1. Introduction
According to the World Health Organization (WHO),
60% of individuals worldwide utilize herbal medicines and 80% of
those living in developing countries rely almost solely on them to
meet their basic medical needs (1).
Physalis angulata Linn. (P. angulata L.) was first
identified and noted in the flora of Libya. P. angulata L.
is a member of the Solanaceae plant (or Nightshade) family
and is widely found in both tropical and subtropical regions. The
Greek word ‘physalis’, which translates to ‘bladder’, is used to
describe the inflated calyx. Popular names for P. angulata
L. include camapu, cutleaf groundcherry, wild tomato, winter
cherry, cow pops, Chinese lantern, mullaca, koropo (in Western
Africa), wild gooseberry and ciplukan (in Indonesia) (2). The extracts or infusions of this plant
are used as antimalarial, anti-asthmatic and for dermatitis
treatments. In addition, in vitro tests have demonstrated
that the extracted phytoconstituents from P. angulata L.
have an anticancer effect against numerous cancer cell lines (Y79,
HeLa, DLD-1, MCF-7 and HGC-27). Furthermore, P. angulata L.
has been employed for a long time as an antipyretic in Japan
(3). In traditional Chinese
medicine, P. angulata L., a species that is widely spread in
the east and southwest areas of China, is frequently used for
antipyretic, anti-inflammatory and diuretic purposes (4).
Tropical Indonesia is home to a large number of
medicinal plants and P. angulata L. grows wild on the slopes
of Mount Kelud in East Java, as well as commercially in Mersi,
Purwokerto, Central Java and a few locations in West Java (5). Ciplukan (P. angulata L.) has
long been used as a traditional medicine to treat a variety of
ailments, including body aches, asthma, diabetes, chickenpox, cough
medication, fever, diarrhea, hypertension and back pain (6).
Over the past 10 years, the benefits of P.
angulata L. as a medicinal plant have been demonstrated both
in vitro and in vivo, with research regarding the
antibacterial, anticancer, antiparasitic, anti-inflammatory,
antifibrotic and antidiabetic properties of P. angulata L.
conducted (Table I).
 | Table IResearch list of Physalis
angulata Linn. as a herbal medicine from 2012 to 2022. |
Table I
Research list of Physalis
angulata Linn. as a herbal medicine from 2012 to 2022.
| Type | No. | Author's/Year | Part | Solvent | Methods | Organism/Organ
test | Dosage | Results | (Refs.) |
|---|
| Antibacterial | 1 | Rivera et
al, 2015 | Calyces | Ethanol 96% | In
vitro | S. aureus
K. pneumoniae P. aeruginosa | 50 µl (1,000
mcg/ml) | Klebsiella
pneumoniae (MIC 94.05±1.94) Staphylococcus aureus (MIC
96.57±1.69) Pseudomonas aeruginosa (MIC 96.87±0.50) | (7) |
| | 2 | Hananto et
al, 2021 | Whole plant | Ethanol 70% | In
vitro | S.
aureus | 20 mg/ml | Staphylococcus
aureus (Zone of Inhibition 17.00±0.0 mm) | (8) |
| | 3 | Pillai et
al, 2022 | Leaves | Petroleum
ether | In
vitro | E. coli
S. aureus | 100 µl-25
mg/ml | Escherichia
coli (MBC=5 mg/ml; MIC 10 mg/ml); Staphylococcus aureus
(MBC=5 mg/ml; MIC 10 mg/ml) | (3) |
| | | | | Ethyl
acetoacetate | | | | Escherichia
coli (MBC=1.25 mg/ml; MIC 2.5 mg/ml); Staphylococcus
aureus (MBC=1.25 mg/ml; MIC 2.5 mg/ml) | |
| | | | | Ethanol | | | | Escherichia
coli (MBC=5 mg/ml; MIC 10 mg/ml); Staphylococcus aureus
(MBC=2.5 mg/ml; MIC 5 mg/ml) | |
| | | | Fruits | Petroleum
Ether | | | 100 µl- 25
mg/ml | Escherichia
coli (MBC=1.25 mg/ml; MIC 2.5 mg/ml) Staphylococcus
aureus (MBC=5 mg/ml; MIC 10 mg/ml); | |
| | | | | Ethyl
Acetoacetate | | | | Escherichia
coli (MBC=1.25 mg/ml; MIC 2.5 mg/ml) Staphylococcus
aureus (MBC=5 mg/ml; MIC 10 mg/ml); | |
| | | | | Ethanol | | | | Escherichia
coli (MBC=5 mg/ml; MIC 10 mg/ml) Staphylococcus aureus
(MBC=5 mg/ml; MIC 10 mg/ml); | |
| | 4 | Dias et al,
2020 | Leaves | Aqueous | In
vitro | S. aureus
L. monocytogenes | 50 µl (50
mg/ml) | Cultivated leaf
extract obtained by decoction: Staphylococcus aureus (agar
diffusion Inhibition zone 13 mm) Listeria monocytogenes
(agar diffusion Inhibition zone 18 mm) Native leaf extract obtained
by decoction: Staphylococcus aureus (agar diffusion
Inhibition zone 8 mm) Listeria monocytogenes (agar diffusion
Inhibition zone 14 mm) | (10) |
| | 5 | Gagare et
al, 2021 | Leaves | Water | In
vitro | S. aureus
P. aeruginosa | 25 µl | Staphylococcus
aureus (agar diffusion Inhibition zone 4 mm) Pseudomonas
aeruginosa (agar diffusion Inhibition zone 2 mm) Escherichia
coli (agar diffusion Inhibition zone 2 mm) | (9) |
| | 6 | Cuong et al,
2020 | Whole plant | Dichlorome- thane
extract | In
vitro | S. aureus
B. subtilis E. coli E. faecalis B.
cereus | | Physalin B: S.
aureus, B. subtilis, E. coli (MIC 128, 64, 32
µg/ml) Physalin D: S. aureus, E. faecalis, B.
subtilis, B. cereus, E. coli (MIC 64, 64, 128,
128, 64 µg/ml) Physalin F: S. aureus, B. subtilis,
B. cereus, E. coli (MIC @ 128 µg/ml) Physalin G :
- | (11) |
| Anticancer | 1 | Pillai et
al, 2022 | Leaves | Ethanolic
extract | In
vitro | DLD-1, HeLa, and
MCF-7 cell lines | 100 mcg/ml | The percentage
viability of Physalis angulata leaf extracts at 100 µg/ml
was observed at 46.23, 33.66, and 51.54 for DLD-1, HeLa, and MCF-7
cell lines, respectively. The leaf extract LC50 values were 90, 44,
and 100 µg/ml for DLD-1, Hela, and MCF-7 cell lines,
respectively. | (3) |
| | | | Fruit | | In
vitro | DLD-1, HeLa, and
MCF-7 cell lines | | The percentage
viability of Physalis angulata fruit extracts at 100 µg/ml
was observed 70, 69.41, and 65.27 for DLD-1, HeLa, and MCF-7 cell
lines, respectively. The fruit extracts LC50 values were 188, 167,
and 157 µg/ml for DLD-1, HeLa, and MCF-7 cell lines,
respectively. | |
| | 2 | Chairissy et
al, 2019 | Leaves | Ethanol | In
vitro | retinoblastoma
cells | 25 µg/ml, 50 µg/ml,
100 µg/ml | Apoptosis 25 µg/ml
1.06±0.31, 50 µg/ml 1.33±0.17, and 100 µg/ml 1.54±0.34
Proliferation 25 µg/ml 87.84±1.01, 50 µg/ml 86.77±1.75, and 100
µg/ml 84.80±1.01 | (13) |
| | 3 | Fang et al,
2021 | Whole plant | Ethanol | In
vitro | HGC-27 cell | 2 µM, 5 µM, 10 µM,
20 µM | IC50 9
µM, G0/G1 phase ratio ↑, G2/M phase ↓, p-CHK2 ↑, cyclin D1 ↓,
cyclin D3↓, CDK4↓, CDK6 ↓ and cyclin E ↓, p-Rb (Ser780) ↓, p-Rb
(Ser795) ↓. Apoptosis 5 µM 18.0±1.0%, 10 µM 36.9±3.7%, and 20
µM 40.6% ±4.8% Caspase 8, 3, 7, PARP ↑ | (17) |
| Antiparasitic | 1 | Silva et al,
2015 | Roots | Aqueous
extract | In
vitro | Leishmania
amazonensis | 25 µg/ml, 50 µg/ml,
100 µg/ml | L.
amazonensis amastigotes IC50: 43.3±10.1 µg/ml L.
amazonensis promastigotes IC50: 39.5±5.1 µg/ml | (23) |
| | 2 | Meira et al,
2013 | Whole plant | Ethanolic
extracts | In
vitro | Trypanosoma
cruzi | | T. cruzi
epimastigotes IC50 5.3-5.8±1.5-1.9 µM T. cruzi
trypomastigotes IC50: 0.68-0.84±0.001-0.004 µM | (20) |
| | 3 | Nogueira et
al, 2013 | Stem | Ethanolic
extracts | In
vitro | Leismania
amazonensis, Leishmania braziliensis | 1.2-100 µg/ml | L.
amazonensis IC50: 5.35±2.50 µg/ml L.
braziliensis IC50: 4.50±1.17 µg/ml | (21) |
| Anti-
inflammatory | 1 | Santo et al,
2019 | Whole plant | Ethanolic
extracts | In vivo | Paw edema | 50 mg/kg and 100
mg/kg | Paw edema ↓, TNF-α
↓, IL-1β↓, COX-2↓, iNOS ↓ | (32) |
| | 2. | Rivera et
al, 2020 | Calyces | Dichlorome- thane
fraction | In vivo | Intestinal
inflammation | 5 and 10 mg/kg | MPO ↓, IL-1β ↓,
TNF-α↓, IL 10 ↑, | (7) |
| | 3. | Arruda et
al, 2021 | Whole plant | Ethanolic
extract | In vivo
& in vitro | Acute lung injury
& HEK293 | | IL-1β↓, TNF-α ↓
IC50 value IL-1β release: Physalin B 0.072±0.011, Physalin D
0.004±0.0008, Physalin F 0.023±0.001, Physalin G 0.015±0.017
IC50 value TNF-α release: Physalin B 0.089±0.019,
Physalin D 0.068±0.09, Physalin F 0.085±0.16, Physalin G
0.138±0.025. | (34) |
| | 4. | Junior et
al, 2014 | Stem | Ethanolic
extract | In vivo | Intestinal
inflammation | 25, 50, 100
mg/kg | MPO↓, ALP activity
↓, IFN-γ ↓, IL-6 ↓, Hsp 70↓, Mapk 3↓, Mapk 9↓, Muc 1↓, Muc2↓, Hpse
expression ↓, edema ↓ | (33) |
| | 5. | Rivera et
al, 2018 | Calyces | Ethanolic
extracts | In vivo | Acute ear
edema | | MPO ↓, edema↓, NO↓,
PGE2 ↓, IL-6 ↓, IL-1β ↓, TNF-α ↓, CCL-2↓ | (7) |
| | 6. | Pereda et
al, 2018 | Aerial Parts | Supercritical CO2
Extraction | In vitro
& Clinical trial | Normal human
epidermal kera- tinocytes, dermal fibroblast, | | TNF α↓, IL-1α ↓,
IL-6 ↓, COX-2 ↓, LOX ↓, Phospholipase A2 ↓, PGE2 ↓, LTB4↓, Histamin
↓, NF-kB ↓ | (53) |
| | 7. | Wang et al,
2021 | Stem &
Leaves | Ethanolic
Extract | In
vitro | RAW 264.7 | | NO ↓, PGE2 ↓, IL-6
↓, TNF α ↓, iNOS↓, COX-2 ↓, NFkB↓ | (16) |
| | 8. | Yen et al,
2019 | Whole plant | Methanolic extract
(Dichlorome- thane fractioned) | In
vitro | RAW 264.7 | | NO ↓ | (27) |
| | 9. | Ukwubile, 2016 | Leaves | Methanolic
extract | In vivo | Paw edema | 400 mg/kg | Mean inhibition
62,71 % | (30) |
| | 10 | Abdul-Nasir- Deen
et al, 2020 | Leaves | Methanolic
extract | In vivo | Paw edema | 30, 100, 300
mg/kg | Prophylactic (2H):
Mean inhibition 64.08±1.75, 60.91±0.62, and 59.12±3.34% Therapeutic
(6H): 89.93±2.47, 82.14±1.14, and 77.48±2.61% | (31) |
| | 11 | Yang et al,
2017 | Stem and Aerial
parts | Methanolic
extract | In
vitro | RAW 264.7 | | TNF-α ↓, IL-6 ↓,
NFkB ↓, | (26) |
| | 12 | Anh et al,
2020 | Whole plants | Methanolic
extract | In
vitro | RAW 264.7 | | INOS ↓, COX-2
↓ | (28) |
| Antifibrotic | 1 | Zhu et al,
2021 | Calyces | Ethanolic
extracts | In vivo
& in vitro | Liver fibrosis HSC
cell | | COL1A1 ↓, αSMA ↓,
TGFβ1 ↓, TIMP-1 ↓ ALT ↑, AST ↑, Fibrous collagen deposition ↓,
fibroplasia ↓, bridging fibrosis ↓, Hydroxypro- line ↑, GLI 1 ↓
HHIP ↓, Cyclin D ↓, Cyclin E ↓, C-MYC ↓ | (37) |
| | 2 | Rohmawaty et
al, 2021 | Aerial parts | Ethanolic
extract | In vivo | Liver fibrosis | 1.11 mg & 2.22
mg | ALT ↓, Histological
fibrosis score ↓, | (36) |
| | 3 | Dewi et al,
2019 | Aerial parts | Ethanolic
extract | Clinical trial | Skin fibrosis | 3x250 mg | MRSS score ↓, PINP
↓ | (38) |
| Antidiabetic | 1 | Raju et al,
2015 | Fruits | Methanolic
extract | In vivo | DM alloxan
induced | 25 & 50
mg/kg | Blood sugar level
↓ | (40) |
| | 2 | Reddy et al,
2014 | Roots | Methanolic
extract | In vivo | DM streptozo- tocin
induced | | Serum glucose level
↓, Triglyceride ↓, Total Cholesterol ↓, VLDL ↓, LDL ↓, SGOT ↓, SGPT
↓, MDA ↓ | (41) |
2. Antibacterial properties
Finding innovative, safer and more cost-effective
treatments that can address the issue of antibiotic resistance has
driven research on the antibacterial properties of P.
angulata L. and its components. Research is ongoing to
determine the impacts of P. angulata L. against a variety of
Gram-positive and Gram-negative bacteria.
P. angulata L. is widely used as a
traditional medicine in Southeast Asian and North and South
American countries, but studies related to in vivo
antibacterial activity in mice and humans have not yet, to the best
of our knowledge, been conducted (2). An ethanol extract of P.
angulata L. calyces suppressed the growth of Staphylococcus
aureus, Klebsiella pneumoniae and Pseudomonas
aeruginosa (7). The growth of
S. aureus was also suppressed by an ethanol extract of P.
angulata L. fruit (8). In
addition, P. angulata L. leaf aqueous extract demonstrated
activity against S. aureus (9) and an aqueous extract of the aerial
parts was effective [minimum inhibitory concentration (MIC) 2.5
mg/ml)] against S. aureus and Listeria monocytogenes
(10). In 2020, Cuong et al
(11) studied four secosteroids,
namely physalin B, D, F and G, obtained from a P. angulata
dichloromethane extract. Physalin B was found to have antibacterial
activity against S. aureus, Bacillus subtilis and
Escherichia coli (E. coli), with MIC values ranging from 32
to 128. Physalin D exhibited antibacterial activity against S.
aureus, B. subtilis, Bacillus cereus and E.
coli, with MIC values ranging from 64 to 128. Physalin F had a
MIC of 128 against S. aureus, B. subtilis, B.
cereus and E. coli, whereas physalin G exhibited no
antimicrobial activity against any of the microorganisms
tested.
The unknown mechanism of action of P.
angulata L. is a potential avenue for future research. In the
future, P. angulata L. could be investigated for
broad-spectrum or narrow-spectrum antibacterial activity.
Preclinical tests are also required to evaluate the role of P.
angulata L. as an antibacterial agent with a more complex
mechanism. Secondary metabolites can alter bacterial cell membrane
functions and structure, impact intermediary metabolism, disrupt
DNA/RNA synthesis and function, interfere with normal cell
communication (quorum sensing) and trigger cytoplasmic coagulation
(12). P. angulata L. could
be used in conjunction with standard drugs to achieve synergism,
which may overcome drug resistance issues, minimize side effects
and enhance drug pharmacokinetics (12).
3. Anticancer properties
Over the last 10 years, the role of P.
angulata L. as an anticancer agent has been investigated using
the Y79, HeLa, DLD-1, MCF-7 and HGC-27 cancer cell lines. In 2019,
research was conducted in Indonesia on the activity of P.
angulata L. on the Y79 (retinoblastoma) cell line. The ethanol
extract of P. angulata L. leaves promoted apoptosis and
lowered the number of live cells at doses of 25, 50 and 100 µg/ml,
with 100 µg/ml causing the greatest increase in apoptosis level
(12). Pillai et al
(3) examined the effects of P.
angulata L. leaf and fruit ethanol extracts on HeLa, DLD-1 and
MCF-7 cells. The fruit extracts had lower median lethal dose
(LD50) values than the leaf extracts, but the leaf
extracts had a stronger cytotoxic action against HeLa cells.
Physalin B, the active component in P.
angulata L may become essential in anticancer therapy (14,15).
There is evidence that physalin B has anticancer activity in a
variety of human solid tumors, including lung, breast, colon,
melanoma and prostate tumors (16).
By altering mitochondrial function, physalin B causes G2/M cell
cycle arrest and cell death in human non-small cell lung cancer
cells (A549) and a cell line for human breast cancer (MCF-7)
affects p53-dependent signaling. The survival and proliferation of
the undifferentiated gastric cancer cell line, HGC-27, and the
ability to produce clones were all inhibited by physalin B, which
induces G0/G1 cell cycle arrest and caspase 8, 3, 7 and poly
(ADP-ribose) polymerase cleavage (17). In 2006, Magalhães et al
(18) conducted in vivo
studies investigating the antitumor activity of P. angulata
L. using mice bearing sarcoma 180 tumor cells, confirming the
antitumor activity of physalin B and D.
The potential role of drug candidates in cell growth
and death is the cornerstone of anticancer research. While cell
proliferation is the process by which cells multiply by expanding
and dividing into two, apoptosis is a mechanism for planned cell
death. However, the effectiveness of P. angulata L. and its
isolates as anticancer drugs must be further studied. Future
research on P. angulata L. as an anticancer agent may focus
on directly preventing cancer cell proliferation by stimulating
phagocytic cells and enhancing natural killer cell activity,
delaying the development of cancer cell appendages by increasing
the production of interferons, interleukins and antibodies in the
bloodstream, removing the tumor tissue from the body and preventing
it from metastasizing by obstructing blood supply to the cancerous
tissue, inducing the inverse transformation of tumor cells into
normal cells, boosting metabolism and protecting normal cells from
changing into cancer cells, increasing appetite, improving sleep
quality and managing pain (19).
4. Antiparasitic properties
Trypanosoma cruzi (T. cruzi),
Leishmania amazonensis (L. amazonensis) and L.
braziliensis have all been studied using P. angulata L.
as an antiparasitic agent. Meira et al (20) conducted T. cruzi in vitro
research using physalin B, D, F and G from P. angulata L.
ethanol extract as candidate agents. According to the results of an
alamar Blue assay, after 24 h treatment, 3.7 g/ml extracts of the
P. angulata L. stem markedly decreased the percentage of
infected cells with T. cruzi. In addition, compared with the
untreated control, the anti-leishmanial impact at 3.7 g/ml
increased after 48 h, and the number of infected macrophages
containing amastigotes of L. amazonensis parasites decreased
by 91.8% (21).
The stem of P. angulata L. acts as an
anti-leishmanial agent (22). The
WHO lists leishmaniasis as a significant tropical disease, ranking
it second only to malaria (23).
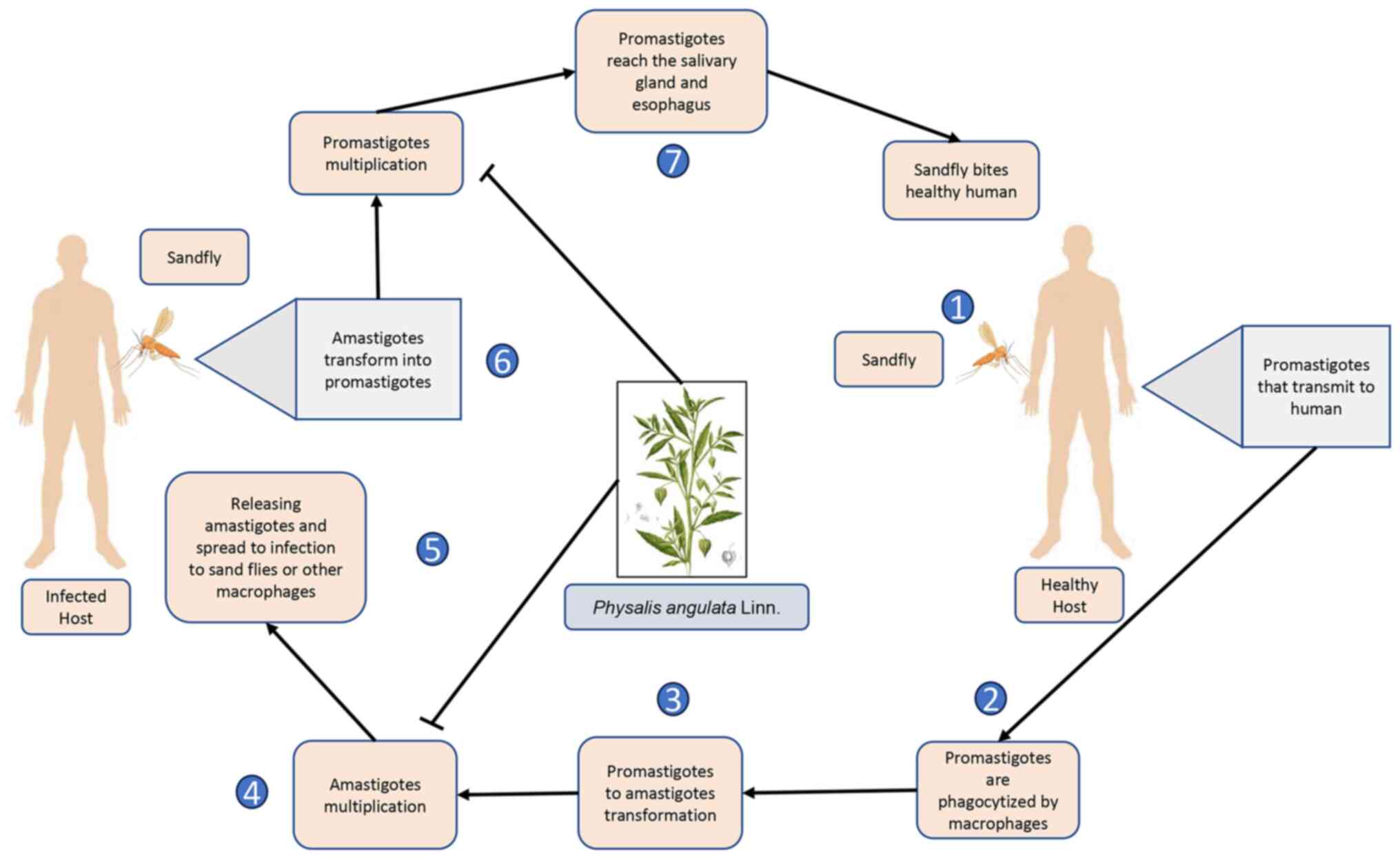
The Leishmania parasites enter the digestive tract of sand
flies (the vector) when it feeds on an infected host and multiply
there as promastigotes. These promastigotes can then be transmitted
to a mammalian host when the sand fly bites a healthy individual.
The parasite multiplies in this mammalian host and settles inside
the macrophages, where it survives and develops. Leishmania
parasites can also persist in amastigote form in a phagolysosomal
chamber. During times of stress, macrophages lyse and are
phagocytosed by new host cells (21). Physalin isolates A, B, D, E, F, G
and H present in the aqueous extract of P. angulata L. roots
induced 99.8% anti-leishmanial activity against L.
amazonensis promastigotes and reduced parasite survival at a
dose of 100 µg/ml extract. Furthermore, P. angulata L.
participates in cell division, cytoskeleton disintegration and
autophagy in promastigotes (23).
P. angulata L. acts as an anti-Leishmania agent by
inhibiting promastigotes multiplication in infected humans and
inhibiting amastigotes multiplication in healthy humans bitten by
sandfly (Fig. 1).
Research opportunities for anti-Leishmania
drug targets could explore several routes, such as effects on
sterol biosynthesis enzymes, thiol metabolism enzymes, the hypusine
pathway, the glycosylphosphatidylinositol pathway, the glycolytic
pathway, the purine salvage pathway, nucleoside transporters,
cyclin-dependent kinases, mitogen-activated protein kinase,
polyamine biosynthesis enzymes, dihydrofolate reductase, peptidase,
topoisomerase, metaspora and glyoxalase systems. Another unique
strategy for directly controlling Leishmania parasites that
dwell in macrophages is the use of macrophage key target drug
delivery systems. Since delivering drugs into macrophages is
difficult, drug carriers such as liposomes, microspheres,
nanoparticles and carbon nanotubes are being investigated.
Additionally, specific receptors expressed by macrophages are also
used to actively deliver drugs (24).
5. Anti-inflammatory properties
Inflammation is a protective response to potentially
harmful stimuli such as allergens and/or injury to tissues.
Inflammation is a complex process that involves various cellular
interactions and can be classified as acute or chronic. Acute
inflammation protects the body by repairing wounds and fighting
microbial invasion, whereas chronic inflammation is distinguished
by the simultaneous destruction and repair of tissues. Macrophages
and lymphocytes are the primary immune cells that infiltrate
chronic inflammatory sites (25).
P. angulata L. has been studied as an
anti-inflammatory agent in vitro, in vivo and in
clinical studies. In the last 10 years, there have been four
studies using RAW 264.7 cells to determine the anti-inflammatory
properties of P. angulata L. and its isolates. Yang et
al (26) isolated physalin E
from the stem and aerial parts of P. angulata L. and
demonstrated that physalin E significantly reduced TNF-α and IL-6
mRNA and protein expression at 12.5, 25.0 and 50.0 M (26,27).
In addition, withaminimin, obtained from dichloromethane extract of
the whole plant of P. angulata L., educed nitric oxide (NO)
generation in RAW 264.7 macrophages stimulated with
lipopolysaccharide (LPS) (27).
Using NO production measurements following 1 lg/ml of LPS
stimulation, the NO inhibition of each of the isolated compounds
was assessed in RAW 246.7 cells. The IC50 values for
physagulin B, physalin B and physagunin R were <1.0 µM, followed
by physalin F (IC50 1.06±0.68 µM), physalucoside A
(IC50 2.69±0.17 µM) and physalin G (IC50
3.74±0.29 µM) (28). Furthermore,
physagulins A, C and H inhibit NO, prostaglandin (PG) E2 and IL-6
production, as well as the expression of inducible NO synthase
(iNOS) and cyclooxygenase-2 (COX-2) proteins and the translocation
of NF-κBo in the nucleus (28,29).
The anti-inflammatory effect of P. angulata
L. leaf methanol extract against carrageenan-induced paw edema was
shown to be dose-dependent, with 62.71% inhibition at 400 mg/kg
compared with 34.31% inhibition for the standard drug (ibuprofen
100 mg/kg) (30). To investigate
the prophylactic anti-inflammatory effects of P. angulata L.
extract, different extract concentrations (30, 100 and 300 mg/kg
body weight) were administered before the paw edema was induced
with carrageenan. The results demonstrated that the mean maximal
swelling at 2 h was significantly (P<0.01) reduced from
69.77±3.83% in the inflamed control group to 64.08±1.75, 60.91±0.62
and 59.12±3.34% in the 30, 100 and 300 mg/kg treatment groups,
respectively. The extracts significantly reduced the mean maximal
swelling (P<0.001) when administered 2 and 6 h after
carrageenan-induced paw edema (curative) (31).
P. angulata L. reduced TNF-α, IL-1β, COX-2
and iNOS mRNA expression and induced a significant reduction in
TNF-α, IL-1β and PGE2 (Fig. 2) paw
edema levels during inflammation (31). LPS-induced NF-κB activation was also
inhibited by physalin E (25).
NF-κB, a promoter-binding immediate early transcriptional
activator, plays a role in immunological, inflammatory and acute
phase responses by regulating the expression of immediate early
inflammatory genes such as TNF-α, IFN-γ, NOS II and intercellular
adhesion molecule (26).
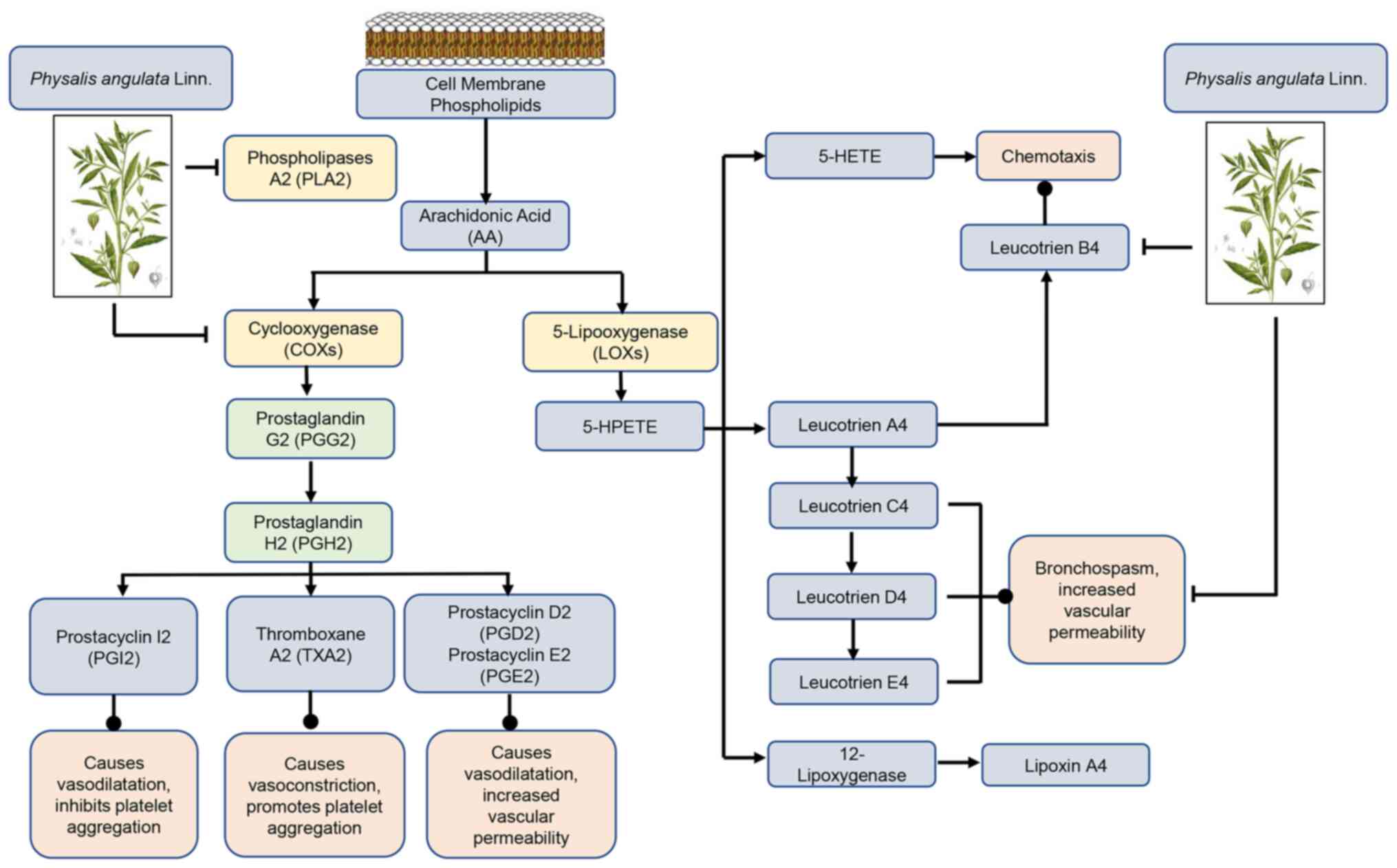 | Figure 2AA metabolism pathways. Esterified AA
on the inner surface of the cell membrane is hydrolyzed to its free
form by PLA2, which is in turn further metabolized by COXs and LOXs
enzymes to a mediator that includes prostanoids, LTs, 5-HPETE,
5-HETE and LXs. Physalis angulata Linn. inhibits the action
of the PLA2, COXs, and Leucotrien B4. AA, arachidonic acid; PLA2,
phospholipase A2; COX, cyclooxygenase; LOX, 5-lipoxygenases; LT,
leukotrienes; 5-HPETE, 5-hydroperoxyeicosatetraenoic acid; 5-HETE,
5-hydroxyeicosatraenoic axid; LX, lipoxin. |
In vivo anti-inflammatory research has also
received attention in the last decade, in which paw edema,
intestinal inflammation and acute lung injury were induced in
experimental animal models. In intestinal inflammation, P.
angulata L. aerial parts improved anti-inflammatory response
throughout 2,4,6-trinitrobenzane sulfonic acid-induced intestinal
damage, modulating oxidative stress, immune response and
inflammatory gene expression (33).
According to a study by Arruda et al (34), physalin D prevents the release of
cytokines, protein accumulation and cell migration caused by ATP,
reduces the edematogenic response and the LPS impact for an
independent glucocorticoid receptor pathway. Furthermore, physalin
D exhibits effective anti-inflammatory activity, low murine
toxicity, good aqueous solubility, as well as pharmacokinetics of
absorption, low liver conversion and high urine and fecal
excretion.
The COX and 5-lipoxygenase pathways are two key
arachidonic acid metabolic processes. The COX process generates the
cyclo-endoperoxides, PGG2 and PGH2, as intermediates. These
cyclo-endoperoxides are then converted into the physiologically
active prostanoid by enzymes. PGs are produced by smooth muscle
cells in blood arteries. PGD2 is a major metabolite of the
cyclooxygenase pathway in mast cells, together with PGE2 causes
vasodilation and promote edema formation. PGE2 is a vasodilator
that stimulates the Gs-protein pathway, whereas PGF2 is a
vasoconstrictor that stimulates the Gq-protein pathway. PGI2 is the
principal arachidonic acid derivative produced by vascular
endothelial cells, and is a strong vasodilator and platelet
adhesion inhibitor that functions via the Gs-protein pathway
(35).
Platelets produce thromboxane A2 (TXA2), a strong
vasoconstrictor that functions via the Gq-protein pathway. TXA2
synthesis increases with inflammation, tissue injury and platelet
activation. When an artery is cut and bleeding, TXA2 enhances
vascular contraction (hemostatic function). In reaction to
inflammation and tissue injury, leukocytes produce leukotrienes
(LTs), such as LTC4. LTC4, like TXA2, is a powerful vasoconstrictor
that functions via the Gq-protein pathway. LTs (and PGs) can also
cause vascular endothelium ‘leakage’, promoting edema during
inflammation. P. angulata L. acts as an anti-inflammatory by
preventing the action of phospholipase, COX and LTB4(35).
P. angulata L. acts as an anti-inflammatory
by inhibiting the cyclooxygenase pathway, thus reducing PGE2. In
addition, P. angulata L. also inhibits the lipo-oxygenase
pathway by reducing LTB4, which is a chemotaxis agent (Fig. 2).
6. Antifibrotic properties
P. angulata L. is an effective acute
anti-inflammatory agent and its potential action against chronic
diseases, such as fibrosis, is also being investigated. Fibrosis is
associated with diseases including the hepatitis virus,
non-alcoholic fatty liver disease, chronic kidney diseases,
idiopathic pulmonary fibrosis, pneumoconiosis and cystic fibrosis
(36). Global disability-adjusted
life-years in 2019 were significantly impacted by fibrosis-related
disorders (36).
Physalin B derived from P. angulata L. has
been proven to be an antifibrosis agent. Physalin B has a potent
antifibrotic effect on activated hematopoietic stem cells (HSCs),
as demonstrated in both in vitro and in vivo studies.
The antifibrotic activity of physalin B on LX-2 cells was examined
using the Cell Counting Kit-8 viability assay, and the results
revealed that the IC50 was 5 µM. Transforming growth
factor β-1 induced HSC proliferation was also inhibited by physalin
B. Furthermore, in vivo studies revealed that physalin B
reduces hepatic injury, as measured by decreased aspartate
aminotransferase and alanine transaminase (ALT) levels (36). Histopathological examination also
demonstrated that physalin B could repair liver fibrosis (37).
In 2019, Dewi et al (38) conducted a study on patients with
scleroderma, which is a fibrosing disease of the skin. P.
angulata L. was administered as an adjuvant therapy at a dose
of 250, 3 times daily for 12 weeks, which reduced the modified
Rodnan skin scores (MRSS) and procollagen type I N-pro-peptide
serum levels of patients. Another study on CCL4-induced liver
fibrosis demonstrated that, in the group that received CCL4, serum
ALT levels were higher and, microscopically, hepatocyte
architecture lost its typical appearance, transparent collagen was
deposited and fiber segmentation formed (36). Significant variations in serum ALT
concentration were observed at the 2.22 mg dose of ethyl acetate
fraction of P. angulata L. along with microscopic histologic
changes, where the Ishak and Metavir scores decreased indicating
healing of the hepatocytes (36-38).
Research is still being conducted on the mechanism
of action of P. angulata L. and its isolates, as well as on
in vitro and in vivo fibrosis models for the heart,
kidneys and lungs. Fibrosis-related in vitro studies may
utilize epithelial cells, endothelial cells, immune cells and
fibroblasts (39). Notable
signaling pathways within in vivo or in vitro studies
involved in fibrotic diseases are growth factors (e.g. fibroblast
growth factors, platelet-derived growth factor, connective tissue
growth factor, and TGF-βs) and related signaling pathways (39). Finding effective therapeutic drugs
is difficult due to the complicated pathophysiology of fibrotic
disorders, which involve several abnormal cells (for example,
epithelial cells, endothelial cells, immune cells and fibroblasts)
and signaling pathways during development of the disease (39).
7. Antidiabetic properties
Raju and Estari (40) demonstrated that fruits from P.
angulata L. reduced blood sugar levels at doses of 25 and 50
mg/kg. The methanolic extract of P. angulata L. roots lowers
blood glucose levels at a dose of 200-400 mg/kg body weight
(40). In addition, withangulatin A
isolated from P. angulata L. fruit has a hypolipidemic
action and lowers blood sugar levels (41). However, further research is needed
to determine the optimal dose of extracts with minimal side
effects. The unclear mechanism of P. angulata L. in reducing
blood sugar levels needs further research. Pharmacology-related
anti-diabetes research could explore the mechanism of insulin
synthesis stimulation and/or secretion, restoration of damaged
pancreatic β cells, improved insulin sensitivity and increased
glucose uptake by fat and muscle cells, insulin mimics, slowing
carbohydrate absorption from the gut, altering glucose metabolizing
enzymes or ameliorating oxidative stress (42).
8. Chemical components of Physalis
angulata Linn (P. angulata L.)
P. angulata L. contains active ingredients
that have medicinal properties. These active substances are: i)
Physalin A in the roots, with antiparasitic properties (23), ii) physalin B in the whole plant,
with anti-inflammatory, antiparasitic, antibacterial, anticancer
and antifibrotic properties (10,16,17,27,29,31,37,43),
iii) physalin D, F, G in the whole plant, with anti-inflammatory,
antiparasitic and antibacterial properties (11,20,23,32,34,43),
iv) physalin E in the whole plant, with anti-inflammatory and
antiparasitic properties (23), v)
physalin H in the root, with antiparasitic properties (20,31),
vi) withangulatin A in the fruit, with antidiabetic properties
(35) and vii) physangulatin A in
the leaves and stems, with anti-inflammatory properties (35). The active substances in P.
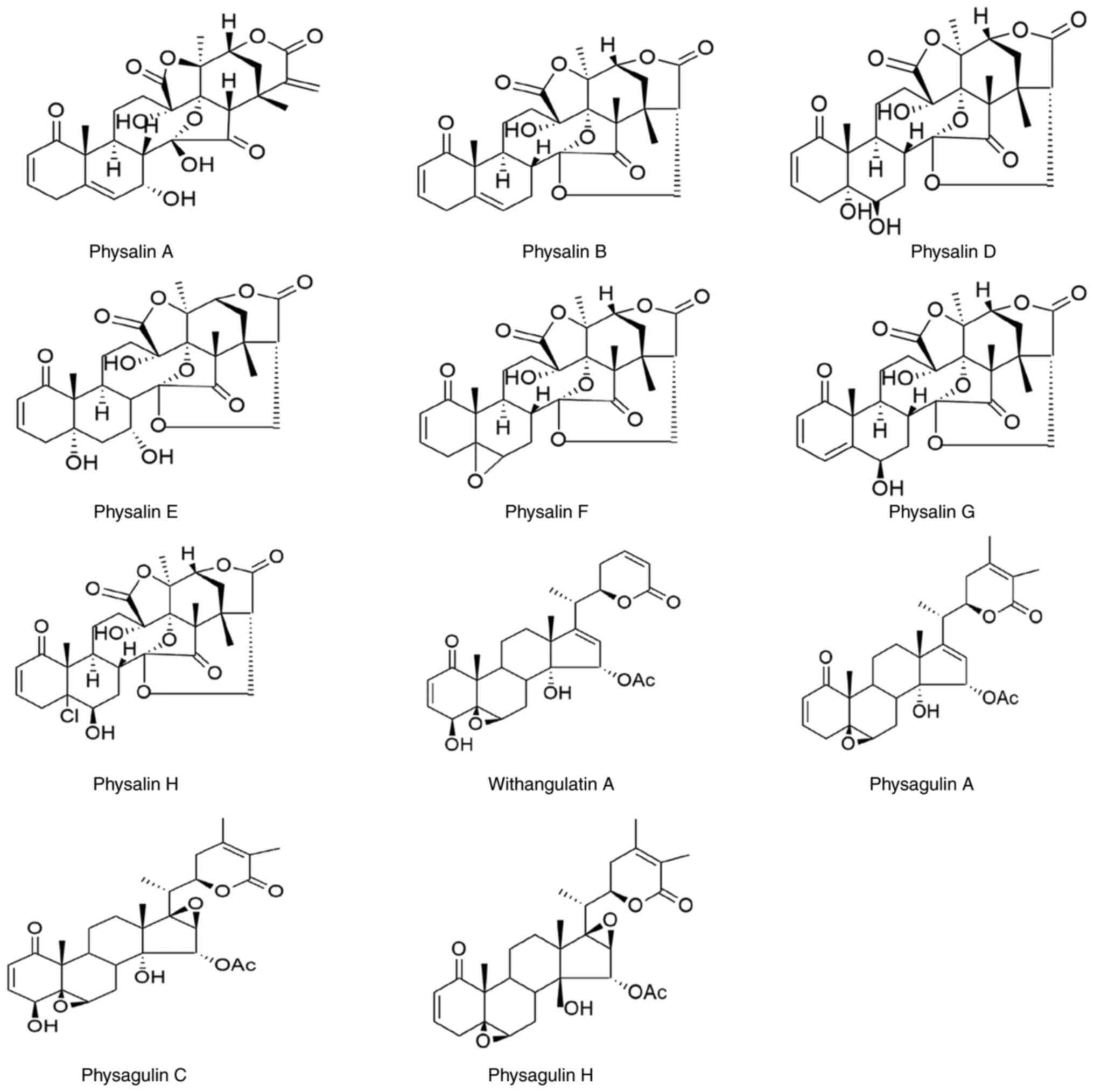
angulata L. are also presented in Table II and Fig. 3.
 | Table IIThe active compounds in Physalis
angulata L. |
Table II
The active compounds in Physalis
angulata L.
| Name of
chemical | Plant part | Activities | (Refs.) |
|---|
| Physalin A | Roots |
Anti-parasitic/antileishmanial | (23) |
| Physalin B | Stem |
Immunomodulatory | (54) |
| | |
Anti-inflammatory | (28,32,43) |
| | Whole plant | Anti-inflammatory,
antiparasitic | (28,32,43) |
| | Root | Antibacterial | (20,23,55) |
| | |
Anticancer/Antifibrosis | (11,17,37) |
| Physalin D | Stem |
Immunomodulatory | (54,56) |
| | Whole plant |
Anti-inflammatory | (32,34,43) |
| | Root | Antiparasitic | (23,55) |
| | | Antibacterial | (11) |
| Physalin E | Root | Antiparasitic | (23) |
| | Whole plant |
Immunomodulatory | (56) |
| | |
Anti-inflammatory | (26,57) |
| Physalin F | Stem |
Immunomodulatory | 54 |
| | Whole plant |
Anti-inflammatory | (28,32,43) |
| | Root | Antiparasitic | (20,23,55) |
| | | Antibacterial | (11) |
| Physalin G | Stem |
Immunomodulatory | (54,56) |
| | Whole plant |
Anti-inflammatory | (28,32,43) |
| | Root | Antiparasitic | (23) |
| | | Antibacterial | (11) |
| Physalin H | Root | Antiparasitic | (23) |
| Withangulatin
A | Fruit | Antidiabetic | (40) |
| Physagulin A | Leaves and
stems |
Anti-inflammatory | (35) |
| Physagulin C | Leaves and
stems |
Anti-inflammatory | (35) |
| Physagulin H | Leaves and
stems |
Anti-inflammatory | (35) |
Clinical study
Over the past 10 years, there has only been one
study of the role of P. angulata L. with human subjects,
namely the study of Dewi et al (38) (2019). The aforementioned study was
about to evaluate the effect of the addition P. angulata L.
extract as adjuvant to scleroderma standard therapy in suppressing
inflammatory, immunological, and fibrosis processes to accelerate
clinical improvement of skin fibrosis based on MRSS in scleroderma
patients. The the degree of disease activity was assessed using the
following biomarkers: Erythrocyte sedimentation rate for
inflammation; serum levels of soluble CD40 ligand (sCD40L) and
B-cell activation factor (BAFF) for immunological biomarkers; and
serum levels of procollagen Type I N-Terminal propeptide (P1NP) for
fibrotic process biomarker (38).
During November 2015-March 2017, 59 scleroderma
patients who met the selection criteria and remained receiving
regular therapy at Cipto Mangunkusumo Hospital and Hasan Sadikin
Hospital in Indonesia participated in a double-blind, randomized
clinical trial. The subjects were randomly allocated into two
groups: the study group (29 patients) received the P.
angulata L. extract 3x250 mg/day for 12 weeks and the placebo
group (30 patients). Examination of MRSS, ESR, P1NP, BAFF and
sCD40L was performed every 4 weeks until the end of the study.
After 12 weeks, MRSS decreased 35.9% in the P. angulata L.
group and 6.3% in the placebo group. Serum P1NP levels were also
decreased in the P. angulata L. group (17.8%) compared with
the placebo group (0.7%). This indicated that P. angulata L.
can therapeutically improve skin fibrosis. The result identified no
correlation between MRSS and the result of ESR value, serum BAFF
and CD40L levels in both groups. To demonstrate that P.
angulata L. has anti-inflammatory properties, more research
utilizing additional inflammatory indicators is required (38).
Based on Dewi's research that P. angulata L.
can therapeutically improve skin fibrosis, research was continued
on other organs. Rohmawaty et al (36) conducted the research on liver of
male adult Wistar rats that induced by carbon tetrachloride (CCl4)
to perform liver fibrosis model.
The aim of the aforementioned study was to determine
if the ethyl acetate fraction of P. angulata L. had an
antifibrotic effect on liver fibrosis. Liver fibrosis was induced
by oral injection of 20% CCl4 twice a week for eight weeks. A total
of four weeks following fibrosis induction, P. angulata L.
ethyl acetate fractions of 1.11 mg (CPL-1) and 2.22 mg (CPL-2) were
administered orally. As a positive control group, vitamin E was
used (36).
The ethyl acetate component of 2.22 mg (CPL-2)
decreased serum alanine aminotransaminase levels (83.95±27.675 vs.
175.23±5.641, P-value <0.05) as compared with the negative
control. Microscopic histopathological changes based on the better
Metavir score (CPL-2 vs. negative control=1.25±1.893 vs.
3.50±0.577; P<0.05) and Ishak score (CPL-2 vs. negative
control=1.50±1.000 vs. 4.75±0.957 P<0.05) were demonstrated.
These findings suggested that the ethyl acetate fraction of P.
angulata L. has an antifibrotic effect (36).
The use of P. angulata L. as an adjuvant
therapy in humans can be provided by calculating the dose. The dose
of P. angulata L. for humans is obtained by calculating the
dose in animals with Laurence Bacharach's coefficient and the yield
of the fraction (36).
9. Extraction process
In the present context, extraction is the process of
separating the parts of a plant that are medicinally active, whilst
utilizing certain solvents and accepted practices. All extraction
procedures have the goal of separating the soluble metabolites of
the plant from its insoluble cellular marc (residue). Preparing
plant samples to preserve the constituent biomolecules before
extraction is the first step in investigating therapeutic plants.
Fresh or dried plant material can be used to extract samples such
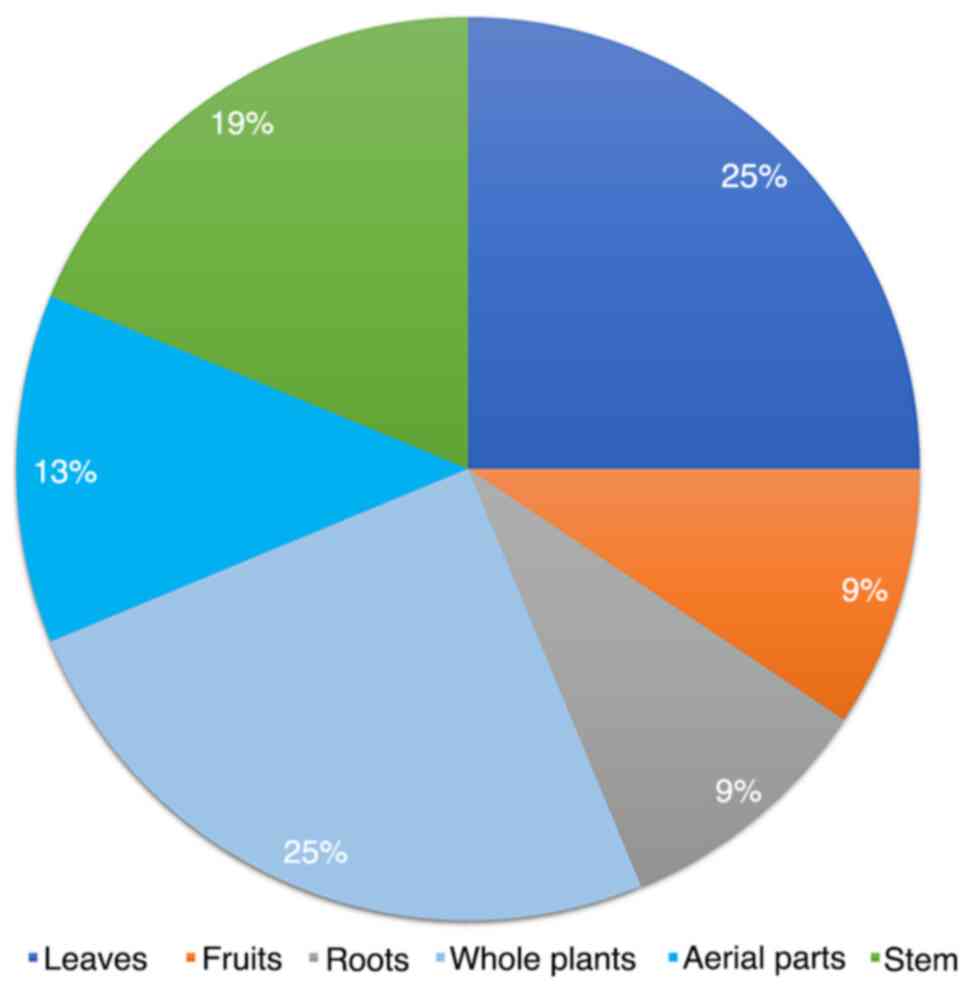
as from the leaves, bark, roots, fruits and flowers (44). Parts of P. angulata L. that
can be utilized include: Whole plants (25%), leaves (25%), stems
(19%), aerial parts (13%), roots (9%) and fruit (9%) (Fig. 4). Sulaiman et al (45) restricted the time between collecting
the medicinal plant and experimental work to a maximum of 3 h to
preserve sample freshness. In most situations, dried samples are
preferred since they require less time to prepare for experiments
(46).
The surface contact between samples and extraction
solvents is increased when the particle size is reduced. Grinding
produces coarser, lower sample sizes whereas pulverized samples
have smaller, more homogeneous particles, which improve the surface
contact with extraction solvents. The optimum particle size for
good extraction is <0.5 mm (44). The particle size has a significant
impact on the use of pectinolytic enzymes that break down cell wall
polysaccharides, as smaller particles increase the activity of
these enzymes (44).
The type of plant, the plant component being
extracted, the makeup of the bioactive chemicals and solvent
accessibility all influence the choice of extraction solvent
(46). In general, non-polar
solvents such as hexane and dichloromethane are used to extract
non-polar substances, while polar solvents such as water, methanol
and ethanol are used to extract polar substances (47-49).
Solvents with increasing polarity are introduced during
fractionation, beginning with n-hexane, the least polar, and ending
with water, the most polar (46,47,50).
The solvents and their polarity are demonstrated in Table III. During fractionation, it is
customary to select five solvents: Two solvents with low polarity
(such as n-hexane and chloroform), two solvents with medium
polarity (such as dichloromethane and n-butanol) and one solvent
with the highest polarity (such as water) (Fig. 5) (46). Water is the ‘greenest’ solvent and
is not only affordable and safe for the environment, but it also
offers the potential for clean processing and pollution avoidance
since it is non-toxic and non-flammable (48,50).
 | Table IIIList of solutions and polarities. |
Table III
List of solutions and polarities.
| No. | Solvent | Polarity |
|---|
| 1. | n-Hexane | 0.009 |
| 2. | Petroleum
ether | 0.117 |
| 3. | Diethyl ether | 0.117 |
| 4. | Ethyl acetate | 0.228 |
| 5. | Chloroform | 0.259 |
| 6. |
Dichloromethane | 0.309 |
| 7. | Acetone | 0.335 |
| 8. | n-Buthanol | 0.586 |
| 9. | Ethanol | 0.654 |
| 10. | Methanol | 0.762 |
| 11. | Water | 1.000 |
When selecting an extraction solvent, the following
factors should be considered: i) Selectivity, the capacity of a
given solvent to separate the inert material from the active
component; ii) safety, the ideal extraction solvent is non-toxic
and non-flammable; iii) price, it should be as affordable as
possible; iv) reactivity, an appropriate extraction solvent should
not react with the extract; v) recovery, it is important to be able
to promptly recover and separate the extraction solvent from the
extract; vi) viscosity, low viscosity is necessary for easy
penetration and vii) the boiling temperature, to avoid heat-related
degradation, the solvent boiling temperature should be as low as
possible (46,47,50).
10. Toxicity studies
Research conducted by Sukandar and Sheba (51) demonstrated that P. angulata
L. extract does not affect the behavior of rats in a single-dose
therapy of up to 5 g/kg body weight and had an LD50 of
>5 g/kg body weight, which is regarded as non-toxic. Sub-chronic
toxicity studies revealed that up to 1 g/kg body weight of P.
angulata L. extract administered for 90 days did not cause
mortality, was not poisonous to organs and had no effect on the
blood cell count, blood biochemistry or urinalysis.
Guideline no. 420 of the Organization for Economic
Co-operation and Development (1997) was used to calculate the acute
toxicity test (LD50) of P. angulata L. methanolic
extracts (51). P. angulata
L. at a dose of 2,000 mg/kg was administered to four groups of 6
albino mice (20-25 g, either sex), and the mortality and general
behavior of the treated animals were observed for 14 days. At the
conclusion of the trial, no fatalities were recorded. The extract
was therefore confirmed to be safe up to a dose of 2,000 mg/kg
(52).
11. Conclusion
P. angulata L. exhibits antibacterial,
anticancer, antiparasitic, anti-inflammatory, antifibrotic and
antidiabetic effects. P. angulata L. extract is safe based
on acute and sub-chronic toxicity data. However, to further
evaluate the safety of P. angulata L. extract, a chronic
toxicity study is required, examining repeated doses or lifetime
exposure.
In the study of medicinal plants, all extraction
stages, including pre-extraction and extraction, are crucial. The
sample preparation steps, such as grinding and drying, have an
impact on the effectiveness and phytochemical components of the
final extractions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AN, ER and AMR performed the literature search and
assisted in drafting and revising the manuscript. All authors have
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kasali FM, Tusiimire J, Kadima JN, Tolo
CU, Weisheit A and Agaba AG: Ethnotherapeutic uses and
phytochemical composition of Physalis peruviana L.: An
overview. ScientificWorldJournal. 2021(5212348)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mahklouf MH: The first record of
Physalis angulata L. (Solanaceae) for the flora of
Libya. Biodiv Res Conserv. 53:67–71. 2019.
|
|
3
|
Ramakrishna Pillai J, Wali AF, Menezes GA,
Rehman MU, Wani TA, Arafah A, Zargar S and Mir TM: Chemical
composition analysis, cytotoxic, antimicrobial and antioxidant
activities of Physalis angulata L.: A comparative study of
leaves and fruit. Molecules. 27(1480)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gao CY, Ma T, Luo J and Kong LY: Three new
cytotoxic withanolides from the Chinese folk medicine Physalis
angulata. Nat Prod Commun. 10:2059–2062. 2015.PubMed/NCBI
|
|
5
|
Hadiyanti N, Supriyadi S and Pardono P:
Keragaman beberapa tumbuhan ciplukan (Physalis spp.) di lereng
gunung kelud, jawa timur. Berita Biologi. 17:135–146. 2018.
|
|
6
|
Fadhli H, Ruska Sl, Furi M, Suhery WN,
Susanti E and Nasution MR: Ciplukan (Physalis angulata L.):
Review tanaman liar yang berpotensi sebagai tanaman obat. J Farm
Indones. 15:134–141. 2023.
|
|
7
|
Rivera DE, Ocampo YC, Castro JP, Caro D
and Franco LA: Antibacterial activity of Physalis angulata
L., Merremia umbellataL., and Cryptostegia grandiflora Roxb. Ex
R.Br. -medicinal plants of the Colombian Northern Coast. Orient
Pharm Exp Med. 15:95–102. 2015.
|
|
8
|
Hananto H, Rahman A and Fahmi M:
Antibacterial activity of ethanolic extract of morel berry
(Physalis angulata L.) towards Staphylococcus aureus.
Malays J Med Health Sci. 17:132–135. 2021.
|
|
9
|
Gagare SB, Chavan SL and Sagade AB:
Antibacterial potential and phytochemical screening of Physalis
angulata and solanum virgianum. Int J Res Biosci Agric Technol.
1:36–40. 2021.
|
|
10
|
Dias FGB, Ferreira MJG, da Silva LMR, de
Sousa Menezes RC and de Figueiredo EAT: Bioaccessibility of the
bioactive compounds and antimicrobial activity of aqueous extracts
of Physalis angulata L. Rev Cienc Agron.
51(e20196619)2020.
|
|
11
|
Cuong LCV, Dat TTH, Nhiem NX, Cuc NT, Yen
DTH and Anh HLT: The anti-microbial activities of secosteroids
isolated from Physalis angulata. Vietnam J Chem. 58:321–326.
2020.
|
|
12
|
Anand U, Jacobo-Herrera N, Altemimi A and
Lakhssassi N: A comprehensive review on medicinal plants as
antimicrobial therapeutics: Potential avenues of biocompatible drug
discovery. Metabolites. 9(258)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chairissy MD, Wulandari LR and Sujuti H:
Pro-apoptotic and anti-proliferative effects of Physalis
angulata leaf extract on retinoblastoma cells. Int J
Ophthalmol. 12:1402–1407. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang Y, Yi L, Wang Q, Xie B, Sha C and
Dong Y: Physalin B suppresses inflammatory response to
lipopolysaccharide in RAW264.7 cells by inhibiting NF-κB signaling.
J Chem. 4(7943140)2018.
|
|
15
|
Guimarães ET, Lima MS, Santos LA, Ribeiro
IM, Tomassini TBC, Ribeiro dos Santos R, dos Santos WLC and Soares
MBP: Activity of physalins purified from Physalis angulata
in in vitro and in vivo models of cutaneous leishmaniasis. J
Antimicrob Chemother. 64:84–87. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang A, Wang S, Zhou F, Li P, Wang Y, Gan
L and Lin L: Physalin B induces cell cycle arrest and triggers
apoptosis in breast cancer cells through modulating p53-dependent
apoptotic pathway. Biomed Pharmacother. 101:334–341.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fang C, Chen C, Yang Y, Li K, Gao R, Xu D,
Huang Y, Chen Z, Liu Z, Chen S, et al: Physalin B inhibits cell
proliferation and induces apoptosis in undifferentiated human
gastric cancer HGC-27 cells. Asia Pac J Clin Oncol. 18:224–231.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Magalhães HI, Veras ML, Torres MR, Alves
AP, Pessoa OD, Silveira ER, Costa-Lotufo LV, de Moraes MO and
Pessoa C: In-vitro and in-vivo antitumour activity of physalins B
and D from Physalis angulata. J Pharm Pharmacol. 58:235–241.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abdulridha MK, Al-Marzoqi AH, Al-Awsi GRL,
Mubarak SMH, Heidarifard M and Ghasemian A: Anticancer Effects of
herbal medicine compounds and novel formulations: A literature
review. J Gastrointest Cancer. 51:765–773. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Meira CS, Guimarães ET, Bastos TM, Moreira
DR, Tomassini TC, Ribeiro IM, Dos Santos RR and Soares MB:
Physalins B and F, seco-steroids isolated from Physalis
angulata L., strongly inhibit proliferation, ultrastructure and
infectivity of Trypanosoma cruzi. Parasitology.
140:1811–1821. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nogueira RC, Rocha VPC, Nonato FR,
Tomassini TC, Ribeiro IM, dos Santos RR and Soares MB: Genotoxicity
and antileishmanial activity evaluation of Physalis angulata
concentrated ethanolic extract. Environ Toxicol Pharmacol.
36:1304–1311. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sangshetti JN, Khan FAK, Kulkarni AA,
Arote R and Patil RH: Antileishmanial drug discovery: Comprehensive
review of the last 10 years. RSC Adv. 5:32376–32415. 2015.
|
|
23
|
da Silva RR, da Silva BJ, Rodrigues AP,
Farias LH, da Silva MN, Alves DT, Bastos GN, do Nascimento JL and
Silva EO: In vitro biological action of aqueous extract from roots
of Physalis angulata against Leishmania
(Leishmania) amazonensis. BMC Complement Altern Med.
15(249)2015.
|
|
24
|
Hassan AA, Khalid HE, Abdalla AH, Mukhtar
MM, Osman WJ and Efferth T: Antileishmanial activities of medicinal
herbs and phytochemicals in vitro and in vivo: An update for the
years 2015 to 2021. Molecules. 27(7579)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ghasemian M, Owlia S and Owlia MB: Review
of anti-inflammatory herbal medicines. Adv Pharmacol Sci.
2016(9130979)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang YJ, Yi L, Wang Q, Xie BB, Dong Y and
Sha CW: Anti-inflammatory effects of physalin E from Physalis
angulata on lipopolysaccharide-stimulated RAW 264.7 cells
through inhibition of NF-κB pathway. Immunopharmacol Immunotoxicol.
39:74–79. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yen PH, Cuong LCV, Dat TTH, Thuy DTQ, Hoa
DTN, Cuc NT, Yen DTH, Thao DT and Anh HLT: Withanolides from the
whole plant of Physalis angulata and their anti-inflammatory
activities. Vietnam J Chem. 57:334–338. 2019.
|
|
28
|
Tuan Anh HL, Le Ba V, Do TT, Phan VK, Pham
Thi HY, Bach LG, Tran MH, Tran Thi PA and Kim YH: Bioactive
compounds from Physalis angulata and their anti-inflammatory
and cytotoxic activities. J Asian Nat Prod Res. 23:809–817.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang L, Lu S, Wang L, Xin M, Xu Y, Wang G,
Chen D, Chen L, Liu S and Zhao F: Anti-inflammatory effects of
three withanolides isolated from Physalis angulata L. in
LPS-activated RAW 264.7 cells through blocking NF-κB signaling
pathway. J Ethnopharmacol. 276(114186)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ukwubile CA and Oise IE: Analgesic and
anti-inflammatory activity of Physalis angulata Linn
(Solanaceae) Leaf methanolic extract in swiss albino mice.
Int Biol Biomed J Autumn. 2:167–70. 2016.
|
|
31
|
Abdul-Nasir-Deen AY, Boakye YD, Osafo N,
Agyare C, Boamah D, Boamah VE and Agyei EK: Anti-inflammatory and
wound healing properties of methanol leaf extract of Physalis
angulata L. S Afr J Bot. 133:124–131. 2020.
|
|
32
|
do Espírito Santo RF, Lima MDS, Juiz PJL,
Opretzka LCF, Nogueira RC, Ribeiro IM, Tomassini TCB, Soares MBP
and Villarreal CF: Physalis angulata concentrated ethanolic
extract suppresses nociception and inflammation by modulating
cytokines and prostanoid pathways. Nat Prod Res. 35:4675–4679.
2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Almeida Junior LD, Quaglio AEV, de Almeida
Costa CAR and Di Stasi LC: Intestinal anti-inflammatory activity of
Ground Cherry (Physalis angulata L.) standardized
CO2 phytopharmaceutical preparation. World J
Gastroenterol. 23:4369–4380. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Arruda JCC, Rocha NC, Santos EG, Ferreira
LGB, Bello ML, Penido C, Costa TEMM, Santos JAA, Ribeiro IM,
Tomassini TCB and Faria RX: Physalin pool from Physalis
angulata L. leaves and physalin D inhibit P2X7 receptor
function in vitro and acute lung injury in vivo. Biomed
Pharmacother. 142(112006)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang B, Wu L, Chen J, Dong L, Chen C, Wen
Z, Hu J, Fleming I and Wang DW: Metabolism pathways of arachidonic
acids: Mechanisms and potential therapeutic targets. Signal
Transduct Target Ther. 6(94)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rohmawaty E, Rosdianto AM, Usman HA,
Saragih WAM, Zuhrotun A, Hendriani R, Wardhana YW, Ekawardhani S,
Wiraswati HL, Agustanti N, et al: Antifibrotic effect of the ethyl
acetate fraction of ciplukan (Physalis angulate Linn.) in
rat liver fibrosis induced by CCI4. J Appl Pharm Sci.
11:175–182. 2021.
|
|
37
|
Zhu X, Ye S, Yu D, Zhang Y, Li J, Zhang M,
Leng Y, Yang T, Luo J, Chen X, et al: Physalin B attenuates liver
fibrosis via suppressing LAP2α-HDAC1-mediated deacetylation of the
transcription factor GLI1 and hepatic stellate cell activation. Br
J Pharmacol. 178:3428–3437. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dewi S, Isbagio H, Purwaningsih EH, Kertia
N, Setiabudy R and Setiati S: A double-blind, randomized controlled
trial of ciplukan (Physalis angulata Linn) extract on skin
fibrosis, inflammatory, immunology, and fibrosis biomarkers in
scleroderma patients. Acta Med Indones. 51:303–310. 2019.PubMed/NCBI
|
|
39
|
Zhao M, Wang L, Wang M, Zhou S, Lu Y, Cui
H, Racanelli AC, Zhang L, Ye T, Ding B, et al: Targeting fibrosis,
mechanisms and cilinical trials. Signal Transduct Target Ther.
7(206)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Raju P and Estari M: Anti-diabetic
activity of compound isolated from Physalis angulata fruit
extracts in alloxan-induced diabetic rats. Am J Sci Med Res.
1:40–43. 2015.
|
|
41
|
Reddy PA, Vijay KR, Reddy GV, Reddy MK and
Reddy YN: Anti-diabetic and hypolipidemic effect of aqueous and
methanolic root extracts of Physalis angulata in
streptozotocin (STZ) induced diabetic rats. Int J Pharm Res
Scholars. 3:402–409. 2014.
|
|
42
|
Kibiti CM and Afolayan AJ: Herbal therapy:
A review of emerging pharmacological tools in the management of
diabetes mellitus in Africa. Pharmacogn Mag. 11 (Suppl
2):S258–S274. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Daltro SRT, Santos IP, Barros PL, Moreira
DRM, Tomassini TCB, Ribeiro IM, Ribeiro Dos Santos R, Meira CS and
Soares MBP: In vitro and in vivo immunomodulatory activity of
Physalis angulata concentrated ethanolic extract. Planta
Med. 87:160–168. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Azwanida NN: A review on the extraction
methods use in medicinal plants, principle, strength, and
limitation. Med Aromat Plants. 4:1–6. 2015.
|
|
45
|
Sulaiman SF, Sajak AAB, Ooi KL, Supriatno
and Seow EM: Effect of solvents in extracting polyphenols
and antioxidants of selected raw vegetables. J Food Compos Anal.
24:506–515. 2011.
|
|
46
|
Abubakar AR and Haque M: Preparation of
medicinal plants: Basic extraction and fractionation procedures for
experimental purposes. J Pharm Bioallied Sci. 12:1–10.
2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Pandey A and Tripathi S: Concept of
standardization, extraction and pre-phytochemical screening
strategies for herbal drug. J Pharmacogn Phytochem. 2:115–119.
2014.
|
|
48
|
Sasidharan S, Chen Y, Saravanan D, Sundram
KM and Yoga Latha L: Extraction, isolation and characterization of
bioactive compounds from plants' extracts. Afr J Tradit Complement
Altern Med. 8:1–10. 2011.PubMed/NCBI
|
|
49
|
Altemimi A, Lakhssassi N, Baharlouei A,
Watson DG and Lightfoot DA: Phytochemicals: Extraction, isolation,
and identification of bioactive compounds from plant extracts.
Plants (Basel). 6(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Das K, Tiwari RKS and Shrivastava DK:
Techniques for evaluation of medicinal plant products as
antimicrobial agent: Current methods and future trends. J Med
Plants Res. 4:104–111. 2010.
|
|
51
|
Sukandar EY and Sheba SH: Acute and
Sub-chronic toxicity studies of combination of Physalis
angulata L. (Cecendet) extract and methylprednisolone on
animals. Int J Integr Health Sci. 7:48–55. 2019.
|
|
52
|
Rathore C, Dutt KR, Sahu S and Deb L:
Antiasthmatic activity of the methanolic extract of Physalis
angulate Linn. J Med Plants Res. 5:5351–5355. 2011.
|
|
53
|
Pereda MDCV, Dieamant G, Nogueira C,
Eberlin S, Facchini G, Mussi L, Polezel MA, Martins-Oliveira D,
Rosa PTV and Di Stasi LC: Sterol-standardized phytopharmaceutical
from ground cherry: Corticoid-like properties on human
keratinocytes and fibroblasts and its effects in a randomized
double-blind placebo-controlled clinical trial. J Cosmet Dermatol.
18:1516–1528. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Vieceli PS, Juiz PJL, Lauria PSS, Couto
RD, Tomassini TCB, Ribeiro IM, Soares MBP and Villarreal CF:
Physalis angulata reduces the progression of chronic
experimental periodontitis by immunomodulatory mechanisms. J
Ethnopharmacol. 273(113986)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Meira CS, Guimarães ET, Dos Santos JA,
Moreira DR, Nogueira RC, Tomassini TC, Ribeiro IM, de Souza CV,
Ribeiro Dos Santos R and Soares MB: In vitro and in vivo
antiparasitic activity of Physalis angulata L. concentrated
ethanolic extract against Trypanosoma cruzi. Phytomedicine.
22:969–974. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
da Silva BJM, Rodrigues APD, Farias LHS,
Hage AAP, Do Nascimento JL and Silva EO: Physalis angulata
induces in vitro differentiation of murine bone marrow cells into
macrophages. BMC Cell Biol. 15(37)2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Pinto NB, Morais TC, Carvalho KMB, Silva
CR, Andrade GM, Brito GAC, Veras ML, Pessoa ODL, Rao VS and Santos
FA: Topical anti-inflammatory potential of Physalin E from
Physalis angulata on experimental dermatitis in mice.
Phytomedicine. 17:740–743. 2010.PubMed/NCBI View Article : Google Scholar
|