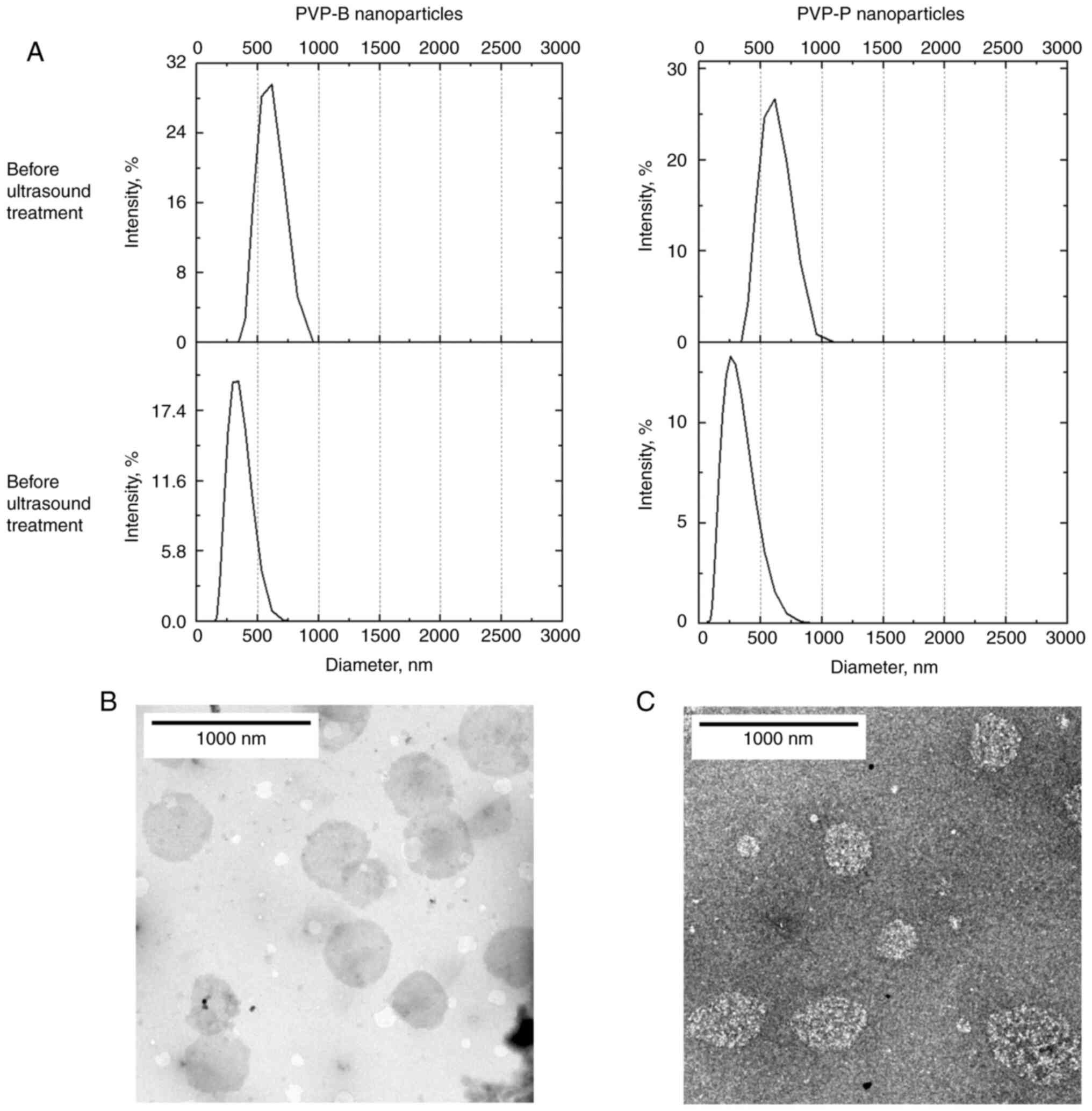
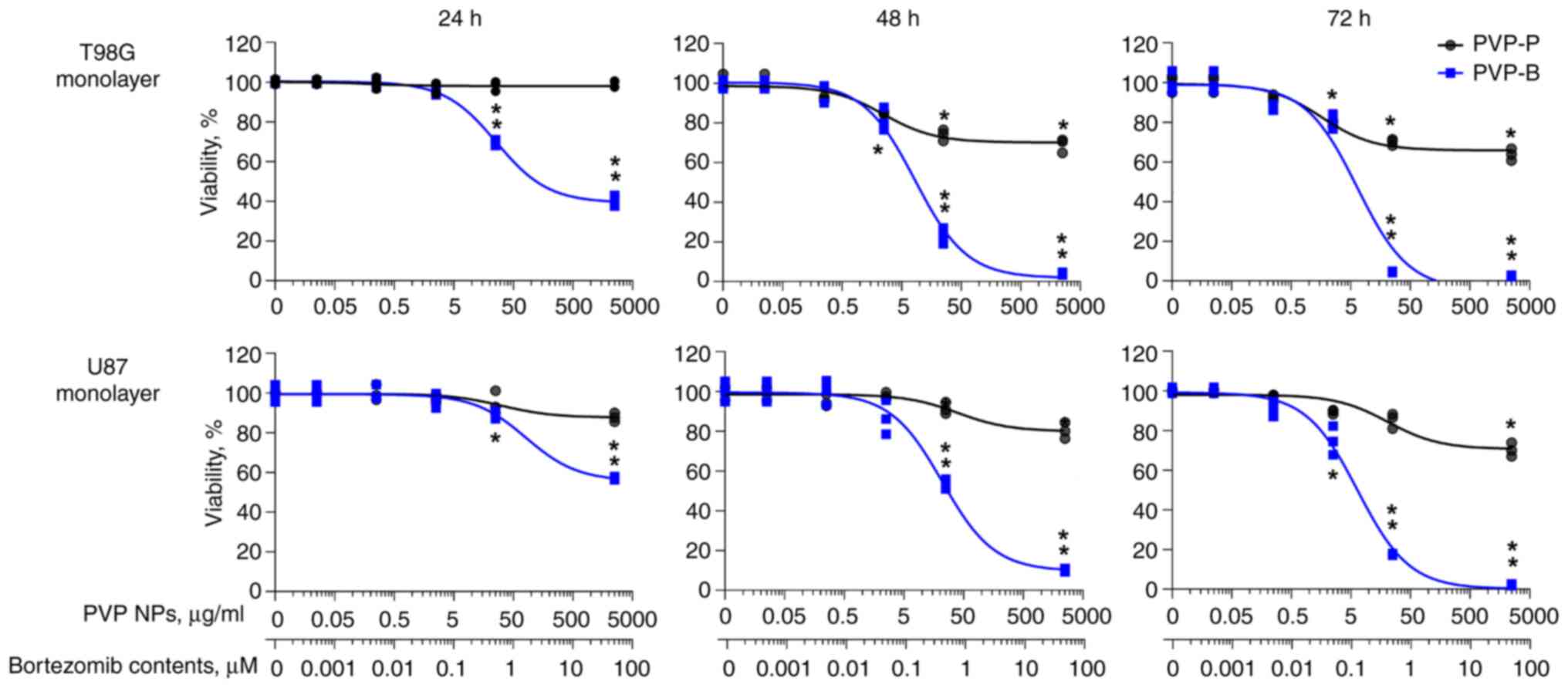
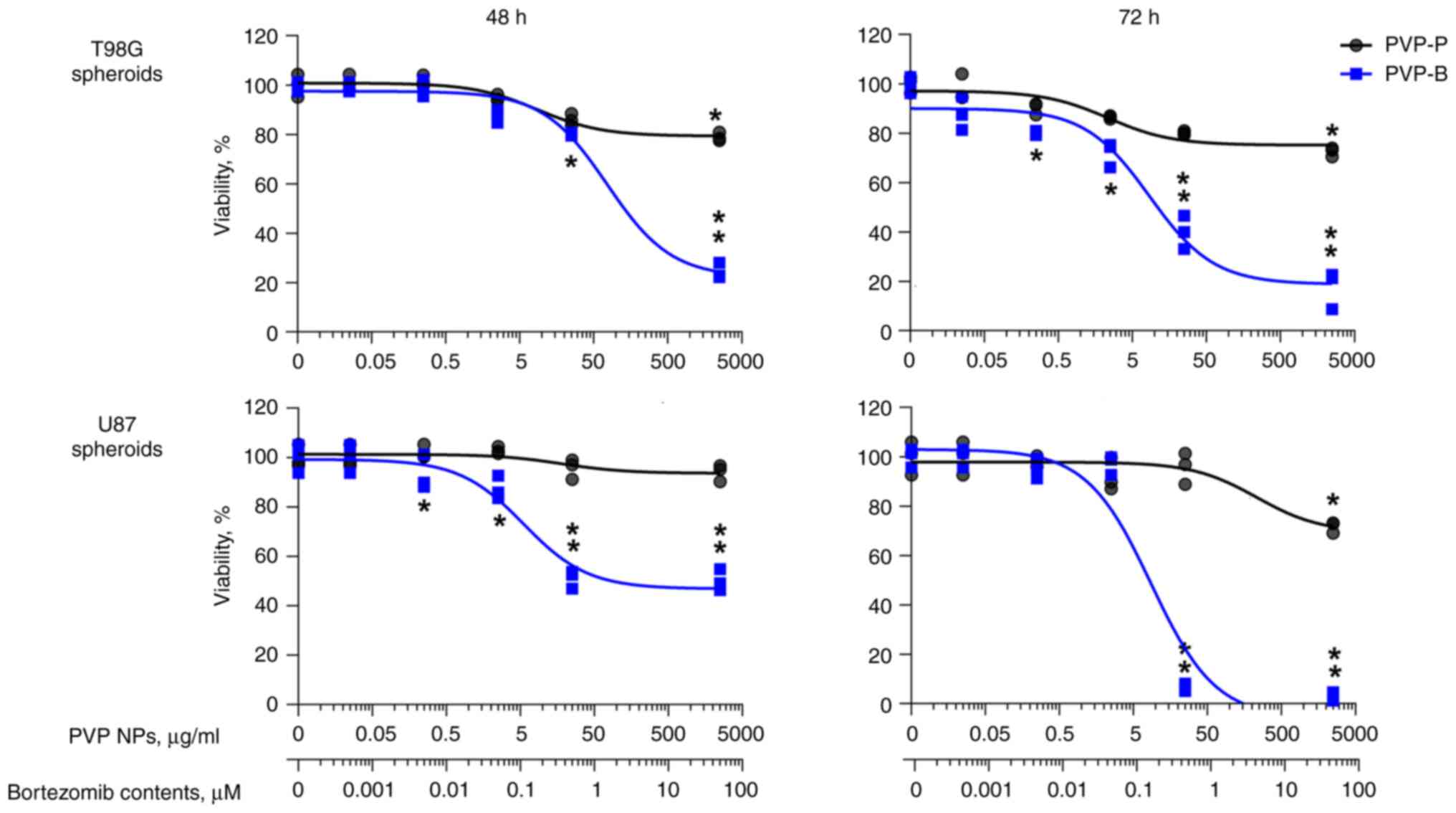
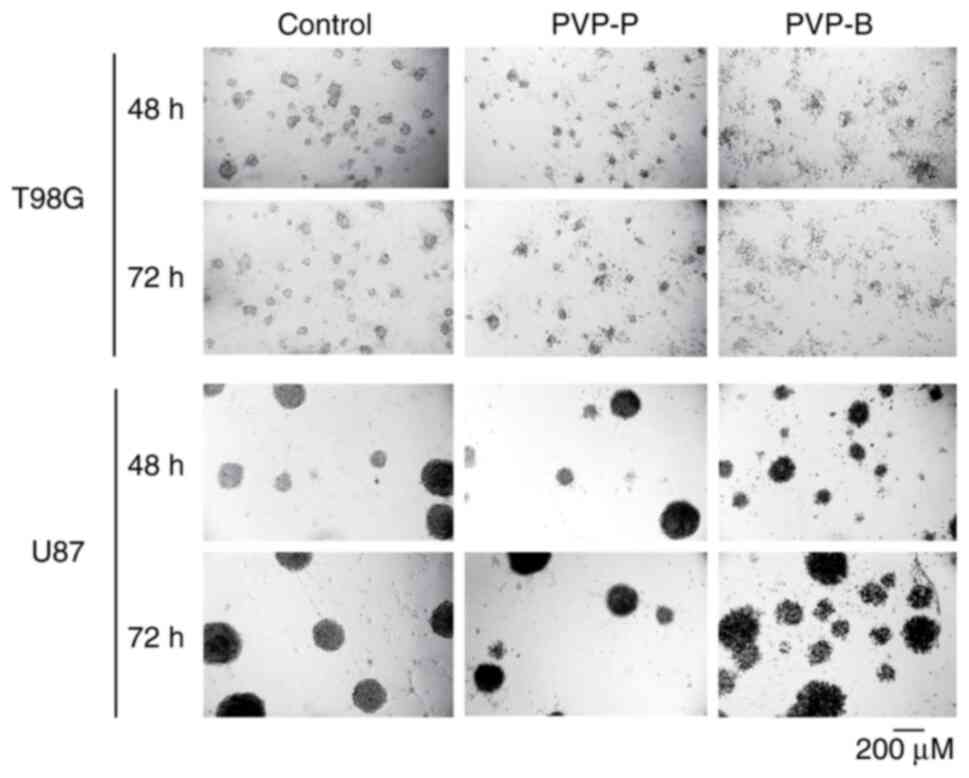
|
1
|
Zavrski I, Jakob C, Schmid P, Krebbel H,
Kaiser M, Fleissner C, Rosche M, Possinger K and Sezer O:
Proteasome: An emerging target for cancer therapy. Anticancer
Drugs. 16:475–481. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Adams J and Kauffman M: Development of the
proteasome inhibitor Velcade (Bortezomib). Cancer Invest.
22:304–311. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Raedler L: Velcade (Bortezomib) receives 2
new FDA indications: For retreatment of patients with multiple
myeloma and for first-line treatment of patients with mantle-cell
lymphoma. Am Health Drug Benefits. 8:135–140. 2015.PubMed/NCBI
|
|
4
|
Cengiz Seval G and Beksac M: The safety of
bortezomib for the treatment of multiple myeloma. Expert Opin Drug
Saf. 17:953–962. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yamamoto S and Egashira N: Pathological
mechanisms of bortezomib-induced peripheral neuropathy. Int J Mol
Sci. 22(888)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tan CRC, Abdul-Majeed S, Cael B and Barta
SK: Clinical pharmacokinetics and pharmacodynamics of bortezomib.
Clin Pharmacokinet. 58:157–168. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu J, Zhao R, Jiang X, Li Z and Zhang B:
Progress on the application of bortezomib and bortezomib-based
nanoformulations. Biomolecules. 12(51)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wen R, Umeano AC, Chen P and Farooqi AA:
Polymer-based drug delivery systems for cancer. Crit Rev Ther Drug
Carrier Syst. 35:521–553. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Waleka E, Stojek Z and Karbarz M: Activity
of povidone in recent biomedical applications with emphasis on
micro- and nano drug delivery systems. Pharmaceutics.
13(654)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kuskov A, Nikitovic D, Berdiaki A,
Shtilman M and Tsatsakis A: Amphiphilic poly-N-vinylpyrrolidone
nanoparticles as carriers for nonsteroidal, anti-inflammatory
drugs: Pharmacokinetic, anti-inflammatory, and ulcerogenic activity
study. Pharmaceutics. 14(925)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Berdiaki A, Kuskov AN, Kulikov PP,
Thrapsanioti LN, Giatagana EM, Stivaktakis P, Shtilman MI,
Tsatsakis A and Nikitovic D: In vitro assessment of
poly-N-Vinylpyrrolidone/acrylic acid nanoparticles biocompatibility
in a microvascular endothelium model. Int J Mol Sci.
23(12446)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Villemson AL, Kuskov AN, Shtilman MI,
Galebskaya LV, Ryumina EV and Larionova NI: Interaction of polymer
aggregates based on stearoyl-poly-N-vinylpyrrolidone with blood
components. Biochemistry (Mosc). 69:621–628. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Berdiaki A, Perisynaki E, Stratidakis A,
Kulikov PP, Kuskov AN, Stivaktakis P, Henrich-Noack P, Luss AL,
Shtilman MM, Tzanakakis GN, et al: Assessment of amphiphilic
poly-N-vinylpyrrolidone nanoparticles' biocompatibility with
endothelial cells in vitro and delivery of an anti-inflammatory
drug. Mol Pharm. 17:4212–4225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yagolovich A, Kuskov A, Kulikov P,
Kurbanova L, Bagrov D, Artykov A, Gasparian M, Sizova S, Oleinikov
V, Gileva A, et al: Amphiphilic poly(N-vinylpyrrolidone)
nanoparticles conjugated with dr5-specific antitumor cytokine dr5-b
for targeted delivery to cancer cells. Pharmaceutics.
13(1413)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Teng Y, Morrison ME, Munk P, Webber SE and
Procházka K: Release kinetics studies of aromatic molecules into
water from block polymer micelles. Macromolecules. 31:3578–3587.
1998.
|
|
16
|
Akasov R, Zaytseva-Zotova D, Burov S, Leko
M, Dontenwill M, Chiper M, Vandamme T and Markvicheva E: Formation
of multicellular tumor spheroids induced by cyclic RGD-peptides and
use for anticancer drug testing in vitro. Int J Pharm. 506:148–157.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kulikov PP, Kuskov AN, Goryachaya AV, Luss
AN and Shtil'man MI: Amphiphilic poly-n-vinyl-2-pyrrolidone:
Synthesis, properties, nanoparticles. Polym Sci Ser D. 10:263–268.
2017.
|
|
18
|
Kuskov AN, Kulikov PP, Luss AL, Goryachaya
AV and Shtil'man MI: Preparation of polymer nanoparticles by
self-assembling of amphiphilic poly-N-vinylpyrrolidone derivatives
in aqueous media. Russ J Appl Chem. 89:1461–1468. 2016.
|
|
19
|
Kozulitsyna TI and Zemskova ZS:
Therapeutic efficiency of ethionamide and prothionamide. Probl
Tuberk. 47:66–69. 1969.PubMed/NCBI(In Russian).
|
|
20
|
Radu IC, Hudita A, Zaharia C, Galateanu B,
Iovu H, Tanasa EV, Georgiana Nitu S, Ginghina O, Negrei C,
Tsatsakis A, et al: Poly(3-hydroxybutyrate-CO-3-hydroxyvalerate)
PHBHV biocompatible nanocarriers for 5-FU delivery targeting
colorectal cancer. Drug Deliv. 26:318–327. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ginghină O, Hudiță A, Zaharia C, Tsatsakis
A, Mezhuev Y, Costache M and Gălățeanu B: Current landscape in
organic nanosized materials advances for improved management of
colorectal cancer patients. Materials (Basel).
14(2440)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hudiță A, Radu IC, Zaharia C, Ion AC,
Ginghină O, Gălățeanu B, Măruțescu L, Grama F, Tsatsakis A,
Gurevich L and Costache M: Bio- and hemo-compatible silk fibroin
PEGylated nanocarriers for 5-fluorouracil chemotherapy in
colorectal cancer: In vitro studies. Pharmaceutics.
13(755)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cicek B, Hacimuftuoglu A, Kuzucu M, Cetin
A, Yeni Y, Genc S, Yildirim S, Bolat I, Kantarci M, Gul M, et al:
Sorafenib alleviates inflammatory signaling of tumor
microenvironment in precancerous lung injuries. Pharmaceuticals
(Basel). 16(221)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gu Z, Wang X, Cheng R, Cheng L and Zhong
Z: Hyaluronic acid shell and disulfide-crosslinked core micelles
for in vivo targeted delivery of bortezomib for the treatment of
multiple myeloma. Acta Biomater. 80:288–295. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang R, Liu Y, Yang Z, Li Y, Rong X, Wang
L, Guo C, Li S, Liu J, Li M and Wu Y: Construction of nanoparticles
based on amphiphilic PEI-PA polymers for bortezomib and paclitaxel
co-delivery. RSC Adv. 5:15453–15460. 2015.
|
|
26
|
Unsoy G, Yalcin S, Khodadust R, Mutlu P,
Onguru O and Gunduz U: Chitosan magnetic nanoparticles for pH
responsive Bortezomib release in cancer therapy. Biomed
Pharmacother. 68:641–648. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
De Santo M, Giovinazzo A, Fava M, Mazzotta
E, De Napoli IE, Greco M, Comandé A, Nigro A, Argurio P, Perrotta
I, et al: Engineered mesoporous silica-based nanoparticles as smart
chemotherapy nanodevice for bortezomib administration†. Mater Chem
Front. 7:216–229. 2023.
|
|
28
|
Yadav S, Sharma AK and Kumar P: Nanoscale
self-assembly for therapeutic delivery. Front Bioeng Biotechnol.
8(127)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shen S, Du XJ, Liu J, Sun R, Zhu YH and
Wang J: Delivery of bortezomib with nanoparticles for basal-like
triple-negative breast cancer therapy. J Control Release.
208:14–24. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu L, Wang S, Qi P, Song S, Yang Y, Shi J
and Han G: Dopamine-modified poly(ε-caprolactone) micelles for pH
controlled delivery of bortezomib. Int J Pharm.
590(119885)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu S, Ono RJ, Yang C, Gao S, Ming Tan JY,
Hedrick JL and Yang YY: Dual pH-responsive shell-cleavable
polycarbonate micellar nanoparticles for in vivo anticancer drug
delivery. ACS Appl Mater Interfaces. 10:19355–19364.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Toda M, Arima Y and Iwata H: Complement
activation on degraded polyethylene glycol-covered surface. Acta
Biomate. 6:2642–2649. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu H, Ye F, Hu M, Yin P, Zhang W, Li Y, Yu
X and Deng Y: Influence of phospholipid types and animal models on
the accelerated blood clearance phenomenon of PEGylated liposomes
upon repeated injection. Drug Deliv. 22:598–607. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Asenjo-Sanz I, Del-Corte M,
Pinacho-Olaciregui J, González-Burgos M, González E, Verde-Sesto E,
Arbe A, Colmenero J and Pomposo JA: Preparation and preliminary
evaluation of povidone single-chain nanoparticles as potential drug
delivery nanocarriers. Med One:. 4(e190013)2019.
|
|
35
|
Roth P, Mason WP, Richardson PG and Weller
M: Proteasome inhibition for the treatment of glioblastoma. Expert
Opin Investig Drugs. 29:1133–1141. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tawfik M, Hadlak S, Götze C, Sokolov M,
Kulikov P, Kuskov A, Shtilman M, Sabel BA and Henrich-Noack P: Live
in-vivo neuroimaging reveals the transport of lipophilic cargo
through the blood-retina barrier with modified amphiphilic
poly-N-vinylpyrrolidone nanoparticles. J Biomed Nanotechnol.
17:846–858. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pensado-López A, Fernández-Rey J, Reimunde
P, Crecente-Campo J, Sánchez L and Torres Andón F: Zebrafish models
for the safety and therapeutic testing of nanoparticles with a
focus on macrophages. Nanomaterials (Basel).
11(1784)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gamble JT, Elson DJ, Greenwood JA, Tanguay
RL and Kolluri SK: The zebrafish xenograft models for investigating
cancer and cancer therapeutics. Biology (Basel).
10(252)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Renieri EA, Sfakianakis DG, Alegakis AA,
Safenkova IV, Buha A, Matović V, Tzardi M, Dzantiev BB, Divanach P,
Kentouri M and Tsatsakis AM: Nonlinear responses to waterborne
cadmium exposure in zebrafish. An in vivo study. Environ Res.
157:173–181. 2017.PubMed/NCBI View Article : Google Scholar
|