid derivative and cyclooxygenase 2
inhibitor, which disrupts the formation of the β-catenin/TCF
complex with DNA and inhibits gene expression (122). Sulindac, a non-steroidal
anti-inflammatory drug (Fig. 1D)
from the group of acetic acid derivatives (123), enhances the degradation of
β-catenin and prevents its translocation to the nucleus, thus
inhibiting the expression of Wnt-target genes in CCs.
Despite the antiglioma potential of cyclooxygenase
inhibitors, there are practically no data concerning their effect
on CSCs. It should not be dismissed that inhibition of
cyclooxygenase enzymes (124) may
suppress the synthesis of Wnt-ligands by microglia cells,
fibroblasts and endothelial cells from the microenvironment of
CSCs. However, these drugs are not the only ones to hinder the
activity of the Wnt signaling pathway in CSCs.
The antibiotic tigecycline (Fig. 1E) stimulates AXIN1 β gene
expression and reduces β-catenin levels in CCs (125). The antiparasitic drug niclosamide
(Fig. 1F) causes LRP6 degradation,
reduces β-catenin content in the nucleus, and inhibits TCF/LEF
factor activity (126). Pyrvinium
pamoate (Fig. 1G) regulates
MGMT gene expression in GB cells, reactivates GSK-3β and
increases the sensitivity of GB cells to TMZ (127). Ivermectin (Fig. 1H) binds to the telomere maintenance
2 (TELO2) protein, which regulates PI3K activity and decreases
β-catenin content in CCs (128).
Particular interest should be paid to a drug from
the riminophenazine group called clofazimine (CFZ),
N,5-bis-(4-chlorophenyl)-3,5-dihydro-3-[(1-methylethyl)imino]-2-phenazinamine,
which was synthesized by Vincent Barry in 1957. CFZ was originally
used as an antimycobacterial agent with proven bactericidal
activity against Hansen's bacillus (Fig. 1I). CFZ inhibits mycobacterial
growth, promotes the formation of reactive oxygen species,
interacts with phospholipids of cell membranes, and disrupts the
ionic equilibrium and energy metabolism of bacterial cells
(129). Its anti-inflammatory
properties combined with its ability to induce the release of
prostaglandins and inhibit phospholipase A2 made it applicable in
the complex treatment of erythema nodosum leprosum (130).
The antineoplastic properties of CFZ against
triple-negative breast cancer are associated with the inhibited
expression of Wnt signaling pathway-related genes, reduction of the
cytoplasmic β-catenin level, and triggering of apoptosis due to
cell cycle arrest in the G2/M-phase in CCs (131). The antineoplastic effect of CFZ
against CCs of different cell lines from colorectal adenocarcinoma
and ovarian cancer is exhibited by the IC50 of the drug,
in the range of 2-10 µm/l, while against different cell lines of
human GB this value is 20-40 µm/l, which may be associated with
both Wnt inhibition and other metabolic effects of the drug
(132).
CFZ partially penetrates into the cerebrospinal
fluid through the intact BBB, but by being a lipophilic substance,
the drug accumulates well in adipose tissue and in monocytes, and
is captured by macrophages (133).
Phagocytosis of CFZ crystals is not accompanied by obvious toxic
manifestations, it suppresses NF-κB, enhances the synthesis of IL-1
receptor antagonist, and induces the M2 activation of macrophages
(134). CFZ, captured and
transported by monocytes, accumulates in the lungs, liver, and
spleen (135), and partially in
the bone marrow. Such findings suggest that CFZ may be a promising
medical agent to be delivered into a tumor with the help of
mononuclear cells, which constitute an important part of the CSC
microenvironment (136).
There is a reasonable assumption that the need for
specific transport through the BBB could decrease the antiglioma
potential of the drug. However, the antiglioma Wnt-inhibitory
effect has been described for valproic acid derivatives,
phenothiazine neuroleptics, olanzapine, amisulpiride and other
drugs (28), which pass through the
BBB with ease. Nevertheless (120), since 2005, >100 studies on the
combination of CT with different drugs have failed. Identifying the
reasons of such outcomes is directly dependent on the need to
regulate the CSC microenvironment, which is, the main factor
contributing to the lower antioglioma potential of pharmacological
agents. However, the development of practical approaches to solving
this problem requires rethinking a number of its important
theoretical aspects.
7. CSC microenvironment, immunotherapy and
immunodeficiency
Since the 1950s, the brain has been considered to be
an immune privileged organ. The microenvironment of CSCs was
associated only with resident microglia cells, which were
considered to originate from cells of the embryonic yolk sac and
not to interact with the immune system after the BBB closure
(137), and mononuclear cells and
other bone marrow immunocytes were not considered to interact with
microglia in any way. However, numerous data suggest otherwise.
It has been demonstrated that microglial cells can
enter deep cervical lymph nodes and interact with T and B
lymphocytes. Each T cell can recognize several hundred fragments of
a single antigen on the antigen-presenting cell membrane together
with class I and class II major histocompatibility complex
molecules. At the same time, B cells can bind intact antigens with
their native structure, indicating an active informational
interaction between bone marrow cells and microglia.
Simultaneously, immunocytes are able to penetrate the brain through
the tumor bloodstream, vascular plexuses, cranial microchannels and
sinuses, and cerebrospinal and interstitial fluid (138), and kill cells containing the
presented antigen. Such findings indicate an important role of the
immune system in the formation of the CSC microenvironment.
Experimental studies conducted in the last decade
have expanded the concept with regard to the participation of
immunocytes in GB pathogenesis. Tumor development in the brain is
accompanied by migration and homing of red bone marrow cells to the
tumor nidus (139). To date,
>80 chemoattractants have been described to draw bone marrow
cells to the tumor nidus through different types of receptors,
including the recognition of stromal cell factor (SDF)1 or
chemokine C-X-C motif ligand 12 (CXCL12), a chemokine of the CXC
subfamily that binds to the CXCR4 receptor on the membrane of
CD45+ cells in the bone marrow, inducing their migration
to the tumor (140). Numerous
studies (141,142) suggest that the involvement of bone
marrow cells in the neoplastic process enriches the population of
immunosuppressive tumor microglia, and is accompanied by a stronger
resistance of CCs to cytostatics.
Production of numerous immunosuppressive cytokines
by neoplastic cells determines the microglia polarization vector,
with a significant proportion of CCs being completely removed
during surgery or destroyed by CRT. It is safe to assume that
vaccination with dead tumor tissue can enhance the antineoplastic
immune response, and the production of exosome-containing microRNAs
and other antitumor factors that can significantly alter the
properties of the CSC microenvironment, resulting in increased
effectiveness of CT. However, the local microenvironment in tumor
recurrence is formed in conditions of overall immunodeficiency that
results from the debilitating effect of PD-ligands,
glucocorticosteroids, radiation and CT (143), and this fact predetermines its
characteristics.
In fact, the exhausted phenotype of immunocytes is
developed due to the GB cells producing PD-L1 and 2, CTLA-4 and
other proteins that inhibit T-cell activity receptors (144). The emergence of a new class of
drugs that prevent PD-ligands from binding to T-cell receptors has
attracted interest (145), but the
effectiveness of nivolumab and other immune checkpoint inhibitors
in the complex treatment of GB was revealed to be low (146), thus suggesting the existence of
other causes of systemic immunosuppression.
Corticosteroids in the complex treatment of GB are
used to counteract cerebral oedema, but their use (147) is associated with inhibition of
lymphocyte proliferation, suppression of migration and interaction
of macrophages with T and B lymphocytes, inhibition of interferon
gamma (IFN-γ) release from macrophages, and reduced antibody
formation. The use of corticosteroids leads to increased blood
glucose levels and dexamethasone-induced leukocytosis (148), which exhausts the red bone marrow
(149) and reduces overall
survival.
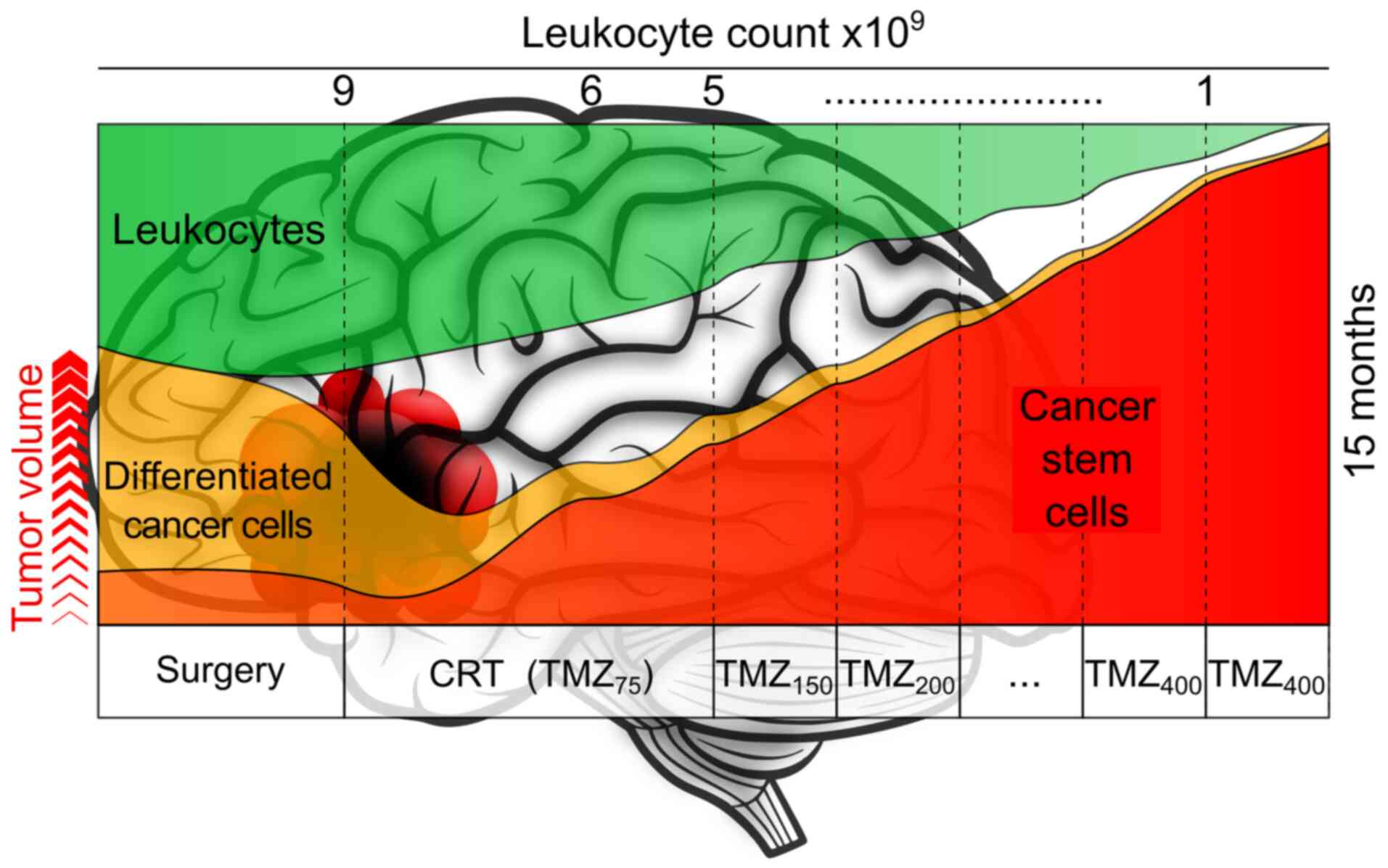
The maximum possible reduction of the number of CCs
in brain tissue is achieved by active use of chemoradiation
therapy. According to a previous study, >30 fractions of
γ-therapy allow lymphocytes to accumulate an average radiation dose
of 2.3 Gy, and the number of CD4+ cells in the organism
decreases by half and remains low for >1 year (150), which is time a patient may not
have. It is likely that higher radiation doses, which are often
used in treatment regimens such as in the case of radiosurgery, may
be accompanied by accumulation of a significantly higher radiation
dose in immunocytes, which induces a bystander effect in the bone
marrow and causes the death of a significant number of immune
cells.
In turn, cytostatics suppress hematopoiesis and
immunopoiesis, contributing to the development of clinically
significant thrombocytopenia and leukopenia, which is generally
considered the only criterion (151) limiting TMZ dose escalation.
Notably, a neutrophil count ≥40% below the norm is a criterion for
a satisfactory prognosis in IDH-wild-type GB (152), while reaching grades 3 and 4
neutropenia is considered a positive prognosis for CT outcome, with
a neutrophil count of <1-109 cells/l, warranting dose
reduction during the subsequent treatment stage.
In light of this, the completion of the main regimen
of GB treatment results in the immunodeficiency of the patient,
while tumor relapse is accompanied by the recruitment of
immunocytes with an ‘exhausted phenotype’ and the formation of an
immunosuppressive CSC microenvironment, which supports tumor
growth, promotes further involvement of the bone marrow in the
neoplastic process and prevents immunotherapy from fulfilling its
antineoplastic potential completely (Fig. 2).
8. HSCs and immunotherapy
Reprogramming of HSCs in the bone marrow during
tumor growth largely contributes to the process of
immunosuppression. Participation of normal stem cells in the tumor
process became a focus of attention after the publication of the
study by Aboody et al (152), which showed the migration of NSCs
to glioma cells. Snyder et al (153) reported the existence of an
association between GB cells and normal stem cells of other types,
in particular with HSCs. HSCs have been demonstrated to have high
mobility towards GB cells (154)
and, when injected into the bloodstream of an animal with glioma,
they migrate to the tumor nidus, where they accumulate in the blood
vessels of the tumor (155),
spread throughout the invasion area, and penetrate into the
necrotic zones of neoplastic tissue.
While migrating into a tumor, HSCs are capable of
interacting with CCs and can exchange a fluorescent stain, which
becomes inextricably connected with intracellular proteins in the
process of staining (156), and
this fact indicates the exchange of information between stem cells
and CCs. The reported mechanisms of such exchange (157) include the fusion of stem cells and
CCs, horizontal transfer of information through tights
intercellular junctions with areas of partial membrane fusion and
cytoplasm unification (158), and
the production of exosomes and other microvesicles containing
fragments of DNA, microRNA and proteins (159) into the external environment. All
this is accompanied by the reprogramming of interacting cells and
their development of new properties.
The significance of reprogramming of HSCs in GB
pathogenesis has barely been studied, but there are reasons to
consider that it is the most important factor of the immune system
inactivation in the tumor process (160). Bone marrow stem cells are known to
be a heterogeneous mixture of subpopulations, having cell elements
with different degrees of maturity, lifespan, gene expression
profiles and epigenetic programs of differentiation (161). Notably, a part of HSCs,
reprogrammed by the tumor, are able to gain advantage over other
HSC clones in bone marrow (162),
which can be accompanied by the expansion of mutant immunocytes
that are tumor tolerant (163).
Theoretically speaking, this is the most important
issue predetermining the final effect of all existing glioma
immunotherapy methods, the scenarios of which are based on the use
of mononuclear CD45+ cells recruited from the bone
marrow into the systemic bloodstream when granulocytic (G-CSF) or
granulocytic-macrophage (GM-CSF) colony-stimulating factors are
administered to patients. According to experimental data (164,165), the introduction of G-CSF into the
organism of experimental animals with injected glial brain tumor
fills the tumor tissue with markers of anti-inflammatory microglia
(166), while the subsequent
introduction of bacterial lipopolysaccharide and IFN-γ (167) promotes the inflammatory M1
activation of macrophages. An even greater antiglioma effect is
achieved when HSCs of a healthy sibling are transplanted into the
organism of an animal with glioma (161).
This fact directly indicates the essential role of
HSCs in the formation of the CSC microenvironment. Reprogramming of
HSCs during their interaction with CSCs and severe leukopenia from
chemoradiation therapy form a vicious circle, creating conditions
for β-catenin accumulation in CSCs and enhancing the lethal
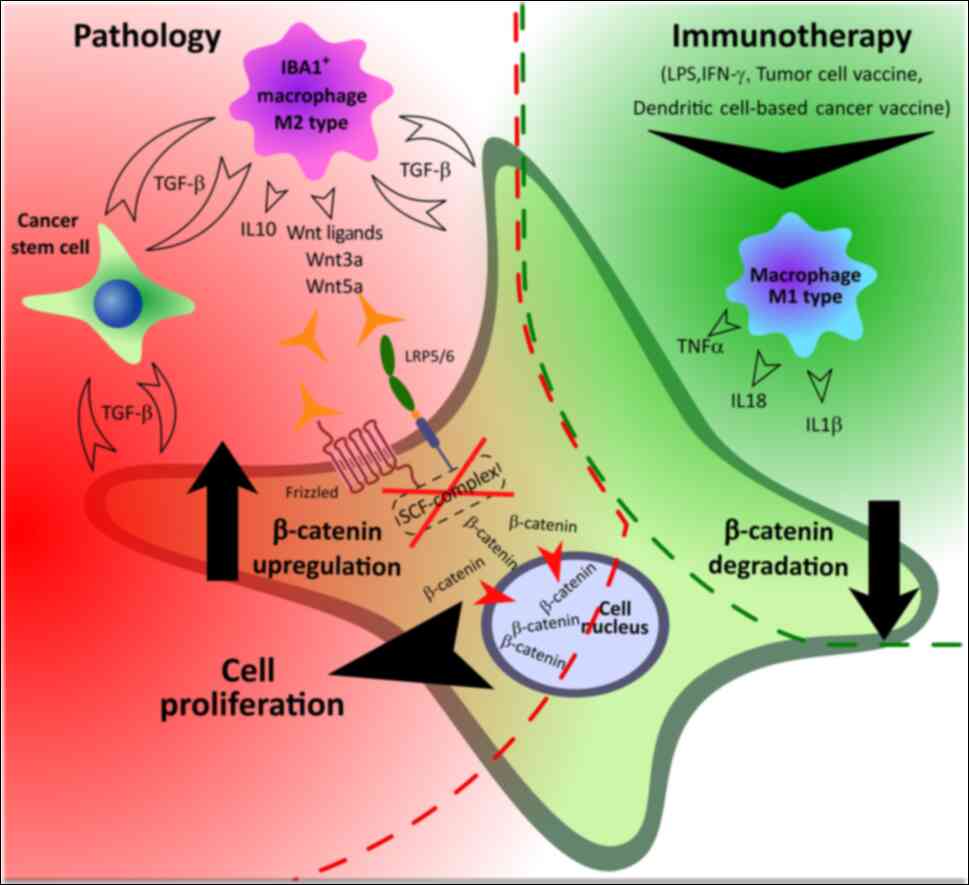
potential of this cell type. In this regard, β-catenin content in
tumors is an important criterion of immunotherapy effectiveness,
and the existing protocols of immunotherapy should be supplemented
with drugs that reduce the level of β-catenin (Fig. 3). At the same time, the construction
of immunotherapy scenarios using HSCs of a healthy relative or
autologous HSCs prepared before the disease, suggests positive
future prospects for the complex treatment of gliomas.
The existing algorithms of adaptive cell-based
immunotherapy of gliomas are still far from reaching this goal.
Adaptive immunotherapy involves the use of autologous immunocytes,
the source of which is a ‘leucoconcentrate’ of mononuclear cells
expressing the leukocyte common antigen CD45+, with
subsequent separation of T-lymphocytes ex vivo, their
additional activation by IL-2 and the targeting of GB cells with
proteins against key antigens, including IL13Rα2, HER2 and
EGFRvIII. The disadvantages of such therapy are clear, since
administration of G-CSF to the patient and stimulation with
antigens of Bacillus Calmette-Guérin, Staphylococcus aureus
or other suppurative microflora may lead to increased brain oedema,
which is a life-threatening condition.
An alternative strategy involves the stimulation of
the immunocytes of a patient ex vivo with antigens of
suppurative microflora and CCs; however, after CT, the bone marrow
of the patient is exhausted, and it is practically impossible to
accumulate the required number of immunocytes during one stage of
leucopheresis. At the same time, repeated administration of G-CSF
to the patient further exhausts the bone marrow and can promote the
formation of an immunosuppressive environment of CSCs.
Attempts have been made to use genetically modified
T cells equipped with chimeric antigen receptors (CARs), in which
sections of antigen-recognition domains, consisting of monoclonal
antibodies, are connected to sections of intracellular T-lymphocyte
signaling domains. The main problem with this technology is the
lack of absolutely perfect target antigens that are completely
tumor-specific, since the antigens IL13Rα2, HER2, EGFRvIII, B7-H3
and CSPG4, which are traditionally used as targets for CAR-T cell
creation, are not specific to CSCs, but much less homogeneously
expressed in tumors.
Attempts to create multivalent CAR-T cells
(167) targeted against the
synNotch receptor and other molecular targets in CSCs have not yet
produced the desired effect, since local immunosuppression remains
the main problem limiting the antitumor potential of CAR-T cells.
Attempts to combine CAR-T cells with microRNAs that locally inhibit
immunosuppressive genes (168)
have not yet exhibited any specific benefits and are associated
with toxicity and BBB penetrability problems (169). Attempts to increase the efficiency
of the targeted delivery of CAR-T cells by binding them to
chlorotoxin, a peptide particularly tropic to CCs (170) that is found in the venom of the
scorpion Leiurus quinquestriatus, as well as to combine
CAR-T therapy with intratumoral delivery of IL-12(171) have not been successful either.
The main disadvantage of adaptive immunotherapy
methods is the use of autologous exhausted immunocytes. Exhaustion
is a state of T-cell dysfunction that occurs in cases of chronic
infections and cancer, and is characterized by decreased effector
function and self-renewal capacity, sustained expression of
inhibitory receptors and a transcriptional state (171) distinct from that of functionally
active effector cells or memory T cells. Exhaustion prevents
optimal control of infections and tumors, but serves as a mechanism
to protect cells from death when they are hyperstimulated by tumor
antigens.
It can be assumed that a high proliferation rate
and a dynamic change of the antigen spectrum of GB cells are
aggression factors that inactivate the immune system. Attempts to
solve this problem using only immune checkpoint inhibitors have not
been successful thus far; in this regard, the use of allogeneic
cytotoxic lymphocytes derived from a healthy donor has been
reported (172), which requires
increasing doses of dexamethasone, revealing the typical
consequences of such therapy. However, this approach deserves
special attention, particularly taking into account the possibility
of regulating the local microenvironment of CSCs by using a
combination of CAR-T cell technology with oncolytic adenoviruses
(173) and immune checkpoint
inhibitors (174).
According to a previous study (175), CAR-T cell therapy poses serious
problems in the treatment of central nervous system tumors, which
emphasizes the problem of the hostile immunosuppressive
microenvironment of CSCs. However, in addition to CAR-T cells,
attempts to use CAR-T natural killer cells or CAR macrophages and
to generate active antineoplastic immunity in patients with GB are
of great interest.
Active immunotherapy involves the development of
antineoplastic immunity in a patient with GB by vaccination with
tumor cell vaccines or incubation of immunocytes with CC lysates
ex vivo, with the subsequent return to the patient in the
form of a dendritic cell vaccine (176). The use of tumor cell vaccines has
numerous advantages, including the fact that systematic vaccination
activates CD8-lymphocytes, the processes of antigen presentation by
macrophages, synthesis of pro-inflammatory cytokines and
modification of the tumor microenvironment (177), which increases the survival rate
of patients with both newly diagnosed and relapsed GB (178).
Autologous or allogeneic CSCs are used as antigens
to create tumor cell vaccines, which can stimulate an immune
response considering the heterogeneity of the neoplastic cell
population (179), which is
particularly relevant for GB. In light of this, a tumor cell
vaccine should include a combination of dead CSCs (for example,
after repeated freezing and thawing of proneural-type CSCs derived
from the first removed tumor) and CSCs of mesenchymal phenotype
obtained by sequential irradiation of autologous CCs, as well as
CSC derivatives from other patients. This approach allows not only
the destruction of CCs, but can also significantly increase
antineoplastic immunity (180) and
allow the formation of local criss-cross intratumor interactions
between CD4+ lymphocytes and other T cells (181), thus leading to increased
production of proinflammatory cytokines and modifying the
microenvironment of CSCs.
It is possible to create vaccines (182) using live genetically modified CCs
producing GM-CSF. Antigen-specific antineoplastic vaccines
producing GM-CSF have been used for tumor treatment for >20
years, and their use predominantly reveals the problem of
leucopenia and immunodeficiency, actually limiting the antiglioma
potential of immunotherapy. A possible method to solve this issue
is the combination of a tumor cell vaccine with transplantation of
HSCs from a haploidentical donor, ideally a sibling.
The common disadvantages of using tumor cell
vaccines are characteristic of the immunotherapy method with
dendritic cell vaccines. Dendritic cells are professional
antigen-presenting cells with high functional plasticity, which
originate from HSCs and show immunostimulatory or immunosuppressive
potential depending on the sequence and combination of
microenvironmental stimuli. The technology for the preparation of
dendritic cell vaccines includes the administration of G-CSF or
GM-CSF to the patient in order to recruit immunocytes into the
bloodstream, subsequent isolation of a fraction of mononuclear
cells containing a sufficient number of CD34+ HSCs,
ex vivo stimulation with CC antigens combined with IL-2 or
IL-4, multiplication in the presence of pro-inflammatory cytokines,
and return to the patient (182).
Antigens for the creation of dendritic cell vaccines include CSCs,
CC lysates, produced CCs and CSCs, exosomes, glioma-associated
peptides, and DNA and RNA fragments (183), but their efficacy in the treatment
of GB is relatively low.
Despite increased individual survival periods of
>23 months that have been reported (184), the overall situation has not
changed even after combining dendritic cell vaccines with immune
checkpoint inhibitors and other immunotherapy methods (185). Therapy with dendritic cell
vaccines results in general immunostimulatory effects in the form
of increased levels of immunosuppressive cytokines and local
infiltration of the tumor stroma by immunocytes. However, the main
challenge of therapy with dendritic cell vaccines is the problem of
immunosuppression, the solution of which is possible only after
creating such vaccines on the basis of healthy HSCs or developing
methods of restoring the patient's own HSCs.
It is the condition of HSCs that predetermines the
final effectiveness of almost all immunotherapy methods, but the
official ClinicalTrails website describes only one study (namely
NCT00014573) suggesting the use of immunotherapy methods while
supporting HSC transplantation in treatment-refractory brain
tumors. The use of autologous HSCs or stem cells of a sibling, and
pharmacological regulation of β-catenin level in HSCs together with
immunotherapy has not been discussed to date.
9. Conclusion
CSCs present a major challenge in GB treatment.
Destruction of these CCs with irradiation and cytostatics appears
to be impossible, and requires certain adjustments of existing GB
treatment strategies towards regulation of CSCs rather than
destruction of CCs. Numerous attempts to solve this problem with
the help of targeted medication have not been successful thus far,
which is usually explained by the plasticity of CSCs, with
β-catenin being the central link in the system of intracellular
signaling pathways, regulating the plasticity of this cell
type.
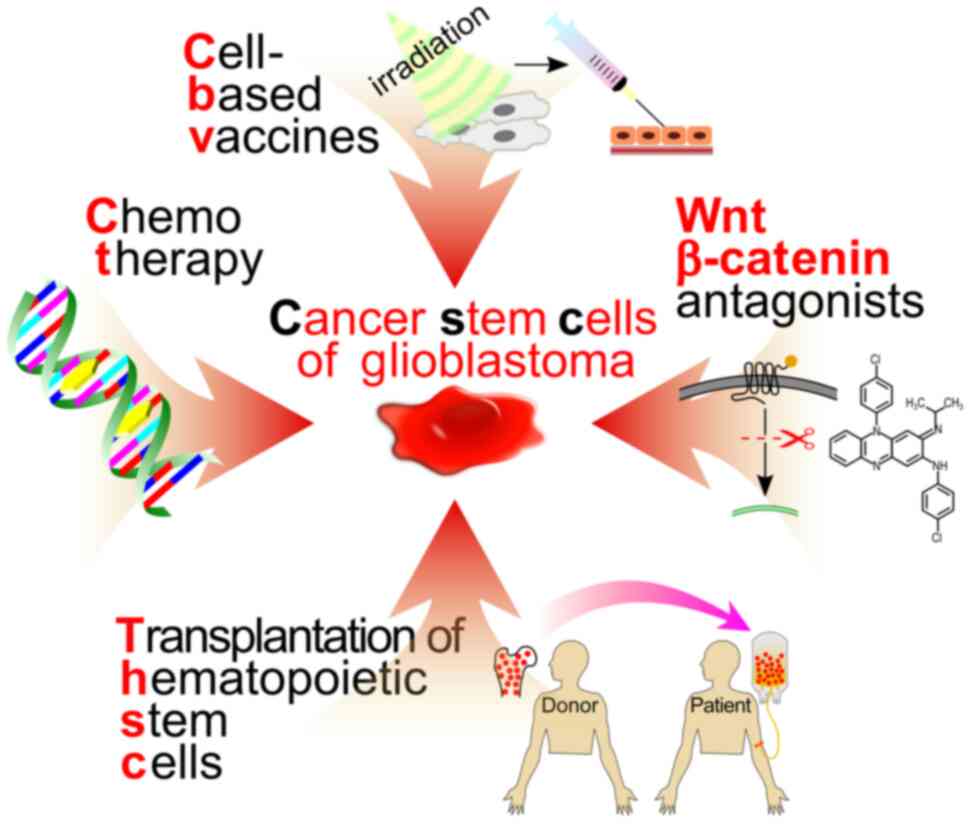
Pharmacological regulation of the β-catenin level
in CSCs using repurposed drugs opens new horizons for the
regulation of the proliferative potential of this cell type. The
antileprosy drug CFZ has the ability to inhibit the intracellular
Wnt/β-catenin signaling pathway. It is characterized by its good
tolerability and has demonstrated antineoplastic activity against
CSCs of a number of aggressive carcinomas, while its antiglioma
potential is virtually unexplored. The ability of CFZ to accumulate
in monocytes makes it very promising to be used as a drug for
targeted delivery to the tumor nidus, due to the transportation
potential of this cell type (Fig.
4).
Particular attention should be paid to the fact
that the β-catenin level in CSCs directly increases under the
influence of an immunosuppressive microenvironment, which is formed
with participation of microgliocytes and monocytes of the exhausted
phenotype, caused by radiation, cytostatics and the reprogramming
effect of the tumor on bone marrow HSCs. Indeed, all existing GB
treatment protocols lead to immunodeficiency, which actually limits
the therapeutic potential of all drugs and technologies, since it
is almost impossible to reduce the β-catenin level without
suppressing Wnt ligand production by the local microenvironment of
HSCs.
The use of CFZ and other repurposed drugs with
demonstrated Wnt-inhibitory activity can reduce the level of
β-catenin in CSCs. In turn, immunotherapy can regulate the
microenvironment of CSCs, resulting in a decrease in Wnt-ligand
synthesis and breaking the aforementioned vicious circle, which
would allow to create a technology of CSC management as part of the
complex immunotherapy of GB. One of the most important tasks in the
creation of such technology is developing immunotherapy scenarios
with the use of tumor cell vaccines containing a heterogeneous
composition of autologous and allogeneic, irradiated and
non-irradiated CCs, which will allow to induce a multidirectional
antineoplastic immune response. This should be enhanced by the
transplantation of healthy sibling HSCs and the administration of
CFZ or other drugs, thus reducing the β-catenin content in CSCs.
Pharmacological regulation of β-catenin content in CSCs and
cellular immunotherapy should be used together, since they are two
sides of the same coin.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AK, OP and IB conceived the study and searched for
relevant articles. AK and IB wrote the original draft, and reviewed
and edited the final manuscript. Data authentication is not
applicable. All authors have read and agreed to the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herrlinger U: News on the horizon in
glioblastoma therapy. ESMO Open. 5(e000601)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schaff LR and Mellinghoff IK: Glioblastoma
and other primary brain malignancies in adults: A review. JAMA.
329:574–587. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Delavar A, Wali AR, Santiago-Dieppa DR, Al
Jammal OM, Kidwell RL and Khalessi AA: Racial and ethnic
disparities in brain tumor survival by age group and tumor type. Br
J Neurosurg. 36:705–711. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-Year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Luo C, Song K, Wu S, Hameed NUF, Kudulaiti
N, Xu H, Qin ZY and Wu JS: The prognosis of glioblastoma: A large,
multifactorial study. Br J Neurosurg. 35:555–561. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yabo YA, Niclou SP and Golebiewska A:
Cancer cell heterogeneity and plasticity: A paradigm shift in
glioblastoma. Neuro Oncol. 24:669–682. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bikfalvi A, da Costa CA, Avril T, Barnier
JV, Bauchet L, Brisson L, Cartron PF, Castel H, Chevet E,
Chneiweiss H, et al: Challenges in glioblastoma research: Focus on
the tumor microenvironment. Trends Cancer. 9:9–27. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang M, Zhang D, Wu JY, Xing K, Yeo E, Li
C, Zhang L, Holland E, Yao L, Qin L, et al: Wnt-mediated
endothelial transformation into mesenchymal stem cell-like cells
induces chemoresistance in glioblastoma. Sci Transl Med.
12(eaay7522)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Barzegar Behrooz A, Talaie Z, Jusheghani
F, Łos MJ, Klonisch T and Ghavami S: Wnt and PI3K/Akt/mTOR survival
pathways as therapeutic targets in glioblastoma. Int J Mol Sci.
23(1353)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Behrooz AB and Syahir A: Could we address
the interplay between CD133, Wnt/β-catenin, and TERT signaling
pathways as a potential target for glioblastoma therapy? Front
Oncol. 11(642719)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Crunkhorn S: Targeting drug-resistant
glioblastoma. Nat Rev Drug Discov. 21(711)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Precilla DS, Kuduvalli SS, Purushothaman
M, Marimuthu P, Muralidharan AR and Anitha TS: Wnt/β-catenin
antagonists: Exploring new avenues to trigger old drugs in
alleviating glioblastoma multiforme. Curr Mol Pharmacol.
15:338–360. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yuan B, Wang G, Tang X, Tong A and Zhou L:
Immunotherapy of glioblastoma: Recent advances and future
prospects. Hum Vaccin Immunother. 18(2055417)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Youngblood MW, Stupp R and Sonabend AM:
Role of Resection in glioblastoma management. Neurosurg Clin N Am.
32:9–22. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
De Biase G, Garcia DP, Bohnen A and
Quiñones-Hinojosa A: Perioperative management of patients with
glioblastoma. Neurosurg Clin N Am. 32:1–8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lu VM, Goyal A, Graffeo CS, Perry A, Burns
TC, Parney IF, Quinones-Hinojosa A and Chaichana KL: Survival
benefit of maximal resection for glioblastoma reoperation in the
temozolomide era: A meta-analysis. World Neurosurg. 127:31–37.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Robin AM, Lee I and Kalkanis SN:
Reoperation for recurrent glioblastoma multiforme. Neurosurg Clin N
Am. 28:407–428. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liang C, Gong J, Zhang B, Meng Z, Li M and
Guo Y: Multiple subtentorial metastasis in diffuse midline glioma
receiving tumor treating fields: A case report and literature
review. Ann Transl Med. 9(1604)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen J, Shi Q, Li S, Zhao Y and Huang H:
Clinical characteristics of glioblastoma with metastatic spinal
dissemination. Ann Palliat Med. 11:506–512. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shah AH, Mahavadi A, Di L, Sanjurjo A,
Eichberg DG, Borowy V, Figueroa J, Luther E, de la Fuente MI,
Semonche A, et al: Survival benefit of lobectomy for glioblastoma:
Moving towards radical supramaximal resection. J Neurooncol.
148:501–508. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ryan JT, Nakayama M, Gleeson I, Mannion L,
Geso M, Kelly J, Ng SP and Hardcastle N: Functional brain imaging
interventions for radiation therapy planning in patients with
glioblastoma: A systematic review. Radiat Oncol.
17(178)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ylanan AMD, Pascual JSG, Cruz-Lim EMD,
Ignacio KHD, Cañal JPA and Khu KJO: Intraoperative radiotherapy for
glioblastoma: A systematic review of techniques and outcomes. J
Clin Neurosci. 93:36–41. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khan L, Soliman H, Sahgal A, Perry J, Xu W
and Tsao MN: External beam radiation dose escalation for high grade
glioma. Cochrane Database Syst Rev. 5(CD011475)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Barbarite E, Sick JT, Berchmans E, Bregy
A, Shah AH, Elsayyad N and Komotar RJ: The role of brachytherapy in
the treatment of glioblastoma multiforme. Neurosurg Rev.
40:195–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vogelius IR and Bentzen SM: Proton vs
photon radiation therapy for glioblastoma: Maximizing information
from trial. Neuro Oncol. 24:849–850. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Malouff TD, Seneviratne DS, Ebner DK,
Stross WC, Waddle MR, Trifiletti DM and Krishnan S: Boron Neutron
capture therapy: A review of clinical applications. Front Oncol.
11(601820)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Laprie A, Tensaouti F and Cohen-Jonathan
Moyal E: Radiation dose intensification for glioblastoma. Cancer
Radiother. 26:894–898. 2022.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
28
|
Tan AC, Ashley DM, López GY, Malinzak M,
Friedman HS and Khasraw M: Management of glioblastoma: State of the
art and future directions. CA Cancer J Clin. 70:299–312.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Woodroffe RW, Zanaty M, Soni N, Mott SL,
Helland LC, Pasha A, Maley J, Dhungana N, Jones KA, Monga V and
Greenlee JDW: Survival after reoperation for recurrent
glioblastoma. J Clin Neurosci. 73:118–124. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zakaria R and Weinberg JS: Challenges
associated with reoperation in patients with glioma. Neurosurg Clin
N Am. 32:129–135. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mathen P, Rowe L, Mackey M, Smart D,
Tofilon P and Camphausen K: Radiosensitizers in the temozolomide
era for newly diagnosed glioblastoma. Neurooncol Pract. 7:268–276.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Herrlinger U, Tzaridis T, Mack F,
Steinbach JP, Schlegel U, Sabel M, Hau P, Kortmann RD, Krex D,
Grauer O, et al: Lomustine-temozolomide combination therapy versus
standard temozolomide therapy in patients with newly diagnosed
glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A
randomised, open-label, phase 3 trial. Lancet. 393:678–688.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hwang K, Lee JH, Kim SH, Go KO, Ji SY, Han
JH and Kim CY: The combination PARP inhibitor olaparib with
temozolomide in an experimental glioblastoma model. In Vivo.
35:2015–2023. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nguyen TTT, Zhang Y, Shang E, Shu C,
Torrini C, Zhao J, Bianchetti E, Mela A, Humala N, Mahajan A, et
al: HDAC inhibitors elicit metabolic reprogramming by targeting
super-enhancers in glioblastoma models. J Clin Invest.
130:3699–3716. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bindra RS: Penetrating the brain tumor
space with DNA damage response inhibitors. Neuro Oncol.
22:1718–1720. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao J, Yang S, Cui X, Wang Q, Yang E,
Tong F, Hong B, Xiao M, Xin L, Xu C, et al: A novel compound
EPIC-0412 reverses temozolomide resistance via inhibiting DNA
repair/MGMT in glioblastoma. Neuro Oncol. 25:857–870.
2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Goel NJ, Bird CE, Hicks WH and Abdullah
KG: Economic implications of the modern treatment paradigm of
glioblastoma: An analysis of global cost estimates and their
utility for cost assessment. J Med Econ. 24:1018–1024.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lauko A, Lo A, Ahluwalia MS and Lathia JD:
Cancer cell heterogeneity & plasticity in glioblastoma and
brain tumors. Semin Cancer Biol. 82:162–175. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Oliver L, Lalier L, Salaud C, Heymann D,
Cartron PF and Vallette FM: Drug resistance in glioblastoma: Are
persisters the key to therapy? Cancer Drug Resist. 3:287–301.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Verhaak RG, Hoadley KA, Purdom E, Wang V,
Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al:
Integrated genomic analysis identifies clinically relevant subtypes
of glioblastoma characterized by abnormalities in PDGFRA, IDH1,
EGFR, and NF1. Cancer Cell. 17:98–110. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Steponaitis G and Tamasauskas A:
Mesenchymal and proneural subtypes of glioblastoma disclose
branching based on GSC associated signature. Int J Mol Sci.
22(4964)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Melhem JM, Detsky J, Lim-Fat MJ and Perry
JR: Updates in IDH-wildtype glioblastoma. Neurotherapeutics.
19:1705–1723. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
French R and Pauklin S: Epigenetic
regulation of cancer stem cell formation and maintenance. Int J
Cancer. 148:2884–2897. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Capper D, Stichel D, Sahm F, Jones DTW,
Schrimpf D, Sill M, Schmid S, Hovestadt V, Reuss DE, Koelsche C, et
al: Practical implementation of DNA methylation and
copy-number-based CNS tumor diagnostics: The Heidelberg experience.
Acta Neuropathol. 136:181–210. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Neftel C, Laffy J, Filbin MG, Hara T,
Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM,
et al: An integrative model of cellular states, plasticity, and
genetics for glioblastoma. Cell. 178:835–849.e21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Garofano L, Migliozzi S, Oh YT, D'Angelo
F, Najac RD, Ko A, Frangaj B, Caruso FP, Yu K, Yuan J, et al:
Pathway-based classification of glioblastoma uncovers a
mitochondrial subtype with therapeutic vulnerabilities. Nat Cancer.
2:141–156. 2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hubert CG and Lathia JD: Seeing the GBM
diversity spectrum. Nat Cancer. 2:135–137. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Richards LM, Whitley OKN, MacLeod G,
Cavalli FMG, Coutinho FJ, Jaramillo JE, Svergun N, Riverin M,
Croucher DC, Kushida M, et al: Gradient of developmental and injury
response transcriptional states defines functional vulnerabilities
underpinning glioblastoma heterogeneity. Nat Cancer. 2:157–173.
2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ensenyat-Mendez M, Íñiguez-Muñoz S, Sesé B
and Marzese DM: iGlioSub: An integrative transcriptomic and
epigenomic classifier for glioblastoma molecular subtypes. BioData
Min. 14(42)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang LB, Karpova A, Gritsenko MA, Kyle JE,
Cao S, Li Y, Rykunov D, Colaprico A, Rothstein JH, Hong R, et al:
Proteogenomic and metabolomic characterization of human
glioblastoma. Cancer Cell. 39:509–528.e20. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Drakulic D, Schwirtlich M, Petrovic I,
Mojsin M, Milivojevic M, Kovacevic-Grujicic N and Stevanovic M:
Current opportunities for targeting dysregulated neurodevelopmental
signaling pathways in glioblastoma. Cells. 11(2530)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Walcher L, Kistenmacher AK, Suo H, Kitte
R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S and
Kossatz-Boehlert U: Cancer stem cells-origins and biomarkers:
Perspectives for targeted personalized therapies. Front Immunol.
11(1280)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bryukhovetskiy A, Shevchenko V, Kovalev S,
Chekhonin V, Baklaushev V, Bryukhovetskiy I and Zhukova M: To the
novel paradigm of proteome-based cell therapy of tumors: Through
comparative proteome mapping of tumor stem cells and
tissue-specific stem cells of humans. Cell Transplant. 23 (Suppl
1):S151–S170. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bryukhovetskiy IS, Dyuizen IV, Shevchenko
VE, Bryukhovetskiy AS, Mischenko PV, Milkina EV and Khotimchenko
YS: Hematopoietic stem cells as a tool for the treatment of
glioblastoma multiforme. Mol Med Rep. 14:4511–4520. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Couturier CP, Nadaf J, Li Z, Baig S, Riva
G, Le P, Kloosterman DJ, Monlong J, Nkili Meyong A, Allache R, et
al: Glioblastoma scRNA-seq shows treatment-induced,
immune-dependent increase in mesenchymal cancer cells and
structural variants in distal neural stem cells. Neuro Oncol.
24:1494–1508. 2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wang X, Zhou R, Xiong Y, Zhou L, Yan X,
Wang M, Li F, Xie C, Zhang Y, Huang Z, et al: Sequential
fate-switches in stem-like cells drive the tumorigenic trajectory
from human neural stem cells to malignant glioma. Cell Res.
31:684–702. 2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Mizrak D, Brittan M and Alison M: CD133:
Molecule of the moment. J Pathol. 214:3–9. 2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zheng Y, Wang L, Yin L, Yao Z, Tong R, Xue
J and Lu Y: Lung cancer stem cell markers as therapeutic targets:
An update on signaling pathways and therapies. Front Oncol.
12(873994)2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Park J, Kim SK, Hallis SP, Choi BH and
Kwak MK: Role of CD133/NRF2 axis in the development of colon cancer
stem cell-like properties. Front Oncol. 11(808300)2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Hefni AM, Sayed AM, Hussien MT, Abdalla AZ
and Gabr AG: CD133 is an independent predictive and prognostic
marker in metastatic breast cancer. Cancer Biomark. 35:207–215.
2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gimple RC, Bhargava S, Dixit D and Rich
JN: Glioblastoma stem cells: Lessons from the tumor hierarchy in a
lethal cancer. Genes Dev. 33:591–609. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Beier CP and Beier D: CD133 negative
cancer stem cells in glioblastoma. Front Biosci (Elite Ed).
3:701–710. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
66
|
Wang Z, Zhang H, Xu S, Liu Z and Cheng Q:
The adaptive transition of glioblastoma stem cells and its
implications on treatments. Signal Transduct Target Ther.
6(124)2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Xie XP, Laks DR, Sun D, Ganbold M, Wang Z,
Pedraza AM, Bale T, Tabar V, Brennan C, Zhou X and Parada LF:
Quiescent human glioblastoma cancer stem cells drive tumor
initiation, expansion, and recurrence following chemotherapy. Dev
Cell. 57:32–46.e8. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Liu J, Gao L, Zhan N, Xu P, Yang J, Yuan
F, Xu Y, Cai Q, Geng R and Chen Q: Hypoxia induced ferritin light
chain (FTL) promoted epithelia mesenchymal transition and
chemoresistance of glioma. J Exp Clin Cancer Res.
39(137)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gao L, Tong S, Liu J, Cai J, Ye Z, Zhou L,
Song P, Li Z, Lei P, Wei H, et al: TMEM2 induces
epithelial-mesenchymal transition and promotes resistance to
temozolomide in GBM cells. Heliyon. 9(e16559)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zhang X, Wang X, Xu R, Ji J, Xu Y, Han M,
Wei Y, Huang B, Chen A, Zhang Q, et al: YM155 decreases
radiation-induced invasion and reverses epithelial-mesenchymal
transition by targeting STAT3 in glioblastoma. J Transl Med.
16(79)2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhang X, Wang X, Xu R, Ji J, Xu Y, Han M,
Wei Y, Huang B, Chen A, Zhang Q, et al: Correction to: YM155
decreases radiation-induced invasion and reverses
epithelial-mesenchymal transition by targeting STAT3 in
glioblastoma. J Transl Med. 19(407)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Huang W, Zhang C, Cui M, Niu J and Ding W:
Inhibition of bevacizumab-induced epithelial-mesenchymal transition
by BATF2 overexpression involves the suppression of Wnt/β-catenin
signaling in glioblastoma cells. Anticancer Res. 37:4285–4294.
2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Coelho BP, Fernandes CFL, Boccacino JM,
Souza MCDS, Melo-Escobar MI, Alves RN, Prado MB, Iglesia RP,
Cangiano G, Mazzaro GR and Lopes MH: Multifaceted WNT signaling at
the crossroads between epithelial-mesenchymal transition and
autophagy in glioblastoma. Front Oncol. 10(597743)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Alkailani MI, Aittaleb M and Tissir F: WNT
signaling at the intersection between neurogenesis and brain
tumorigenesis. Front Mol Neurosci. 15(1017568)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Rajakulendran N, Rowland KJ, Selvadurai
HJ, Ahmadi M, Park NI, Naumenko S, Dolma S, Ward RJ, So M, Lee L,
et al: Wnt and Notch signaling govern self-renewal and
differentiation in a subset of human glioblastoma stem cells. Genes
Dev. 33:498–510. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Rim EY, Clevers H and Nusse R: The Wnt
pathway: From signaling mechanisms to synthetic modulators. Annu
Rev Biochem. 91:571–598. 2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Sareddy GR, Pratap UP, Viswanadhapalli S,
Venkata PP, Nair BC, Krishnan SR, Zheng S, Gilbert AR, Brenner AJ,
Brann DW and Vadlamudi RK: PELP1 promotes glioblastoma progression
by enhancing Wnt/β-catenin signaling. Neurooncol Adv.
1(vdz042)2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Latour M, Her NG, Kesari S and Nurmemmedov
E: WNT signaling as a therapeutic target for glioblastoma. Int J
Mol Sci. 22(8428)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Tang C, Guo J, Chen H, Yao CJ, Zhuang DX,
Wang Y, Tang WJ, Ren G, Yao Y, Wu JS, et al: Gene mutation
profiling of primary glioblastoma through multiple tumor biopsy
guided by 1H-magnetic resonance spectroscopy. Int J Clin Exp
Pathol. 8:5327–5335. 2015.PubMed/NCBI
|
|
80
|
Morris LG, Ramaswami D and Chan TA: The
FAT epidemic: A gene family frequently mutated across multiple
human cancer types. Cell Cycle. 12:1011–1012. 2013.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Fargeas CA, Lorico A and Corbeil D:
Commentary: Could we address the interplay between CD133,
Wnt/β-catenin, and TERT signaling pathways as a potential target
for glioblastoma therapy? Front Oncol. 11(712358)2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Shevchenko V, Arnotskaya N, Zaitsev S,
Sharma A, Sharma HS, Bryukhovetskiy A, Pak O, Khotimchenko Y and
Bryukhovetskiy I: Proteins of Wnt signaling pathway in cancer stem
cells of human glioblastoma. Int Rev Neurobiol. 151:185–200.
2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Manoranjan B, Chokshi C, Venugopal C,
Subapanditha M, Savage N, Tatari N, Provias JP, Murty NK, Moffat J,
Doble BW and Singh SK: A CD133-AKT-Wnt signaling axis drives
glioblastoma brain tumor-initiating cells. Oncogene. 39:1590–1599.
2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Jhanwar-Uniyal M, Wainwright JV, Mohan AL,
Tobias ME, Murali R, Gandhi CD and Schmidt MH: Diverse signaling
mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a
formidable relationship. Adv Biol Regul. 72:51–62. 2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Shahcheraghi SH, Tchokonte-Nana V, Lotfi
M, Lotfi M, Ghorbani A and Sadeghnia HR: Wnt/beta-catenin and
PI3K/Akt/mTOR signaling pathways in glioblastoma: Two main targets
for drug design: A review. Curr Pharm Des. 26:1729–1741.
2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Liu N, Guo XH, Liu JP and Cong YS: Role of
telomerase in the tumor microenvironment. Clin Exp Pharmacol
Physiol. 47:357–364. 2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Abad E, Graifer D and Lyakhovich A: DNA
damage response and resistance of cancer stem cells. Сancer Lett.
474:106–117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Fan D, Yue Q, Chen J, Wang C, Yu R, Jin Z,
Yin S, Wang Q, Chen L, Liao X, et al: Reprogramming the
immunosuppressive microenvironment of IDH1 wild-type glioblastoma
by blocking Wnt signaling between microglia and cancer cells.
Oncoimmunology. 10(1932061)2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Hao J, Han X, Huang H, Yu X, Fang J, Zhao
J, Prayson RA, Bao S and Yu JS: Sema3C signaling is an alternative
activator of the canonical WNT pathway in glioblastoma. Nat Commun.
14(2262)2023.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Montemurro N, Pahwa B, Tayal A, Shukla A,
De Jesus Encarnacion M, Ramirez I, Nurmukhametov R, Chavda V and De
Carlo A: Macrophages in recurrent glioblastoma as a prognostic
factor in the synergistic system of the tumor microenvironment.
Neurol Int. 15:595–608. 2023.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Chan MK, Chung JY, Tang PC, Chan AS, Ho
JY, Lin TP, Chen J, Leung KT, To KF, Lan HY and Tang PM: TGF-β
signaling networks in the tumor microenvironment. Cancer Lett.
550(215925)2022.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Yun EJ, Kim S, Hsieh JT and Baek ST:
Wnt/β-catenin signaling pathway induces autophagy-mediated
temozolomide-resistance in human glioblastoma. Cell Death Dis.
11(771)2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Matias D, Predes D, Niemeyer Filho P,
Lopes MC, Abreu JG, Lima FRS and Moura Neto V:
Microglia-glioblastoma interactions: New role for Wnt signaling.
Biochim Biophys Acta Rev Cancer. 1868:333–340. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Tao W, Chu C, Zhou W, Huang Z, Zhai K,
Fang X, Huang Q, Zhang A, Wang X, Yu X, et al: Dual role of WISP1
in maintaining glioma stem cells and tumor-supportive macrophages
in glioblastoma. Nat Commun. 11(3015)2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Bayik D and Lathia JD: Cancer stem
cell-immune cell crosstalk in tumor progression. Nat Rev Cancer.
21:526–536. 2021.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Omuro A: Immune-checkpoint inhibitors for
glioblastoma: What have we learned? Arq Neuropsiquiatr. 80 (5 Suppl
1):S266–S269. 2022.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Verdugo E, Puerto I and Medina MÁ: An
update on the molecular biology of glioblastoma, with clinical
implications and progress in its treatment. Cancer Commun (Lond).
42:1083–1111. 2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Sautter L, Hofheinz R, Tuettenberg J,
Grimm M, Vajkoczy P, Groden C, Schmieder K, Hochhaus A, Wenz F and
Giordano FA: Open-label phase II evaluation of imatinib in primary
inoperable or incompletely resected and recurrent glioblastoma.
Oncology. 98:16–22. 2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Kim JY, Jo Y, Oh HK and Kim EH: Sorafenib
increases tumor treating fields-induced cell death in glioblastoma
by inhibiting STAT3. Am J Cancer Res. 10:3475–3486. 2020.PubMed/NCBI
|
|
100
|
Alamón C, Dávila B, García MF, Sánchez C,
Kovacs M, Trias E, Barbeito L, Gabay M, Zeineh N, Gavish M, et al:
Sunitinib-containing carborane pharmacophore with the ability to
inhibit tyrosine kinases receptors FLT3, KIT and PDGFR-β, exhibits
powerful in vivo anti-glioblastoma activity. Cancers (Basel).
12(3423)2020.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Reardon DA, Desjardins A, Vredenburgh JJ,
O'Rourke DM, Tran DD, Fink KL, Nabors LB, Li G, Bota DA, Lukas RV,
et al: Rindopepimut with bevacizumab for patients with relapsed
EGFRvIII-expressing glioblastoma (ReACT): Results of a double-blind
randomized phase II trial. Clin Cancer Res. 26:1586–1594.
2020.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Padovan M, Eoli M, Pellerino A, Rizzato S,
Caserta C, Simonelli M, Michiara M, Caccese M, Anghileri E,
Cerretti G, et al: Depatuxizumab mafodotin (Depatux-M) plus
temozolomide in recurrent glioblastoma patients: Real-world
experience from a multicenter study of italian association of
neuro-oncology (AINO). Cancers (Basel). 13(2773)2021.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Lassman AB, Pugh SL, Wang TJC, Aldape K,
Gan HK, Preusser M, Vogelbaum MA, Sulman EP, Won M, Zhang P, et al:
Depatuxizumab mafodotin in EGFR-amplified newly diagnosed
glioblastoma: A phase III randomized clinical trial. Neuro Oncol.
25:339–350. 2023.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Cloughesy T, Finocchiaro G, Belda-Iniesta
C, Recht L, Brandes AA, Pineda E, Mikkelsen T, Chinot OL, Balana C,
Macdonald DR, et al: Randomized, double-blind, placebo-controlled,
multicenter phase II study of onartuzumab plus bevacizumab versus
placebo plus bevacizumab in patients with recurrent glioblastoma:
efficacy, safety, and hepatocyte growth factor and
O6-methylguanine-DNA methyltransferase biomarker
analyses. J Clin Oncol. 35:343–351. 2017.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Cloughesy TF, Drappatz J, de Groot J,
Prados MD, Reardon DA, Schiff D, Chamberlain M, Mikkelsen T,
Desjardins A, Ping J, et al: Phase II study of cabozantinib in
patients with progressive glioblastoma: Subset analysis of patients
with prior antiangiogenic therapy. Neuro Oncol. 20:259–267.
2018.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Zhang T, Xin Q and Kang JM: Bevacizumab
for recurrent glioblastoma: A systematic review and meta-analysis.
Eur Rev Med Pharmacol Sci. 25:6480–6491. 2021.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Wen PY, Rodon JA, Mason W, Beck JT,
DeGroot J, Donnet V, Mills D, El-Hashimy M and Rosenthal M: Phase
I, open-label, multicentre study of buparlisib in combination with
temozolomide or with concomitant radiation therapy and temozolomide
in patients with newly diagnosed glioblastoma. ESMO Open.
5(e000673)2020.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Rosenthal M, Clement PM, Campone M,
Gil-Gil MJ, DeGroot J, Chinot O, Idbaih A, Gan H, Raizer J, Wen PY,
et al: Buparlisib plus carboplatin or lomustine in patients with
recurrent glioblastoma: A phase Ib/II, open-label, multicentre,
randomised study. ESMO Open. 5(e000672)2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Kaley TJ, Panageas KS, Pentsova EI,
Mellinghoff IK, Nolan C, Gavrilovic I, DeAngelis LM, Abrey LE,
Holland EC, Omuro A, et al: Phase I clinical trial of temsirolimus
and perifosine for recurrent glioblastoma. Ann Clin Transl Neurol.
7:429–436. 2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Schiff D, Jaeckle KA, Anderson SK, Galanis
E, Giannini C, Buckner JC, Stella P, Flynn PJ, Erickson BJ,
Schwerkoske JF, et al: Phase 1/2 trial of temsirolimus and
sorafenib in the treatment of patients with recurrent glioblastoma:
North central cancer treatment group study/alliance N0572. Cancer.
124:1455–1463. 2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Lin Z, Xu H, Yang R, Li Z, Zheng H, Zhang
Z, Peng J, Zhang X, Qi S, Liu Y and Huang G: Effective treatment of
a BRAF V600E-mutant epithelioid glioblastoma patient by
vemurafenib: a case report. Anticancer Drugs. 33:100–104.
2022.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Herrera-Rios D, Li G, Khan D, Tsiampali J,
Nickel AC, Aretz P, Hewera M, Suwala AK, Jiang T, Steiger HJ, et
al: A computational guided, functional validation of a novel
therapeutic antibody proposes Notch signaling as a clinical
relevant and druggable target in glioma. Sci Rep.
10(16218)2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Bogdahn U, Hau P, Stockhammer G,
Venkataramana NK, Mahapatra AK, Suri A, Balasubramaniam A, Nair S,
Oliushine V, Parfenov V, et al: Targeted therapy for high-grade
glioma with the TGF-β2 inhibitor trabedersen: Results of a
randomized and controlled phase IIb study. Neuro Oncol. 13:132–142.
2011.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Hadizadeh M, AminJafari A, Parvizpour S
and Ghasemi S: Novel targets to overcome antiangiogenesis therapy
resistance in glioblastoma multiforme: Systems biology approach and
suggestion of therapy by galunisertib. Cell Biol Int. 46:1649–1660.
2022.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Wick A, Desjardins A, Suarez C, Forsyth P,
Gueorguieva I, Burkholder T, Cleverly AL, Estrem ST, Wang S, Lahn
MM, et al: Phase 1b/2a study of galunisertib, a small molecule
inhibitor of transforming growth factor-beta receptor I, in
combination with standard temozolomide-based radiochemotherapy in
patients with newly diagnosed malignant glioma. Invest New Drugs.
38:1570–1579. 2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Hsu SY, Lee SC, Liu HC, Peng SF, Chueh FS,
Lu TJ, Lee HT and Chou YC: Phenethyl isothiocyanate suppresses the
proinflammatory cytokines in human glioblastoma cells through the
PI3K/Akt/NF-κB signaling pathway in vitro. Oxid Med Cell Longev.
2022(2108289)2022.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Li S, He Y, Chen K, Sun J, Zhang L, He Y,
Yu H and Li Q: RSL3 drives ferroptosis through NF-κB pathway
activation and GPX4 depletion in glioblastoma. Oxid Med Cell
Longev. 2021(2915019)2021.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Volmar MNM, Cheng J, Alenezi H, Richter S,
Haug A, Hassan Z, Goldberg M, Li Y, Hou M, Herold-Mende C, et al:
Cannabidiol converts NF-κB into a tumor suppressor in glioblastoma
with defined antioxidative properties. Neuro Oncol. 23:1898–1910.
2021.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Navone SE, Guarnaccia L, Cordiglieri C,
Crisà FM, Caroli M, Locatelli M, Schisano L, Rampini P, Miozzo M,
La Verde N, et al: Aspirin affects tumor angiogenesis and
sensitizes human glioblastoma endothelial cells to temozolomide,
bevacizumab, and sunitinib, impairing vascular endothelial growth
factor-related signaling. World Neurosurg. 120:e380–e391.
2018.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Kast RE: Adding high-dose celecoxib to
increase effectiveness of standard glioblastoma chemoirradiation.
Ann Pharm Fr. 79:481–488. 2021.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Yin D, Jin G, He H, Zhou W, Fan Z, Gong C,
Zhao J and Xiong H: Celecoxib reverses the glioblastoma
chemo-resistance to temozolomide through mitochondrial metabolism.
Aging (Albany NY). 13:21268–21282. 2021.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Pantovic A, Bosnjak M, Arsikin K, Kosic M,
Mandic M, Ristic B, Tosic J, Grujicic D, Isakovic A, Micic N, et
al: In vitro antiglioma action of indomethacin is mediated via
AMP-activated protein kinase/mTOR complex 1 signalling pathway. Int
J Biochem Cell Biol. 83:84–96. 2017.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Allani SK, Weissbach H and Lopez Toledano
MA: Sulindac induces differentiation of glioblastoma stem cells
making them more sensitive to oxidative stress. Neoplasma.
65:376–388. 2018.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Qiu J, Shi Z and Jiang J: Cyclooxygenase-2
in glioblastoma multiforme. Drug Discov Today. 22:148–156.
2017.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Li H, Jiao S, Li X, Banu H, Hamal S and
Wang X: Therapeutic effects of antibiotic drug tigecycline against
cervical squamous cell carcinoma by inhibiting Wnt/β-catenin
signaling. Biochem Biophys Res Commun. 467:14–20. 2015.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Wieland A, Trageser D, Gogolok S, Reinartz
R, Höfer H, Keller M, Leinhaas A, Schelle R, Normann S, Klaas L, et
al: Anticancer effects of niclosamide in human glioblastoma. Clin
Cancer Res. 19:4124–4136. 2013.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Li H, Liu S, Jin R, Xu H, Li Y, Chen Y and
Zhao G: Pyrvinium pamoate regulates MGMT expression through
suppressing the Wnt/β-catenin signaling pathway to enhance the
glioblastoma sensitivity to temozolomide. Cell Death Discov.
7(288)2021.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Liu Y, Fang S, Sun Q and Liu B:
Anthelmintic drug ivermectin inhibits angiogenesis, growth and
survival of glioblastoma through inducing mitochondrial dysfunction
and oxidative stress. Biochem Biophys Res Commun. 480:415–421.
2016.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Hajikhani B, Nasiri MJ, Hosseini SS,
Khalili F, Karimi-Yazdi M, Hematian A, Nojookambari NY, Goudarzi M,
Dadashi M and Mirsaeidi M: Clofazimine susceptibility testing of
Mycobacterium avium complex and Mycobacterium abscessus: A
meta-analysis study. J Glob Antimicrob Resist. 26:188–193.
2021.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Park L, Wallace CE, Vasile G and Buckley
C: A case of lepromatous leprosy with erythema nodosum leprosum.
Cureus. 15(e33846)2023.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Ahmed K, Koval A, Xu J, Bodmer A and
Katanaev VL: Towards the first targeted therapy for triple-negative
breast cancer: Repositioning of clofazimine as a
chemotherapy-compatible selective Wnt pathway inhibitor. Cancer
Lett. 449:45–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Xu J, Koval A and Katanaev VL: Beyond
TNBC: Repositioning of clofazimine against a broad range of
Wnt-dependent cancers. Front Oncol. 10(602817)2020.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Keswani RK, Tian C, Peryea T, Girish G,
Wang X and Rosania GR: Repositioning clofazimine as a
macrophage-targeting photoacoustic contrast agent. Sci Rep.
6(23528)2016.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Trexel J, Yoon GS, Keswani RK, McHugh C,
Yeomans L, Vitvitsky V, Banerjee R, Sud S, Sun Y, Rosania GR and
Stringer KA: Macrophage-mediated clofazimine sequestration is
accompanied by a shift in host energy metabolism. J Pharm Sci.
106:1162–1174. 2017.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Yoon GS, Sud S, Keswani RK, Baik J,
Standiford TJ, Stringer KA and Rosania GR: Phagocytosed clofazimine
biocrystals can modulate innate immune signaling by inhibiting TNFα
and boosting IL-1RA secretion. Mol Pharm. 12:2517–2527.
2015.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Geribaldi-Doldán N, Fernández-Ponce C,
Quiroz RN, Sánchez-Gomar I, Escorcia LG, Velásquez EP and Quiroz
EN: The role of microglia in glioblastoma. Front Oncol.
10(603495)2021.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Castellani G, Croese T, Peralta Ramos JM
and Schwartz M: Transforming the understanding of brain immunity.
Science. 380(eabo7649)2023.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Rustenhoven J and Kipnis J: Brain borders
at the central stage of neuroimmunology. Nature. 612:417–429.
2022.PubMed/NCBI View Article : Google Scholar : De Leo A, Ugolini
A and Veglia F: Myeloid cells in glioblastoma microenvironment.
Cells 10: 18, 2020.
|
|
139
|
Lewellis SW and Knaut H: Attractive
guidance: How the chemokine SDF1/CXCL12 guides different cells to
different locations. Semin Cell Dev Biol. 23:333–340.
2012.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Giordano FA, Link B, Glas M, Herrlinger U,
Wenz F, Umansky V, Brown JM and Herskind C: Targeting the
post-irradiation tumor microenvironment in glioblastoma via
inhibition of CXCL12. Cancers (Basel). 11(272)2019.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Wang S, Chen C, Li J, Xu X, Chen W and Li
F: The CXCL12/CXCR4 axis confers temozolomide resistance to human
glioblastoma cells via up-regulation of FOXM1. J Neurol Sci.
414(116837)2020.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Dolina JS, Van Braeckel-Budimir N, Thomas
GD and Salek-Ardakani S: CD8+ T cell exhaustion in
cancer. Front Immunol. 12(715234)2021.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Belk JA, Daniel B and Satpathy AT:
Epigenetic regulation of T cell exhaustion. Nat Immunol.
23:848–860. 2022.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Yang T, Kong Z and Ma W: PD-1/PD-L1 immune
checkpoint inhibitors in glioblastoma: Clinical studies, challenges
and potential. Hum Vaccin Immunother. 17:546–553. 2021.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Arrieta VA, Dmello C, McGrail DJ, Brat DJ,
Lee-Chang C, Heimberger AB, Chand D, Stupp R and Sonabend AM:
Immune checkpoint blockade in glioblastoma: From tumor
heterogeneity to personalized treatment. J Clin Invest.
133(e163447)2023.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Caramanna I, de Kort JM, Brandes AA, Taal
W, Platten M, Idbaih A, Frenel JS, Wick W, Preetha CJ, Bendszus M,
et al: Corticosteroids use and neurocognitive functioning in
patients with recurrent glioblastoma: Evidence from European
organization for research and treatment of cancer (EORTC) trial
26101. Neurooncol Pract. 9:310–316. 2022.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Pitter KL, Tamagno I, Alikhanyan K,
Hosni-Ahmed A, Pattwell SS, Donnola S, Dai C, Ozawa T, Chang M,
Chan TA, et al: Corticosteroids compromise survival in
glioblastoma. Brain. 139:1458–1471. 2016.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Klement RJ and Champ CE: Corticosteroids
compromise survival in glioblastoma in part through their elevation
of blood glucose levels. Brain. 140(e16)2017.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Yovino S, Kleinberg L, Grossman SA,
Narayanan M and Ford E: The etiology of treatment-related
lymphopenia in patients with malignant gliomas: Modeling radiation
dose to circulating lymphocytes explains clinical observations and
suggests methods of modifying the impact of radiation on immune
cells. Cancer Invest. 31:140–144. 2013.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Kut C and Kleinberg L: Radiotherapy,
lymphopenia and improving the outcome for glioblastoma: A narrative
review. Chin Clin Oncol. 12(4)2023.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Saito T, Sugiyama K, Hama S, Yamasaki F,
Takayasu T, Nosaka R, Muragaki Y, Kawamata T and Kurisu K:
Prognostic importance of temozolomide-induced neutropenia in
glioblastoma, IDH-wildtype patients. Neurosurg Rev. 41:621–628.
2018.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Aboody KS, Brown A, Rainov NG, Bower KA,
Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, et al:
Neural stem cells display extensive tropism for pathology in adult
brain: Evidence from intracranial gliomas. Proc Natl Acad Sci USA.
97:12846–12851. 2000.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Snyder EY, Park KI, Flax JD, Liu S,
Rosario CM, Yandava BD and Aurora S: Potential of neural
‘stem-like’ cells for gene therapy and repair of the degenerating
central nervous system. Adv Neurol. 72:121–132. 1997.PubMed/NCBI
|
|
154
|
Bryukhovetskiy IS, Mischenko PV, Tolok EV,
Zaitcev SV, Khotimchenko YS and Bryukhovetskiy AS: Directional
migration of adult hematopoeitic progenitors to C6 glioma in
vitro. Oncol Lett. 9:1839–1844. 2015.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Hass R, von der Ohe J and Dittmar T:
Hybrid formation and fusion of cancer cells in vitro and in vivo.
Cancers (Basel). 13(4496)2021.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Goldenberg DM: Horizontal transmission of
malignancy by cell-cell fusion. Expert Opin Biol Ther. 12 (Suppl
1):S133–S139. 2012.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Sun Z, Wang L, Zhou Y, Dong L, Ma W, Lv L,
Zhang J and Wang X: Glioblastoma stem cell-derived exosomes enhance
stemness and tumorigenicity of glioma cells by transferring Notch1
protein. Cell Mol Neurobiol. 40:767–784. 2020.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Khan F, Pang L, Dunterman M, Lesniak MS,
Heimberger AB and Chen P: Macrophages and microglia in
glioblastoma: Heterogeneity, plasticity, and therapy. J Clin
Invest. 133(e163446)2023.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Wang G, Zhong K, Wang Z, Zhang Z, Tang X,
Tong A and Zhou L: Tumor-associated microglia and macrophages in
glioblastoma: From basic insights to therapeutic opportunities.
Front Immunol. 13(964898)2022.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Bryukhovetskiy IS, Mischenko PV, Tolok EV,
Zaitcev SV, Khotimchenko YS and Bryukhovetskiy AS: Directional
migration of adult hematopoeitic progenitors to C6 glioma in
vitro. Oncol Lett. 9:1839–1844. 2015.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Lee-Six H, Øbro NF, Shepherd MS, Grossmann
S, Dawson K, Belmonte M, Osborne RJ, Huntly BJP, Martincorena I,
Anderson E, et al: Population dynamics of normal human blood
inferred from somatic mutations. Nature. 561:473–478.
2018.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Jaiswal S: Clonal hematopoiesis and
nonhematologic disorders. Blood. 136:1606–1614. 2020.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Kast RE, Hill QA, Wion D, Mellstedt H,
Focosi D, Karpel-Massler G, Heiland T and Halatsch ME:
Glioblastoma-synthesized G-CSF and GM-CSF contribute to growth and
immunosuppression: Potential therapeutic benefit from dapsone,
fenofibrate, and ribavirin. Tumour Biol.
39(1010428317699797)2017.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Bryukhovetskiy I, Manzhulo I, Mischenko P,
Milkina E, Dyuizen I, Bryukhovetskiy A and Khotimchenko Y: Cancer
stem cells and microglia in the processes of glioblastoma
multiforme invasive growth. Oncol Lett. 12:1721–1728.
2016.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Zaitsev S, Sharma HS, Sharma A, Manzhulo
I, Polevshchikov A, Kudriavtsev I, Khotimchenko Y, Pak O,
Bryukhovetskiy A and Bryukhovetskiy I: Pro-inflammatory
modification of cancer cells microsurroundings increases the
survival rates for rats with low differentiated malignant glioma of
brain. Int Rev Neurobiol. 151:253–279. 2020.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Choe JH, Watchmaker PB, Simic MS, Gilbert
RD, Li AW, Krasnow NA, Downey KM, Yu W, Carrera DA, Celli A, et al:
SynNotch-CAR T cells overcome challenges of specificity,
heterogeneity, and persistence in treating glioblastoma. Sci Transl
Med. 13(eabe7378)2021.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Meister H, Look T, Roth P, Pascolo S,
Sahin U, Lee S, Hale BD, Snijder B, Regli L, Ravi VM, et al:
Multifunctional mRNA-based CAR T cells display promising antitumor
activity against glioblastoma. Clin Cancer Res. 28:4747–4756.
2022.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Maggs L, Cattaneo G, Dal AE, Moghaddam AS
and Ferrone S: CAR T cell-based immunotherapy for the treatment of
glioblastoma. Front Neurosci. 15(662064)2021.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Wang D, Starr R, Chang WC, Aguilar B,
Alizadeh D, Wright SL, Yang X, Brito A, Sarkissian A, Ostberg JR,
et al: Chlorotoxin-directed CAR T cells for specific and effective
targeting of glioblastoma. Sci Transl Med.
12(eaaw2672)2020.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Agliardi G, Liuzzi AR, Hotblack A, De Feo
D, Núñez N, Stowe CL, Friebel E, Nannini F, Rindlisbacher L,
Roberts TA, et al: Intratumoral IL-12 delivery empowers CAR-T cell
immunotherapy in a pre-clinical model of glioblastoma. Nat Commun.
12(444)2021.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Brown CE, Rodriguez A, Palmer J, Ostberg
JR, Naranjo A, Wagner JR, Aguilar B, Starr R, Weng L, Synold TW, et
al: Off-the-shelf, steroid-resistant, IL13Rα2-specific CAR T cells
for treatment of glioblastoma. Neuro Oncol. 24:1318–1330.
2022.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Wang G, Zhang Z, Zhong K, Wang Z, Yang N,
Tang X, Li H, Lu Q, Wu Z, Yuan B, et al: CXCL11-armed oncolytic
adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram
tumor microenvironment in glioblastoma. Mol Ther. 31:134–153.
2023.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Ghouzlani A, Kandoussi S, Tall M, Reddy
KP, Rafii S and Badou A: Immune checkpoint inhibitors in human
glioma microenvironment. Front Immunol. 12(679425)2021.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Gatto L, Franceschi E, Di Nunno V, Maggio
I, Lodi R and Brandes AA: Engineered CAR-T and novel CAR-based
therapies to fight the immune evasion of glioblastoma: Gutta cavat
lapidem. Expert Rev Anticancer Ther. 21:1333–1353. 2021.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Bryukhovetskiy I: Cell-based immunotherapy
of glioblastoma multiforme. Oncol Lett. 23(133)2022.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Baharom F, Ramirez-Valdez RA, Khalilnezhad
A, Khalilnezhad S, Dillon M, Hermans D, Fussell S, Tobin KKS,
Dutertre CA, Lynn GM, et al: Systemic vaccination induces
CD8+ T cells and remodels the tumor microenvironment.
Cell. 185:4317–4332.e15. 2022.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Liau LM, Ashkan K, Brem S, Campian JL,
Trusheim JE, Iwamoto FM, Tran DD, Ansstas G, Cobbs CS, Heth JA, et
al: Association of autologous tumor lysate-loaded dendritic cell
vaccination with extension of survival among patients with newly
diagnosed and recurrent glioblastoma: A phase 3 prospective
externally controlled cohort trial. JAMA Oncol. 9:112–121.
2023.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Keskin DB, Anandappa AJ, Sun J, Tirosh I,
Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E,
et al: Neoantigen vaccine generates intratumoral T cell responses
in phase Ib glioblastoma trial. Nature. 565:234–239.
2019.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Chen KS, Reinshagen C, Van Schaik TA,
Rossignoli F, Borges P, Mendonca NC, Abdi R, Simon B, Reardon DA,
Wakimoto H and Shah K: Bifunctional cancer cell-based vaccine
concomitantly drives direct tumor killing and antitumor immunity.
Sci Transl Med. 15(eabo4778)2023.PubMed/NCBI View Article : Google Scholar
|
|
180
|
Wang J, Weiss T, Neidert MC, Toussaint NC,
Naghavian R, Sellés Moreno C, Foege M, Tomas Ojer P, Medici G,
Jelcic I, et al: Vaccination with designed neopeptides induces
intratumoral, cross-reactive CD4+ T-cell responses in glioblastoma.
Clin Cancer Res. 28:5368–5382. 2022.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Yu TW, Chueh HY, Tsai CC, Lin CT and Qiu
JT: Novel GM-CSF-based vaccines: One small step in GM-CSF gene
optimization, one giant leap for human vaccines. Hum Vaccin
Immunother. 12:3020–3028. 2016.PubMed/NCBI View Article : Google Scholar
|
|
182
|
Li L, Zhou J, Dong X, Liao Q, Zhou D and
Zhou Y: Dendritic cell vaccines for glioblastoma fail to complete
clinical translation: Bottlenecks and potential countermeasures.
Int Immunopharmacol. 109(108929)2022.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Liau LM, Ashkan K, Tran DD, Campian JL,
Trusheim JE, Cobbs CS, Heth JA, Salacz M, Taylor S, D'Andre SD, et
al: First results on survival from a large phase 3 clinical trial
of an autologous dendritic cell vaccine in newly diagnosed
glioblastoma. J Transl Med. 16(142)2018.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Zhu P, Li SY, Ding J, Fei Z, Sun SN, Zheng
ZH, Wei D, Jiang J, Miao JL, Li SZ, et al: Combination
immunotherapy of glioblastoma with dendritic cell cancer vaccines,
anti-PD-1 and poly I:C. J Pharm Anal. 13:616–624. 2023.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Medikonda R, Dunn G, Rahman M, Fecci P and
Lim M: A review of glioblastoma immunotherapy. J Neurooncol.
151:41–53. 2021.PubMed/NCBI View Article : Google Scholar
|