Introduction
The prominent antitumor potential of proteasome
inhibitors was discovered in 1999 and immediately attracted
attention of scientists and medical oncologists (1). The first-in-class proteasome inhibitor
bortezomib (Velcade®) became one of the most rapidly
developed antitumor agents in recent history with 8 years from
initial synthesis in 1995 to US Food and Drug Administration
approval in 2003 for treatment of multiple myeloma (2). Further in 2006, bortezomib was
approved for treatment of mantle cell lymphoma (3). Nevertheless, a set of limitations
narrow the broadening of bortezomib's wide use beyond the treatment
hematological malignancies. Similar to conventional
chemotherapeutic drugs, proteasomal inhibitors including bortezomib
exhibit the pronounced dose-limiting toxicities, such as
thrombocytopenia and peripheral neuropathy (4,5).
Bortezomib non-specifically binds to plasma proteins unselectively
and is extensively metabolized by hepatic cytochrome P450 family
enzymes, which attenuates its tissue penetration (6,7). Low
bioavailability, partly explained by poor solubility in aqueous
solutions due to high hydrophobic properties, together with high
toxicity to normal tissues limit its application in therapeutic
regimens for the treatment of solid tumors.
Polymers are extensively studied as perspective
systems for drug delivery due to their tunable properties, optimal
pharmacokinetics, biodegradability and safety. Polymer nanocarriers
with immobilized drugs show off increased efficiency due to their
favorable delivery, ability of administration with poorly soluble
active substances, increased biocompatibility and bioavailability,
low effective doses, controlled release and prolonged effect of the
drugs from the polymeric system (8). Therefore, nanodelivery approach can
contribute to the improvement of bortezomib tissue distribution and
reduction of its adverse effects. In this regard, polymer
poly(N-vinylpyrrolidone) (PVP) has unique properties, extensively
reviewed in the literature (9),
which make it suitable for nanodelivery applications and namely
flexible design. It has favorable solubility in both inorganic and
organic solvents, ability to carry both hydrophilic and hydrophobic
substances, lack of toxicity, and it is eco-friendly.
Previously, it has been demonstrated by the authors
that polymeric micelles composed of amphiphilic PVP are suitable
for the delivery of a wide variety of therapeutic molecules
encapsulated in their core, with high hydrophobicity as a main
requirement. For example, PVP micelles recently acted as
nanocarriers for the anti-inflammatory non-steroidal drug
indomethacin (10), demonstrated
stability in blood serum and excellent blood compatibility,
quaintly without initiating the complement cascade or decreasing
the potential of the system (11,12).
On the contrary, they appeared to protect the endothelium (13).
A technique for production of PVP-based micelles
loaded with prothionamide, an anti-tuberculosis drug without
anticancer effect, has been previously described. It was used as a
highly hydrophobic core for micelles bearing antitumor
receptor-specific protein based on human cytokine TRAIL on their
surface (14). In the present
study, the use of a similar technology for encapsulation of the
proteasome inhibitor bortezomib into micellar nanoparticles
composed of amphiphilic PVP was pioneered. The production and
characterization of the bortezomib-loaded micellar nanoparticles,
investigation of toxicity in vitro in human 2D and 3D models
of human glioblastoma, and in vivo in zebrafish embryos, was
further reported. The newly obtained PVP-B micelles can become a
perspective nanoplatform for improved delivery of bortezomib,
broadening the range of its applications to the treatment of solid
tumors.
Materials and methods
Materials and reagents
AIBN (2,2'-azobisisobutyronitrile), 1,4-dioxane and
VP (N-vinyl-2-pyrrolidone) were obtained from Acros (https://www.thermofisher.com/ru/en/home/chemicals/acros-organics.html).
Stearoyl chloride, DMSO (dimethylsulfoxide), potassium
tert-butylate, prothionamide and MTT reagent
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide)
were purchased from Sigma-Aldrich; Merck KGaA. Bortezomib was
obtained from Santa Cruz Biotechnology, Inc. Dulbecco's Modified
Eagle Medium (DMEM), trypsin-versene solution and PBS were
purchased from PanEco. Fetal bovine serum (FBS) was purchased from
HyClone; Cytiva. Cyclo-RGDfK(TPP) peptide was a kind gift of
Professor S. Burov (Cytomed JSC, St-Petersburg, Russia).
Production of the micelles
The amphiphilic micellar nanoparticles were produced
as previously described (14).
Briefly, amphiphilic poly-N-vinylpyrrolidone with number-average
molecular weight of 6 kDa and one end stearoyl hydrophobic group
was synthesized via radical monomer polymerization in dioxane.
Stearoyl chloride was used both as a regulator of the chain growth
and a chain-transfer agent. Further, polymerization reaction
between VP monomer, stearoyl chloride and initiator (dissolved in
dioxane) was held for 2 h at 80˚C. The synthesized polymer was
dissolved in 5 volumes of double deionized water, dialyzed in a
molecular weight cut-off of 12 kDa Dialysis Cassette Slide-A-Lyzer
(Thermo Fisher Scientific, Inc.) against double deionized water for
72 h, and freeze-dried using Martin Christ GmbH device. The
titration analysis and vapor pressure osmometry using Knauer device
and polystyrene standards in toluene solution were applied for
determination of PVP number-average molecular weight.
Emulsification was applied to manufacture the
micelles with incorporated prothionamide or bortezomib. Polymer
suspension in water was mixed with prothionamide or bortezomib
solution in chloroform and homogenized by ultrasound for 12 min
under supercooling. Further, chloroform was evaporated using
Heidolph Hei-VAP Value Digital device. Non-encapsulated drug was
deleted by centrifugation at for 5 min at 4,000 x g using Sigma
4-5L centrifuge.
Drug loading characteristics of
amphiphilic PVP nanoparticles
The content of bortezomib or prothionamide in
drug-loaded polymeric nanoparticles and efficiency of encapsulation
process were evaluated by determining of non-encapsulated drug
quantity left in supernatant after drug-loaded nanoparticles
preparation and centrifugation.
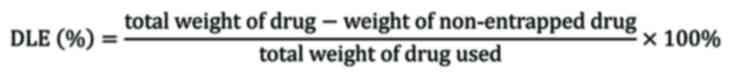
The drug loading capacity (DLC) and drug loading
efficiency (DLE) of the amphiphilic PVP nanoparticles was
determined using a spectrophotometer (Unico 2802 UV-vis). It was
confirmed that residual amounts of water in the system did not
affect this calibration (15). The
absorbance wavelength for bortezomib evaluation was 269 nm, and for
prothionamide estimation absorbance wavelength was 294 nm. DLC of
the obtained preparations was determined using following
equation:
DLE was estimated using following equation:
Dynamic light scattering (DLS) and
zeta potential measurements
The DLS and zeta potential measurements were carried
out using a Malvern Zetasizer Nano ZS (Malvern Panalytical Ltd.).
The NPs were placed into 70 µl cuvettes (BRAND GmbH) or Malvern
1070 folded capillary cells (Malvern Panalytical Ltd.) for the
determination of size and zeta potential, respectively. The
measurement duration was determined automatically for each run. The
temperature was set to 25˚C.
To assess the influence of ultrasonic treatment on
the size of the NPs, the suspensions were treated using a 60W
KDL-1.3L ultrasound cleaner for 15 min on ice.
Transmission electron microscopy
(TEM)
TEM measurements were carried out using a JEM-1400
(JEOL, Ltd.) microscope operating at 120 kV. The formvar/carbon TEM
grids were treated by a glow-discharge device Emitech K100X at 25
mA for 30 sec. The samples were deposited on the grid surface
without fixative for 1 min, then the grids were blotted and stained
by 1% uranyl acetate.
PVP-B cytotoxicity in vitro
Human glioblastoma cell line T98G (cat. no.
CRL-1690), glioblastoma of unknown origin U87 (cat. no. HTB-14) and
normal human dermal fibroblasts (cat. no. PCS-201-012) were
obtained from the American Type Culture Collection. All cells were
tested negative for mycoplasma contamination. Cells were cultivated
in DMEM cell culture medium containing 10% FBS at 37˚C and 5%
CO2. Detachment was held by trypsin-versene solution. To
create a 3D in vitro model, cells were seeded in 96-well
plates (5,000 cells/well), and after the cells were attached to the
bottom, 40 µM cyclo-RGDfK(TPP) peptide in DMEM with 10% FBS was
added, as previously described (16). The multicellular tumor spheroids
were formed after 72 h.
Cytotoxic activity of the developed formulations was
examined using MTT test. The monolayer cells or tumor spheroids
were treated with tested substances for indicated time periods.
Further, 0.05% (w/v) MTT reagentwas added to each well and
incubated for 4 h at 37˚C. The formed formazan crystals were
resuspended in DMSO and absorbance at 570 nm was detected using
iMark Microplate Reader (Bio-Rad Laboratories, Inc.). The viability
was expressed in % of control using the formula: (OD of sample-OD
of background)/(OD of control-OD of background) x100%.
PVP-B toxicity in vivo in
zebrafish
Adult wild-type zebrafish Danio rerio AB were
maintained in aquariums with an aeration and recirculation system
at 28˚C, pH 6.5-7.5, with 14-h light/10-h dark photoperiod. The
fish were fed twice a day according to conventional recommendations
by zebrafish feed. For spawning, sexually mature 3-months old
zebrafish were used. Embryos were collected and placed in zebrafish
E3 embryo water (5 mM NaCl, 0.33 mM MgSO4 х
7H2O, 0.33 mM CaCl2, 0.17 mM KCl, 0.1%
methylene blue).
Unfertilized eggs and embryos 24 h after
fertilization that had significant developmental defects were
detected under a Nexcope NSZ-810 light microscope and removed from
the experiment. Experimental embryos were mechanically
dechorionized with tweezers and placed in 24-well culture plates (2
embryos per well, total 1 ml of solution per well). The
bortezomib-loaded PVP-B nanoparticles were added in a concentration
range of 0.03-30 mg/ml in each well. Each well was performed in
triplicate (n=6 per group). The embryos were examined 24 [48 h
post-fertilization; (hpf)] and 72 (96 hpf) h after the addition of
PVP-B; developmental disorders and delays, morphological changes,
including irregular shape of the yolk sacs, impaired tail
development and decreased motor activity were recorded. To estimate
the range of mortality from 0 to 100%, embryonic deaths were
recorded at 24 (48 hpf) and 72 (96 hpf) h after addition of PVP-B.
Lethal concentration 50% (LC50) was calculated at the
end of the experiment from cumulative mortality by regression
analysis using Graphpad Prism version 8.01 software
(Dotmatics).
Zebrafish embryos were euthanized at the age of 120
hpf using a bleach solution of 1 part sodium hypochlorite 6.15% to
5 parts water according to the Guidelines for Use of Zebrafish in
the NIH Intramural Research Program accepted in 2009 (https://oacu.oir.nih.gov/system/files/media/file/2023-08/b17_zebrafish.pdf),
in accordance with the AVMA Guidelines on Euthanasia: 2020
Edition.
Statistical analysis
The obtained data represented normal distribution
and were displayed as the mean ± SD. The experiments were held in
three replicates no less than three times. In cell experiments,
statistical differences were analyzed by unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference. LC50 was determined by non-linear regression
analysis in Graphpad Prism version 8.01 (Dotmatics).
Results
Production and characterization of
bortezomib-loaded nanoparticles
The amphiphilic polymers composed of PVP with
molecular weight of 11 kDa were synthesized by earlier developed
original one-step method with 80-90% yield (17,18).
The core of the self-assembled micelles was loaded with proteasomal
inhibitor bortezomib by the emulsion method, which involved
ultrasonic treatment of polymer solution in water jointly with
bortezomib solution in chloroform. Thus, micellar PVP-B
nanoparticles encapsulating bortezomib were manufactured. To
compare the properties of micelles depending on loaded substances,
in addition to PVP-B, the previously characterized particles PVP-P
were loaded with a model substance prothionamide in similar
conditions (14). The
prothionamide, an antituberculosis drug (19), was chosen due to its high
hydrophobicity for stabilization of the hydrophobic core of the
PVP-micelles. PVP-P nanoparticles served as a model object to
correctly distinguish the effect of bortezomib in PVP-B, since
hollow PVP micelles that are not stabilized by a hydrophobic core
may have different characteristics.
The encapsulation efficiency was 12.36 mg/g PVP for
bortezomib, and 97.47 mg/g PVP for prothionamide. As the initial
content of bortezomib and prothionamide in regard to PVP polymer
was relatively low (~1 and 10% mass, respectively), it was possible
to prepare final drug-loaded nanoparticles with DLE close to 100%,
which was important in terms of rational usage of rather expensive
biologically active substances, and more accurate evaluation of
drug content in final nanoparticles preparations.
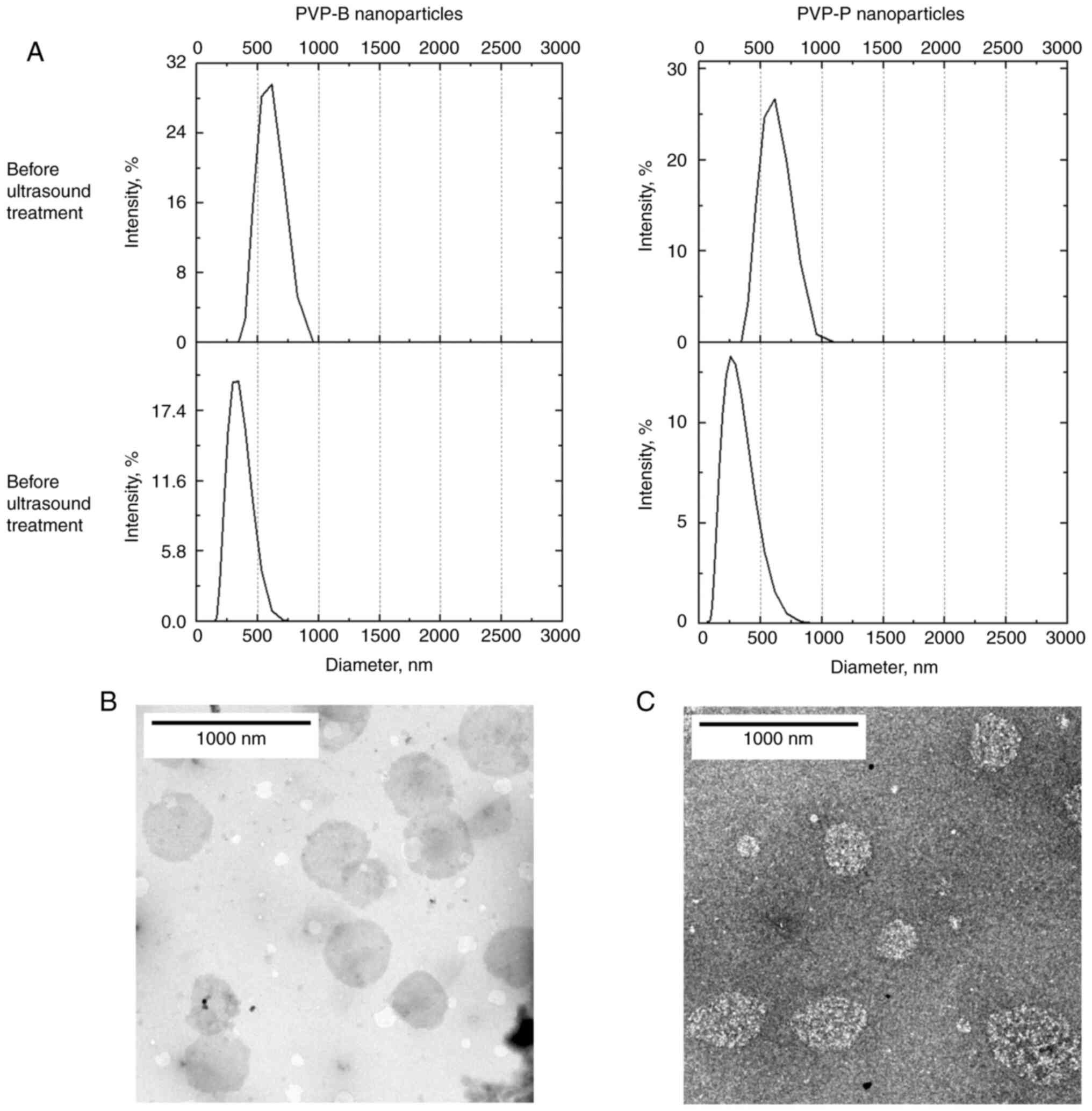
According to DLS, the typical size of the PVP-B
nanoparticles was similar to the size of the control PVP-P
nanoparticles. The peak position on the intensity distributions was
570 and 590 nm, respectively (Fig.
1A). Upon the ultrasound treatment, the peak position shifted
down to 290-330 nm (Fig. 1A), and
then it did not change for at least 2 days of storage at 4˚C. No
precipitation was observed after ultrasound treatment.
Consequently, all the substance remained encapsulated within the
particles, indicating that the DLC of both PVP-B and PVP-P has not
changed. The similarity of the two studied types of NPs indicated
that in the current case the NP size is determined by the polymer
rather than by the drug incorporated in it. When TEM was used to
visualize the NPs, they appeared as roughly spherical objects with
submicron size (Fig. 1B and
C). All main properties of
drug-loaded nanoparticles, including Z-average hydrodynamic
diameter, DLC, DLE and ζ-potential, are presented in Table I.
 | Table IMain properties of the drug-loaded
amphiphilic PVP NPs. |
Table I
Main properties of the drug-loaded
amphiphilic PVP NPs.
| Drug-loaded
amphiphilic PVP NPs | Z-average
hydrodynamic diameter, nm | Drug loading
capacity, % mass | Drug loading
efficiency, % mass | ζ-potential,
mV |
|---|
| PVP-B | 570 | 1,2 | 99,8 | -7.7±0.4 |
| PVP-P | 590 | 9,8 | 98,3 | -12.7±0.6 |
In vitro cytotoxicity evaluation of
nanoparticles
The cytotoxicity of the obtained micelles was
evaluated in vitro in monolayer cell culture (2D) and
multicellular spheroids (3D) of human glioblastoma cell lines U87
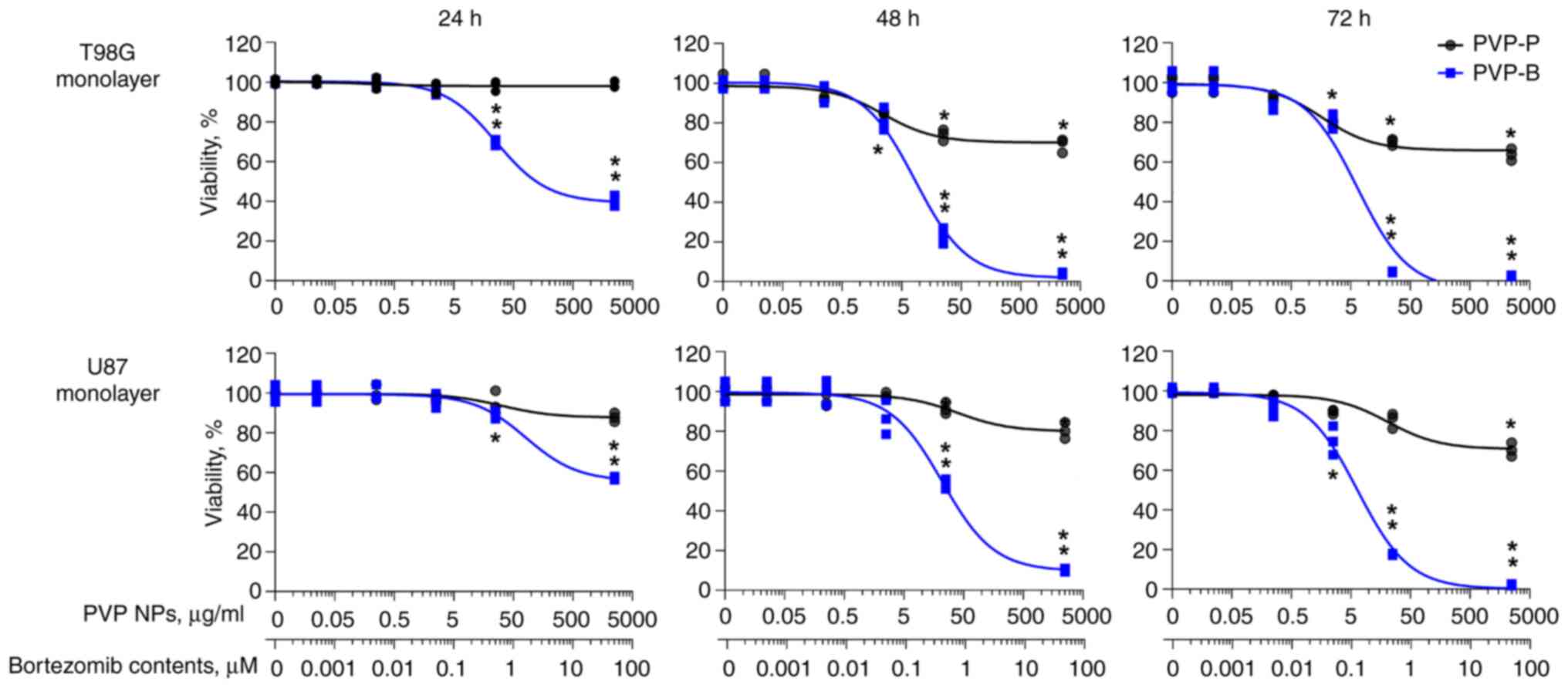
and T98G. PVP-B exhibited significant time- and
concentration-dependent cytotoxicity, reaching the maximal effect
after 72-h incubation. In monolayer T98G cells, IC50 was
6.4±1.6 µg/ml (amounting to 204.8±5.1 nM of bortezomib contents).
In monolayer U87 cells, IC50 was 6.5±0.8 µg/ml
(208.0±2.6 nM bortezomib) (Fig.
2).
As far as prothionamide was not reported to obtain
antitumor activity, therefore prothionamide-loaded PVP-P micelles
were used as control for PVP-B. In the present experiments, PVP-P
did not obtain significant cytotoxicity in both glioma cell lines
(Figs. 2 and 3). To ensure the reliability of the
results, the cytotoxicity of individual substances (PVP, bortezomib
and prothionamide) was examined. The cytotoxicity of
bortezomib-loaded PVP-B was lower than that of free bortezomib
(IC50=20.3±1.3 nM) (Fig.
S1), considering the time required to release the substance
from the hydrophobic core of the micelles. In support of the
current results, neither hollow PVP polymer (Fig. S2), nor free prothionamide (Fig. S3) demonstrated signs of cytotoxic
activity.
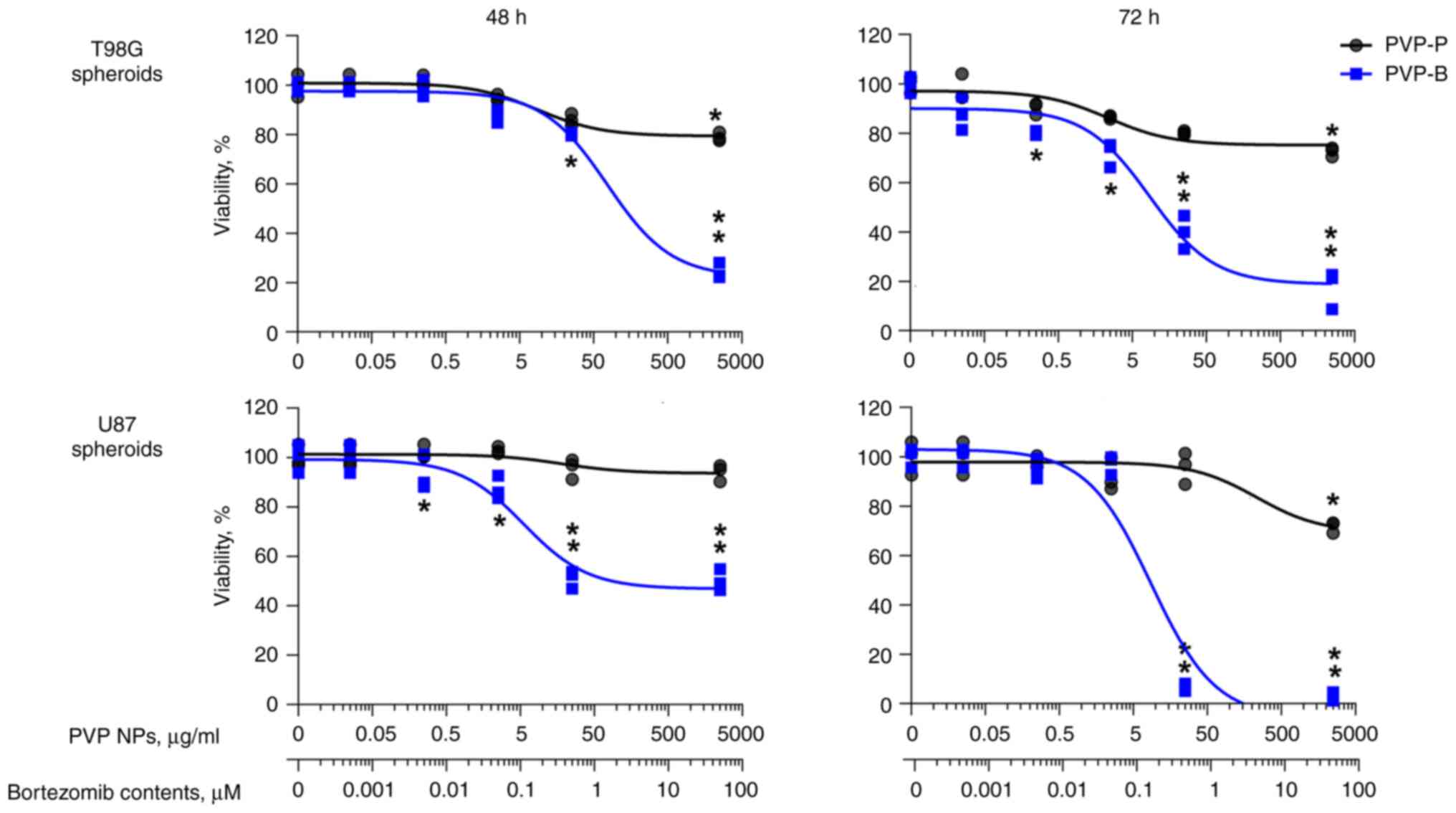
After 72-h incubation, PVP-B almost totally
inhibited the viability of 3D tumor spheroids generated from human
glioblastoma cells T98G (IC50=8.5±2.7 µg/ml PVP-B,
amounting to 272.0±8.6 nM of bortezomib contents) and U87 cells
(IC50=8.7±0.9 µg/ml PVP-B, 278.4±2.9 nM bortezomib)
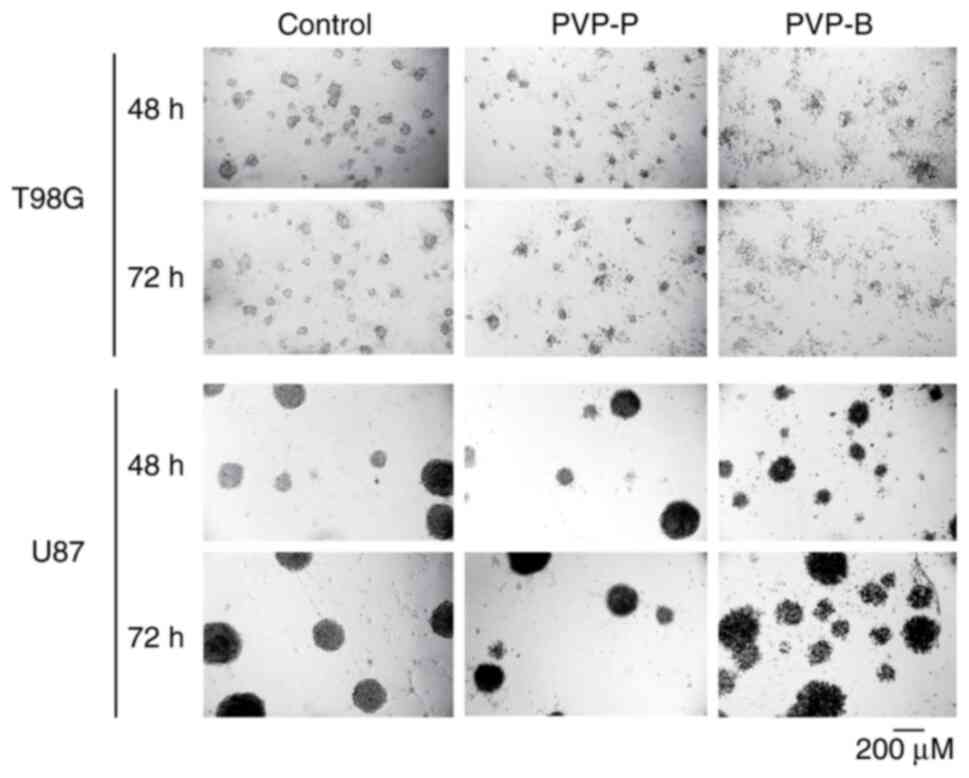
(Fig. 3). The morphology of
spheroids demonstrated significant fragmentation, indicating cell
death after incubation with PVP-B, but not with PVP-P (Fig. 4).
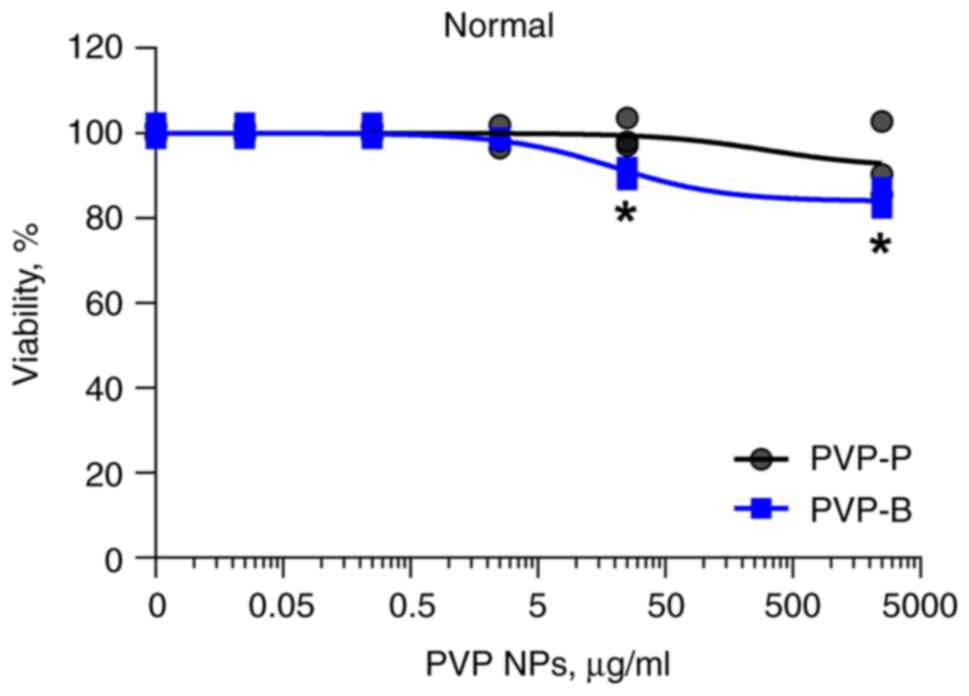
Additionally, the cytotoxicity of PVP-P and PVP-B
was evaluated in normal cells. After 72-h incubation, neither of
them revealed significant cytotoxicity in monolayer culture of
normal human dermal fibroblasts (Fig.
5).
Evaluation of PVP-B toxicity in vivo
in zebrafish
The in vivo toxicity of the bortezomib-loaded
PVP-B nanoparticles was tested in zebrafish Danio rerio. Signs of
acute toxicity were noted upon adding PVP-B to final concentration
of PVP-B 30 mg/ml, manifested in increased motor activity of the
tail and embryo death within the first h. When PVP-B was added at
concentrations of 10 mg/ml, an increase in tail motor activity was
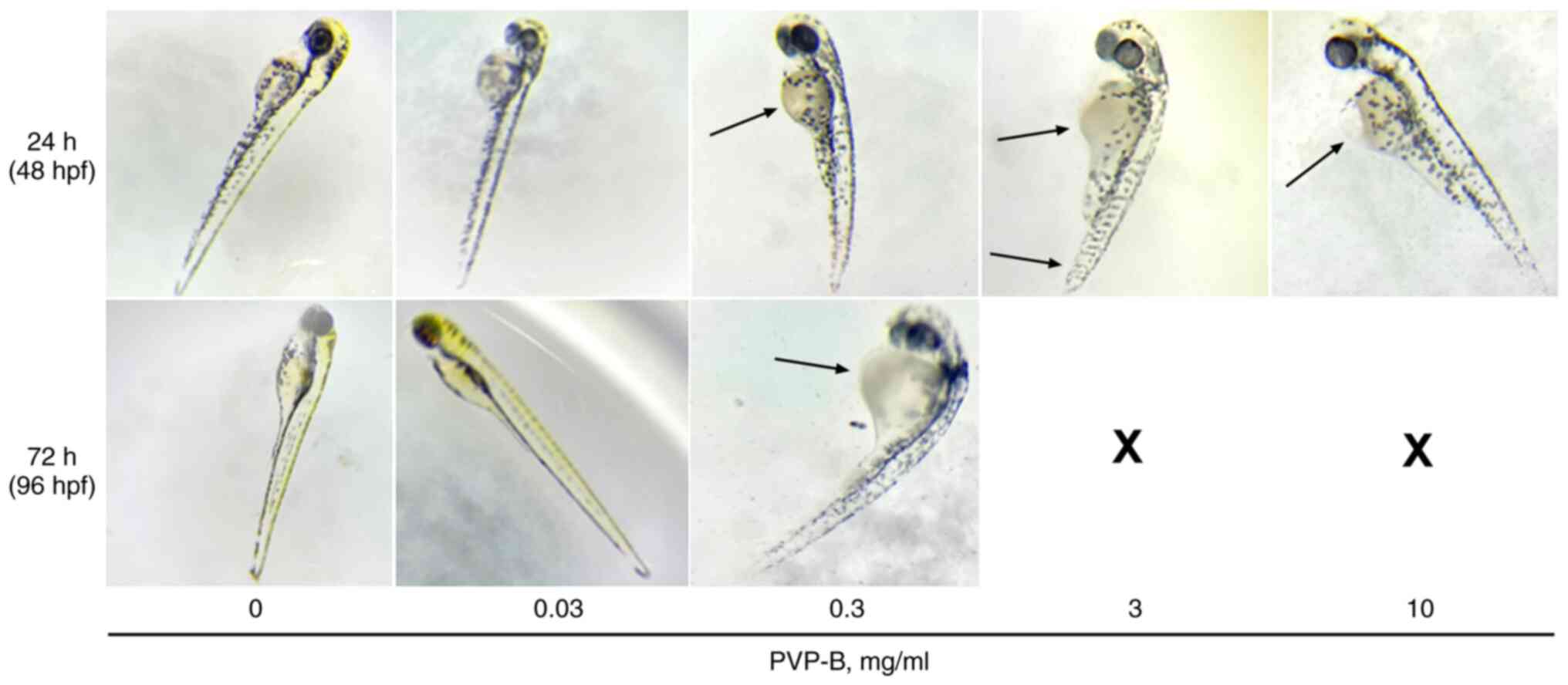
observed, indicating the irritant toxic effect. A delay in tail
development and spinal deformations were noted at PVP-B
concentration of 3 mg/ml after 24-h incubation (Fig. 6). Death of 100% embryos was noted
after 72-h incubation with 30, 10 and 3 mg/ml PVP-B, whereas at 0.3
mg/ml PVP-B, 83.3% of embryos succumbed. After 72-h incubation with
0.3 mg/ml PVP-B, developmental delays were observed in the
surviving embryos. At concentrations 0.3, 3 and 10 mg/ml, PVP-B
caused an enlargement of the yolk sac (Fig. 6). At 0.03 mg/ml PVP-B, no visible
signs of toxicity were noted; 5 out of 6 (83.3%) embryos were alive
both after 24 and 72-h incubation.
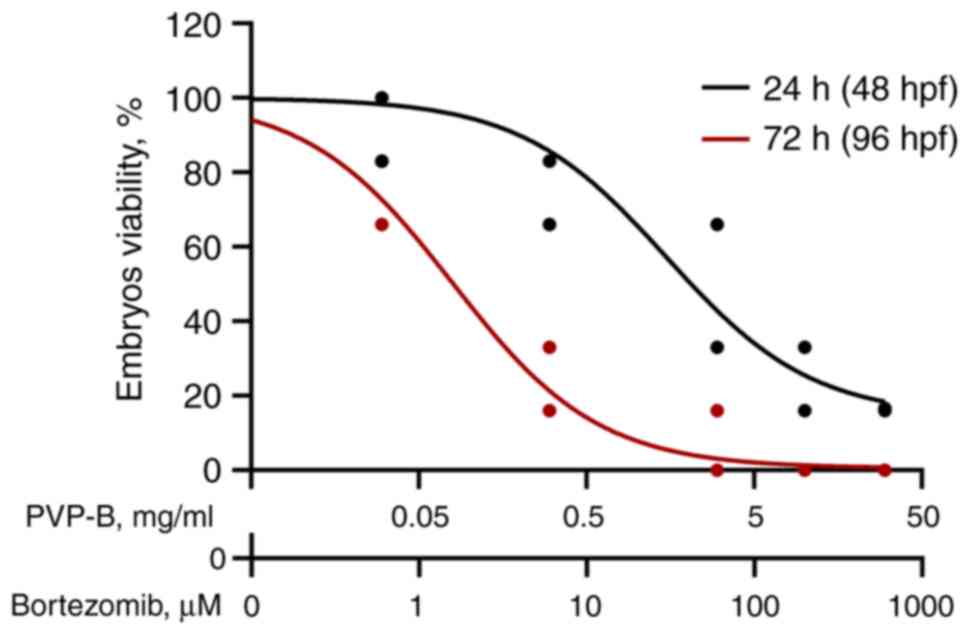
Therefore, the PVP-B toxicity was
concentration-dependent, with mortality rising as the concentration
increased. The LC50 was 0.1±0.011 mg/ml, as determined
by regression analysis (Fig. 7),
which is an order of magnitude higher compared with the cytotoxic
doses in cancer cells.
Discussion
It is of great necessity for anticancer therapy to
both expand the range of active substances delivered to tumors by
nanoparticles, and broaden the range of cancer types that can be
treated with nanosized antitumor drugs (20-23).
Due to poor pharmacokinetic properties, proteasome inhibition with
bortezomib is approved only for the treatment of hematologic
malignancies. However, if the poor serum stability and the side
effects will be addressed, it may have therapeutic potential as
part of a combinatorial strategy for the treatment of solid tumors,
particularly brain cancer. A number of nanoscaled systems have been
developed for delivery of bortezomib, such as liposomes, polymeric
micelles, nanogels, dendrimers and biomimetic materials (7). For example, Gu et al (24) encapsulated bortezomib into
hyaluronic acid-shelled and core-disulfide-crosslinked
biodegradable micelles (HA-CCMs-BP) (24). Zhang et al (25) reported the co-delivery of bortezomib
with paclitaxel in branched polyethyleneimine and palmitic acid
nanoparticles. Unsoy et al (26) manufactured chitosan coated
superparamagnetic iron oxide nanoparticles for magnetic targeting
of bortezomib. De Santo et al (27) recently applied mesoporous
silica-based nanodevice for bortezomib administration. Taking into
account considerations of biocompatibility and safety of use,
self-assembled polymer micelles have attracted remarkable attention
due to their versatility, scalability and suitability for wide
range of applications (28).
However, most of the previously reported polymeric micellar
formulations bearing bortezomib contained polyethylene glycol (PEG)
in their composition. For example, nanoparticles composed of
amphiphilic copolymer PEG-block-poly(d,l-lactide) (29), PEG-poly(ε-caprolactone) (30), or pH-responsive diblock copolymers
of PEG and catechol-functionalized polycarbonate with acid-labile
acetal bond as a linker (31).
Previously, there has been increasing concern about PEG regarding
its tendency to activate complement (32) or accelerated blood clearance upon
repeated injection (33).
PVP derivatives (Povidone K12, Kollidon®,
Plasdone™,) are widely used in pharmaceuticals as
excipients, blood substitutes, and inactive ingredients in
intra-articular and intravenous drugs. In addition, povidone
single-chain nanoparticles have been suggested as suitable for
delivery of anticancer compounds cisplatin and lovastatin (34). In a previous study by the authors,
the production of PVP micelles loaded with model substance
prothionamide to stabilize the hydrophobic core was reported
(14).
In the present study, antitumor drug bortezomib was
loaded in the core of PVP micelles, aiming at reducing its toxicity
and providing prolonged action. The specific activity was first
assessed in the most common in vitro model of monolayer cell
lines. The glioblastoma cells were chosen due to the prospects for
the use of proteasome inhibitors against this aggressive brain
tumor, provided that the problem of effective delivery of the drug
to the lesion site is solved (35).
Importantly, it has been recently demonstrated by
the authors that amphiphilic PVP nanoparticles loaded with
lipophilic cargo are able to cross the blood-retina barrier
(36). This suggests that a similar
effect may be observed at the blood-brain barrier, justifying the
selection of glioblastoma cell lines as a model object for
cytotoxicity testing. In the present experiments, loading of
bortezomib into micelles protected cells from immediate toxicity by
prolonged drug release, since the cytotoxic activity of
PVP-encapsulated bortezomib was time-delayed. Expectedly, 3D tumor
spheroids were more resistant to PVP-B treatment compared with 2D
monolayer tumor cell culture, indicating that they mimic solid
tumors in an improved way compared with monolayer cells.
Additionally, the in vivo toxicity of the
PVP-B nanoparticles was tested in zebrafish Danio rerio
embyos. This model is distinguished by its accessibility and
ability to quickly screen the toxicological and pharmacological
effects of various substances, including nanoparticles, while
reflecting in vivo metabolism issues in an improved way
compared with in vitro models (37). Of note, the calculated
LC50 of the PVP-B by an order of magnitude exceeded the
IC50 in cancer cells in vitro, both 2D and 3D.
This indicated that PVP-B micelles may have a therapeutic window
between effective dose and dose-limiting toxicity. However,
zebrafish model carry a set of limitations, such as methodology of
drug dosing, translation of administration, distribution,
metabolism and excretion characteristics, and a set of specific
biological characteristics which are different from mammals
(38,39). Therefore, further in vivo
experiments in mammals, including orthotopic intracranial xenograft
model of human glioblastoma, will provide additional information
regarding pharmacokinetic parameters, antitumor efficacy and
safety. Additionally, since the ability of PVP-micelles to
immobilize targeted protein has been previously demonstrated
(14), this work may be continued
in production of the micelles loaded with bortezomib and
surface-modified with protein ligand for targeted drug delivery and
a synergistic antitumor effect.
In conclusion, novel amphiphilic micellar PVP
nanoparticles with encapsulated bortezomib may provide a safe and
effective alternative to existing therapeutic regimens involving
proteasome inhibitors due to high efficacy and possibly reduced
side effects.
Supplementary Material
Cytotoxicity of free bortezomib in
monolayer T98G and U87 human glioblastoma cell lines evaluated by
MTT test after 24 and 72 h of incubation. Calculated
IC50 was 20.3±1.3 nM. *P<0.05 and
**P<0.01 by Student's t-test.
Cytotoxicity of amphiphilic PVP
polymer in monolayer T98G and U87 human glioblastoma cell lines
evaluated by MTT test after 72 h of incubation. PVP, poly
(N-vinylpyrrolidone).
Cytotoxicity of free prothionamide in
monolayer U87 human glioblastoma cell line evaluated by MTT test
after 72 h of incubation.
Acknowledgements
The TEM, DLS and zeta potential measurements were
carried out using the equipment purchased on account of the
Lomonosov MSU Development Program to 2020. The authors are grateful
to Professor Sergey Burov (Cytomed JSC, St-Petersburg, Russia) for
providing cyclic RGD-peptide.
Funding
Funding: The present study was supported by Russian Science
Foundation (grant no. 23-15-00468;
https://rscf.ru/project/23-15-00468/).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ANK and AVY conceptualized the study. PPK, DVB, EVK
and IIK curated data. ANK, DVB, AVY, MEG and IIK performed formal
analysis. ANK, AVY, DAS and MEG acquired funding. PPK, DVB, PAP,
EVK, AAI and IIK conducted investigation. ANK, DAS, AMT, MEG, DVB,
AVY, EAM and VSP developed methodology. ANK and AVY performed
project administration. ANK, DVB, MEG, EAM, AMT and VSP provided
resources. ANK supervised the study. PPK, DVB, AVY, AEN, PCS, AMT
and VSP validated data. DVB, EVK, IIK, AEN and AVY visualized data.
AVY, DVB, IIK, AEN and PCS wrote the original draft. ANK, DVB, VSP,
AMT, DAS and MEG wrote, reviewed and edited the manuscript. All
authors read and approved the final version of the manuscript. ANK
and AVY confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Experiments performed on animals were carried out in
accordance with the European Convention for the Protection of
Vertebrate Animals Used for Experimental and Other Scientific
Purposes. Experimental protocol was approved (approval no. 03p; May
16, 2023) by local ethics committee of the N.N. Blokhin National
Medical Research Center of Oncology (Moscow, Russia).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zavrski I, Jakob C, Schmid P, Krebbel H,
Kaiser M, Fleissner C, Rosche M, Possinger K and Sezer O:
Proteasome: An emerging target for cancer therapy. Anticancer
Drugs. 16:475–481. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Adams J and Kauffman M: Development of the
proteasome inhibitor Velcade (Bortezomib). Cancer Invest.
22:304–311. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Raedler L: Velcade (Bortezomib) receives 2
new FDA indications: For retreatment of patients with multiple
myeloma and for first-line treatment of patients with mantle-cell
lymphoma. Am Health Drug Benefits. 8:135–140. 2015.PubMed/NCBI
|
|
4
|
Cengiz Seval G and Beksac M: The safety of
bortezomib for the treatment of multiple myeloma. Expert Opin Drug
Saf. 17:953–962. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yamamoto S and Egashira N: Pathological
mechanisms of bortezomib-induced peripheral neuropathy. Int J Mol
Sci. 22(888)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tan CRC, Abdul-Majeed S, Cael B and Barta
SK: Clinical pharmacokinetics and pharmacodynamics of bortezomib.
Clin Pharmacokinet. 58:157–168. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu J, Zhao R, Jiang X, Li Z and Zhang B:
Progress on the application of bortezomib and bortezomib-based
nanoformulations. Biomolecules. 12(51)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wen R, Umeano AC, Chen P and Farooqi AA:
Polymer-based drug delivery systems for cancer. Crit Rev Ther Drug
Carrier Syst. 35:521–553. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Waleka E, Stojek Z and Karbarz M: Activity
of povidone in recent biomedical applications with emphasis on
micro- and nano drug delivery systems. Pharmaceutics.
13(654)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kuskov A, Nikitovic D, Berdiaki A,
Shtilman M and Tsatsakis A: Amphiphilic poly-N-vinylpyrrolidone
nanoparticles as carriers for nonsteroidal, anti-inflammatory
drugs: Pharmacokinetic, anti-inflammatory, and ulcerogenic activity
study. Pharmaceutics. 14(925)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Berdiaki A, Kuskov AN, Kulikov PP,
Thrapsanioti LN, Giatagana EM, Stivaktakis P, Shtilman MI,
Tsatsakis A and Nikitovic D: In vitro assessment of
poly-N-Vinylpyrrolidone/acrylic acid nanoparticles biocompatibility
in a microvascular endothelium model. Int J Mol Sci.
23(12446)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Villemson AL, Kuskov AN, Shtilman MI,
Galebskaya LV, Ryumina EV and Larionova NI: Interaction of polymer
aggregates based on stearoyl-poly-N-vinylpyrrolidone with blood
components. Biochemistry (Mosc). 69:621–628. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Berdiaki A, Perisynaki E, Stratidakis A,
Kulikov PP, Kuskov AN, Stivaktakis P, Henrich-Noack P, Luss AL,
Shtilman MM, Tzanakakis GN, et al: Assessment of amphiphilic
poly-N-vinylpyrrolidone nanoparticles' biocompatibility with
endothelial cells in vitro and delivery of an anti-inflammatory
drug. Mol Pharm. 17:4212–4225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yagolovich A, Kuskov A, Kulikov P,
Kurbanova L, Bagrov D, Artykov A, Gasparian M, Sizova S, Oleinikov
V, Gileva A, et al: Amphiphilic poly(N-vinylpyrrolidone)
nanoparticles conjugated with dr5-specific antitumor cytokine dr5-b
for targeted delivery to cancer cells. Pharmaceutics.
13(1413)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Teng Y, Morrison ME, Munk P, Webber SE and
Procházka K: Release kinetics studies of aromatic molecules into
water from block polymer micelles. Macromolecules. 31:3578–3587.
1998.
|
|
16
|
Akasov R, Zaytseva-Zotova D, Burov S, Leko
M, Dontenwill M, Chiper M, Vandamme T and Markvicheva E: Formation
of multicellular tumor spheroids induced by cyclic RGD-peptides and
use for anticancer drug testing in vitro. Int J Pharm. 506:148–157.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kulikov PP, Kuskov AN, Goryachaya AV, Luss
AN and Shtil'man MI: Amphiphilic poly-n-vinyl-2-pyrrolidone:
Synthesis, properties, nanoparticles. Polym Sci Ser D. 10:263–268.
2017.
|
|
18
|
Kuskov AN, Kulikov PP, Luss AL, Goryachaya
AV and Shtil'man MI: Preparation of polymer nanoparticles by
self-assembling of amphiphilic poly-N-vinylpyrrolidone derivatives
in aqueous media. Russ J Appl Chem. 89:1461–1468. 2016.
|
|
19
|
Kozulitsyna TI and Zemskova ZS:
Therapeutic efficiency of ethionamide and prothionamide. Probl
Tuberk. 47:66–69. 1969.PubMed/NCBI(In Russian).
|
|
20
|
Radu IC, Hudita A, Zaharia C, Galateanu B,
Iovu H, Tanasa EV, Georgiana Nitu S, Ginghina O, Negrei C,
Tsatsakis A, et al: Poly(3-hydroxybutyrate-CO-3-hydroxyvalerate)
PHBHV biocompatible nanocarriers for 5-FU delivery targeting
colorectal cancer. Drug Deliv. 26:318–327. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ginghină O, Hudiță A, Zaharia C, Tsatsakis
A, Mezhuev Y, Costache M and Gălățeanu B: Current landscape in
organic nanosized materials advances for improved management of
colorectal cancer patients. Materials (Basel).
14(2440)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hudiță A, Radu IC, Zaharia C, Ion AC,
Ginghină O, Gălățeanu B, Măruțescu L, Grama F, Tsatsakis A,
Gurevich L and Costache M: Bio- and hemo-compatible silk fibroin
PEGylated nanocarriers for 5-fluorouracil chemotherapy in
colorectal cancer: In vitro studies. Pharmaceutics.
13(755)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cicek B, Hacimuftuoglu A, Kuzucu M, Cetin
A, Yeni Y, Genc S, Yildirim S, Bolat I, Kantarci M, Gul M, et al:
Sorafenib alleviates inflammatory signaling of tumor
microenvironment in precancerous lung injuries. Pharmaceuticals
(Basel). 16(221)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gu Z, Wang X, Cheng R, Cheng L and Zhong
Z: Hyaluronic acid shell and disulfide-crosslinked core micelles
for in vivo targeted delivery of bortezomib for the treatment of
multiple myeloma. Acta Biomater. 80:288–295. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang R, Liu Y, Yang Z, Li Y, Rong X, Wang
L, Guo C, Li S, Liu J, Li M and Wu Y: Construction of nanoparticles
based on amphiphilic PEI-PA polymers for bortezomib and paclitaxel
co-delivery. RSC Adv. 5:15453–15460. 2015.
|
|
26
|
Unsoy G, Yalcin S, Khodadust R, Mutlu P,
Onguru O and Gunduz U: Chitosan magnetic nanoparticles for pH
responsive Bortezomib release in cancer therapy. Biomed
Pharmacother. 68:641–648. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
De Santo M, Giovinazzo A, Fava M, Mazzotta
E, De Napoli IE, Greco M, Comandé A, Nigro A, Argurio P, Perrotta
I, et al: Engineered mesoporous silica-based nanoparticles as smart
chemotherapy nanodevice for bortezomib administration†. Mater Chem
Front. 7:216–229. 2023.
|
|
28
|
Yadav S, Sharma AK and Kumar P: Nanoscale
self-assembly for therapeutic delivery. Front Bioeng Biotechnol.
8(127)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shen S, Du XJ, Liu J, Sun R, Zhu YH and
Wang J: Delivery of bortezomib with nanoparticles for basal-like
triple-negative breast cancer therapy. J Control Release.
208:14–24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu L, Wang S, Qi P, Song S, Yang Y, Shi J
and Han G: Dopamine-modified poly(ε-caprolactone) micelles for pH
controlled delivery of bortezomib. Int J Pharm.
590(119885)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu S, Ono RJ, Yang C, Gao S, Ming Tan JY,
Hedrick JL and Yang YY: Dual pH-responsive shell-cleavable
polycarbonate micellar nanoparticles for in vivo anticancer drug
delivery. ACS Appl Mater Interfaces. 10:19355–19364.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Toda M, Arima Y and Iwata H: Complement
activation on degraded polyethylene glycol-covered surface. Acta
Biomate. 6:2642–2649. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu H, Ye F, Hu M, Yin P, Zhang W, Li Y, Yu
X and Deng Y: Influence of phospholipid types and animal models on
the accelerated blood clearance phenomenon of PEGylated liposomes
upon repeated injection. Drug Deliv. 22:598–607. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Asenjo-Sanz I, Del-Corte M,
Pinacho-Olaciregui J, González-Burgos M, González E, Verde-Sesto E,
Arbe A, Colmenero J and Pomposo JA: Preparation and preliminary
evaluation of povidone single-chain nanoparticles as potential drug
delivery nanocarriers. Med One:. 4(e190013)2019.
|
|
35
|
Roth P, Mason WP, Richardson PG and Weller
M: Proteasome inhibition for the treatment of glioblastoma. Expert
Opin Investig Drugs. 29:1133–1141. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tawfik M, Hadlak S, Götze C, Sokolov M,
Kulikov P, Kuskov A, Shtilman M, Sabel BA and Henrich-Noack P: Live
in-vivo neuroimaging reveals the transport of lipophilic cargo
through the blood-retina barrier with modified amphiphilic
poly-N-vinylpyrrolidone nanoparticles. J Biomed Nanotechnol.
17:846–858. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pensado-López A, Fernández-Rey J, Reimunde
P, Crecente-Campo J, Sánchez L and Torres Andón F: Zebrafish models
for the safety and therapeutic testing of nanoparticles with a
focus on macrophages. Nanomaterials (Basel).
11(1784)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gamble JT, Elson DJ, Greenwood JA, Tanguay
RL and Kolluri SK: The zebrafish xenograft models for investigating
cancer and cancer therapeutics. Biology (Basel).
10(252)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Renieri EA, Sfakianakis DG, Alegakis AA,
Safenkova IV, Buha A, Matović V, Tzardi M, Dzantiev BB, Divanach P,
Kentouri M and Tsatsakis AM: Nonlinear responses to waterborne
cadmium exposure in zebrafish. An in vivo study. Environ Res.
157:173–181. 2017.PubMed/NCBI View Article : Google Scholar
|