Introduction
Vaginal dryness can be a sign of vulvovaginal
atrophy, also known as the genitourinary syndrome of menopause.
Vulvovaginal atrophy has key medical and psychological
consequences, such as vulvovaginal pain, dyspareunia, urinary
incontinence, less sexual desire and satisfaction, more difficulty
reaching orgasm, as well as depression and anxiety, and is the most
common symptom during menopause. Unlike hot flashes and night
sweats, which resolve spontaneously, symptoms of vaginal dryness
affecting the lower urinary tract develop over time. The prevalence
of vaginal dryness increases in the years following menopause and
causes symptoms such as itching, burning and pain during
intercourse. Furthermore, it is estimated that ~17% of female
patients between the ages of 17 and 50 years experience vaginal
dryness and pain during sexual contact, which leads to anxiety and
decreased libido (1). Its
prevalence ranges from 36 to almost 84% and it is often
underdiagnosed and undertreated (2,3). The
condition may also occur earlier in perimenopausal women who take
antiestrogenic medications or who have decreased levels of estrogen
(4).
Currently, estrogen therapy, which is approved for
the treatment of vaginal atrophy, is often associated with adverse
effects and multiple contraindications in menopausal patients.
Among these, metabolic imbalance, mood swings, bloating and risk of
developing ovarian cancer are the most common (5,6).
Furthermore, long-term use of estrogen therapy may lead to breast
cancer and should therefore be limited (7). During the reproductive age, ovaries
produce a large number of circulating estrogens (3,6).
Normally, vaginal walls are lubricated by cervical-vaginal fluid
produced by the cervix at the top of the vagina. Lower levels of
estrogen during the postmenopausal period decrease sexual desire
and may affect all layers of the vagina, leading to narrowing of
the vagina, loss of rugae, keratinization of the surface and
thinning of the vaginal epithelium. A thin vaginal epithelium leads
to increased susceptibility to trauma, with clinical signs such as
bleeding, petechiae and ulceration with any type of pressure,
including sexual activity or a simple gynecological maneuver
(3,7).
In addition to the onset of menopause, estrogen
levels decrease significantly due to other causes such as birth or
breastfeeding, cancer treatment, surgical removal of the ovaries
and anti-estrogen drugs used to treat uterine fibroids and
endometriosis. Other causes of vulvovaginal atrophy include
Sjogren's syndrome, allergies, cold medicines, antidepressants,
vaginal washings, anxiety and stress overload (6,8).
The main symptoms of vulvovaginal atrophy include
vaginal dryness and reduced lubrication during sexual activity,
urge incontinence, inflammation, itching, discomfort, atypical
vaginal discharge and dyspareunia, which affect the quality of life
(9). In addition, recurrent urinary
tract infections have frequently been reported (5,10).
Urinary complaints in postmenopausal patients should
be managed following physical examination and laboratory diagnostic
testing, including serum estrogen levels and Papanicolaou smear.
Differential diagnosis should be considered to eliminate vaginal
infection (candidiasis, trichomoniasis or bacterial vaginosis) or
other conditions that cause chronic vaginal and vulvar itching,
discharge or pain such as irritants and vulvovaginal dermatoses
(11). Irritants that can cause
chronic vaginal itching include perfumes, locally applied
lubricants or cosmetic soaps. Vulvovaginal dermatoses that may
cause similar symptoms include lichen sclerosus, planus and simplex
chronicus.
The principal therapeutic target in the management
of vaginal atrophy is the relief of its symptoms, particularly
vaginal dryness. Treatment strategies are primarily based on the
use of moisturizers and lubricants, including physical therapy,
low-dose vaginal estrogen therapy, vaginal dehydroepiandrosterone
and oral ospemifene, with a more modern approach including the use
of vaginal lasers (12,13). Patients who experience problems with
natural vaginal lubrication due to hormonal changes often benefit
from estrogen therapy. In addition to estrogen treatment, or in
case of side effects, preparations with moisturizing agents that
help introduce and maintain water in the vaginal mucosa are
recommended.
Non-hormonal treatments include vaginal/topical
moisturizers and lubricants, such as a combination of Hippophaë
rhamnoides oil, hyaluronic acid, glycogen, collagen,
isoflavones and vitamins (14,15).
Lubricants provide short-term relief and are typically used for
vaginal dryness during intercourse, whereas moisturizers have
long-lasting effects and may be used every 2-3 days (16).
Hormonal replacement therapy may be systemic (oral
estrogen replacement) or localized (intravaginal/topical estrogen,
intravaginal releasing rings and vaginal dehydroepiandrosterone).
Estrogenic therapy is considered the most effective treatment for
vaginal atrophy, dryness and dyspareunia in patients with estrogen
deficiency. On the other hand, estrogen therapy is known for
increased risk of stroke and thromboembolism. In addition, estrogen
therapy should be administered with caution in patients who survive
hormone-sensitive cancer as the systemic absorption of estrogen can
stimulate the proliferation of breast cancer cells. Although
systemic estrogen therapy improves symptoms of vaginal atrophy,
systemic doses are higher than those used for topical application
and should be administered only if other menopausal symptoms
requiring treatment are present (3,7,17).
Post-marketing studies are primarily used to
determine whether medical devices are effective and safe in a
non-controlled, real-life setting. Halova is a medical device in
the form of ovules with local action, intended for use as an
adjuvant in the healing, re-epithelialization or calming of
wounded, atrophic or irritated vaginal mucosa. The primary
objective of this study was to observe the tolerability of Halova
ovules in treating vaginal dryness and restoring the natural
lubrication of the vaginal mucosa. The secondary objective was to
evaluate the performance of the medical device by clinical
examination in decreasing the symptoms of vulvovaginal atrophy,
evaluation of endometrium thickness and vaginal pH. Additionally,
the degree of patient satisfaction was assessed using a 5-point
Likert Scale (18). There is need
for insights on alternative therapeutic strategies for symptoms,
sexual function and quality of life of patients with vulvovaginal
atrophy and postmenopausal sexual dysfunction.
Materials and methods
Study design
The present study was designed as part of a medical
device post-marketing clinical follow-up, involving routine care
from a number of clinical practices. The study had an open-label,
multicenter, non-randomized, real-world evidence study design. The
data were collected between March and July 2022. The clinical sites
and locations are listed in Table
I.
 | Table IClinical practices and locations. |
Table I
Clinical practices and locations.
| Practice | City |
|---|
| Societatea
comerciala Pan Medical SRL | Sibiu |
| Gynecological
Office of Dr Ispasoiu Corina | Sibiu |
| Natisan Medical
Center | Pitesti |
| Gynecological
Office of Dr Rădulescu G Mihaela Elena | Ramnicu
Valceea |
| Medical Office of
Dr Saleh K Majed | Craiova |
| Hospital MedLife
Humanitas Cluj-Napoca | Cluj-Napoca |
| Gynecological
Office of Dr. Ioana Trotea Targu Jiu | Targu Jiu |
| Medical Office of
Dr Surpanelu Oana | Iasi |
| Medsan | Cluj |
| Medical Clinic of
Dr Cioata Ionel Trifon | Timisoara |
| Clinical Hospital
‘Dr. Ion Cantacuzino’ Bucharest | Bucharest |
| Tulcea County
Emergency Hospital | Tulcea |
| Clinical Hospital
‘Dr. Ion Cantacuzino’ Bucharest | Bucharest |
| Clinical iMed Sibiu
Oftalmologie, Obstetrica-Ginecologie | Sibiu |
| Medical Office of
Obstetrics and Gynecology of Dr Popescu Dragos SRL | Sibiu |
| Gynecological
Office of Dr Iliescu Irina | Iasi |
| Medical office of
Obstetrics and Gynecology of Dr Sterie Ionut SRL | Tulcea |
| Medical Office of
Dr Todorut Florina | Timisoara |
Participants
The participant population included female patients
aged 18-70 years of Caucasian ethnicity with clinical
manifestations associated with the following conditions: Dryness in
the vaginal region, perimenopause, vulvovaginal atrophy, menopausal
disorder or vaginal prolapse. A total of 249 patients were
evaluated; 179 patients received Halova ovules as monotherapy,
while 70 used Halova ovules in association with vaginal lubricants
(polytherapy). Subjects with psoriasis, vitiligo, plantar ulcers,
lipoid necrobiosis, granuloma annulare and vulvar or cervical
cancer were excluded. The sample size initially included a total of
249 subjects, with baseline characteristics shown in Table II.
 | Table IIBaseline demographic data for sample
population. |
Table II
Baseline demographic data for sample
population.
| Baseline
characteristic | n | % |
|---|
| Age, years | | |
|
<50 | 77 | 32.08 |
|
≥50 | 163 | 67.91 |
| Female | 240 | 100.00 |
| Caucasian | 240 | 100.00 |
| Menopausal
status | | |
|
Premenopausal | 42 | 17.92 |
|
Perimenopausal | 35 | 14.58 |
|
Menopausal | 162 | 67.50 |
| Physical
activity | | |
|
Yes | 49 | 20.42 |
|
No | 191 | 79.58 |
| Sexual
activity | | |
|
Yes | 141 | 58.75 |
|
No | 99 | 41.25 |
The study involved 18 Romanian specialist physicians
as investigators, each with 4-20 patients undergoing treatment with
Halova ovules. Endometrial evaluation was performed by transvaginal
ultrasonography. The total duration of the study was 30 days. The
medical device was applied once daily, during days 1-10.
Prospective data were collected, including the
initial diagnosis, transvaginal ultrasonography, vaginal pH value,
vaginal symptoms and adverse events. Endometrial thickness on
transvaginal ultrasound were as follows: ˃5 mm, absent; 4.1-5 mm,
mild thickness; 3.1-4.0 mm, moderate thickness; 2.1-3 mm for
serious thickness; and ≤2, mm severe. To assess pH, a piece of
litmus paper was placed on the lateral vaginal wall until moist. A
pH ≥4.6 indicated vulvovaginal atrophy, assuming the patient did
not have bacterial vaginosis (if tests and wet mount were performed
to exclude infection with Gardnerella vaginalis). Primary
and secondary outcomes were collected at baseline and after 30
days.
Medical device
Halova is a medical device manufactured by Perfect
Care Manufacturing S.R.L (European Medical Device Regulation device
identification no. 5944754000754). Intravaginal administration is
intended to promote and accelerate hydration, healing,
epithelialization and/or soothing of injured, atrophic or irritated
vaginal mucosa. Halova vaginal ovules are composed of sodium
hyaluronate (5 mg), marigold extract (60 mg), vitamin E (10 mg),
aloe vera oil (60 mg), semi-synthetic glycerides (1,587 mg),
lanolin (50 mg), silicon dioxide (25 mg) and xylitol (3 mg).
The ovules melt evenly in the vaginal mucosa,
forming a cream that contributes to restoring normal lubrication of
the vaginal mucosa and helps preserve normal pH and vaginal flora.
Halova ovules are intended for use in adult patients (including
those in menopause) and are indicated for vaginal dryness caused by
age, various pathologies or other drug treatments, relief of pain
and discomfort during sexual intercourse, balancing of vaginal
flora and vaginal pH preservation. Halova ovules contain
ingredients that promote or accelerate hydration, healing,
re-epithelialization and/or soothing of injured, atrophic or
irritated vaginal mucosa.
Hyaluronic acid is an alternative to non-hormonal
treatment for signs of vaginal atrophy and dyspareunia. Sodium
hyaluronate retains a large amount of water, provides moisture to
the vaginal tissue and is an effective treatment for vulvovaginal
discomfort (19). Hyaluronic acid
is contained in Halova at a concentration of 5 mg/ovule and is used
to promote hydration of dry vaginal mucosa and re-epithelialization
of damaged tissue. The mechanism of action of hyaluronic acid with
a high molecular mass at the level of the vaginal mucosa is
realized by formation of an extracellular matrix with water trapped
in the structure (20).
Calendula officinalis extract (3.3%) is used
to prevent contamination with exogenous bacteria during handling of
the medical device and to prevent microbiological contamination of
the fat base with gram-negative bacteria and fungi.
Xylitol serves as a nutritional substrate. The ovule
base is composed of a mixture of fatty base, lanolin and oily
extract of aloe vera. Vitamin E prevents oxidative degradation of
the fatty base (21).
The medical device is in the form of ovules of 1.8 g
each, ovoid in shape, white or pale yellow, with a smooth
appearance, without spots of color or areas with agglomerated
powders. In the longitudinal section, the ovules have a homogeneous
appearance, without agglomeration of particles and air bubbles. The
medical device was administered for 10 days to patients meeting the
eligibility criteria.
Ethical and regulatory
considerations
Written consent for participation in the study was
obtained from all patients. Owing to legal considerations (General
Data Protection Regulation Directive effective from May 21, 2018,
in all European Union countries), patients or their legal
representatives have an absolute right to request that their data
be removed from the study database. A notified Body (ENTE
CERTIFICATIONE MACCHINE SRL) reviewed the post-marketing clinical
follow-up plan, including ethical considerations. As the present
study was a post-marketing clinical follow-up study, ethics
approval was not required.
The study was conducted according to the Guide to
Medical Devices: ‘Post-market clinical follow-up studies and
International Society for Pharmacoepidemiology (2015) Guidelines
for ‘Good Pharmacoepidemiology Practices (GPP)’.
The data collection and study procedures were
conducted following the ethical principles of the Declaration of
Helsinki (2013. Data were stored according to Annex E of ISO
14155:2020, Good Clinical Practice in Clinical investigation of
medical devices for human subjects (22-25).
The study and its details are registered at
clinicaltrials.gov (ID no. NCT05654610).
Primary objectives
The primary objective was to evaluate the
tolerability of Halova ovules in treating vaginal dryness and
restoring natural lubrication of the vaginal mucosa.
Secondary objectives
The secondary objectives were to investigate the
performance of the medical device by clinical examination, along
with the degree of patient satisfaction (5-point Likert scale).
Statistical analysis
All statistical analyses were performed using
Microsoft Excel Analysis ToolPak version 16.69.1, Excel Windows 10.
P<0.05 was considered to indicate a statistically significant
difference. The quality and completeness of data were preliminarily
assessed. To examine treatment effect over time, Fisher's exact
test was performed for categorical variables and the Mann-Whitney U
test was used for non-normally distributed variables.
Results
Clinical performance
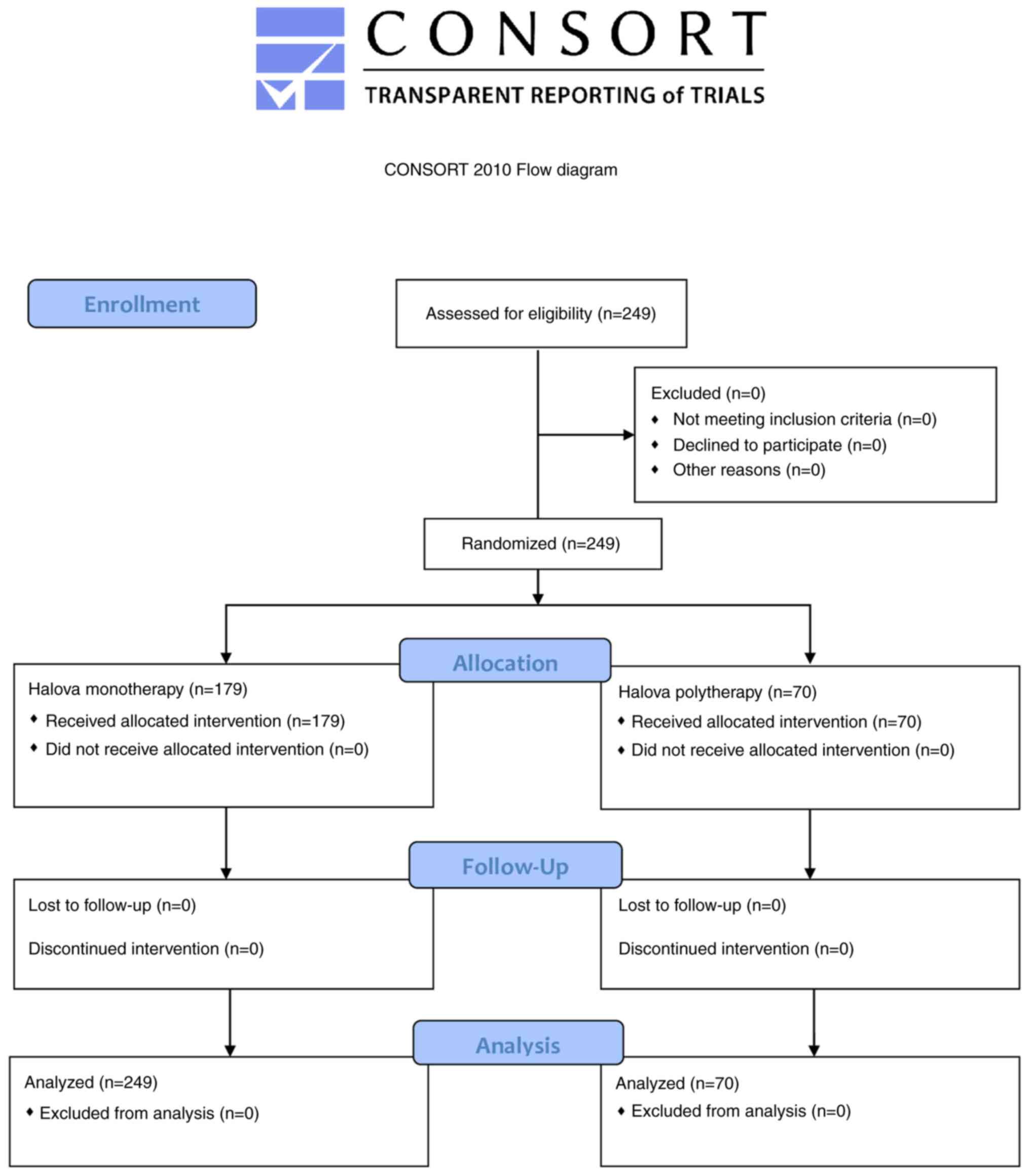
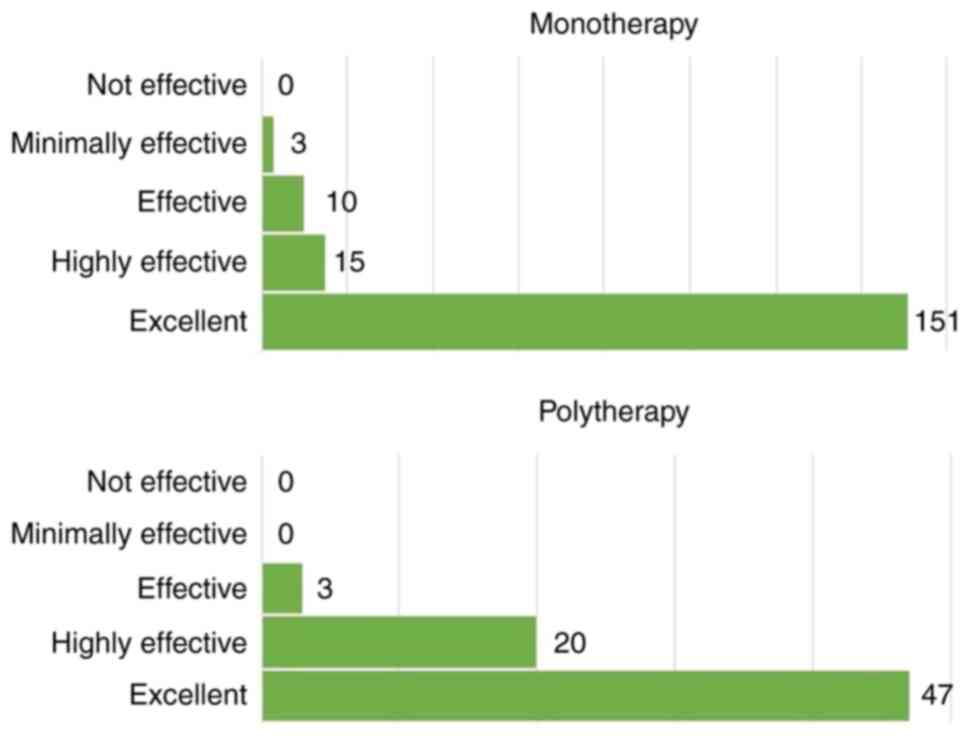
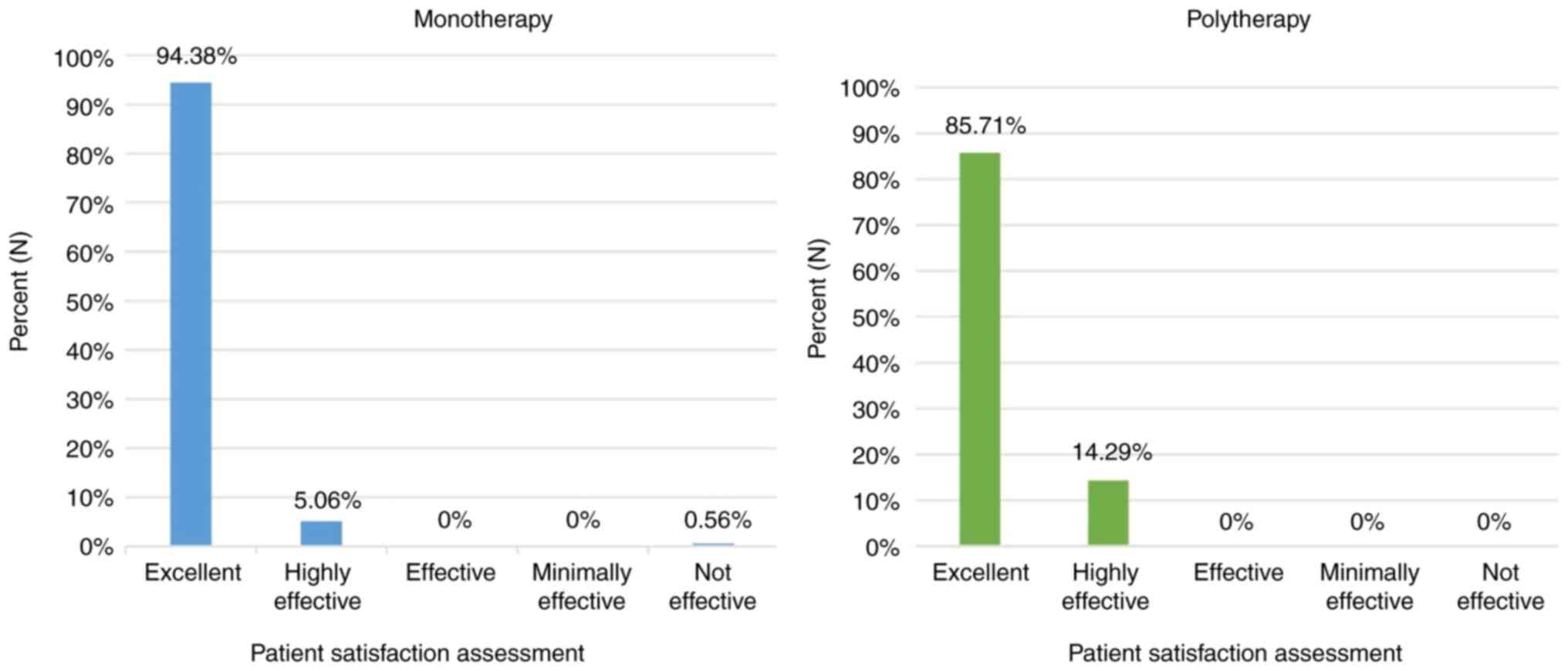
CONSORT diagram is shown in Fig. 1. In the monotherapy group, the
treatment was rated by the majority of patients as ‘excellent’
(84.35%); in the polytherapy group, the treatment was rated as
‘excellent’ or ‘highly effective’ for the majority of patients
(95.71%; Fig. 2).
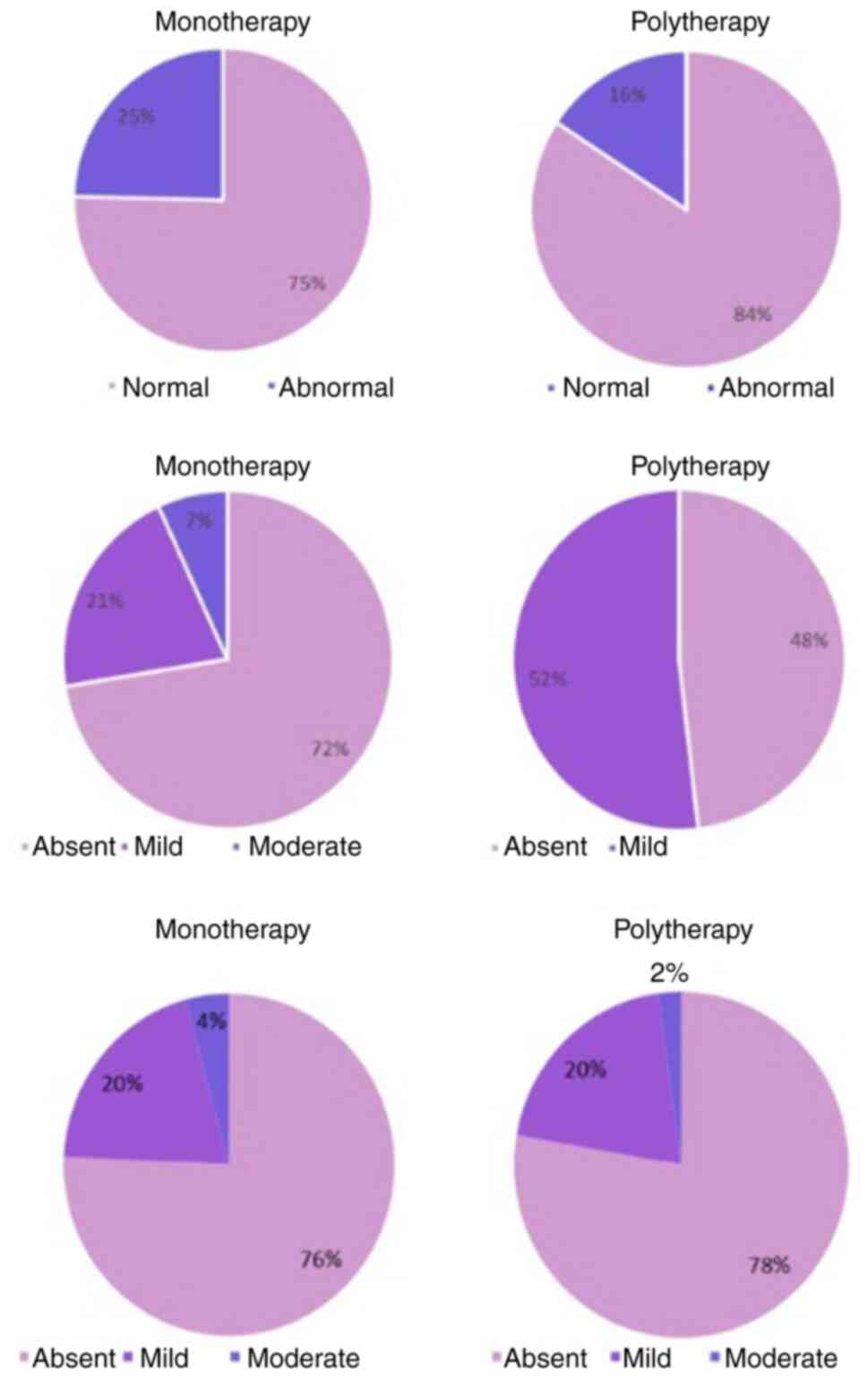
Vaginal pH evaluation
The vaginal pH levels of the patients were evaluated
after 30 days (Fig. 3). Normal
vaginal pH was noted in 75% of patients treated with monotherapy
and in 84% of patients receiving polytherapy.
Dyspareunia symptoms
Dyspareunia symptoms were evaluated using a 5-point
scale, from ‘absent’ to ‘severe’ (Fig.
3). In patients using Halova ovules as monotherapy, 72%
reported absent dyspareunia; in patients receiving polytherapy, 48%
reported absent and 52% mild dyspareunia.
Endometrial thickness evaluation
Ultrasound was performed to determine endometrial
thickness (Fig. 3). A notable
proportion (76% in the monotherapy and 78% from the polytherapy
group) of patients exhibited an endometrial thickness rating of
‘absent’. ‘Mild’ and ‘moderate’ endometrial thickness accounted for
20 and 4%, respectively, of the study participants in the
monotherapy group. In polytherapy, the percentages were similar,
with 78% of patients with a rating of absent, 20% with a rating of
mild and 2% with a rating of moderate.
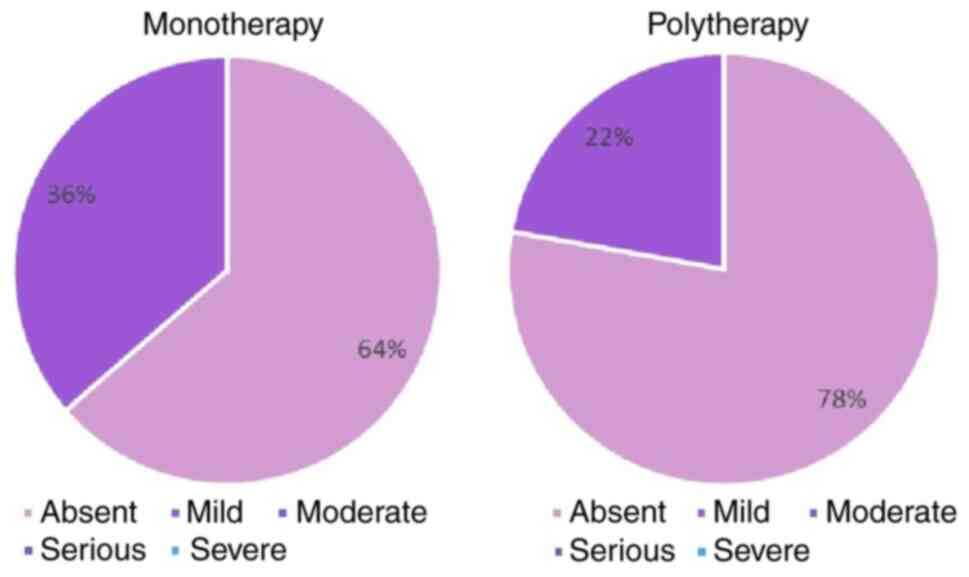
Dysuria symptoms
A total of 64% of patients receiving monotherapy
reported absent dysuria (Fig. 4).
When Halova was administered as polytherapy, 78% of the study
participants reported absent dysuria.
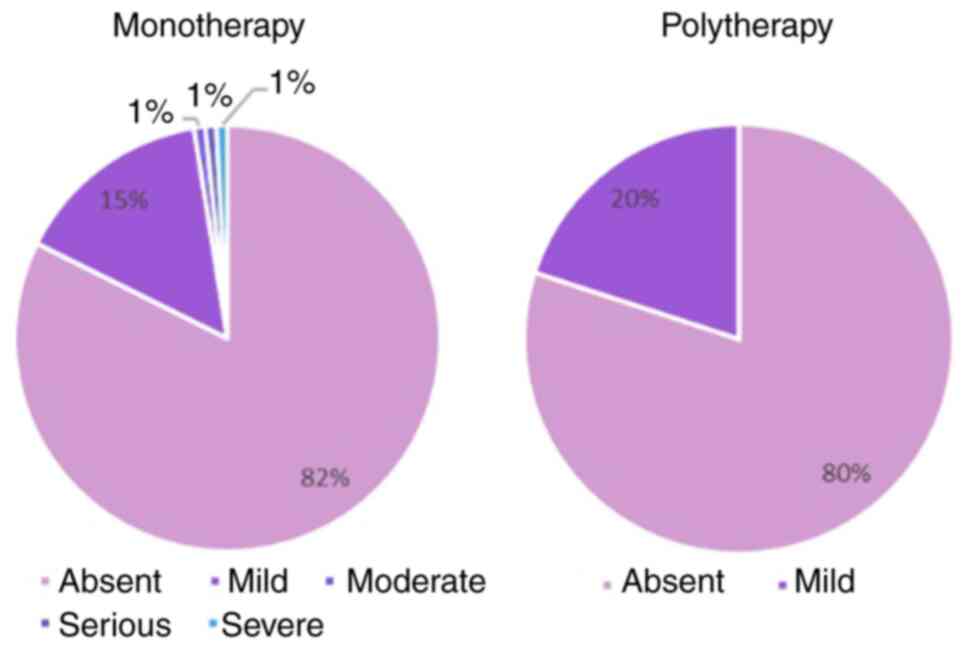
Vaginal dryness symptoms
Halova decreased vaginal dryness, with no vaginal
dryness reported at in 82% in the monotherapy group and 80% of the
patients from the polytherapy group respectively (Fig. 5).
Patient satisfaction
A total of 94.38% of patients treated with Halova
monotherapy rated the treatment as ‘excellent’ (Fig. 6).
Discussion
Vulvovaginal atrophy and its primary symptoms
(vaginal dryness and dyspareunia) are closely associated with
female sexuality. Vaginal dryness can be severe and distressing
enough to affect daily activity, sexual desire, and normal sexual
intercourse (26). A total of ~40%
of the women with vaginal atrophy report dyspareunia (27). Dyspareunia is defined as the
recurrent pain that occurs during sexual intercourse. This has
negative effects on sexuality and sexual function. Studies report
that dyspareunia and postmenopausal vaginal health are taboo topics
for a substantial number of patients (28,29).
Both healthcare professionals and patients may find it difficult to
approach the subject of sexual problems associated with menopause
and vulvovaginal atrophy (29).
With the administration of Halova ovules, the
symptoms of dyspareunia were decreased, thus favoring normal sexual
function in affected patients. The beneficial effects of decreasing
vaginal dryness can be explained by the local action of sodium
hyaluronate. Multiple studies have confirmed the advantages of
using topical sodium hyaluronate to treat vaginal dryness since it
is a modern, safe and well-tolerated product (30,31).
Owing to its highly anionic properties, sodium hyaluronate can
attract water to swell, create volume, and provide structural
support, thereby acting as a topical lubricant (32). Sodium hyaluronate is the salt form
of hyaluronic acid, with a smaller molecular structure that
increases stability and resistance to oxidation. Water solubility
is associated with better skin and mucosal penetration, leading to
better hydration. The mechanical protection of the vaginal
endothelium is ensured by its high viscosity (33). Sodium hyaluronate has been used
since 1980 for the treatment of various other diseases, such as dry
eye, joint diseases, cystitis, atopic dermatitis, cataract
extraction and osteoarthritis and as a filler for skin wrinkles
(34,35). It provides a high safety profile
that has been previously studied in postmenopausal patients
(30).
During menopause, there is a decrease in the number
of epithelial cells and glycogen production, which translates to
less glucose converted to lactic acid. Lactic acid is important for
maintaining a highly acidic pH and sustaining the activity of
lactobacilli. The increase in vaginal pH in the absence of the
lactic acid leads to alteration of the vaginal microflora, allowing
the onset of urinary tract infections and vaginitis (36,37). A
pH ≥5 is associated with vaginal atrophy and typical signs and
symptoms, such as labial thinning, pale and dry vaginal mucosa and
vulvovaginal erythema with or without bleeding (25). Poor sleep, cardiometabolic symptoms,
muscle and joint pain and mood changes affect ~80% of women during
diminishing estrogenic activity (26).
Halova is efficient in maintaining a healthy vaginal
pH. The normal vaginal pH (3.8-4.5) is key for its protective role
in blocking yeast and bacterial multiplication. Thus, the
supportive role of the medical device in preventing vaginal
infection was demonstrated based on its effective role in
correction of unbalanced vaginal pH, with 88.79% of patients in the
mono- and 95.35% in the polytherapy arm reporting normal vaginal
pH.
The therapeutic indications of the device are linked
to the treatment of both postmenopausal and non-menopausal vaginal
dryness, relief of pain and discomfort during sexual intercourse,
balance of vaginal flora, treatment of vaginal pH disturbances and
boosting vaginal lubrication (38).
Vitamin E, due to its rich composition of phytoestrogens, is a key
element in stabilizing estrogen levels and can improve menopausal
symptoms including hot flashes, irritability, insomnia, dizziness,
palpitations, shortness of breath, and vaginal dryness. When
applied locally, it favors healing and re-epithelialization through
its nourishing and moisturizing effects. A recent review by
Porterfield et al (27)
summarized evidence for vaginal vitamin E efficacy in reducing
patient-reported genitourinary symptoms in healthy postmenopausal
patients compared with placebo or vaginal estrogen. A review by
Feduniw et al (28)
concluded that vitamin E might be an option for standard hormone
therapy and may be an option to treat symptomatic patients with
contraindications to estrogen.
Fractional CO2 laser has been proposed as
alternative treatment in patients with vaginal atrophy with
significant success rates and short-term adverse effects but a
possible disadvantage is discomfort related to probe introduction,
extraction and laser impulse transmission (39). Also, Schiavi et al (31) reported the efficacy of a medical
device containing purified bovine colostrum in improving
vulvovaginal atrophy, sexual function, urinary symptoms and quality
of life in postmenopausal patients when applied topically.
There are concerns associated with the treatment of
vaginal atrophy and its symptoms in patients with breast cancer.
The increasing use of aromatase inhibitors has led to an increased
incidence of vaginal atrophy, with an impact on the quality of life
of patients with breast cancer (40). However, systemic or topical hormonal
therapy is contraindicated in patients with breast cancer. Thus,
Halova might be a therapeutic alternative in these patients and in
patients in whom local estrogenic treatment is controversial, such
as those with uterine cancer or a history of deep vein thrombosis
or pulmonary embolism, stroke or myocardial infarction or blood
clotting disorder (41). In the
present study, recurrence of symptoms was not evaluated after the
treatment period. An ancillary study should be designed to detect
long-term performance.
A treatment consisting of 10 ovules of Halova was
administered to 249 patients. Clinical endpoints were collected
before and after the treatment. The results related to clinical
performance, vaginal pH, vaginal symptoms indicate the device
performance after 10 days of treatment.
Halova notably alleviated symptoms such as vaginal
dryness, dyspareunia and dysuria while restoring the normal pH,
thus harnessing the protective features of the vaginal microbiome.
Halova applied intravaginally once per day for 10 days, may be a
suitable treatment option for vulvovaginal atrophy and urogenital
complaints in patients of reproductive age and postmenopausal
status. The medical device was safe and effective in alleviating
signs and symptoms of atrophic vaginitis in postmenopausal
patients, such as dysuria, dyspareunia and vaginal dryness.
The medical device demonstrated anti-atrophic
activity in the genitourinary tract, resulting in notably improved
symptoms associated with normal sexual functioning. Future research
is needed to confirm its long-term tolerability and performance
long-term as well as during pregnancy. Limitations of the present
study include the absence of a control group, short treatment
follow-up, and heterogeneity of the sample population. Halova may
be a promising treatment for conditions such as endometriosis or
cystitis in treating symptoms such as dyspareunia, dysuria and
pelvic pain; further studies are required to determine its efficacy
in treating such conditions.
Acknowledgements
The authors would like to thank Mr. Alexandru-Remus
Pinta Romania for performing the statistical analysis and Mr.
Adrian Pocola (both MDX Research, Timisoara, Romania) for technical
support provided during data management collection.
Funding
Funding: Perfect Care Distribution S.R.L. (www.perfectcare.eu)
provided medical devices and partial grant support for data
management services, grant no HLV01/2022.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IP, RP and DGI designed the study, and wrote the
manuscript. IP and CT performed data collection and analysis. RP
reviewed and edited the manuscript. IP, RAO and AAA interpreted
data. All authors have read and approved the final manuscript. DGI
and IP XX and XX confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All participants provided written consent to the
collection of their study data. The study was performed in
accordance with the Declaration of Helsinki. Ethics approval was
not required due to the nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sarmento ACA, Costa APF, Vieira-Baptista
P, Giraldo PC, Eleutério J Jr and Gonçalves AK: Genitourinary
syndrome of menopause: Epidemiology, physiopathology, clinical
manifestation and diagnostic. Front Reprod Health.
3(779398)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cagnacci A, Venier M, Xholli A, Paglietti
C, Caruso S and ANGEL Study: Female sexuality and vaginal health
across the menopausal age. Menopause. 27:14–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alvisi S, Gava G, Orsili I, Giacomelli G,
Baldassarre M, Seracchioli R and Meriggiola MC: Vaginal health in
menopausal women. Medicina (Kaunas). 55(615)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Moral E, Delgado JL, Carmona F, Caballero
B, Guillán C, González PM, Suárez-Almarza J, Velasco-Ortega S and
Nieto C: as the writing group of the GENISSE study. Genitourinary
syndrome of menopause. Prevalence and quality of life in Spanish
postmenopausal women. The GENISSE study. Climacteric. 21:167–173.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Portman DJ and Gass MLS: Vulvovaginal
Atrophy Terminology Consensus Conference Panel. Genitourinary
syndrome of menopause: New terminology for vulvovaginal atrophy
from the international society for the study of women's sexual
health and the North American menopause society. Menopause.
21:1063–1068. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goncharenko V, Bubnov R, Polivka J, Zubor
P, Biringer K, Bielik T, Kuhn W and Golubnitschaja O: Vaginal
dryness: Individualised patient profiles, risks and mitigating
measures. EPMA J. 10:73–79. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Donders GGG, Bellen G, Grinceviciene S,
Ruban K and Vieira-Baptista P: Aerobic vaginitis: No longer a
stranger. Res Microbiol. 168:845–858. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Waetjen LE, Crawford SL, Chang PY, Reed
BD, Hess R, Avis NE, Harlow SD, Greendale GA, Dugan SA and Gold EB:
Study of Women's Health Across the Nation (SWAN). Factors
associated with developing vaginal dryness symptoms in women
transitioning through menopause: A longitudinal study. Menopause.
25:1094–1104. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mac Bride MB, Rhodes DJ and Shuster LT:
Vulvovaginal atrophy. Mayo Clin Proc. 85:87–94. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Calleja-Agius J and Brincat MP: The
urogenital system and the menopause. Climacteric. 18 (Suppl
1):S18–S22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
O'Connell TX, Nathan LS, Satmary WA and
Goldstein AT: Non-neoplastic epithelial disorders of the vulva. Am
Fam Physician. 77:321–326. 2008.PubMed/NCBI
|
|
12
|
Palacios S, Mejía A and Neyro JL:
Treatment of the genitourinary syndrome of menopause. Climacteric.
18 (Suppl 1):S23–S29. 2015.
|
|
13
|
Shifren JL: Genitourinary syndrome of
menopause. Clin Obstet Gynecol. 61:508–516. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tersigni C, Di Simone N, Tempestilli E,
Cianfrini F, Russo R, Moruzzi MC, Amar ID, Fiorelli A, Scambia G
and Villa P: Non-hormonal treatment of vulvo-vaginal
atrophy-related symptoms in post-menopausal women. J Obstet
Gynaecol. 35:835–838. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Castelo-Branco C, Cancelo MJ, Villero J,
Nohales F and Juliá MD: Management of post-menopausal vaginal
atrophy and atrophic vaginitis. Maturitas. 52 (Suppl 1):S46–S52.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Potter N and Panay N: Vaginal lubricants
and moisturizers: A review into use, efficacy, and safety.
Climacteric. 24:19–24. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bleibel B and Nguyen H: Vaginal Atrophy.
StatPearls Publishing, Treasure Island, FL, 2023. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK559297/.
|
|
18
|
Robinson J: Likert Scale. In: Encyclopedia
of Quality of Life and Well-Being Research. Michalos AC (ed).
Springer, Dordrecht, pp3620-3621, 2014. Available from: https://doi.org/10.1007/978-94-007-0753-5_1654.
|
|
19
|
Dos Santos CCM, Uggioni MLR, Colonetti T,
Colonetti L, Grande AJ and Da Rosa MI: Hyaluronic acid in
postmenopause vaginal atrophy: A systematic review. J Sex Med.
18:156–166. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Garantziotis S and Savani RC: Hyaluronan
biology: A complex balancing act of structure, function, location
and context. Matrix Biol. 78-79:1–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vitamin E: Halova Ovule. Available from:
https://halova.ro/beneficiile-utilizarii-halova-ovule/vitamina-e/.
|
|
22
|
Guidance-MDCG Endorsed Documents and Other
Guidance-European Commission. 2023. Available from: https://health.ec.europa.eu/medical-devices-sector/new-regulations/guidance-mdcg-endorsed-documents-and-other-guidance_en.
|
|
23
|
Guidelines for Good Pharmacoepidemiology
Practices (GPP)-International Society for Pharmacoepidemiology.
Available from: https://www.pharmacoepi.org/resources/policies/guidelines-08027/.
|
|
24
|
ISO 14155:2020. Clinical Investigation of
Medical Devices for Human Subjects. Available from: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/16/71690.html.
|
|
25
|
WMA-The World Medical Association-WMA
Declaration of Helsinki-Ethical Principles for Medical Research
Involving Human Subjects. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/.
|
|
26
|
Goldstein I and Alexander JL: Practical
aspects in the management of vaginal atrophy and sexual dysfunction
in perimenopausal and postmenopausal women. J Sex Med. 2 (Suppl
3):S154–S165. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Porterfield L, Wur N, Delgado ZS, Syed F,
Song A and Weller SC: Vaginal vitamin E for treatment of
genitourinary syndrome of menopause: A systematic review of
randomized controlled trials. J Menopausal Med. 28:9–16.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Feduniw S, Korczyńska L, Górski K,
Zgliczyńska M, Bączkowska M, Byrczak M, Kociuba J, Ali M and
Ciebiera M: The effect of vitamin E supplementation in
postmenopausal women-a systematic review. Nutrients.
15(160)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Heidari M, Ghodusi M, Rezaei P, Kabirian
Abyaneh S, Sureshjani EH and Sheikhi RA: Sexual function and
factors affecting menopause: A systematic review. J Menopausal Med.
25:15–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Stute P: Is vaginal hyaluronic acid as
effective as vaginal estriol for vaginal dryness relief? Arch
Gynecol Obstet. 288:1199–1201. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schiavi MC, Di Tucci C, Colagiovanni V,
Faiano P, Giannini A, D'Oria O, Prata G, Perniola G, Monti M, Zullo
MA, Muzi L and Benedetti Panici P: A medical device containing
purified bovine colostrum (Monurelle Biogel) in the treatment of
vulvovaginal atrophy in postmenopausal women: Retrospective
analysis of urinary symptoms, sexual function, and quality of life.
Low Urin Tract Symptoms. 11:O11–O15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lin Q, Song B, Zhong Y, Yin H, Li Z, Wang
Z, Cheong KL, Huang R and Zhong S: Effect of sodium hyaluronate on
antioxidant and anti-ageing activities in caenorhabditis elegans.
Foods. 12(1400)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dovedytis M, Liu ZJ and Bartlett S:
Hyaluronic acid and its biomedical applications: A review. Eng
Regen. 1:102–113. 2020.
|
|
34
|
Ling PX, Liang H, He YL and Zhang TM: The
application of sodium hyaluronate in joint diseases. Zhongguo Xiu
Fu Chong Jian Wai Ke Za Zhi. 16:1–4. 2002.PubMed/NCBI(In Chinese).
|
|
35
|
Altman RD: Intra-articular sodium
hyaluronate in osteoarthritis of the knee. Semin Arthritis Rheum.
30 (Suppl 1):S11–S18. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cardozo L: Postmenopausal cystitis. BMJ.
313(129)1996.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Baber R: Treating menopausal women: Have
we lost our way? Aust N Z J Obstet Gynaecol. 61:493–495.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Halova benefits. Halova Ovule. Available
from: https://halova.ro/beneficiile-utilizarii-halova-ovule/.
|
|
39
|
Di Donato V, D'Oria O, Scudo M, Prata G,
Fischetti M, Lecce F, Schiavi MC, Giannini A, Muzii L, Battaglia F,
et al: Safety evaluation of fractional CO2 laser
treatment in post-menopausal women with vaginal atrophy: A
prospective observational study. Maturitas. 135:34–39.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Moegele M, Buchholz S, Seitz S, Lattrich C
and Ortmann O: Vaginal estrogen therapy for patients with breast
cancer. Geburtshilfe Frauenheilkd. 73:1017–1022. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tavani A and La Vecchia C: The adverse
effects of hormone replacement therapy. Drugs Aging. 14:347–357.
1999.PubMed/NCBI View Article : Google Scholar
|