High-density lipoprotein (HDL), an important
lipoprotein in the body, plays a key protective and regulatory role
via reverse cholesterol transport. HDL participate in the transport
of cholesterol from extrahepatic tissues to the liver for
metabolism (11). The primary
function of HDL is to transport excess cholesterol from tissues and
cells to the liver for metabolism and excretion (12). HDL can prevent cardiovascular
disease by removing excess cholesterol in the arterial blood vessel
walls and reducing the risk of atherosclerosis. Lee-Rueckert et
al (12) confirmed that the
content of HDL is negatively correlated with onset of coronary
heart disease. Antioxidants such as vitamin E and glutathione are
abundant in HDL, which can maintain the integrity of vascular
endothelial cells, neutralize peroxides to protect low-density
lipoprotein (LDL) from oxidation and reduce the occurrence of
atherosclerosis and arterial inflammation (13,14).
HDL can reduce the inflammatory response by regulating adhesion and
activation of inflammatory cells to maintain integrity and
elasticity of the blood vessel wall and prevent cardiovascular
disease such as atherosclerosis (15). Moreover, HDL can maintain normal
flow of blood by inhibiting platelet aggregation and activation of
coagulation factors (16), HDL also
can regulate the formation and dissolution of thrombi in the blood
by promoting the release of fibrinogen activators (17). In conclusion, antioxidant,
anti-inflammatory and anticoagulant agents prevent atherosclerosis
and decrease the risk of cardiovascular disease. Therefore,
maintaining appropriate levels of HDL is key in maintaining
cardiovascular health. However, whether M. pneumoniae
infection can affect the blood HDL levels and the underlying
mechanism require further investigation.
Cytokines participating in both humoral and cellular
immunity act as bridges within the immune system to ensure the
coordination of these responses to defend the body against
pathogens (26,27). In humoral immunity, cytokines such
as IL-4, IL-5 and IL-6 secreted by Th cells can promote the
differentiation of B lymphocytes into antibody-producing cells
(plasma cells) and contribute to antibody production and antigen
neutralization (28-30).
In cellular immunity, cytokines such as IFN-γ secreted by activated
T lymphocytes enhance the cytotoxic activity of cells such as CTLs
and natural killer cells (NK) involved in cellular immune responses
(24). In brief, cytokines are key
mediators in both the clearance of pathogens and inflammation,
however, the effect on the host tissue is complicated and cannot be
predicted accurately due to the diversity of cellular and tissue
environments (31,32). Moreover, the immune complexes
comprising the antigen and corresponding antibody following M.
pneumoniae infection can effectively activate the complement
system, leading to removal of invasive microorganisms and immune
damage of host multiple systems (33-35).
He et al (36) demonstrated
common antigen components between M. pneumoniae and host
cells resulting in the evasion of host immune surveillance, hence
contributing to long-term survival of M. pneumoniae.
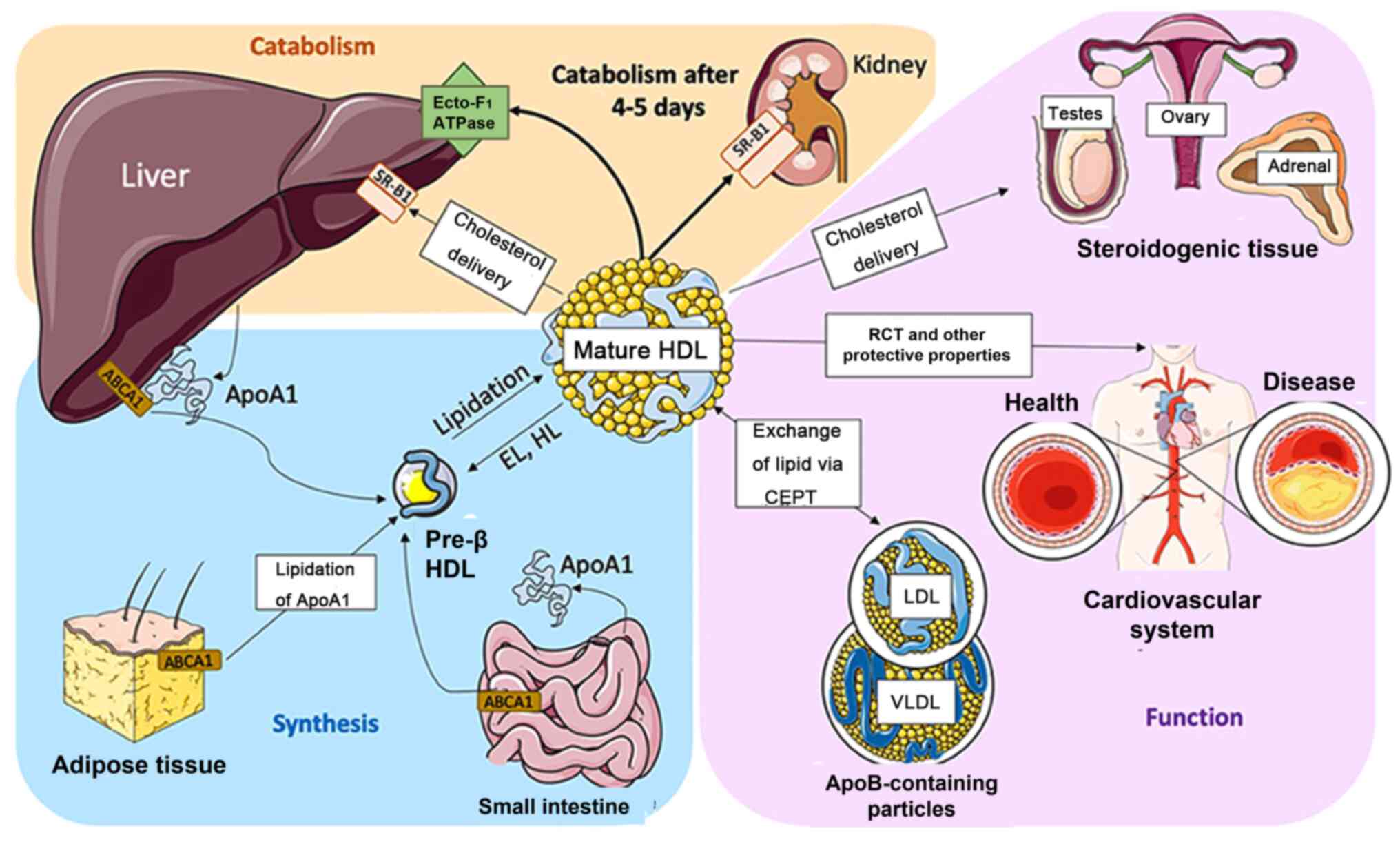
The primary role of HDL is to transport cholesterol
from peripheral tissue such as the cardiovascular system to
steroidogenic tissues comprising testes, ovaries, and adrenal gland
to product hormone. Moreover, it can exchange lipids with Apo
B-containing particles such as LDL and very LDL (VLDL) via
cholesterol ester transfer protein. Furthermore, HDL participates
in reverse cholesterol transport, producing a marked
antiatherogenic effect. Specifically, it can transport cholesterol
from foam cells to the liver, metabolize it into bile and excrete
it out of the body (40).
The protective role of HDL has been demonstrated in
numerous types of diseases, such as metabolic (41) and cardiovascular disease (42) and cancer (43). Mitochondria are dynamic organelles
that supply energy to the body and dysfunction is related to
numerous kinds of disease, such as maternally inherited diabetes
and deafness, the syndrome of metabolic defects, and autosomal
dominant optic atrophy (44). Zheng
et al (45) suggested that
the occurrence and development of numerous types of diseases can be
prevented and reversed via the role of HDL in preserving
mitochondrial structure and function. Hence, understanding the
mechanism concerning the effect of HDL on mitochondria will be
beneficial for clarifying the pathogenesis of diseases including
metabolic defects and providing new ideas for treatment. Moreover,
HDL serves an important protective role in acute pancreatitis by
inhibiting acinar cell pyroptosis (46). As a type of cell death, pyroptosis
may contribute to initiation, progression, exacerbation and
complications of atherosclerosis associated with activation of
signal transducer, such as STAT3(47). Thus, targeting pyroptosis may be a
treatment method for atherosclerosis. Nevertheless, the
relationships between HDL and mitochondria and pyroptosis are
unclear.
Oxidative stress occurs when there is an imbalance
between production of ROS and the ability of body to neutralize
them; factors including infection can contribute to oxidative
stress (95,96). During infection, immune cells
produce ROS to combat pathogens; excessive or prolonged
inflammation can further increase oxidative stress (97). ROS, highly reactive molecules, can
damage various cellular components including DNA, lipid and
proteins (98). Oxidative stress
triggered by M. pneumoniae infection oxidizes lipid
components of HDL such as phospholipids and cholesterol, which
results in structural changes in the lipoprotein and decreases its
ability to function effectively (99,100).
As a result, the ability of HDL to remove excess cholesterol from
cells and prevent formation of fatty deposits in blood vessels is
impaired. Moreover, oxidized HDL loses anti-inflammatory and
antioxidant functions, hence, it is unable to remove oxidized LDL
and oxidized products existing in cells (101,102). Additionally, oxidized HDL can be
pro-inflammatory and pro-atherogenic, which promotes development of
atherosclerosis through hardening and narrowing of the arteries
(103). Maintaining balance
between antioxidants and ROS is key for preserving integrity and
functionality of HDL. While there is evidence suggesting that
infections influence HDL-related metabolic enzyme activities, more
research is needed to establish a direct link between M.
pneumoniae infection and HDL metabolism (102,103).
The cellular composition of heart, a vital organ,
includes cardiomyocytes, fibroblasts, myofibroblasts and
inflammatory cells such as macrophages (104,105). Myofibroblasts synthesize
extracellular matrix to replace dead cardiomyocytes, however, the
regeneration rate of cardiomyocytes is low (106,107). The heart can work effectively in
with a small number of dead cells, but if the death is wide and
severe, the heart cannot repair itself, resulting in arrhythmia and
heart failure (108,109). The cardiovascular system, also
known as the circulatory system, comprises the heart, arteries,
capillaries and veins, and serves key functions in the maintenance
of normal life activities. Specifically, it can supply organs and
tissues with oxygen and nutrients and transport waste to excretory
organs (110).
Several studies have validated the association
between HDL and cardiovascular risk and considered the cholesterol
in HDL as a key element to predict cardiovascular disease (72,111,112). However, it is uncertain whether
causality between HDL and cardiovascular risk exists. At present,
clinical trials associated with increasing HDL concentration and
promoting HDL function have not yet been completed (103,109,111,113). Further research is needed to
explore treatment methods to changing the metabolism and function
of HDL (113). Additionally, the
molecular mechanism of HDL expression is complicated and may
involve multiple proteins, bioactive lipids and non-coding RNA
(113).
Certain antiphospholipid antibodies such as
anticardiolipin antibody and lupus anticoagulant are found in the
blood of patients with cardiovascular system disease and can be
raised during M. pneumoniae infection through interaction
between M. pneumoniae cell wall components and human
phospholipids (39). However, the
potential mechanism underlying how M. pneumoniae infection
regulates occurrence and development of disease related to the
cardiovascular system is incompletely understood. At present,
direct links between M. pneumoniae infection and long-term
cardiovascular health are not clear (8). While respiratory infections caused by
M. pneumoniae trigger an acute inflammatory response,
whether the association between the infection and cardiovascular
complications result from inflammatory response is unclear
(114). Consequently, identifying
the mechanisms underlying cell death and cardiac damage during
M. pneumoniae infection is of importance.
There are preventive or treatment strategies
proposed to improve prognosis of patients with pneumonia caused by
M. pneumoniae and decrease the incidence of cardiovascular
disease. Statins, angiotensin-converting enzyme (ACE) inhibitors
and angiotensin receptor blockers (ARBs) are commonly used and
effective drugs (8,129,131). Feldman and Anderson (133) demonstrated that the long-term use
of statins contributes to lower incidence of pneumonia and
associated cardiac injury. Moreover, statins inhibit the activity
of β-hydroxy-β-methylglutaryl-CoA reductase to reduce cholesterol
levels and stimulate the liver to uptake LDL from the blood, which
has a notable impact on preventing cardiovascular diseases
especially coronary heart disease (134,135). Alexander et al (136) claimed that ACE inhibitors can
reduce the risk of community-acquired pneumonia hospitalization and
mortality. ACE is involved in generation and regulation of
angiotensin, while ACE inhibitors decrease the generation of
angiotensin II, thus, lowering blood pressure, improving heart
function, and alleviating cardiovascular burden (137,138). ARB can block angiotensin II
receptors, leading to vasodilation and decreased blood pressure
(139). ARBs are commonly
prescribed as a treatment for hypertension (140,141), heart failure (142) and certain kidney conditions
(143). However, the side effects
of these drugs cannot be ignored; for example, statins increase the
death risk of sepsis or ventilator-associated pneumonia (144). Drug selection and combination
therapy are crucial for treatment of diseases de Gomensoro et
al (145) suggested that
immunizations can reduce the risk of major cardiovascular
complications related to infection. With the mutation and outbreak
of influenza, the American Heart Association and American College
of Cardiology has recommended influenza vaccination for patients
with cardiovascular disease such as coronary atherosclerosis
(146). According to Mohseni et
al (147), Behrouzi et
al (148) and Saade et
al (149), influenza
vaccination can reduce cardiovascular complication and
cardiovascular-related mortality. Besides the influenza
vaccination, pneumococcal vaccination also serves a protective role
among high cardiovascular risk populations (150). However, the mechanism and
specificity of vaccines against M. pneumoniae is unclear; it
is an urgent need to clarify whether targeted vaccination decreases
the incidence of cardiovascular complications (151).
Not applicable.
Funding: The present study was supported by Health Commission of
Shanxi Province (grant no. 2020147).
Not applicable.
TS and LK designed the study and wrote the
manuscript. YaL, TL, and YuL reviewed and edited the manuscript.
Data authentication is not applicable. All authors have read and
approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hurst L: Bacteriology. Scientific
e-Resources, 2019.
|
|
2
|
Atkinson TP and Waites KB: Mycoplasma
pneumoniae infections in childhood. Pediatr Infect Dis J.
33:92–94. 2014.
|
|
3
|
Kashyap S and Sarkar M: Mycoplasma
pneumonia: Clinical features and management. Lung India.
27(75)2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li ZJ, Zhang HY, Ren LL, Lu QB, Ren X,
Zhang CH, Wang YF, Lin SH, Zhang XA, Li J, et al: Etiological and
epidemiological features of acute respiratory infections in China.
Nat Commun. 12(5026)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dumke R and Ziegler T: Long-term low rate
of macrolide-resistant Mycoplasma pneumoniae strains in
Germany. Antimicrob Agents Chemother. 63:e00455–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Baseman JB and Tully JG: Mycoplasmas:
Sophisticated, reemerging, and burdened by their notoriety. Emerg
Infect Dis. 3:21–32. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Waites KB and Talkington DF: Mycoplasma
pneumoniae and its role as a human pathogen. Clin Microbiol
Rev. 17:697–728. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Restrepo MI and Reyes LF: Pneumonia as a
cardiovascular disease. Respirology. 23:250–259. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang J, Zhao H, Yuan H, Zhu F and Zhou W:
Prevalence and association of mycoplasma infection in the
development of coronary artery disease. Braz J Biol.
83(e246385)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rezaee-Zavareh MS, Tohidi M, Sabouri A,
Ramezani-Binabaj M, Sadeghi-Ghahrodi M and Einollahi B: Infectious
and coronary artery disease. ARYA Atheroscler.
12(41)2016.PubMed/NCBI
|
|
11
|
Nazir S, Jankowski V, Bender G, Zewinger
S, Rye KA and van der Vorst EPC: Interaction between high-density
lipoproteins and inflammation: Function matters more than
concentration! Adv Drug Deliv Rev. 159:94–119. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee-Rueckert M, Escola-Gil JC and Kovanen
PT: HDL functionality in reverse cholesterol transport-Challenges
in translating data emerging from mouse models to human disease.
Biochim Biophys Acta. 1861:566–583. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Abd El-Aal HAHM: Lipid peroxidation
end-products as a key of oxidative stress: effect of antioxidant on
their production and transfer of free radicals. Submitted: 11
November 2011 Published: 29 August 2012. doi: 10.5772/45944.
|
|
14
|
Tousoulis D, Andreou I, Antoniades C,
Tentolouris C and Stefanadis C: Role of inflammation and oxidative
stress in endothelial progenitor cell function and mobilization:
Therapeutic implications for cardiovascular diseases.
Atherosclerosis. 201:236–247. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Adegbola P, Aderibigbe I, Hammed W and
Omotayo T: Antioxidant and anti-inflammatory medicinal plants have
potential role in the treatment of cardiovascular disease: A
review. Am J Cardiovasc Dis. 7(19)2017.PubMed/NCBI
|
|
16
|
Ouweneel AB and Van Eck M: Lipoproteins as
modulators of atherothrombosis: From endothelial function to
primary and secondary coagulation. Vascul Pharmacol. 82:1–10.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mineo C and Shaul PW: Novel biological
functions of high-density lipoprotein cholesterol. Circ Res.
111:1079–1090. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang J, Xia C, Sharma A, Gaba GS and
Shabaz M: Chest CT findings and differential diagnosis of
Mycoplasma pneumoniae pneumonia and Mycoplasma
pneumoniae combined with streptococcal pneumonia in children. J
Healthc Eng. 2021(8085530)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu J, Ye Y, Chen X, Xiong L, Xie W and Liu
P: Insight into the pathogenic mechanism of Mycoplasma
pneumoniae. Curr Microbiol. 80(14)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jiang Z, Li S, Zhu C, Zhou R and Leung
PHM: Mycoplasma pneumoniae infections: pathogenesis and
vaccine development. Pathogens. 10(119)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rank RG: Role of the immune response, in
Microbiology of chlamydia. CRC Press. pp217-234, 2019.
|
|
22
|
Porsch F, Mallat Z and Binder CJ: Humoral
immunity in atherosclerosis and myocardial infarction: From B cells
to antibodies. Cardiovasc Res. 117:2544–2562. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Stelmach I, Podsiadłowicz-Borzecka M,
Grzelewski T, Majak P, Stelmach W, Jerzyńska J, Popławska M and
Dziadek J: Humoral and cellular immunity in children with
Mycoplasma pneumoniae infection: A 1-year prospective study.
Clin Diagn Lab Immunol. 12:1246–1250. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nutt SL and Huntington ND: Cytotoxic T
lymphocytes and natural killer cells, in Clinical Immunology.
Elsevier. pp247-259.e1, 2019.
|
|
25
|
Zhu X and Zhu J: CD4 T helper cell subsets
and related human immunological disorders. Int J Mol Sci.
21(8011)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Delves PJ and Roitt IM: The immune system.
First of two parts. N Engl J Med. 343:37–49. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rich RR and Chaplin DD: The human immune
response, in Clinical Immunology. Elsevier. pp3-17.e1, 2019.
|
|
28
|
Parija SC: Immune response, in Textbook of
Microbiology and Immunology. Springer. pp211-226, 2023.
|
|
29
|
Takatsu K: Cytokines involved in B-cell
differentiation and their sites of action. Proc Soc Exp Biol Med.
215:121–133. 1997.PubMed/NCBI View Article : Google Scholar
|
|
30
|
de Araújo-Souza PS, Hanschke SC and Viola
JP: Epigenetic control of interferon-gamma expression in CD8 T
cells. J Immunol Res. 2015(849573)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mendoza L: A network model for the control
of the differentiation process in Th cells. Biosystems. 84:101–114.
2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pan W, Wang Q and Chen Q: The cytokine
network involved in the host immune response to periodontitis. Int
J Oral Sci. 11(30)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang J, Hooper WC, Phillips DJ and
Talkington DF: Cytokines in Mycoplasma pneumoniae
infections. Cytokine Growth Factor Rev. 15:157–168. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sahu SK, Kulkarni DH, Ozanturk AN, Ma L
and Kulkarni HS: Emerging roles of the complement system in
host-pathogen interactions. Trends Microbiol. 30:390–402.
2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Noris M and Remuzzi G: Overview of
complement activation and regulation. Semin Nephrol. 33:479–492.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
He J, Liu M, Ye Z, Tan T, Liu X, You X,
Zeng Y and Wu Y: Insights into the pathogenesis of Mycoplasma
pneumoniae (Review). Mol Med Rep. 14:4030–4036. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Esposito S, Argentiero A, Gramegna A and
Principi N: Mycoplasma pneumoniae: A pathogen with unsolved
therapeutic problems. Expert Opin Pharmacother. 22:1193–1202.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Narita M: Pathogenesis of extrapulmonary
manifestations of Mycoplasma pneumoniae infection with
special reference to pneumonia. J Infect Chemother. 16:162–169.
2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Narita M: Classification of extrapulmonary
manifestations due to Mycoplasma pneumoniae infection on the
basis of possible pathogenesis. Front Microbiol.
7(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jomard A and Osto E: High density
lipoproteins: Metabolism, function, and therapeutic potential.
Front Cardiovasc Med. 7(39)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xepapadaki E, Nikdima I, Sagiadinou EC,
Zvintzou E and Kypreos KE: HDL and type 2 diabetes: the chicken or
the egg? Diabetologia. 64:1917–1926. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ben-Aicha S, Badimon L and Vilahur G:
Advances in HDL: Much more than lipid transporters. Int J Mol Sci.
21(732)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ganjali S, Banach M, Pirro M, Fras Z and
Sahebkar A: HDL and cancer-causality still needs to be confirmed?
Update 2020. Semin Cancer Biol. 73:169–177. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chan DC: Mitochondrial dynamics and its
involvement in disease. Annu Rev Pathol. 15:235–259.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zheng A, Li H, Feng Z and Liu J:
Integrative analyses reveal Tstd1 as a potential modulator of HDL
cholesterol and mitochondrial function in mice. Cells.
10(2976)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lu Y, Li B, Wei M, Zhu Q, Gao L, Ma N, Ma
X, Yang Q, Tong Z, Lu G and Li W: HDL inhibits pancreatic acinar
cell NLRP3 inflammasome activation and protect against acinar cell
pyroptosis in acute pancreatitis. Int Immunopharmacol.
125(110950)2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wei Y, Lan B, Zheng T, Yang L, Zhang X,
Cheng L, Tuerhongjiang G, Yuan Z and Wu Y: GSDME-mediated
pyroptosis promotes the progression and associated inflammation of
atherosclerosis. Nat Commun. 14(929)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Webb NR: High-density lipoproteins and
serum amyloid A (SAA). Curr Atheroscler Rep. 23(7)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Malle E and De Beer FC: Human serum
amyloid A (SAA) protein: A prominent acute-phase reactant for
clinical practice. Eur J Clin Invest. 26:427–435. 1996.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ganjali S, Blesso CN, Banach M, Pirro M,
Majeed M and Sahebkar A: Effects of curcumin on HDL functionality.
Pharmacol Res. 119:208–218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bonacina F, Pirillo A, Catapano AL and
Norata GD: HDL In immune-inflammatory responses: Implications
beyond cardiovascular diseases. Cells. 10(1061)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Szabo G, Romics L Jr and Frendl G: Liver
in sepsis and systemic inflammatory response syndrome. Clin Liver
Dis. 6:1045–1066. 2002.PubMed/NCBI View Article : Google Scholar
|
|
53
|
von Eckardstein A, Nordestgaard BG,
Remaley AT and Catapano AL: High-density lipoprotein revisited:
Biological functions and clinical relevance. Eur Heart J.
44:1394–1407. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Khovidhunkit W, Kim MS, Memon RA,
Shigenaga JK, Moser AH, Feingold KR and Grunfeld C: Effects of
infection and inflammation on lipid and lipoprotein metabolism:
Mechanisms and consequences to the host. J Lipid Res. 45:1169–1196.
2004.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Luo H, He J, Qin L, Chen Y, Chen L, Li R,
Zeng Y, Zhu C, You X and Wu Y: Mycoplasma pneumoniae lipids
license TLR-4 for activation of NLRP3 inflammasome and autophagy to
evoke a proinflammatory response. Clin Exp Immunol. 203:66–79.
2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ramos EI, Das K, Harrison AL, Garcia A,
Gadad SS and Dhandayuthapani S: Mycoplasma genitalium and M.
pneumoniae regulate a distinct set of protein-coding genes in
epithelial cells. Front Immunol. 12(738431)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Felder KM, Carranza PM, Gehrig PM,
Roschitzki B, Barkow-Oesterreicher S, Hoelzle K, Riedel K, Kube M
and Hoelzle LE: Insights into the gene expression profile of
uncultivable hemotrophic Mycoplasma suis during acute infection,
obtained using proteome analysis. J Bacteriol. 194:1505–1514.
2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Schmidl SR, Otto A, Lluch-Senar M, Piñol
J, Busse J, Becher D and Stülke J: A trigger enzyme in
Mycoplasma pneumoniae: Impact of the
glycerophosphodiesterase GlpQ on virulence and gene expression.
PLoS Pathog. 7(e1002263)2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sharma NK, Ferreira BL, Tashima AK,
Brunialti MKC, Torquato RJS, Bafi A, Assuncao M, Azevedo LCP and
Salomao R: Lipid metabolism impairment in patients with sepsis
secondary to hospital acquired pneumonia, a proteomic analysis.
Clin Proteomics. 16(29)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Trinder M, Genga KR, Kong HJ, Blauw LL, Lo
C, Li X, Cirstea M, Wang Y, Rensen PCN, Russell JA, et al:
Cholesteryl ester transfer protein influences high-density
lipoprotein levels and survival in sepsis. Am J Respir Crit Care
Med. 199:854–862. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Adorni MP, Ronda N, Bernini F and Zimetti
F: High density lipoprotein cholesterol efflux capacity and
atherosclerosis in cardiovascular disease: Pathophysiological
aspects and pharmacological perspectives. Cells.
10(574)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ahn KK, Kwon D, Jung K, Ha Y, Seo MJ, Kim
SH, Kim MY, Cho KD, Lee BH and Chae C: Identification of
interleukin-1, tumor necrosis factor-α, and interleukin-6
expression in lungs from pigs naturally infected with Mycoplasma
hyopneumoniae by in situ hybridization. J Vet Med Sci. 71:441–445.
2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Su X, Zhang G, Cheng Y and Wang B: New
insights into the emerging effects of inflammatory response on HDL
particles structure and function. Mol Biol Rep. 48:5723–5733.
2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Murphy AJ, Woollard KJ, Hoang A,
Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D
and Chin-Dusting J: High-density lipoprotein reduces the human
monocyte inflammatory response. Arterioscler Thromb Vasc Biol.
28:2071–2077. 2008.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Udomkarnjananun S, Takkavatakarn K,
Praditpornsilpa K, Nader C, Eiam-Ong S, Jaber BL and Susantitaphong
P: Hepatitis B virus vaccine immune response and mortality in
dialysis patients: a meta-analysis. J Nephrol. 33:343–354.
2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Murgia F, Giagnoni F, Lorefice L, Caria P,
Dettori T, D'Alterio MN, Angioni S, Hendren AJ, Caboni P, Pibiri M,
et al: Sex hormones as key modulators of the immune response in
multiple sclerosis: A review. Biomedicines. 10(3107)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Li J, Song CL, Wang T, Ye YL, Du JR, Li SH
and Zhu JM: Etiological and epidemiological characteristics of
severe acute respiratory infection caused by multiple viruses and
Mycoplasma pneumoniae in adult patients in Jinshan,
Shanghai: A pilot hospital-based surveillance study. PLoS One.
16(e0248750)2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Blasi F: Atypical pathogens and
respiratory tract infections. Eur Respir J. 24:171–182.
2004.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wang HH, Garruti G, Liu M, Portincasa P
and Wang DQ: Cholesterol and lipoprotein metabolism and
atherosclerosis: Recent advances in reverse cholesterol transport.
Ann Hepatol. 16:27–42. 2018.
|
|
70
|
Higuchi ML, Sambiase N, Palomino S,
Gutierrez P, Demarchi LM, Aiello VD and Ramires JA: Detection of
Mycoplasma pneumoniae and chlamydia pneumoniae in ruptured
atherosclerotic plaques. Braz J Med Biol Res. 33:1023–1026.
2000.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kumar V: Pulmonary innate immune response
determines the outcome of inflammation during pneumonia and
sepsis-associated acute lung injury. Front Immunol.
11(1722)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rosenson RS, Brewer HB Jr, Ansell BJ,
Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR and Webb NR:
Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat
Rev Cardiol. 13:48–60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Yu H: HDL and scavenger receptor class B
Type I (SRBI). Adv Exp Med Biol. 1377:79–93. 2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Esteve E, Ricart W and Fernández-Real JM:
Dyslipidemia and inflammation: An evolutionary conserved mechanism.
Clin Nutr. 24:16–31. 2005.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Tan M, Ye J, Zhao M, Ke X, Huang K and Liu
H: Recent developments in the regulation of cholesterol transport
by natural molecules. Phytother Res. 35:5623–5633. 2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Tosheska Trajkovska K and Topuzovska S:
High-density lipoprotein metabolism and reverse cholesterol
transport: Strategies for raising HDL cholesterol. Anatol J
Cardiol. 18(149)2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Baranova IN, Bocharov AV, Vishnyakova TG,
Chen Z, Birukova AA, Ke Y, Hu X, Yuen PST, Star RA, Birukov KG, et
al: Class B scavenger receptors BI and BII protect against
LPS-Induced acute lung injury in mice by mediating LPS. Infect
Immun. 89(e0030121)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Webb NR, Connell PM, Graf GA, Smart EJ, de
Villiers WJ, de Beer FC and van der Westhuyzen DR: SR-BII, an
isoform of the scavenger receptor BI containing an alternate
cytoplasmic tail, mediates lipid transfer between high density
lipoprotein and cells. J Biol Chem. 273:15241–15248.
1998.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Glatz JFC and Luiken JJFP: Dynamic role of
the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid
uptake and utilization. J Lipid Res. 59:1084–1093. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Catapano AL, Pirillo A, Bonacina F and
Norata GD: HDL in innate and adaptive immunity. Cardiovasc Res.
103:372–383. 2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Meyer Sauteur PM, Theiler M, Buettcher M,
Seiler M, Weibel L and Berger C: Frequency and clinical
presentation of mucocutaneous disease due to Mycoplasma
pneumoniae infection in children with community-acquired
pneumonia. JAMA Dermatol. 156:144–150. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Segovia JA, Chang TH, Winter VT, Coalson
JJ, Cagle MP, Pandranki L, Bose S, Baseman JB and Kannan TR: NLRP3
is a critical regulator of inflammation and innate immune cell
response during Mycoplasma pneumoniae infection. Infect
Immun. 86:e00548–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Rafieian-Kopaei M, Setorki M, Doudi M,
Baradaran A and Nasri H: Atherosclerosis: Process, indicators, risk
factors and new hopes. Int J Prev Med. 5(927)2014.PubMed/NCBI
|
|
84
|
Grao-Cruces E, Lopez-Enriquez S, Martin ME
and Montserrat-de la Paz S: High-density lipoproteins and immune
response: A review. Int J Biol Macromol. 195:117–123.
2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Pirillo A, Catapano AL and Norata GD: HDL
in infectious diseases and sepsis. Handb Exp Pharmacol.
224:483–508. 2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Stasi A, Franzin R, Fiorentino M,
Squiccimarro E, Castellano G and Gesualdo L: Multifaced roles of
HDL in sepsis and SARS-CoV-2 infection: Renal implications. Int J
Mol Sci. 22(5980)2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Feingold KR: The bidirectional link
between HDL and COVID-19 infections. J Lipid Res.
62(100067)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Shimamoto T, Yamamichi N, Gondo K,
Takahashi Y, Takeuchi C, Wada R, Mitsushima T and Koike K: The
association of Helicobacter pylori infection with serum lipid
profiles: An evaluation based on a combination of meta-analysis and
a propensity score-based observational approach. PLoS One.
15(e0234433)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Ossoli A, Pavanello C and Calabresi L:
High-density lipoprotein, lecithin: Cholesterol acyltransferase,
and atherosclerosis. Endocrinol Metab (Seoul). 31:223–229.
2016.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Reisinger AC, Schuller M, Sourij H,
Stadler JT, Hackl G, Eller P and Marsche G: Impact of sepsis on
high-Density lipoprotein metabolism. Front Cell Dev Biol.
9(795460)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Säemann MD, Poglitsch M, Kopecky C,
Haidinger M, Hörl WH and Weichhart T: The versatility of HDL: A
crucial anti-inflammatory regulator. Eur J Clin Invest.
40:1131–1143. 2010.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Chen L, Zhao ZW, Zeng PH, Zhou YJ and Yin
WJ: Molecular mechanisms for ABCA1-mediated cholesterol efflux.
Cell Cycle. 21:1121–1139. 2022.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Zannis VI, Chroni A and Krieger M: Role of
apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med.
84:276–294. 2006.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Jacobo-Albavera L, Domínguez-Pérez M,
Medina-Leyte DJ, González-Garrido A and Villarreal-Molina T: The
role of the ATP-binding cassette A1 (ABCA1) in human disease. Int J
Mol Sci. 22(1593)2021.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Yin K, Liao DF and Tang CK: ATP-binding
membrane cassette transporter A1 (ABCA1): A possible link between
inflammation and reverse cholesterol transport. Mol Med.
16:438–449. 2010.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Bhattacharya S: Reactive oxygen species
and cellular defense system. Free Radicals in human health and
disease, pp17-29, 2015.
|
|
97
|
Lauridsen C: From oxidative stress to
inflammation: Redox balance and immune system. Poult Sci.
98:4240–4246. 2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Juan CA, Pérez de la Lastra JM, Plou FJ
and Pérez-Lebeña E: The chemistry of reactive oxygen species (ROS)
revisited: Outlining their role in biological macromolecules (DNA,
lipids and proteins) and induced pathologies. Int J Mol Sci.
22(4642)2021.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Zhang X, Zhou Y, Tang H, Zhao D and Liu F:
Immunosuppression reduces lung injury caused by Mycoplasma
pneumoniae infection. Sci Rep. 9(7147)2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Tabet F and Rye KA: High-density
lipoproteins, inflammation and oxidative stress. Clin Sci (Lond).
116:87–98. 2009.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Zhang Q, Jiang Z and Xu Y: HDL and
Oxidation, in HDL Metabolism and Diseases. Springer pp63-77,
2022.
|
|
102
|
Podrez EA: Anti-oxidant properties of
high-density lipoprotein and atherosclerosis. Clin Exp Pharmacol
Physiol. 37:719–725. 2010.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Seidman MA, Mitchell RN and Stone JR:
Pathophysiology of atherosclerosis, in Cellular and molecular
pathobiology of cardiovascular disease. Elsevier pp221-237,
2014.
|
|
104
|
Lafuse WP, Wozniak DJ and Rajaram MVS:
Role of cardiac macrophages on cardiac inflammation, fibrosis and
tissue repair. Cells. 10(51)2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Dobaczewski M, Gonzalez-Quesada C and
Frangogiannis NG: The extracellular matrix as a modulator of the
inflammatory and reparative response following myocardial
infarction. J Mol Cell Cardiol. 48:504–511. 2010.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Kurose H: Cardiac fibrosis and
fibroblasts. Cells. 10(1716)2021.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Laflamme MA and Murry CE: Heart
regeneration. Nature. 473:326–335. 2011.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Patel P and Karch J: Regulation of cell
death in the cardiovascular system. Int Rev Cell Mol Biol.
353:153–209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
European Heart Rhythm Association; Heart
Rhythm Society. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman
B, Fromer M, Gregoratos G, Klein G, et al: ACC/AHA/ESC 2006
guidelines for management of patients with ventricular arrhythmias
and the prevention of sudden cardiac death: A report of the
American College of Cardiology/American Heart Association Task
Force and the European Society of Cardiology Committee for Practice
Guidelines (Writing Committee to Develop Guidelines for Management
of Patients With Ventricular Arrhythmias and the Prevention of
Sudden Cardiac Death). J Am Coll Cardiol. 48:e247–e346.
2006.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Aaronson PI, Ward JP and Connolly MJ: The
cardiovascular system at a glance. John Wiley & Sons, 2020.
|
|
111
|
Nicholls SJ and Nelson AJ: HDL and
cardiovascular disease. Pathology. 51:142–147. 2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Giammanco A, Noto D, Barbagallo CM, Nardi
E, Caldarella R, Ciaccio M, Averna MR and Cefalù AB:
Hyperalphalipoproteinemia and beyond: The role of HDL in
cardiovascular diseases. Life. 11(581)2021.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Rohatgi A, Westerterp M, von Eckardstein
A, Remaley A and Rye KA: HDL in the 21st century: A multifunctional
roadmap for future HDL research. Circulation. 143:2293–2309.
2021.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Lee KY: Pediatric respiratory infections
by Mycoplasma pneumoniae. Expert Rev Anti Infect Ther.
6:509–521. 2008.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Pothineni NVK, Subramany S, Kuriakose K,
Shirazi LF, Romeo F, Shah PK and Mehta JL: Infections,
atherosclerosis, and coronary heart disease. Eur Heart J.
38:3195–3201. 2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Dickson BC and Gotlieb AI: Towards
understanding acute destabilization of vulnerable atherosclerotic
plaques. Cardiovasc Pathol. 12:237–248. 2003.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Halvorsen B, Otterdal K, Dahl TB,
Skjelland M, Gullestad L, Øie E and Aukrust P: Atherosclerotic
plaque stability-what determines the fate of a plaque? Prog
Cardiovasc Dis. 51:183–194. 2008.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Li B, Xia Y and Hu B: Infection and
atherosclerosis: TLR-dependent pathways. Cell Mol Life Sci.
77:2751–2769. 2020.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Espinola-Klein C, Rupprecht HJ,
Blankenberg S, Bickel C, Kopp H, Victor A, Hafner G, Prellwitz W,
Schlumberger W and Meyer J: Impact of infectious burden on
progression of carotid atherosclerosis. Stroke. 33:2581–2586.
2002.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Husain K, Hernandez W, Ansari RA and
Ferder L: Inflammation, oxidative stress and renin angiotensin
system in atherosclerosis. World J Biol Chem. 6:209–217.
2015.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Badimon L and Vilahur G: Thrombosis
formation on atherosclerotic lesions and plaque rupture. J Intern
Med. 276:618–632. 2014.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Libby P: The changing landscape of
atherosclerosis. Nature. 592:524–533. 2021.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Krafft C and Christy C: Mycoplasma
pneumonia in children and adolescents. Pediatr Rev. 41:12–19.
2020.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Lewes D, Rainford DJ and Lane WF:
Symptomless myocarditis and myalgia in viral and Mycoplasma
pneumoniae infections. Br Heart J. 36(924)1974.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Zhao Y, Ma G and Yang X: HDAC5 promotes
Mycoplasma pneumoniae-induced inflammation in macrophages
through NF-κB activation. Life Sci. 221:13–19. 2019.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Bays HE, Taub PR, Epstein E, Michos ED,
Ferraro RA, Bailey AL, Kelli HM, Ferdinand KC, Echols MR, Weintraub
H, et al: Ten things to know about ten cardiovascular disease risk
factors. Am J Prev Cardiol. 5(100149)2021.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Buttar HS, Li T and Ravi N: Prevention of
cardiovascular diseases: Role of exercise, dietary interventions,
obesity and smoking cessation. Exp Clin Cardiol. 10:229–249.
2005.PubMed/NCBI
|
|
128
|
Zhu Y, Chunmei L, Paihe D, Jianping W,
Xiaowei W and Weihua M: Epidemiological investigation and analysis
of Mycoplasma pneumoniae infection. Nanoscie Nanotechnol
Lett. 11:424–427. 2019.
|
|
129
|
Atkinson TP, Balish MF and Waites KB:
Epidemiology, clinical manifestations, pathogenesis and laboratory
detection of Mycoplasma pneumoniae infections. FEMS
Microbiol Rev. 32:956–973. 2008.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Guillon A, Arafa EI, Barker KA, Belkina
AC, Martin I, Shenoy AT, Wooten AK, Lyon De Ana C, Dai A, Labadorf
A, et al: Pneumonia recovery reprograms the alveolar macrophage
pool. JCI Insight. 5(e133042)2020.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Watson A and Wilkinson TMA: Respiratory
viral infections in the elderly. Ther Adv Respir Dis.
15(1753466621995050)2021.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Lv YT, Sun XJ, Chen Y, Ruan T, Xu GP and
Huang JA: Epidemic characteristics of Mycoplasma pneumoniae
infection: A retrospective analysis of a single center in Suzhou
from 2014 to 2020. Ann Transl Med. 10(1123)2022.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Feldman C and Anderson R: Anderson,
Prevalence, pathogenesis, therapy, and prevention of cardiovascular
events in patients with community-acquired pneumonia. Pneumonia
(Nathan). 8(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Lee M and Lee BC: Statins. Stroke
Revisited: Dyslipidemia in Stroke. pp77-89, 2021.
|
|
135
|
Das UN: Essential fatty acids and their
metabolites could function as endogenous HMG-CoA reductase and ACE
enzyme inhibitors, anti-arrhythmic, anti-hypertensive,
anti-atherosclerotic, anti-inflammatory, cytoprotective, and
cardioprotective molecules. Lipids Health Dis. 7(37)2008.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Alexander DP, Nickman NA, Chhibber A,
Stoddard GJ, Biskupiak JE and Munger MA: Angiotensin-converting
enzyme inhibitors reduce community-acquired pneumonia
hospitalization and mortality. Pharmacotherapy. 42:890–897.
2022.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Bakhle Y: How ACE inhibitors transformed
the renin-angiotensin system. Br J Pharmacol. 177:2657–2665.
2020.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Belova L: Angiotensin II-generating
enzymes. Biochemistry (Moscow). 65:1337–1345. 2000.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Javanmard SH, Heshmat-Ghahdarijani K and
Vaseghi G: Angiotensin-converting-enzyme inhibitors (ACE
inhibitors) and angiotensin II receptor blocker (ARB) use in
COVID-19 prevention or treatment: A paradox. Infection Control Hosp
Epidemiol. 42:118–119. 2021.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Siragy H: Angiotensin II receptor
blockers: Review of the binding characteristics. Am J Cardiol.
84:3–8. 1999.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Hjermitslev M, Grimm DG, Wehland M,
Simonsen U and Krüger M: Azilsartan medoxomil, an angiotensin II
receptor antagonist for the treatment of hypertension. Basic Clin
Pharmacol Toxicol. 121:225–233. 2017.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Dasgupta C and Zhang L: Angiotensin II
receptors and drug discovery in cardiovascular disease. Drug Discov
Today. 16:22–34. 2011.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Kobori H, Mori H, Masaki T and Nishiyama
A: Angiotensin II blockade and renal protection. Curr Pharm Design.
19:3033–3042. 2013.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Sibila O, Restrepo MI and Anzueto A: What
is the best antimicrobial treatment for severe community-acquired
pneumonia (including the role of steroids and statins and other
immunomodulatory agents). Infectious Dis Clin. 27:133–147.
2013.PubMed/NCBI View Article : Google Scholar
|
|
145
|
de Gomensoro E, Del Giudice G and Doherty
TM: Challenges in adult vaccination. Ann Med. 50:181–192.
2018.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Warren-Gash C, Smeeth L and Hayward AC:
Influenza as a trigger for acute myocardial infarction or death
from cardiovascular disease: A systematic review. Lancet Infect
Dis. 9:601–610. 2009.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Mohseni H, Kiran A, Khorshidi R and Rahimi
K: Influenza vaccination and risk of hospitalization in patients
with heart failure: A self-controlled case series study. Eur Heart
J. 38:326–333. 2017.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Behrouzi B, Bhatt DL, Cannon CP, Vardeny
O, Lee DS, Solomon SD and Udell JA: Association of influenza
vaccination with cardiovascular risk: A meta-analysis. JAMA Netw
Open. 5(e228873)2022.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Saade EA, Abul Y, McConeghy K, Edward
Davidson H, Han L, Joyce N, Canaday DH, Hsueh L, Bosco E and
Gravenstein S: High-dose influenza vaccines for the prevention of
hospitalization due to cardiovascular events in older adults in the
nursing home: Post-hoc analysis of a cluster-randomized trial.
Vaccine. 40:6700–6705. 2022.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Jaiswal V, Ang SP, Lnu K, Ishak A, Pokhrel
NB, Chia JE, Hajra A, Biswas M, Matetic A, Dhatt R and Mamas MA:
Effect of pneumococcal vaccine on mortality and cardiovascular
outcomes: A systematic review and meta-analysis. J Clin Med.
11(3799)2022.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Mahmood M, Javaid A, Shahid F and Ashfaq
UA: Rational design of multimeric based subunit vaccine against
Mycoplasma pneumonia: Subtractive proteomics with immunoinformatics
framework. Infect Genet Evol. 91(104795)2021.PubMed/NCBI View Article : Google Scholar
|