1. Introduction
Melasma is a persistent condition characterized by
excessive melanin production in the skin (1). Multiple investigations utilizing the
melasma quality of life scale (MelasQoL) score have observed a
decline in the overall quality of life attributed to melasma
(2-4).
However, severity of melasma measured by the melasma area severity
index score (MASI) does not yield any statistically significant
impact on quality of life (5,6).
Melasma is observed across diverse ethnicities and geographical
regions, with higher prevalence among populations with darker skin
tones residing in regions characterized by high levels of solar
radiation compared with individuals with lighter skin tones
(4,7). Individuals with light brown skin
tones, particularly those of Latino and Asian descent, tend to
exhibit hyperpigmentation more frequently. The prevalence of
pigmented phenotypes is higher among the populations of Southeast
Asians, Middle East Asians, Mediterranean Africans, Hispanic
Americans and Brazilians (7). The
incidence of this condition in dermatological clinic patients in
Southeast Asia ranges from 0.25 to 4.0%, but previous studies have
shown higher prevalence of up to 40% in the general population
(2,4,6).
Melasma is associated with cellular malfunction in
melanocytes, which are responsible for pigment production (2). Melasma can be induced by various
factors, including ultraviolet (UV) light exposure, hormonal
fluctuations, genetic predisposition, racial background and the use
of cosmetic products (3,5). UV radiation has the potential to
induce generation of free radicals, initiating the production of
reactive oxygen species (ROS) and resulting in DNA damage (8). Melanocytes serve a pivotal part in the
process of melanogenesis by serving as the primary site for
synthesis of melanin (9). During a
typical melanogenesis process, melanin is conveyed to the outer
layer of keratinocytes to protect against DNA harm caused by UV
radiation (10). Excessive
stimulation of melanogenesis can lead to the development of
pigmentation problems (11). The
process of melanin formation is initiated by generation of ROS,
specifically hydrogen peroxide and quinone intermediates (12). UV light directly induce
melanogenesis in melanocytes and activates signaling pathways on
numerous cell types, such as keratinocytes, mast cells and
fibroblasts (8). Moreover, UV
radiation has the potential to induce cutaneous inflammation,
resulting in erythema of the skin (13). This arises because of augmentation
of blood circulation in the skin, which triggers the activation of
NF-κB transcription in immune cells, specifically keratinocytes and
dermal fibroblasts. The activation of NF-κB leads to the production
and release of cytokines, including IL-6 and TNF-α. This triggers
manufacture and secretion of proteases, specifically matrix
metalloproteinases (MMPs), which have the potential to cause damage
to collagen in the skin (14).
The extent of skin damage caused by UV radiation is
contingent upon the specific wavelength of light (10). UVA radiation induces melanin
production primarily in the basal layer of skin cells, whereas UVB
radiation promotes melanin dispersion across the epidermis. UVC,
which is the most potent oncogenic kind of UV light, has a minimal
impact on pigmentation (15,16).
However, UV radiation can stimulate all components of the
corticosteroid hormone axis, including glucosteroidogenesis
(17). The stimulation is
influenced by the wavelength of light, with the UVC spectrum, which
has the shortest wavelength, having the greatest impact (15). UVB has less effect, whereas UVA has
either no effect or only moderate impacts, limited to increases in
corticotropin-releasing hormone (CRH) and endorphin peptides
(15). UVB radiation increases the
amounts of ß-endorphin in the skin. It is hypothesized that these
responses may have evolved to protect against harmful UV radiation
and pigmentary actions, which are dependent on ß-endorphin and
regulated by p53(18). UVB
radiation increases expression and activity of the α-melanocyte
stimulating hormone (α-MSH) and melanocortin 1 receptor (MC1R), as
well as the expression of pro-opiomelanocortin (POMC) and the
generation of POMC peptides, such as α-MSH, ß-endorphin and
adrenocorticotropic hormone (ACTH) (18,19).
This is hypothesized to regulate the pigmentation of mammalian
skin, protect it from UV-induced damage and affect immunological
responses in the skin (16). UVB
radiation additionally triggers production of corticotropin hormone
and urocortin while altering the expression of type 1 CRH receptor
(15).
In addition to UV exposure, pigmentary abnormality
can arise because of chronic inflammation, mechanical trauma to the
skin and irregular production of α-MSH (8,20).
This is facilitated by a sequence of biological mechanisms wherein
melanocytes generate skin pigments, known as melanin, across
numerous levels of the dermis (21). The regulation of melanin production
in melanocytes involves the microphthalmia-associated transcription
factor (MITF), as well as enzymes specific to melanocytes, namely,
tyrosinase, tyrosinase-related protein 1 (TRP-1) and 2(22). The primary objective in preventing
skin problems, such as hyperpigmentation, is to target inhibition
of melanogenic enzymes and MITF (23).
Nevertheless, management of hyperpigmentation
conditions, such as melasma, typically necessitates a protracted
duration for effective treatment (2). The extended length of treatment is
associated with diminished patient satisfaction (20). Various treatment modalities
necessitate both topical and oral interventions (11). The utilization of bleaching
compounds, such as corticosteroids, hydroquinone, monobenzyl
hydroquinone, tretinoin and mercury, has been prohibited in
numerous regions (21). One example
is hydroquinone 2%, a depigmenting agent employed for an extensive
period due to its ability to impede activity of tyrosinase, the
primary enzyme involved in melanogenesis (20). However, use of hydroquinone 2% is
controversial as it induces irritation and is linked to the
development of skin malignancies (24). In the context of oral therapy,
certain antioxidants, such as vitamin C and glutathione, have been
demonstrated to be effective in treatment of melasma (25).
Because melasma is a chronic and recurrent
hyperpigmentation disease, protecting the skin from triggers is the
best course of action (1). The
prevention of hyperpigmentation can be achieved by strategies such
as applying sunscreen, utilizing antioxidants and taking vitamins
and nutrients (26). The role of
nutrition in promoting skin health has been widely recognized as it
offers numerous advantages such as preventing premature aging and
reducing the risk of skin disease (24,27,28).
‘Nutricosmetic’ refers to food that contains specific components,
such as vitamins, peptides, polysaccharides, polyphenols, coenzyme
Q10, polyunsaturated fatty acid (PUFA) and carotenoids, which are
intended to enhance cosmetic outcomes (24). Carotenoids can be acquired from a
diverse range of dietary sources, including natural food sources
and supplementary forms (29).
Carotenoids play a notable role in human nutrition as they serve as
precursors to vitamin A (28).
Carotenoids have preventive attributes that safeguard the skin
against oxidative free radical harm, as well as from high-energy
sources, such as UV radiation (24). Carotenoids are present in both
topical and oral formulations, with the most prevalent variants
being β- and α-carotene, lycopene, lutein, zeaxanthin and α- and
β-cryptoxanthin (30).
The mechanism by which carotenoids exert their
antioxidant activity is hypothesized to involve prevention of lipid
peroxidation and the scavenging of singlet oxygen (31). These potent antioxidants may inhibit
deposition of pigments by inhibiting the enzyme tyrosinase,
decreasing inflammation and interfering with the generation of free
radicals (32). The administration
of carotenoids in oral formulations facilitates dissemination of
their systemic effects to the dermis and epidermis, resulting in a
decrease in migration of pigments from the epidermis to the dermis
(33). Carotenoids possess high
efficacy as natural antioxidants (28,29).
O2 quenching has been demonstrated to be highly
effective as a scavenging agent, particularly for carotenoids with
11 conjugated double bonds (29).
Carotenoids also function as chemical quenchers of singlet oxygen,
undergoing changes such as oxidation or oxygenation (28,29).
Carotenoids are involved in three widely recognized primary
mechanisms for scavenging free radicals: Transfer of electrons
between carotenoids and free radicals; direct addition of free
radicals to carotenoids and hydrogen atom transfer to carotenoids
(24,34,35).
Carotenoid radical products have potential to undergo additional
changes, resulting in secondary carotenoid derivatives with varying
levels of reactivity (29). This is
key because the newly produced carotenoid species may cease to
function as effective antioxidants and may become potentially
damaging, pro-oxidant agents (24,31).
Carotenoids can be derived from fruit extracts, such
as those obtained from red fruit (Pandanus Conoideus Lam.).
The consumption of red fruit, which exerts anti-diabetic,
antitumor, anti-inflammatory, and anti-atherosclerosis, is
prevalent in the Papua region of Indonesia (36-41).
Several studies have indicated that red fruit has a significant
concentration of carotenoids and tocopherols (36,37,39,42-44).
To the best of our knowledge, however, the amount of research
studying the impact of carotenoids found in red fruit on skin
health remains limited. The lack of studies may be attributed to
the fact that red fruit is exclusively found in Indonesia. The
primary objective of the present literature review was to assess
the potential role of red fruit as an anti-pigmentation agent in
the prevention of melasma.
2. Melanogenesis and melasma treatment
The skin can respond to numerous stimulatory signals
through a cutaneous neuroendocrine system (45). The skin contains parts of the
hypothalamic-pituitary-adrenocortical (HPA) axis, affected by
environmental stresses and associated with skin functions,
including melanogenesis (45,46).
Exposure to stressful events triggers activation of the central HPA
axis (47,48). Under stress, the body produces and
releases CRH, which, in turn, enhances the expression of POMC
(49,50). POMC undergoes enzymatic conversion
to produce ACTH and other melanocortin peptides, including α-MSH
(48,50). CRH has been detected in the human
skin and promotes ACTH synthesis and release (49,51).
ACTH attaches to MC2R in the adrenal cortex, which triggers
production and release of glucocorticoids into the bloodstream
(50,51). This leads to a range of
physiological effects such as regulating metabolism, reducing
inflammation, and suppressing the immune response (49-51).
Hence, it is key to determine the skin's ability to control
processing of POMC-derived peptides and to examine variations in
this process between keratinocytes and melanocytes (19,50).
Multiple signaling molecules/ligands are necessary
throughout melanocyte formation (17,52).
The involvement of specific G-protein-coupled receptors (GPCRs) in
transformation of melanocytes may be because of their crucial role
in the development and maintenance of melanocytes (51-53).
GPCRs play significant roles in the physiology of melanocyte
lineage, influencing all stages of development and functions of
mature melanocytes (52,53). GPCR ligands are found in the skin
and control melanocyte homeostasis, which includes pigmentation
regulation (52). Endothelins (ETs)
and endothelin receptor type B (EDNRB) are involved in melanocyte
transformation and the advancement of melanoma (53). The ET system comprises three closely
related short peptides (ET-1, 2 and 3), two GPCRs (EDNRA and EDNRB)
and two proteinases endothelin-converting enzyme 1 and 2 (52,53).
Melanocytes have a receptor called MC1R, which
regulates melanogenesis. MC1R is a member of a minor group of GPCRs
that are divided into five distinct subtypes that play a key role
in essential physiological functions (19,49,51).
MC1R is the sole melanocortin receptor that is present in
melanocytes (50,53). MC1R is a receptor belonging to class
A and is linked to Gs protein (46). MC1R interacts with α-MSH, which is
produced from pro-opiomelanocortin (18,19). A
number of enzymes act as prohormone convertases on POMC leading to
the generation of numerous hormones, such as ACTH, α-MSH, β-MSH,
γ-MSH, β-endorphin (β-END) and β-lipotropic hormone (47,53).
Various POMC-derived peptides are generated dependent of tissue
origin and these peptides exert effects on distinct melanocortin
receptors (16,54,55).
POMC is locally expressed in the skin (18,19,50).
The processing of POMC in human skin is similar to that in the
hypothalamus as ACTH, α-MSH and β-END have been detected in
melanocytes and keratinocytes and skin biopsies of rats (49,51,53).
Melanogenesis refers to the biological process of
synthesizing melanin pigments, predominantly by specialized cells
known as melanocytes (56). The
principal role of melanocytes is synthesis of melanin pigments
(57). Melanin pigments are
regulated by positive and negative regulators (16). The positive regulators are MC1R
ligands with melanocortins and ACTH (16). The negative regulator is locally
produced agouti signaling protein, which acts as an antagonist to
melanocortins by binding to the same or separate sites on MC1R,
causing a switch from eumelanogenesis to pheomelanogenesis and
inhibiting melanogenesis (16).
Melanocytes within the epidermis are enveloped by
keratinocytes, with an estimated ratio of 1 melanocyte to 36
keratinocytes (9). These
melanocytes effectively transfer melanin pigment to the surrounding
keratinocytes (58). Melanocytes
have melanosomes, which are organelles resembling lysosomes at the
subcellular level (59). These
melanosomes are responsible for synthesis and storage of melanin
pigments, which are distributed to the neighboring keratinocytes
(56). The regulation of
melanogenesis involves coordination of several signaling networks,
including the Wnt/β-catenin, PI3K/Akt, cAMP/protein kinase (PK) A,
and stem cell factor (SCF)/c-kit-mediated signaling pathways
(60).
Wnt signaling serves a critical role in melanocyte
development (61,62). Research has demonstrated that
canonical Wnt signaling, specifically Wnt1 and Wnt3a, has crucial
roles in the development of melanocytes (62,63). A
typical canonical Wnt pathway molecule, promoted melanogenesis of
melanocytes via the up-regulation of the expression of MITF,
tyrosinase and TRP (56,62,63).
The initiation of Wnt receptor complexes induces substitution of
the versatile glycogen synthase kinase-3β, resulting in buildup of
β-catenin (61). The stable form of
β-catenin is translocated to the nucleus, where it upregulates
production of MITF, thereby promoting melanogenesis (63). The upregulation of Wnt expression
either via the canonical or non-canonical signaling pathway happens
in a gradual manner inside the hyperpigmented skin regions of
individuals with melasma (56).
An increased intracellular concentration of cAMP
facilitates the activation of PKA, which, in turn, phosphorylates
CREB and CREB binding protein (64). This phosphorylation results in the
upregulation of MITF expression (65). The activation of melanogenic gene
promoters by MITF leads to upregulation of melanogenesis (56). A study investigating the dominant
negative mutant of MITF, which lacks the transactivation domain,
demonstrated the essential role of MITF in cAMP-induced stimulation
of tyrosinase production (66).
During melanogenesis, tyrosine and levodopa serve as
substrates for tyrosinase (12,56).
In addition, they serve as bioregulatory agents for other cellular
activities (11,67). These processes encompass development
of dendrites and the promotion of cell migration, achieved by
reducing activity of PKC (68).
Although PKC serves a regulatory role in melanogenesis, cAMP is the
most important biochemical regulator (12,26,58).
Furthermore, control of melanogenesis is associated with the p38
MAPK and PI3K/AKT signaling pathways (22). The process of phosphorylating p38
MAPK leads to upregulation of MITF and tyrosinase, hence inducing
the activation of melanin production (69). In addition, activation of the
PI3K/AKT pathway leads to a decrease in melanin synthesis by
downregulating MITF, tyrosinase and TRPs) (56). Conversely, inhibiting the PI3K/AKT
pathway enhances melanin formation by activating MITF and inducing
expression of tyrosinase (70).
The involvement of this signaling system in
physiological adaptations of the skin to environmental variables,
such as exposure to UV radiation, is widely acknowledged (12,56,71,72).
The most important function of the skin is as a physical barrier,
which is determined by its location between internal and external
environments (45). The epidermal
pigmentary system protects skin from the damaging effect of solar
radiation (45). Research has
demonstrated that UV radiation stimulates the activation of the
MC1R (46), which may be why the
skin darkening effects of α-MSH and ACTH are particularly
noticeable in sun-exposed parts of the skin (68). UV radiation can also induce the
release of POMC peptides in the skin (48,53).
Studies have demonstrated that cultured keratinocytes generate
α-MSH and ACTH when exposed to UV radiation (46,58,73).
POMC peptides may serve as both paracrine and autocrine mediators
in the tanning response, as evidenced by similar reactions in
melanocytes (46). Moreover, these
peptides are not limited to melanogenesis; they may serve a role in
other processes related to the pigmentary response, such as
enhancing the branching of melanocytes and promoting interactions
with keratinocytes and extracellular matrix (49,50).
As aforementioned, POMC peptides may have a key function in
coordinating the events that take place during a pigmentary
response (46). The expression of
POMC varies in many situations such as normal hair development,
production of immune cytokines, presence of skin disease or
exposure to UV radiation (46). The
activation of the MC1R by α-MSH or ACTH leads to an elevation in
cAMP synthesis (74). This
indirectly triggers a shift from the creation of pheomelanin to the
synthesis of eumelanin (67). In
addition to the α-MSH-MC1R signaling route, the SCF/receptor
tyrosine kinase Kit (SCF/c-Kit) pathway serves a role in melanocyte
pigmentation and development by activating the MITF transcription
factor, specifically the M-MITF isoform that is exclusive to the
melanocyte lineage (56). The
induction of pigmentation by UV and visible light is facilitated by
release of SCF (75). SCF acts as a
ligand for the tyrosine kinase receptor c-KIT, thereby initiating
downstream actions that result in proliferation of melanocytes
(10). The secretion of 19 SCF,
often referred to as mast cell growth factor, is observed in human
keratinocytes and fibroblasts (64). The dermal manifestation of melasma
is characterized by upregulation of SCF due to prolonged exposure
to UV radiation, leading to skin inflammation and activation of
fibroblasts (11). This
upregulation of SCF serves a key role in the stimulation of
melanogenesis, resulting in an increase in melanin production
(69).
The management of melasma poses a challenge and
necessitates the implementation of long-term therapy strategies
involving topical medicines (1).
The outcomes frequently yield dissatisfaction, and the utilization
of topical medications may lead to notable adverse responses such
as skin irritation, redness, and dryness (4). The proposed first-line topical
treatment for this pigmentary condition is a triple combination of
hydroquinone, retinoic acid and corticosteroids (1). Numerous compounds such as kojic acid,
arbutin, cysteamine, that impede the process of melanogenesis have
been created (1,25,76).
The assessment of melasma involves numerous evaluation tools,
including the MASI and modified MASI score, MelasQoL, colorimetry
and mexametry (4,67). Melasma mostly affects parts of the
skin that are exposed to sunlight, particularly in female subjects
in their reproductive years due to hormonal imbalance (2). There is currently no known cure for
this condition, which has a substantial negative impact on overall
quality of life (2,4,6). This
impact includes a decrease in self-esteem, leading to social
challenges, heightened anxiety and symptoms of depression (6).
The absence of a universally effective medicine
results in a preference for combination treatment (67). Various therapeutic modalities are
available for treatment of pigmentation disorders (11). These include topical hypopigmenting
agents, utilization of laser technology, microneedling techniques,
administration of chemical peels, utilization of radiofrequency
devices, and the use of oral drugs (77). Moreover, it is imperative for
patients to refrain from activities or circumstances that may
worsen their condition (12).
Promising strategies encompass the reduction of both
local and systemic oxidative stress, stabilization of mast cells in
the upper dermis, reduction of melanogenesis without causing damage
to melanocytes, removal of epidermal melanin without inducing
inflammatory responses, reversal of senescence and induction of
autophagy (64). Owing to the
incomplete understanding of the etiology of melasma, there are
potential opportunities for the advancement of novel therapeutic
approaches that target mechanisms responsible for persistent
pigmentation, as opposed to only reducing melanin production and
eliminating melanin from the outer layer of the skin (11). Treatment of this condition should
involve a comprehensive approach that integrates photoprotective
agents, antioxidant therapies, skin-lightening agents, exfoliants
and resurfacing procedures in instances of severe manifestation
(26,77). Numerous novel oral, topical and
combination medications have been developed and require clinical
trials to validate their effectiveness and safety (25).
Endogenous photoprotection through the consumption
of dietary elements, such as carotenoids, has been the topic of
study (30). The greatest
proportion of dietary carotenoids is derived from fruits and
vegetables, which serve a crucial role in nutritional intake
(28). Humans rely on regular
availability of carotenoids as they serve as precursors for vitamin
A (29). This vitamin is needed for
various bodily functions, including eyesight and cell signaling
(24). Carotenoid pigments serve as
a protective mechanism for the photosynthetic system in plants by
effectively dissipating surplus energy (31). Moreover, multiple lines of evidence
substantiate the hypothesis that carotenoids play a crucial role in
safeguarding human skin against diseases generated by UV radiation
(24,29,30).
Enhancing the carotenoid concentration in plants may enhance the
nutritional value of food derived from them as essential cellular
signaling systems and defensive mechanisms are typically preserved
across organisms in natural environments (28).
3. Carotenoid effects on skin health
The effect of carotenoids from various plants on
skin health has been widely studied (28,70,78-81).
β-carotene can absorb UVB rays and serve as a ROS scavenger to
reduce the production of inflammatory cytokines (31). β-carotene is a potent antioxidant
due to its ability to neutralize singlet oxygen, which decreases
skin aging due to sun exposure. In tissue, β-carotene is a source
of vitamin A (82). Research
regarding the effect of carotenoids on skin health is shown in
Table I.
 | Table ICarotenoid effects on skin
health. |
Table I
Carotenoid effects on skin
health.
| First author,
year | Methods | Results | (Refs.) |
|---|
| Juturu et
al, 2016 | In a randomized,
double-blind, placebo-controlled clinical trial for 12 weeks with
50 participants using lutein supplements, zeaxanthin was measured
with a chromameter. | Carotenoid content
in lutein and zeaxanthin isomers protects the skin from
sunlight | (81) |
| Hashemi-Shahri
et al, 2018 | Spectrophotometric
evaluation was conducted to assess impact of crocetin on activity
of intracellular and mushroom tyrosinase, as well as melanin
concentration. Protein levels of tyrosinase and MITF were assessed
in control and crocetin-treated cells. Anti-oxidative activity was
also assessed | Crocetin, a
naturally occurring carotenoid, inhibits the action of tyrosinase
and MITF | (79) |
| Lee et al,
2018 | Anti-pigmentation
effects mediated by CE were assessed by three-dimensional rebuilt
pigmented epidermis model, Fontana-Masson staining and melanin
content assays. | Extract derived
from CE inhibits mRNA and protein levels of
microphthalmia-associated transcription factor, tyrosinase,
tyrosinase-related protein-1, and tyrosinase-related protein-2 and
suppresses the phosphorylation of protein kinase A and
extracellular signal-related kinase, both of which serve key roles
as upstream regulators in melanogenesis. Efficacy of extracts from
Chlamydomonas reinhardtii plant to mitigate pigmentation was
validated | (78) |
| Phacharapiyangkul
et al, 2021 | Analysis of
molecular process within B16F10 murine melanoma cells. | Significant
inhibitory effect of MPE on melanogenesis in B16F10 cells
stimulated with α-MSH. The concentration-dependent effect of MPE on
suppression of MITF and tyrosinase expression was observed The
inhibitory effect of Musa sapientum, which contains
β-carotene, on melanogenesis in α-MSH-induced B16F10 cells was
demonstrated. | (70) |
Clinical research by Juturu et al (81)on lutein and zeaxanthin (L/Zi) isomers
showed skin color brightening and improved skin structure: The
study was a clinical trial conducted over a 12-week supplementation
period, employing a randomized, double-blind and placebo-controlled
design. A total of 50 individuals who were in good health were
selected to participate, with 46 of them successfully completing
the research. The participants consisted of both males and females,
ranging in age from 18 to 45 years. All individuals had mild to
moderate dry skin. The skin type was categorized according to the
Fitzpatrick skin type II-IV scale (2,3). The
participants were given either a daily oral dietary supplement
consisting of 10 mg L and 2 mg Zi isomers [3R,3'R-Zi and 3R,3'S
(meso)-Zi] or a placebo. The measurement of the least erythemal
dose and skin lightening (L*) was conducted using
Chromameter®. The individual typological angle (ITA˚)
was computed, based on L* value and sallowness (b* value) of the
skin. Furthermore, subjective evaluations were documented (81).
The skin-lightening action of L/Zi may be attributed
to its ability to block high-energy blue light rays found in both
sunshine and indoor lighting (83).
In addition, as a UV absorber/filter, it may promote inhibition of
tyrosinase and boost antioxidant capacity (80). The pigmentation of the skin is
determined by the presence and distribution of different forms of
melanin, namely, pheomelanin and eumelanin (16). L/Zi decreases inflammation and
inhibits the activity of free radicals, hence decreasing the
production of both forms of melanin (81). A decrease in eumelanin results in an
increase in the L* value, whereas a decrease in pheomelanin leads
to a decrease in b* value (81).
Consequently, this will result in an augmentation of the ITA˚,
causing a brightening effect on the entire complexion (81). When the L/Zi is ingested, it enters
the dermis and then the epidermis, causing a decrease in the depth
of pigmentation in the skin (81).
Numerous ongoing investigations are examining the precise method by
which it affects skin whitening and pigmentation (80,81,83). L
and Zi possess carotenoid properties that inhibit melanin pathways,
decrease cytokine levels and enhance antioxidant activity inside
the skin (81).
Hashemi-Shahri et al (79) discovered crocetin (a carotenoid) in
saffron plants has antioxidant and anti-melanogenesis effects in
melanoma culture cells (79). The
aforementioned study was undertaken to investigate the
anti-tyrosinase effects of crocetin, given its well-documented
antioxidant activity in several studies and the significant role it
plays as an antioxidant (32,79,84).
At the protein level, crocetin decreases expression
of tyrosinase and MITF (79).
Therefore, crocetin may be proposed as a promising dermatological
depigmenting agent in the formulation of skin care products
(85). The presence of antioxidants
derived from natural sources that possess anti-tyrosinase activity
serves a significant role in reducing skin damage caused by
melanogenesis (33). Phytochemicals
serve as precursors to produce molecules that exhibit decreased
toxicity in comparison with synthetic substances (86). Crocetin is a naturally occurring
carotenoid and a constituent of saffron (Crocus sativus L.)
(87). Crocetin is a diterpene
dicarboxylic acid possessing symmetrical characteristics, featuring
seven double bonds and four methyl groups. The glycosylated form of
crocetin, known as crocin, accounts for ~94% of the total crocetin
content in saffron and the remaining 6% exists in the free form
(79). The aforementioned study
showed that crocetin effectively suppresses expression of
tyrosinase and MITF proteins in comparison with control cells when
administered at concentrations of 1, 2, 4, 8 and 16 µM (79). Tyrosinase is a key enzyme involved
in melanogenesis in the skin, where it plays a role in controlling
formation of melanin (58). The
decrease in melanin synthesis in cultivated B16 melanoma cells is
hypothesized to be associated with inhibition of tyrosinase
activity (79). Alternatively,
degradation of MITF indicates suppression of TRPs and production of
tyrosinase (79). The levels of
tyrosinase protein directly influence the degree of melanin
synthesis in cells (26).
The participation of dioxygen at the dinuclear
copper ions located at the active site of tyrosinase may influence
production of ROS during the melanin synthesis process (69). Hence, substances that impede the
generation of both free radicals and the activity of tyrosinase
might augment protection of the skin from oxidative stress and
hyperpigmentation (79). The
reduction in tyrosinase and MITF protein levels caused by crocetin,
together with its antioxidant properties, indicates its potential
as an agent for inhibiting melanogenesis (79).
The anti-melanogenic effects of plant extracts
derived from Chlamydomonas reinhardtii (CE) were examined by
Lee et al (78). The study
evaluated the probable mechanisms underlying the inhibitory impact
of CE on B16F10 melanoma cells and normal human epidermal
melanocyte cells and human skin-equivalent models (78). CE is a microalgae species containing
antioxidants, phenolic components and pigments such as carotenoids
(78). Multiple studies have
demonstrated that carotenoids effectively scavenge ROS and can
provide protection against the generation of ROS by UV radiation,
as well as hyperpigmentation in melanocytes (24,28,31,32,44,88).
The aforementioned study revealed a dose-dependent decrease in
cellular melanin content as a result of treatment with CE extract
(78). Nevertheless, this did not
arise from a direct inhibition of tyrosinase activity, suggesting
that CE extract may operate via alternative inhibition pathways not
reliant on the enzyme catalytic activities (32,57,78).
The expression of additional genes necessary for the production of
melanin, such as TRP-1 and TRP-2, was similarly decreased (78). One potential explanation could be
that the extract has a suppressive impact on the protein function,
as tyrosinase, Trp-1 and Trp-2 all have MITF as a shared
transcription factor (28,32,57,78).
CREB phosphorylation enhances its ability to attach to the MITF
promoter (78). The aforementioned
study clearly demonstrated that the treatment with the extract
effectively prevented phosphorylation of PKA and CREB in
α-MSH-stimulated B16F10 cells (78). This indicates that downregulation of
MITF by the CE extract was achieved by inhibiting the PKA/CREB
pathway triggered by α-MSH (78).
Subsequent investigations will elucidate the precise processes and
molecular target of CE extract-induced suppression of the PKA/CREB
pathway in melanocytes. UV irradiation induces melanogenesis by
phosphorylating and activating ERK in human melanocytes, but JNK
and p38 remain unaffected (14,71,78).
Another source of carotenoids that are well known
for their antioxidant properties is banana (Musa sapientum;
MS) (70,89). Phacharapiyangkul et al
(70) demonstrated a significant
inhibitory effect of MS extract (MPE) on melanogenesis in B16F10
cells stimulated with α-MSH. The concentration-dependent inhibition
of MITF and tyrosinase expression is observed upon treatment with
MPE (70). Furthermore, MPE results
in a substantial concentration-dependent reduction in the levels of
melanosome transfer protein indicators such as Rab27a and Pmel17)
(70). The aforementioned study
observed a reduction in the heightened phosphorylation of AKT in
B16F10 cells following treatment with MPE. In addition, MPE
treatment resulted in alterations in microtubule-associated protein
1 light chain 3-II and p62, which are recognized as indicators of
autophagy (70,73). The aforementioned study suggested
that MPE has the potential to serve as a viable treatment for
inhibiting melanogenesis. This inhibitory impact may be achieved by
the modulation of the AKT pathway, leading to a decrease in MITF
expression and suppression of tyrosinase enzyme family production
(70). The aforementioned study
suggested that MPE has potential as a depigmenting agent in
cosmeceuticals (24,31,32,70).
MPE containing β-carotene has benefits as a suppressor of
melanogenesis in α-MSH-induced B16F10 cells associated with the AKT
signaling pathway (34,70). MPE inhibits melanogenesis by
inhibiting melanosome transport and autophagy (70). There also studies that show that MPE
effectively inhibits melanin production in B16F10 mouse and G361
human melanoma cells (89,90).
4. Carotenoid content in red fruit and its
potential role as an anti-pigmentation agent
Red fruit (Pandanus conoideus Lam.) is a
traditional fruit from Papua that is high in antioxidant content.
Table II summarizes the potential
anti-pigmentation role of carotenoids in red fruit. Red fruit oil
(RFO) has high carotenoid and tocopherol contents (42,91,92).
Roreng et al (92) showed
that carotenoids (pro-vitamin A) in red fruit extracts have high
bioavailability. Red fruit contains high levels of bioactive
compounds, including flavonoids 238.63 mg/100 g quercetin
equivalent, tannins 600.71 mg/100 g tannic acid equivalent; vitamin
C (958.18 mg/100 g), β-carotene (287,416.99 µg/100 g) and
antioxidants 372.15 mg/l Garlic acid equivalent antioxidant
capacity (GAEAC) (93). The
variation in RFO antioxidant content depends on where red fruit is
grown.
 | Table IICarotenoid contents in red fruits and
its potential role as an anti-pigmentation agent. |
Table II
Carotenoid contents in red fruits and
its potential role as an anti-pigmentation agent.
| First author,
year | Methods | Results | (Refs.) |
|---|
| Roreng et
al, 2014 | Carotenoid
depletion and repletion to evaluate the carotenoid bioavailability
of red fruit extract and determine retinol accumulation factor in
rat liver. | Carotenoids in red
fruit extract are absorbed, metabolized and stored. The retinol
accumulation factor was 49.2 and relative bioavailability with pure
β-carotene was 86.52%. | (92) |
| Dumaria et
al, 2018 | Experimental study
using post-test- only group design. Cavia porcellus was
exposed to UVB, basic cream, 4% hydroquinone or 10% red fruit
extract cream. The amount of melanin was compared using the
percentage of the pixel area | 10% red fruit
extract cream prevents increase in amount of melanin in guinea pig
skin exposed to UVB light as effectively as 4% hydro- quinone
cream | (93) |
| Sugianto et
al, 2019 | A total of 30 male
Wistar rats were examined for MMP-1 expression and MDA levels. RFO
was identified with β-carotene and tocopherol content phytochemical
screening assay. Identification of β-carotene and tocopherol by
TLC, UVB irradiation, RT-PCR and TBARS assay | RFO contains
tocopherol and β-carotene, which can reduce MMP-1 gene expression
but has no significant effect on MDA levels | (44) |
| Wulansari et
al, 2020 | HLPC analysis was
performed using an HLPC system. | Concentration of
β-carotene varied between 193.9 and 1,003.8 µg/ml, whereas the
concentration of β-cryptoxanthin ranged from 3.3 to 48.9 µg/ml | (91) |
Current research regarding the potential advantages
of red fruit for promoting skin health remains limited. To the best
of our knowledge, over the last decade, only two articles that
specifically examined the advantages of red fruit in relation to
skin health have been published (44,93).
The aforementioned study on red fruits explores the advantages of
consuming red fruit in relation to its anti-inflammatory,
anticancer and antioxidative stress capabilities. However, the
aforementioned investigations have shown encouraging findings,
indicating that red fruit may enhance skin health. Sugianto et
al (44) conducted in
vivo research on rat skin exposed to UVB light and it was
proven that RFO contained β-carotene and tocopherol, which
decreased expression of the MMP-1 gene. The aforementioned study
used 30 male Wistar rats grouped as follows: P0, no treatment,
whereas; P1, UVB light; P2, UVB light and 0.5 ml/200 g body weight
(BW) RFO; P3, UVB light and 1 ml/200 g BW RFO and P4, UVB light and
2 ml/200 g BW RFO. The expression of MMP-1 and the levels of
malondialdehyde were assessed. RFO can be characterized by its
β-carotene and tocopherol levels. The aforementioned experiment
used a phytochemical screening assay to identify presence of
flavonoids, phenolics, triterpenoids, saponins, tannins, steroids
and alkaloids in RFO; identification of β-carotene and tocopherol
was performed using thin layer chromatography, malondialdehyde
levels were tested with thiobarbituric acid reactive substances
assay and reverse transcription-quantitative PCR was used to see
MMP-1 mRNA expression (44).
Research on anti-melanocyte effects conducted on
Cavia porcellus skin exposed to UVB light by Dumaria et
al (93) revealed that 10% red
fruit extract cream could prevent an increase in amount of melanin
in guinea pig skin exposed to UVB rays as effectively as 4%
hydroquinone. The aforementioned experimental study employed
post-test-only control group design. The subjects were divided into
three groups of 10 guinea pigs as follows: Group 1 (control group),
UVB radiation and standard cream; group 2, UVB radiation and
topical cream containing 4% hydroquinone and group 3, UVB radiation
and cream containing 10% red fruit extract. The cumulative UVB
dosage administered over 2 weeks was 390 mJ/cm2. The
quantification of melanin content was performed by determining the
proportion of the pixel area occupied by melanin and compared with
all epidermal tissues. The aforementioned study revealed that group
1 exhibited the highest proportion of melanin area, with a mean
value of 19.78±3.79%. The melanin area percentage in group 3 was
found to be 1.25±0.76%, whereas in group 2, it was 0.85±0.37%.
There were statistically significant variations in the proportion
of melanin observed between group 1 and groups 2 and 3. There was
no statistically significant difference in the proportion of
melanin between group 2 and group 3(93).
Antioxidants inhibit generation of ROS, which can
initiate the melanogenesis process (94), thus inhibiting UVB-induced
melanogenesis (12,26,58).
The red fruit extract in a previous study contained 372.15 mg/l
GAEAC (93). Red fruits contain
flavonoids that directly inhibit the enzyme tyrosinase, making them
potential anti-pigmentation agents when exposed to UV rays. Tannins
have antioxidant properties and anti-tyrosinase activity (93). UVB radiation does not lead to an
increase in melanin formation due to suppression of the melanin
synthesis process (93). Topical
treatment such as cream has a high antioxidant content (84) which is effective in mitigating the
impact of ROS on the skin (94).
Furthermore, incorporation of antioxidants in cream can enhance
skin hydration and decrease water loss through the skin. The
findings of the aforementioned study indicate that the red fruit
extract in ointment as topical treatment effectively inhibits the
melanin production in marmot skin exposed to UVB radiation
(93).
Wulansari et al (91) showed that the bioactivity of red
fruit is influenced by its natural antioxidant content, including
carotenoids, α-tocopherol and unsaturated fatty acids. The
aforementioned study involved the collection of five distinct
cultivars of red fruit, specifically Maler, Bergum, Wesi, Uaghelu
and Kenen, originating from various places in Papua. High
performance liquid chromatography analysis was employed to assess
the carotenoid content, specifically β-carotene and
β-cryptoxanthin, in red fruit oil of the five clones. The
concentration of β-carotene varied between 193.9 and 1003.8 µg/ml,
whereas the concentration of β-cryptoxanthin ranged from 3.3 to
48.9 µg/ml. Red fruit exhibits a notable concentration of
carotenoids, with β-carotene being recognized for its antioxidant
properties (42,44). In addition, β-cryptoxanthin exerts
preventive effects against lung cancer, emphysema and osteoporosis
(91).
The aforementioned studies demonstrate the
potential role of carotenoids in red fruit as an anti-pigmentation
agent via several pathways such as PKA, ERK, AKT, MMP1 and MITF.
These pathways lead to pigmentation process through collagen
degradation and tyrosinase, which regulates synthesis of melanin.
Carotenoids in red fruit have a potential role in inhibiting the
aforementioned pathways (Fig.
1).
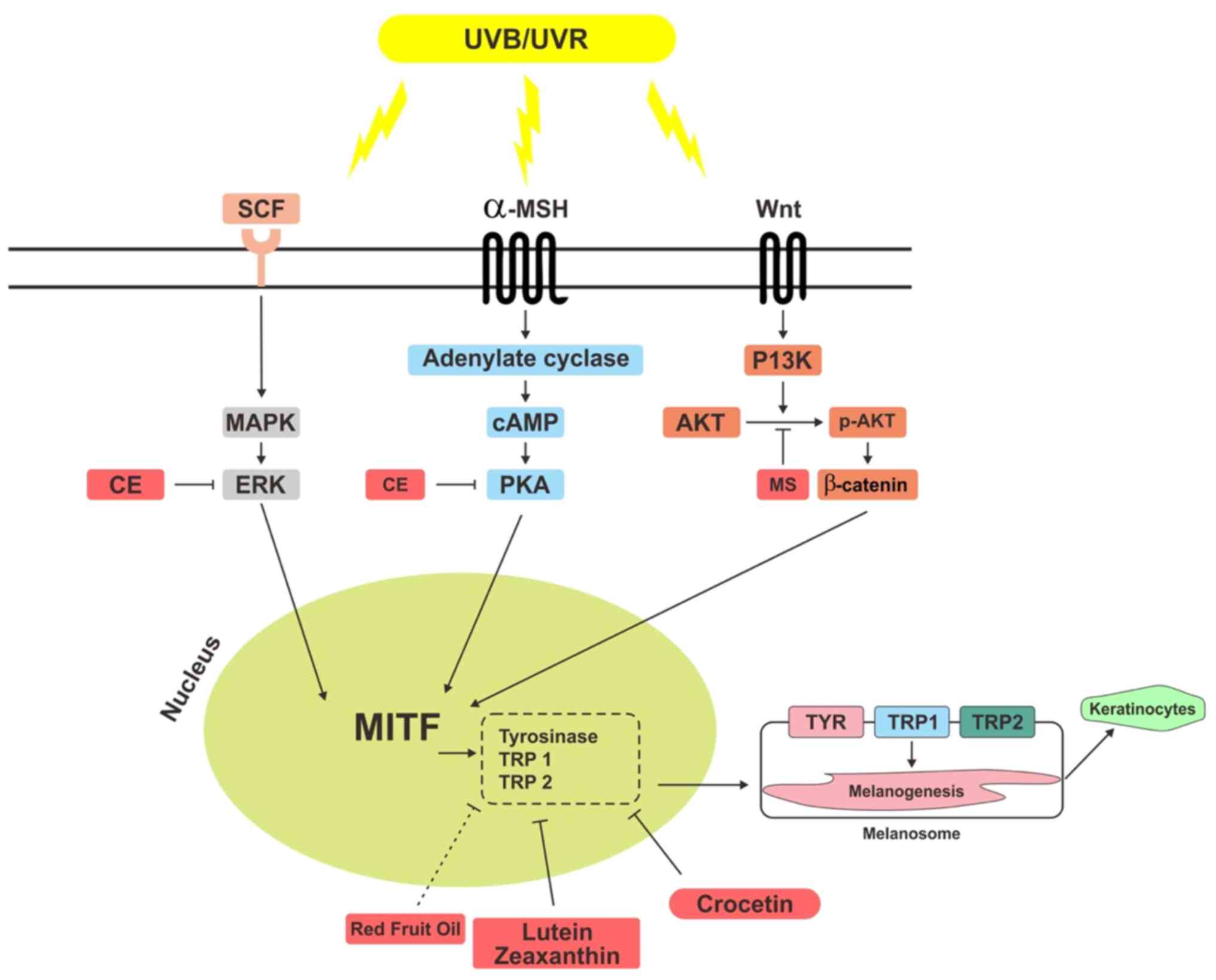 | Figure 1Potential mechanisms of carotenoids
in red fruit as an anti-pigmentation agent. RFO contains
carotenoids inhibit MITF by suppressing tyrosinase. Lutein,
zeaxanthin, crocetin, CE, and MS have carotenoid contents that
inhibit ERK, PKA, and PI3K/AKT pathways, thereby inhibiting
activation of MITF. MITF, microphthalmia-associated transcription
factor; CE, Chlamydomonas reinhardtii; MS, musa
sapientum; PKA, protein kinase A; UVR, Ultraviolet Radiation;
SCF, stem cell factor; MSH, melanocyte stimulating hormone; p-,
phosporylated; TRP, tyrosinase related protein; TYR,
tyrosinase. |
5. Conclusion
Skin protection is the primary prevention method
for melasma. Consuming carotenoids has benefits for the skin as
melanogenesis inhibition occurs via PKA, ERK, and AKT signaling
pathway, which can reduce tyrosinase activity as well as MITF gene
expression. Red fruit (Pandanus conoideus Lam.) contains
high amounts of carotenoids. This fruit also has benefits as an
antioxidant and anti-inflammatory agent and it has the possibility
of being an anti-pigmentation agent that can inhibit melanogenesis,
similar to other plants that contain carotenoids. Further in
vitro and in vivo research needs to be conducted on the
potential of red fruit against melanogenesis.
Acknowledgements
Not applicable.
Funding
Funding: The proofreading service and publication fee for the
present study was supported by Universitas Kristen Maranatha.
Availability of data and materials
Not applicable.
Authors' contributions
ST and JWG conceived the study and performed the
literature review. HR and RL performed the literature review. All
authors wrote the manuscript. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Piętowska Z, Nowicka D and Szepietowski
JC: Understanding melasma-how can pharmacology and cosmetology
procedures and prevention help to achieve optimal treatment
results? A narrative review. Int J Environ Res Public Health.
19(12084)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Handel AC, Miot LDB and Miot HA: Melasma:
A clinical and epidemiological review. An Bras Dermatol.
89:771–782. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhu Y, Zeng X, Ying J, Cai Y, Qiu Y and
Xiang W: Evaluating the quality of life among melasma patients
using the MELASQoL scale: A systematic review and meta-analysis.
PLoS One. 17(e0262833)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Majid I and Aleem S: Melasma: Update on
epidemiology, clinical presentation, assessment, and scoring. J
Skin Stem Cell. 8(e120283)2022.
|
|
5
|
Jusuf NK, Putra IB and Mahdalena M: Is
there a correlation between severity of melasma and quality of
life? Open Access Maced J Med Sci. 7(2615)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yalamanchili R, Shastry V and Betkerur J:
Clinico-epidemiological study and quality of life assessment in
melasma. Indian J Dermatol. 60(519)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsai J and Chien AL: Photoprotection for
skin of color. Am J Clin Dermatol. 23:195–205. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Espósito ACC, Brianezi G, de Souza NP,
Miot LDB, Marques MEA and Miot HA: Exploring pathways for sustained
melanogenesis in facial melasma: An immunofluorescence study. Int J
Cosmet Sci. 40:420–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cichorek M, Wachulska M, Stasiewicz A and
Tymińska A: Skin melanocytes: Biology and development. Postepy
Dermatol Alergol. 30:30–41. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Espósito ACC, Cassiano DP, da Silva CN,
Lima PB, Dias JAF, Hassun K, Bagatin E, Miot LDB and Miot HA:
Update on melasma-part I: Pathogenesis. Dermatol Ther (Heidelb).
12:1967–1988. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maddaleno AS, Camargo J, Mitjans M and
Vinardell MP: Melanogenesis and melasma treatment. Cosmetics.
8(82)2021.
|
|
12
|
Slominski RM, Sarna T, Płonka PM, Raman C,
Brożyna AA and Slominski AT: Melanoma, melanin, and melanogenesis:
The Yin and Yang relationship. Front Oncol.
12(842496)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ansary TM, Hossain MR, Kamiya K, Komine M
and Ohtsuki M: Inflammatory molecules associated with ultraviolet
radiation-mediated skin aging. Int J Mol Sci.
22(3974)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Calniquer G, Khanin M, Ovadia H,
Linnewiel-Hermoni K, Stepensky D, Trachtenberg A, Sedlov T,
Braverman O, Levy J and Sharoni Y: Combined effects of carotenoids
and polyphenols in balancing the response of skin cells to UV
irradiation. Molecules. 26(1931)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Slominski AT, Zmijewski MA, Plonka PM,
Szaflarski JP and Paus R: How UV light touches the brain and
endocrine system through skin, and why. Endocrinology.
159(1992)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Slominski A, Tobin DJ, Shibahara S and
Wortsman J: Melanin pigmentation in mammalian skin and its hormonal
regulation. Physiol Rev. 84:1155–1228. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Skobowiat C, Sayre RM, Dowdy JC and
Slominski AT: Ultraviolet radiation regulates cortisol activity in
a waveband-dependent manner in human skin ex vivo. Br J Dermatol.
168:595–601. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC
and Slominski A: Cutaneous hypothalamic-pituitary-adrenal axis
homolog: Regulation by ultraviolet radiation. Am J Physiol
Endocrinol Metab. 301:E484–E493. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schiller M, Brzoska T, Böhm M, Metze D,
Scholzen TE, Rougier A and Luger TA: Solar-simulated ultraviolet
radiation-induced upregulation of the melanocortin-1 receptor,
proopiomelanocortin, and alpha-melanocyte-stimulating hormone in
human epidermis in vivo. J Invest Dermatol. 122:468–476.
2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Artzi O, Horovitz T, Bar-Ilan E, Shehadeh
W, Koren A, Zusmanovitch L, Mehrabi JN, Salameh F, Isman Nelkenbaum
G, Zur E, et al: The pathogenesis of melasma and implications for
treatment. J Cosmet Dermatol. 20:3432–3445. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nautiyal A and Wairkar S: Management of
hyperpigmentation: Current treatments and emerging therapies.
Pigment Cell Melanoma Res. 34:1000–1014. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim HJ, Kim JS, Woo JT, Lee IS and Cha BY:
Hyperpigmentation mechanism of methyl 3,5-di-caffeoylquinate
through activation of p38 and MITF induction of tyrosinase. Acta
Biochim Biophys Sin (Shanghai). 47:548–556. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tuerxuntayi A, Liu YQ, Tulake A, Kabas M,
Eblimit A and Aisa HA: Kaliziri extract upregulates tyrosinase,
TRP-1, TRP-2 and MITF expression in murine B16 melanoma cells. BMC
Complement Altern Med. 14(166)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Meléndez-Martínez AJ, Stinco CM and
Mapelli-Brahm P: Skin carotenoids in public health and
nutricosmetics: The emerging roles and applications of the UV
radiation-absorbing colourless carotenoids phytoene and
phytofluene. Nutrients. 11(1093)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cassiano DP, Espósito ACC, da Silva CN,
Lima PB, Dias JAF, Hassun K, Miot LDB, Miot HA and Bagatin E:
Update on melasma-part II: Treatment. Dermatol Ther (Heidelb).
12:1989–2012. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Solano F: Photoprotection and skin
pigmentation: Melanin-related molecules and some other new agents
obtained from natural sources. Molecules. 25(1537)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cao C, Xiao Z, Wu Y and Ge C: Diet and
skin aging-from the perspective of food nutrition. Nutrients.
12(870)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Saini RK, Prasad P, Lokesh V, Shang X,
Shin J, Keum YS and Lee JH: Carotenoids: Dietary sources,
extraction, encapsulation, bioavailability, and health benefits-A
review of recent advancements. Antioxidants (Basel).
11(795)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rivera-Madrid R, Carballo-Uicab VM,
Cárdenas-Conejo Y, Aguilar-Espinosa M and Siva R: Overview of
carotenoids and beneficial effects on human health. In:
Carotenoids: Properties, Processing and Applications. Elsevier,
Amsterdam, pp1-40, 2020.
|
|
30
|
Balić A and Mokos M: Do we utilize our
knowledge of the skin protective effects of carotenoids enough?
Antioxidants (Basel). 8(259)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fiedor J and Burda K: Potential role of
carotenoids as antioxidants in human health and disease. Nutrients.
6:466–488. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hoang HT, Moon JY and Lee YC: Natural
antioxidants from plant extracts in skincare cosmetics: Recent
applications, challenges and perspectives. Cosmetics.
8(106)2021.
|
|
33
|
Nahhas AF, Abdel-Malek ZA, Kohli I,
Braunberger TL, Lim HW and Hamzavi IH: The potential role of
antioxidants in mitigating skin hyperpigmentation resulting from
ultraviolet and visible light-induced oxidative stress.
Photodermatol Photoimmunol Photomed. 35:420–428. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wertz K, Hunziker PB, Seifert N, Riss G,
Neeb M, Steiner G, Hunziker W and Goralczyk R: beta-Carotene
interferes with ultraviolet light A-induced gene expression by
multiple pathways. J Invest Dermatol. 124:428–434. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hadden WL, Watkins RH, Levy LW, Regalado
E, Rivadeneira DM, Van Breemen RB and Schwartz SJ: Carotenoid
composition of marigold (Tagetes erecta) flower extract used as
nutritional supplement. J Agric Food Chem. 47:4189–4194.
1999.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xia N, Schirra C, Hasselwander S,
Förstermann U and Li H: Red fruit (Pandanus conoideus Lam)
oil stimulates nitric oxide production and reduces oxidative stress
in endothelial cells. J Funct Foods. 51:65–74. 2018.
|
|
37
|
Sugiritama LW, Dewi Ratnayanti IGA, Sri
Wiryawan IGN, Ika Wahyuniari IA, Linawati NM and Arijana IGKN:
Effect of Red Fruit Oil (Pandanus conoideus Lam) on animal
model of preeclampsia. Int J Sci Res. 5:1770–1773. 2016.
|
|
38
|
Sumarsono P, Widjiati W and Susilowati S:
Red fruit oil increases trophoblast cells and decreases caspase-9
expression in placenta of lead exposed mice. Univ Med.
35(110)2016.
|
|
39
|
Schirra C, Xia N, Schüffler A, Heck A,
Hasselwander S, Förstermann U and Li H: Phosphorylation and
activation of endothelial nitric oxide synthase by red fruit
(Pandanus conoideus Lam) oil and its fractions. J
Ethnopharmacol. 251(112534)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ratnawati H, Chandra Y and Kho E:
Anticancer effect of red fruit fractions toward breast cancer in
T47D cell and oral squamous cancer in KB cell. In: Proceedings of
the 4th International Conference on Life Sciences and Biotechnology
(ICOLIB 2021). Atlantis Press International BV, Dordrecht,
pp330-340, 2023.
|
|
41
|
Astuti Y and Dewi LLR: Pengaruh ekstrak
buah merah (Pandanus conoideus L.) terhadap kadar glukosa
darah. The effect of red fruit extract (Pandanus conoideus
L.) to the blood glucose level. Mutiara Medika Edisi Khusus. 7:1–6.
2007.
|
|
42
|
Heriyanto Gunawan IA, Fujii R, Maoka T,
Shioi Y, Kameubun KMB, Limantara L and Brotosudarmo TP: Carotenoid
composition in buah merah (Pandanus conoideus Lam.), an
indigenous red fruit of the Papua Islands. J Food Compos Anal.
96(103722)2021.
|
|
43
|
Suprijono MM, Sujuti H, Kurnia D and
Widjanarko SB: Absorption, distribution, metabolism, excretion, and
toxicity evaluation of Papua red fruit flavonoids through a
computational study. In: IOP Conference Series: Earth and
Environmental Science. vol. 475. Institute of Physics Publishing,
pp012078, 2020.
|
|
44
|
Sugianto M, Achadiyani A and Nugraha GI:
Antioxidant effects of red fruit oil on MMP-1 gene expression and
malondialdehyde levels on skin exposed to UVB rays. Mol Cell Bio
Scie. 3(100)2019.
|
|
45
|
Slominski A and Wortsman J:
Neuroendocrinology of the skin1. Endocr Rev. 21:457–487. 2000.
|
|
46
|
Slominski AT, Zmijewski MA, Zbytek B,
Tobin DJ, Theoharides TC and Rivier J: Key role of CRF in the skin
stress response system. Endocr Rev. 34:827–884. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bocheva G, Slominski RM and Slominski AT:
Neuroendocrine aspects of skin aging. Int J Mol Sci.
20(2798)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pang S, Wu H, Wang Q, Cai M, Shi W and
Shang J: Chronic stress suppresses the expression of cutaneous
hypothalamic-pituitary-adrenocortical axis elements and
melanogenesis. PLoS One. 9(e98283)2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Slominski A, Wortsman J, Luger T, Paus R
and Solomon S: Corticotropin releasing hormone and
proopiomelanocortin involvement in the cutaneous response to
stress. Physiol Rev. 80:979–1020. 2000.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rousseau K, Kauser S, Pritchard LE,
Warhurst A, Oliver RL, Slominski A, Wei ET, Thody AJ, Tobin DJ and
White A: Proopiomelanocortin (POMC), the ACTH/melanocortin
precursor, is secreted by human epidermal keratinocytes and
melanocytes and stimulates melanogenesis. FASEB J. 21:1844–1856.
2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Slominski A, Zbytek B, Szczesniewski A,
Semak I, Kaminski J, Sweatman T and Wortsman J: CRH stimulation of
corticosteroids production in melanocytes is mediated by ACTH. Am J
Physiol Endocrinol Metab. 288:E701–E706. 2005.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Raymond JH, Aktary Z, Larue L and Delmas
V: Targeting GPCRs and their signaling as a therapeutic option in
melanoma. Cancers (Basel). 14(706)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Slominski AT, Zmijewski MA, Skobowiat C,
Zbytek B, Slominski RM and Steketee JD: Sensing the environment:
Regulation of local and global homeostasis by the skin's
neuroendocrine system. Adv Anat Embryol Cell Biol. 212:1–115.
2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Slominski AT, Slominski RM, Raman C, Chen
JY, Athar M and Elmets C: Neuroendocrine signaling in the skin with
a special focus on the epidermal neuropeptides. Am J Physiol Cell
Physiol. 323:C1757–C1776. 2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Böhm M and Grässel S: Role of
proopiomelanocortin-derived peptides and their receptors in the
osteoarticular system: From basic to translational research. Endocr
Rev. 33:623–651. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
D'Mello SAN, Finlay GJ, Baguley BC and
Askarian-Amiri ME: Signaling pathways in melanogenesis. Int J Mol
Sci. 17(1144)2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Merecz-Sadowska A, Sitarek P, Stelmach J,
Zajdel K, Kucharska E and Zajdel R: Plants as modulators of
melanogenesis: Role of extracts, pure compounds and patented
compositions in therapy of pigmentation disorders. Int J Mol Sci.
23(14787)2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bento-Lopes L, Cabaço LC, Charneca J, Neto
MV, Seabra MC and Barral DC: Melanin's journey from melanocytes to
keratinocytes: Uncovering the molecular mechanisms of melanin
transfer and processing. Int J Mol Sci. 24(11289)2023.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Le L, Sirés-Campos J, Raposo G, Delevoye C
and Marks MS: Melanosome biogenesis in the pigmentation of
mammalian skin. Integr Comp Biol. 61:1517–1545. 2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Fu C, Chen J, Lu J, Yi L, Tong X, Kang L,
Pei S, Ouyang Y, Jiang L, Ding Y, et al: Roles of inflammation
factors in melanogenesis (Review). Mol Med Rep. 21:1421–1430.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ng L, Kaur P, Bunnag N, Suresh J, Sung
ICH, Tan QH, Gruber J and Tolwinski NS: WNT signaling in disease.
Cells. 8(826)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhang J, Li Y, Wu Y, Yang T, Yang K, Wang
R, Yang J and Guo H: Wnt5a inhibits the proliferation and
melanogenesis of melanocytes. Int J Med Sci. 10:699–706.
2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lin X, Meng X and Lin J: The possible role
of Wnt/β-catenin signalling in vitiligo treatment. J Eur Acad
Dermatol Venereol. 37:2208–2221. 2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Liu W, Chen Q and Xia Y: New mechanistic
insights of melasma. Clin Cosmet Investig Dermatol. 16:429–442.
2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hsiao JJ and Fisher DE: The roles of
microphthalmia-associated transcription factor and pigmentation in
melanoma. Arch Biochem Biophys. 563:28–34. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kim H, Kim I, Dong Y, Lee IS, Kim JS, Kim
JS, Woo JT and Cha BY: Melanogenesis-inducing effect of
cirsimaritin through increases in microphthalmia-associated
transcription factor and tyrosinase expression. Int J Mol Sci.
16:8772–8788. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
da Cunha MG and da Silva Urzedo AP:
Melasma: A review about pathophysiology and treatment. In:
Pigmentation Disorders-Etiology and Recent Advances in Treatments.
IntechOpen, 2023.
|
|
68
|
Slominski A, Zmijewski MA and Pawelek J:
L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators
of melanocyte functions. Pigment Cell Melanoma Res. 25:14–27.
2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Niu C and Aisa HA: Upregulation of
melanogenesis and tyrosinase activity: Potential agents for
vitiligo. Molecules. 22(1303)2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Phacharapiyangkul N, Thirapanmethee K,
Sa-ngiamsuntorn K, Panich U, Lee CH and Chomnawang MT: The ethanol
extract of Musa sapientum Linn. Peel inhibits melanogenesis
through AKT signaling pathway. Cosmetics. 8(70)2021.
|
|
71
|
D'Orazio J, Jarrett S, Amaro-Ortiz A and
Scott T: UV radiation and the skin. Int J Mol Sci. 14:12222–12248.
2013.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kamiński K, Kazimierczak U and Kolenda T:
Oxidative stress in melanogenesis and melanoma development. Contemp
Oncol (Pozn). 26:1–7. 2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hseu YC, Vudhya Gowrisankar Y, Wang LW,
Zhang YZ, Chen XZ, Huang PJ, Yen HR and Yang HL: The in vitro and
in vivo depigmenting activity of pterostilbene through induction of
autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in
keratinocytes via Nrf2-mediated antioxidant pathways. Redox Biol.
44(102007)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Herraiz C, Martínez-Vicente I and Maresca
V: The α-melanocyte-stimulating hormone/melanocortin-1 receptor
interaction: A driver of pleiotropic effects beyond pigmentation.
Pigment Cell Melanoma Res. 34:748–761. 2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Yardman-Frank JM and Fisher DE: Skin
pigmentation and its control: From ultraviolet radiation to stem
cells. Exp Dermatol. 30:560–571. 2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Panzella L and Napolitano A: Natural and
bioinspired phenolic compounds as tyrosinase inhibitors for the
treatment of skin hyperpigmentation: Recent advances. Cosmetics.
6(57)2019.
|
|
77
|
Grimes PE, Ijaz S, Nashawati R and Kwak D:
New oral and topical approaches for the treatment of melasma. Int J
Womens Dermatol. 5:30–36. 2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Lee A, Kim JY, Heo J, Cho DH, Kim HS, An
IS, An S and Bae S: The inhibition of melanogenesis via the PKA and
ERK signaling pathways by Chlamydomonas reinhardtii extract
in B16F10 melanoma cells and artificial human skin equivalents. J
Microbiol Biotechnol. 28:2121–2132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Hashemi-Shahri SH, Golshan A, Mohajeri SA,
Baharara J, Amini E, Salek F, Sahebkar A and Tayarani-Najaran Z:
ROS-scavenging and anti-tyrosinase properties of crocetin on B16F10
murine melanoma cells. Anticancer Agents Med Chem. 18:1064–1069.
2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Roberts RL, Green J and Lewis B: Lutein
and zeaxanthin in eye and skin health. Clin Dermatol. 27:195–201.
2009.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Juturu V, Bowman J and Deshpande J:
Overall skin tone and skin-lightening-improving effects with oral
supplementation of lutein and zeaxanthin isomers: A double-blind,
placebo-controlled clinical trial. Clin Cosmet Investig Dermatol.
9:325–332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Arct J and Mieloch M: β-carotene in skin
care. Pol J Cosmetol. 19:206–213. 2016.
|
|
83
|
Madaan T, Choudhary AN, Gyenwalee S,
Thomas S, Mishra H, ariq M, Vohora D and Talegaonkar S: Lutein, a
versatile phyto-nutraceutical: An insight on pharmacology,
therapeutic indications, challenges and recent advances in drug
delivery. PharmaNutrition. 5:64–75. 2017.
|
|
84
|
Babbush K, Babbush R and Khachemoune A:
The therapeutic use of antioxidants for melasma. J Drugs Dermatol.
19:788–792. 2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Mzabri I, Addi M and Berrichi A:
Traditional and modern uses of saffron (Crocus sativus).
Cosmetics. 6(63)2019.
|
|
86
|
Kumar A, P N, Kumar M, Jose A, Tomer V, Oz
E, Proestos C, Zeng M, Elobeid T, K S and Oz F: Major
phytochemicals: Recent advances in health benefits and extraction
method. Molecules. 28(887)2023.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Zhao C, Kam HT, Chen Y, Gong G, Hoi MP,
Skalicka-Woźniak K, Dias ACP and Lee SM: Crocetin and its glycoside
crocin, two bioactive constituents from Crocus sativus L.
(saffron), differentially inhibit angiogenesis by inhibiting
endothelial cytoskeleton organization and cell migration through
VEGFR2/SRC/FAK and VEGFR2/MEK/ERK signaling pathways. Front
Pharmacol. 12(675359)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Ćetković GS, Djilas SM, Čanadanović-Brunet
JM and Tumbas VT: Antioxidant properties of marigold extracts. Food
Res Int. 37:643–650. 2004.
|
|
89
|
Vu HT, Scarlett CJ and Vuong QV: Phenolic
compounds within banana peel and their potential uses: A review. J
Funct Foods. 40:238–248. 2018.
|
|
90
|
Youryon P and Supapvanich S:
Physicochemical quality and antioxidant changes in ‘Leb Mue Nang’
banana fruit during ripening. Agric Nat Resour. 51:47–52. 2017.
|
|
91
|
Wulansari D, Wawo AH and Agusta A:
Carotenoid content of five accessions red fruit (Pandanus
conoideus Lam.) oil. IOP Conf Ser Earth Environ Sci.
591(012033)2020.
|
|
92
|
Roreng M, Palupi N and Prangdimurti E:
Carotenoids from red fruit (Pandanus conoideus Lam.) extract
are bioavailable: A study in rats. IOSR J Pharm. 4:11–16. 2014.
|
|
93
|
Dumaria CH, Wiraguna A and Pangkahila W:
Krim ekstrak buah merah (Pandanus conoideus) 10% sama
efektifnya dengan krim hidrokuinon 4% dalam mencegah peningkatan
jumlah melanin kulit marmut (Cavia porcellus) yang dipapar
sinar ultraviolet B. J Biomed. 10:85–91. 2018.
|
|
94
|
Freitas JV, Junqueira HC, Martins WK,
Baptista MS and Gaspar LR: Antioxidant role on the protection of
melanocytes against visible light-induced photodamage. Free Radic
Biol Med. 131:399–407. 2019.PubMed/NCBI View Article : Google Scholar
|















