Introduction
Nuclear terrorism or power plant accidents risk
serious radiation damage (1). An
emergency radiation medical system capable of responding to such
accidents must be established. Humans and rodents exposed to
high-dose ionizing radiation (IR) develop acute radiation syndrome
(ARS). In humans, hematopoietic ARS occurs at radiation doses >1
Gy and the severity of the syndrome is directly proportional to the
dose of absorbed radiation (2-4).
The white blood cell count decreases, predisposing people to
infection. When humans are exposed to 6-8 Gy radiation,
gastrointestinal ARS induces intestinal mucosa disintegration,
along with hematopoietic ARS (3).
In 1999, three patients experienced ARS caused by a critical
nuclear accident at the JCO nuclear fuel processing facility in
Tokai-mura, Japan. Two of these patients died of intestinal injury
(5-7).
Predicting risk of lethal intestinal injury is essential when
high-dose exposure is suspected. Intestinal tissue removal is
necessary to assess damage to intestinal epithelial cells cau2sed
by radiation. Hence, it has been difficult to assess intestinal
damage in a noninvasive manner. Therefore, a less invasive method
that can objectively evaluate the degree of intestinal injury is
required.
MicroRNAs (miRNAs or miRs) are endogenous, small
non-coding RNAs that regulate cellular processes such as
proliferation and growth, differentiation, programmed cell death,
cell cycle progression and tissue development (8). Studies have detected miRNAs in body
fluids such as serum, plasma and urine (9-11);
these miRNAs in body fluids have been proposed as biomarkers for
various physiological responses and pathological stages of cancers
and neurodegenerative disease. For example, miR-122 is specific to
the liver and appears in blood in large amounts in liver cancer.
Therefore, miR-122 has attracted attention as a biomarker for liver
cancer (12).
Intestinal epithelial cells are radiosensitive; when
humans or mice are exposed to IR doses >10 Gy, cell death is
induced (13-15).
IR-induced cell death forms extracellular vesicles called apoptotic
bodies or induces extracellular leakage of various intracellular
components such as proteins and enzymes (16). Our previous study demonstrated that
the release of miR-375-3p from pancreatic β cells increases
following high-dose IR exposure (17). Similarly, miRNAs in intestinal
epithelial cells are released following IR exposure and may
infiltrate blood and feces; to the best of our knowledge, however,
no previous study has confirmed this. Therefore, the present study
aimed to identify miRNAs excreted in serum or feces as high-dose IR
biomarker candidates using an IR-induced intestinal injury mouse
model.
Materials and methods
Mice
A total of eight male 7-week-old C57BL/6NJcl (body
weight: 23.2±1.0 g) mice were obtained from CLEA Japan. All mice
were given access to a solid diet CE-2 (CLEA Japan) and water ad
libitum and were housed in a conventional animal room with
12/12-h light/dark cycles at room temperature and humidity 40-50%.
Up to five mice were housed/cage and bedding, feed and water were
changed weekly. Mice were observed 2-3 times/day for monitoring; no
abnormalities in mouse health or behavior were observed. Mice were
allowed to acclimatize for 1 week before irradiation. Blood and
small intestine samples from all mice were collected under
anesthesia with isoflurane (Pfizer, Inc.). Small animal anesthesia
machines (Muromachi Kikai Co., Ltd.) were used to anesthetize the
mice. Isoflurane at 4-5% was used for induction and maintained at
2-3%. After anesthesia, 0.5-1.0 ml blood was drawn from the heart
and mice were promptly cervically dislocated for euthanasia. The
time-lapse from the start of the anesthesia to the end of blood
collection was <10 min/animal. Death was confirmed by
respiratory and cardiac arrest. Small intestine samples were
collected after the death of the mice. Blood samples were placed in
a BD MicroTainer® SST (Becton, Dickinson and Company) and the
coagulated blood was centrifuged at 6,000 x g for 3 min at room
temperature for serum separation. Feces samples were directly
collected in tubes. The Hirosaki University Ethics Committee for
Animal Experiments approved the experiments (approval no. G12003),
which were conducted under the Hirosaki University Guidelines for
Animal Experiments.
X-ray irradiation
Following 1 week acclimatization, mice were exposed
to X-rays (MBR-1520R-3 X-ray machine; Hitachi Ltd.) at 1.0 Gy/min
(150 kVp, 20 mA, 0.5 mm aluminum and 0.3 mm copper filters). Mice
were fixed in a circular mouse holder (Natsume Seisakusho Co.,
Ltd.) and irradiated uniformly with X-rays while rotating the
holder. The mice in the irradiated group were irradiated with 10
Gy, while those in the non-irradiated group were not irradiated.
Blood, feces and small intestine samples were collected 3 days
after exposure to X-rays.
TUNEL assay
TUNEL assay was performed to confirm tissue damage
in the small intestine caused by exposure to 10 Gy X-rays. For
tissue analyses, the small intestine was fixed with 4%
paraformaldehyde solution in Ca2+ and
Mg2+-free Dulbecco's phosphate-buffered saline [D-PBS
(-)]at pH 7.2 for 2 days at room temperature. The fixed small
intestine was embedded in paraffin. Sections were cut to 4 µm and
placed on glass slides. Paraffin-embedded sections were
deparaffinized with xylene and ethanol after which they were washed
with D-PBS (-). Cell death analysis was performed using the
DeadEnd™ Fluorometric TUNEL System (Promega Corporation) according
to the manufacturer's instructions. To label fragmented DNA with
fluorescein-12-dUTP, small intestinal tissue was incubated with the
reaction solution for 1 h at 37˚C. ProLong Gold Antifade Reagent
with 4',6-diamidino-2-phenylindole (Thermo Fisher Scientific, Inc.)
was used for nuclear staining and mounting at room temperature. The
stained tissues were examined using a confocal laser scanning
microscope LSM710 (Carl Zeiss GmbH). At least five fields of view
per sample were observed.
Total RNA extraction
Total RNA from the small intestine, serum and feces
were extracted using the Isogen II reagent and ethachinmate (both
Nippon Gene Co., Ltd.) according to the manufacturer's
instructions. Total RNA was extracted from drinking water and feed
used as controls. RNA concentrations from the small intestine were
assessed using NanoDrop spectrophotometer (NanoDrop Technologies;
Thermo Fisher Scientific, Inc.). RNA samples had 260/280 nm
absorbance ratios of 1.8-2.0. RNA concentration of serum and feces
was measured using Quant-iT RiboGreen RNA Reagent and Fluoroskan
Ascent (both Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The quality of total RNAs was
confirmed using the Agilent 2100 Bioanalyzer and Agilent RNA 6000
Pico kit (both Agilent Technologies, Inc.), according to the
manufacturer's instructions.
Microarray analysis
Cyanine 3 (Cy3)-labeled miRNAs were synthesized from
total RNAs of irradiated and non-irradiated samples (small
intestine, serum and feces; all n=4) using the miRNA Complete
Labeling Reagent and Hyb kit (cat. no. 5190-0456; Agilent
Technologies, Inc.). SurePrint G3 mouse miRNA microarray slides
(8x60 K, Ver.21.0; cat. no. G4872A; Agilent Technologies, Inc.)
were hybridized with Cy3-labeled miRNA in hybridization solution
prepared with Gene Expression Hybridization kit (Agilent
Technologies, Inc.), according to the manufacturer's instructions.
Cy3 fluorescence signals were obtained using the SureScan
microarray scanner and processed using Feature Extraction version
10.7 software (both Agilent Technologies, Inc.) according to the
manufacturer's instructions. The expression data obtained were
processed using GeneSpring GX14.5 software (Agilent Technologies,
Inc.) to normalize all values to the 90% shift on the respective
microarrays, followed by the normalization of the median expression
of all samples. miRNAs with expression change >2.0-fold were
selected. miRNA accession numbers were confirmed in miRbase
(mirbase.org/). The obtained microarray data were
registered with Gene Expression Omnibus (ncbi.nlm.nih.gov/geo/) (accession no. GSE247876). To
predict target genes of miRNAs and pathways, TargetScan Mouse
(targetscan.org/mmu_72/) and WikiPathways
(wikipathways.org/) analyses were performed using
the GeneSpring 14.5 software (Agilent Technologies, Inc.). Pathway
data of Mus musculus were downloaded from WikiPathways
(/data.wikipathways.org/current/gpml/).
Statistical analysis
Target genes of miRNAs were searched by TargetScan
and pathway predictions related to the target genes were searched
on WikiPathways in GeneSpring 14.5 software with cut-off value of
P<0.05.
Results
Small intestine damage in mice exposed
to 10 Gy X-rays
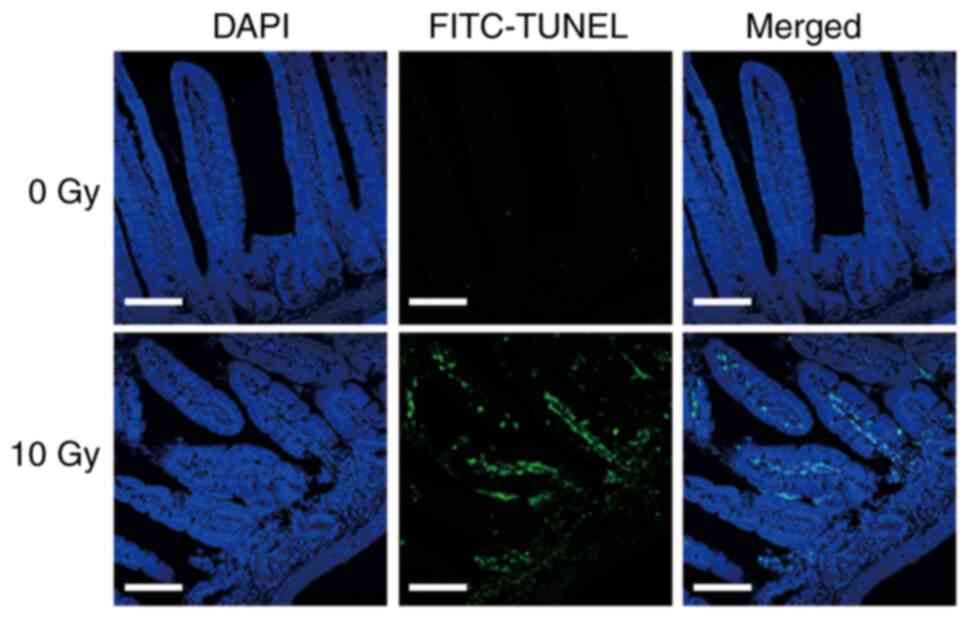
Small intestinal damage following 10 Gy X-ray
irradiation was confirmed by TUNEL assay. At 72 h after radiation
exposure, green fluorescent TUNEL labeling increased and showed
positive signals, especially in small intestinal pit sites rich in
small intestinal epithelial stem cells (Fig. 1). This indicated that cell death was
induced in the small intestines of mice exposed to 10 Gy
X-rays.
Small RNAs are detected in mouse serum
or feces
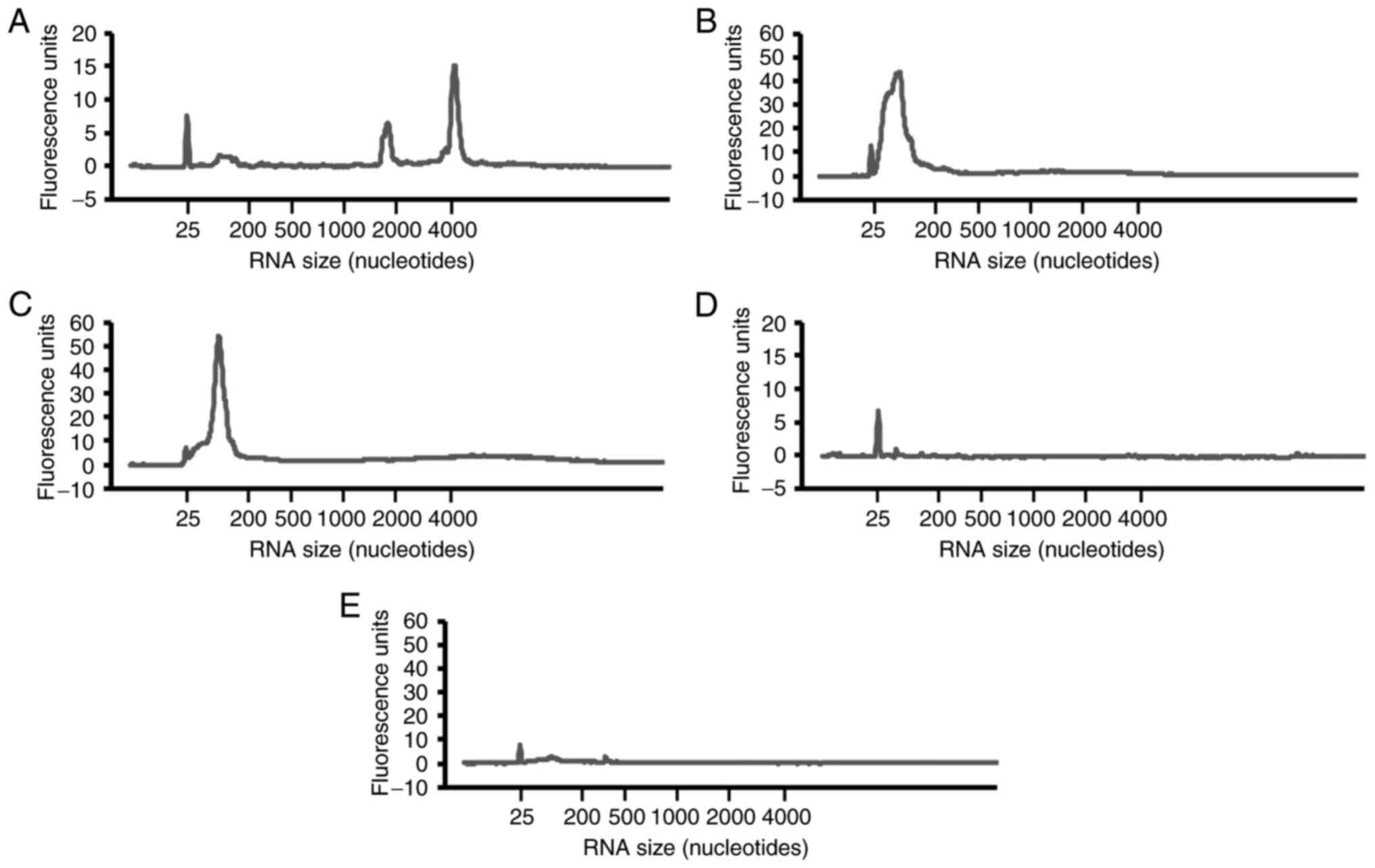
Total RNAs were extracted from the small intestine,
serum and feces. Peaks of 18 and 28 S ribosomal RNAs were detected
in total RNAs from the small intestines (Fig. 2A), whereas these peaks were not
detected in serum and feces. However, a peak of small RNAs of
25-200 nucleotides was detected in serum and feces (Fig. 2B and C). Almost no RNA peak was detected in the
drinking water and animal feed used as a control (Fig. 2D and E). This demonstrated the presence of small
RNAs in mouse serum and feces.
miRNA expression in the serum or feces
of mice exposed to 10 Gy X-rays
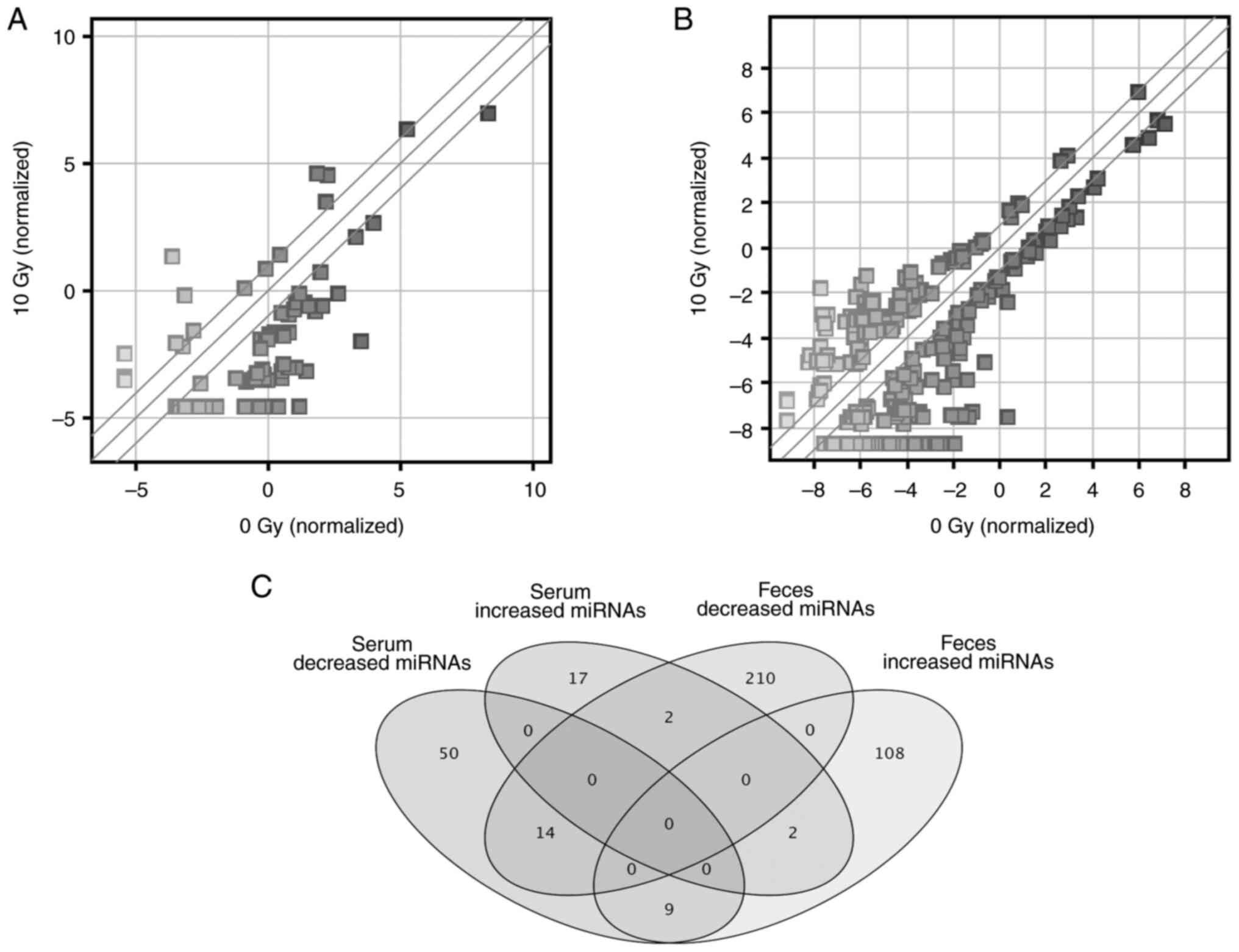
Microarray analysis was performed to examine miRNAs
expressed in serum and feces. In serum, 21 and 73 miRNAs were up-
and downregulated >2.0-fold in the 10 Gy irradiation compared
with the non-irradiated group, respectively (Fig. 3A; Table
SI). In feces, 119 and 226 miRNAs increased and decreased more
than 2.0-fold in the 10 Gy irradiation compared with the
non-irradiated group, respectively (Fig. 3B; Table
SII). Venn diagram of these miRNAs is presented in Fig. 3C and a breakdown of these miRNAs is
shown in Table I.
 | Table IUp- and downregulated miRs in serum
and feces of mice exposed to 10 Gy X-ray irradiation. |
Table I
Up- and downregulated miRs in serum
and feces of mice exposed to 10 Gy X-ray irradiation.
| Serum expression | Fecal expression | miR |
|---|
| Upregulated | Upregulated | miR-375-3p,
miR-574-3p |
| Upregulated | Downregulated | miR-500-3p,
miR-3076-5p |
| Downregulated | Upregulated | let-7i-5p, miR-25-3p,
miR-27b-3p, miR-29c-3p, miR-129-1-3p, miR-468-3p, miR-486a-5p,
miR-669n, miR-7016-5p |
| Downregulated | Downregulated | miR-140-3p,
miR-181a-5p, miR-223-3p, miR-361-5p, miR-758-5p, miR-2916-5p,
miR-3091-5p, miR-3113-5p, miR-6899-5p, miR-6938-5p, miR-6946-5p,
miR-6961-5p, miR-7066-5p, miR-7687-5p |
Using TargetScan, 646 genes were predicted as a
targets of up- and 1,306 of downregulated miRNAs in serum (data not
shown). In feces, 926 and 499 genes were predicted as target genes
of up- and downregulated miRNAs, respectively (data not shown).
Using a threshold P-value of 0.05, the WikiPathways
analysis detected 107 and 140 pathways using 646 and 1,306 target
genes predicted for the 21 and 73 miRNAs in serum, respectively. In
addition, 128 and 91 pathways were detected by pathway analysis
performed using 926 and 499 predicted target genes of the 119 and
226 miRNAs in feces, respectively. Tables II and III present the top 20 pathways involving
predicted target genes of increased miRNAs in serum or feces; serum
and feces shared 14 pathways.
 | Table IITop 20 pathways involving predicted
target genes of upregulated microRNAs in serum. |
Table II
Top 20 pathways involving predicted
target genes of upregulated microRNAs in serum.
| Pathway name | Pathway ID | P-value | Target gene
count |
|---|
|
Mm_Non-odorant_GPCRs | WP1396_69993 |
3.58x1029 | 14 |
|
Mm_Focal_Adhesion-PI3K-Akt-mTOR-signaling_pathway | WP2841_94308 |
3.58x1029 | 14 |
|
Mm_MAPK_signaling_pathway | WP493_78412 |
4.58x1023 | 11 |
|
Mm_mRNA_processing | WP310_78419 |
4.96x1021 | 10 |
| Mm_PluriNetWork | WP1763_89515 |
5.35x1019 | 9 |
|
Mm_EGFR1_Signaling_Pathway | WP572_82883 |
5.35x1019 | 9 |
|
Mm_IL-3_Signaling_Pathway | WP373_69196 |
6.22x1015 | 7 |
|
Mm_Chemokine_signaling_pathway | WP2292_97515 |
6.22x1015 | 7 |
|
Mm_Regulation_of_Actin_Cytoskeleton | WP523_71326 |
6.22x1015 | 7 |
|
Mm_MicroRNAs_in_Cardiomyocyte_Hypertrophy | WP1560_70037 |
6.22x1015 | 7 |
|
Mm_GPCRs,_Class_A_Rhodopsin-like | WP189_79710 |
6.22x1015 | 7 |
|
Mm_Focal_Adhesion | WP85_94410 |
6.69x1013 | 6 |
|
Mm_IL-2_Signaling_Pathway | WP450_89849 |
6.69x1013 | 6 |
|
Mm_ESC_Pluripotency_Pathways | WP339_94309 |
6.69x1013 | 6 |
|
Mm_Myometrial_Relaxation_and_Contraction_Pathways | WP385_95806 |
6.69x1013 | 6 |
|
Mm_Purine_metabolism | WP2185_101822 |
6.69x1013 | 6 |
|
Mm_Odorant_GPCRs | WP1397_82866 |
7.18x1011 | 5 |
|
Mm_Insulin_Signaling | WP65_88446 |
7.18x1011 | 5 |
|
Mm_Alpha6-Beta4_Integrin_Signaling_Pathway | WP488_72049 |
7.18x1011 | 5 |
| Mm_Apoptosis | WP1254_95784 |
7.18x1011 | 5 |
 | Table IIITop 20 pathways involving predicted
target genes of upregulated microRNAs in feces. |
Table III
Top 20 pathways involving predicted
target genes of upregulated microRNAs in feces.
| Pathway name | Pathway ID | P-value | Target gene
count |
|---|
|
Mm_mRNA_processing | WP310_78419 | <0.001 | 27 |
|
Mm_Focal_Adhesion | WP85_94410 |
1.77x1034 | 18 |
|
Mm_Focal_Adhesion-PI3K-Akt-mTOR-signaling_pathway | WP2841_94308 |
1.34x1032 | 17 |
|
Mm_PluriNetWork | WP1763_89515 |
5.79x1027 | 14 |
|
Mm_Non-odorant_GPCRs | WP1396_69993 |
3.28x1023 | 12 |
|
Mm_EGFR1_Signaling_Pathway | WP572_82883 |
3.28x1023 | 12 |
|
Mm_GPCRs,_Class_A_Rhodopsin-like | WP189_79710 |
2.47x1021 | 11 |
|
Mm_Insulin_Signaling | WP65_88446 |
1.85x1019 | 10 |
|
Mm_Delta-Notch_Signaling_Pathway | WP265_69189 |
1.85x1019 | 10 |
|
Mm_MicroRNAs_in_Cardiomyocyte_Hypertrophy | WP1560_70037 |
1.85x1019 | 10 |
|
Mm_Myometrial_Relaxation_and_Contraction_Pathways | WP385_95806 |
1.39x1017 | 9 |
|
Mm_Integrin-mediated_Cell_Adhesion | WP6_97547 |
1.39x1017 | 9 |
|
Mm_Odorant_GPCRs | WP1397_82866 |
1.39x1017 | 9 |
|
Mm_Chemokine_signaling_pathway | WP2292_97515 |
1.39x1017 | 9 |
|
Mm_Metapathway_biotransformation | WP1251_94721 |
1.04x1015 | 8 |
|
Mm_Kit_Receptor_Signaling_Pathway | WP407_69079 |
1.04x1015 | 8 |
|
Mm_Spinal_Cord_Injury | WP2432_102465 |
1.04x1015 | 8 |
|
Mm_MAPK_signaling_pathway | WP493_78412 |
7.81x1014 | 7 |
|
Mm_Regulation_of_Actin_Cytoskeleton | WP523_71326 |
7.81x1014 | 7 |
|
Mm_IL-3_Signaling_Pathway | WP373_69196 |
7.81x1014 | 7 |
There were four upregulated miRNAs with signal
intensity >100 in the small intestine in serum and 19 in feces
(Table IV). mir-375-3p was
detected in both serum and feces. Therefore, these miRNAs in serum
and/or feces may be derived from the small intestine.
 | Table IVCandidate upregulated miRs in serum
and feces derived from small intestine of mice exposed to 10 Gy
X-ray irradiation. |
Table IV
Candidate upregulated miRs in serum
and feces derived from small intestine of mice exposed to 10 Gy
X-ray irradiation.
| Sample | miRs in small
intestine (raw signal >100) |
|---|
| Serum | miR-23b-3p,
miR-24-3p, miR-27a-3p, miR-375-3p |
| Feces | let-7i-5p,
miR-103-3p, miR-107-3p, miR-148a-3p, miR-19b-3p, miR-200b-3p,
miR-200c-3p, miR-25-3p, miR-27b-3p, miR-29a-3p, miR-29c-3p,
miR-30c-5p, miR-3473a, miR-3473b, miR-375-3p, miR-3968, miR-494-3p,
miR-690, miR-8110 |
Discussion
The present study identified four miRNAs in serum
and 19 in feces derived from the small intestine that represent
novel high-dose radiation exposure candidate biomarkers. In
particular, miR-375-3p was upregulated in serum and feces after 10
Gy X-ray exposure and may be a candidate biomarker to estimate
intestinal injury.
Large amounts of small RNAs were present in
supernatant of cultured cells and serum, which is consistent with
previous results (17-19).
Feed and drinking water contained little RNA. Therefore, most
miRNAs detected in serum and feces were derived from murine tissue.
Our previous study demonstrated that miR-375-3p is abundant in the
digestive tract (including the small intestine) (17). Because feces are in direct contact
with the digestive tract (including the small and large intestine),
it was hypothesized that radiation-induced injury of digestive
tract cells would result the leakage of miRNAs, including
miR-375-3p (which is abundant in the digestive tract (17)) into the feces. Therefore, it was
hypothesized that upregulated miRNAs, including miR-375-3p, in
feces originated from the gastrointestinal tract.
Here, 10 Gy irradiation increased the expression of
21 miRNAs in serum and 119 miRNAs in feces >2.0-fold. miR-375-3p
was increased in both serum and feces after 10 Gy exposure. In our
previous study, serum miR-375-3p was highly expressed in the
pancreas, small intestine, and colon and the expression of this
miRNA increased following exposure to 7 Gy in mice (17). This suggests that upregulated
miR-375-3p in serum is derived from the pancreas and small
intestine. Therefore, in the present study, following 10 Gy
exposure, upregulated miR-375-3p in the serum was likely derived
from the leakage from the pancreas and small intestine.
miR-375-3p is primarily expressed in β-cell islets
of the pancreas and plays an important role in the complex
regulatory network of pancreatic development and insulin secretion
(20-23).
Here, the insulin signaling pathway was one of the top 20 pathways
associated with predicted targeted genes of upregulated miRNAs in
serum and feces. The increase in miR-375-3p in serum may be due to
pancreatic or small intestinal damage. Hence, impairment of the
insulin signaling pathway is also expected to occur. Fendler et
al (24) reported that a
combination of three serum miRNAs (miR-133b, miR-215 and miR-375)
predicts radiation-induced fatality in mice and macaques. With
upregulated miR-375-3p in feces suggesting intestinal injury and
increased miR-375-3p in serum, this miRNA may be a biomarker
capable of predicting the lethal dose of radiation exposure in
humans.
Fecal calprotectin has been reported as a biomarker
of radiation exposure (25). Fecal
calprotectin is used for adjuvant diagnosis of ulcerative colitis
and Crohn's disease and it is measured via fluorescence enzyme
immunoassay (26-28).
Calprotectin is a calcium- and zinc-binding heterodimer of 36.5 kDa
that belongs to the S100 family and is present on the surface of
monocytes and macrophages, facilitating recruitment to the site of
inflammation. The synthesis of calprotectin is increased during the
inflammatory process (27).
Calprotectin has been reported as a biomarker of acute radiation
enteritis caused by radiation treatment of prostate cancer
(29,30). To evaluate sensitivity and
specificity, comparative analyses of the usefulness of fecal
calprotectin and the 19 miRNAs identified in the present study
(including miR-375-3p) will be necessary.
The present study compared irradiated and
non-irradiated mice. Although 10 Gy irradiation caused intestinal
damage, the present study did not compare the degree of intestinal
damage and miRNA expression in the small intestine following
exposure to various doses of radiation. Serum miRNAs may reflect
damage at various tissue sites, while fecal miRNAs are thought to
be primarily of intestinal origin. Future studies should compare
intestinal damage caused by various radiation doses and miRNA
expression levels. The tissue specificity of the miRNAs detected in
this study should also be examined to determine if they are
biomarkers specific for intestinal damage.
miRNAs released by radiation exposure may be
degraded in body fluids. miRNAs bound to miRNA-binding proteins
and/or miRNAs internalized in extracellular vesicles are not
degraded and remain in body fluids (31). miRNAs in extracellular vesicles may
be transferred to other cells and cause damage and/or alter their
function (32).
The detection of small RNA was not performed in
irradiated samples. Our previous study examined changes in serum
RNA at lethal doses of 7 Gy irradiation at 0, 24, 48 and 72 h after
irradiation and found no increase in overall RNA levels (17). Similarly, there should be no change
in total yield in feces; this should be investigated in future.
When humans are exposed to high doses of radiation,
treatments such as hematopoietic factors and stem cell
transplantation are effective in restoring bone marrow (33). However, if intestinal damage is
severe, recovery is difficult and an indicator to assess degree of
intestinal damage is needed to triage patients. Future studies
should investigate the potential role of such indicators as a
biomarker for early detection of intestinal disorder.
Here, miR-375-3p levels were increased in serum and
feces samples by high-dose radiation exposure. Future studies
should compare miR-375-3p with existing markers of intestinal
damage and confirm the present results using specimens from
patients with colorectal cancer undergoing radiotherapy to
determine whether miR-375-3p may be a biomarker of early intestinal
damage.
Supplementary Material
Serum miRs in mice exposed to 10 Gy
X-ray irradiation.
Fecal miRs in mice exposed to 10 Gy
X-ray irradiation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Japan Society for
the Promotion of Science KAKENHI (grant nos. 21H04844 and
17K19779).
Availability of data and materials
The data generated in the present study may be found
in the Gene Expression Omnibus under accession number GSE247876 or
at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE247876.
Authors' contributions
MC performed experiments and wrote the manuscript.
HU, HK and IN performed experiments. All authors have read and
approved the final manuscript. MC and HU confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
All experiments were performed in accordance with
the Guidelines for Animal Experimentation of Hirosaki University.
The procedures were approved and monitored by the Animal Research
Committee of Hirosaki University (approval no. G12003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fliedner TM: Nuclear terrorism: the role
of hematology in coping with its health consequences. Curr Opin
Hematol. 13:436–444. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Berger ME, Christensen DM, Lowry PC, Jones
OW and Wiley AL: Medical management of radiation injuries: Current
approaches. Occup Med (Lond). 56:162–172. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Macià I, Garau M, Lucas Calduch A and
López EC: Radiobiology of the acute radiation syndrome. Rep Pract
Oncol Radiother. 16:123–130. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Singh VK, Newman VL and Seed TM:
Colony-stimulating factors for the treatment of the hematopoietic
component of the acute radiation syndrome (H-ARS): A review.
Cytokine. 71:22–37. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tanaka SI: Summary of the JCO criticality
accident in Tokai-mura and a dose assessment. J Radiat Res. 42
(Suppl 42):S1–S9. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sasaki MS, Hayata I, Kamada N, Kodama Y
and Kodama S: Chromosome aberration analysis in persons exposed to
low-level radiation from the JCO criticality accident in
Tokai-mura. J Radiat Res. 42 (Suppl 42):S107–S116. 2001.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Asano S: Current status of hematopoietic
stem cell transplantation for acute radiation syndromes. Int J
Hematol. 95:227–231. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gulyaeva LF and Kushlinskiy NE: Regulatory
mechanisms of microRNA expression. J Transl Med.
14(143)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Backes C, Meese E and Keller A: Specific
miRNA disease biomarkers in blood, serum and plasma: Challenges and
prospects. Mol Diagn Ther. 20:509–518. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Foye C, Yan IK, David W, Shukla N,
Habboush Y, Chase L, Ryland K, Kesari V and Patel T: Comparison of
miRNA quantitation by nanostring in serum and plasma samples. PLoS
One. 12(e0189165)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Stuopelyte K, Daniunaite K, Bakavicius A,
Lazutka JR, Jankevicius F and Jarmalaite S: The utility of
urine-circulating miRNAs for detection of prostate cancer: Br J.
Cancer. 115:707–715. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hayes CN and Chayama K: MicroRNAs as
Biomarkers for Liver Disease and Hepatocellular Carcinoma. Int J
Mol Sci. 17(280)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Potten CS, Merritt A, Hickman J, Hall P
and Faranda A: Characterization of radiation-induced apoptosis in
the small intestine and its biological implications. Int J Radiat
Biol. 65:71–78. 1994.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Potten CS, Wilson JW and Booth C:
Regulation and significance of apoptosis in the stem cells of the
gastrointestinal epithelium. Stem Cells. 15:82–93. 1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jeong BK, Song JH, Jeong H, Choi HS, Jung
JH, Hahm JR, Woo SH, Jung MH, Choi BH, Kim JH and Kang KM: Effect
of alpha-lipoic acid on radiation-induced small intestine injury in
mice. Oncotarget. 7:15105–15117. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Albanese J and Dainiak N: Modulation of
intercellular communication mediated at the cell surface and on
extracellular, plasma membrane-derived vesicles by ionizing
radiation. Exp Hematol. 31:455–464. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chiba M, Monzen S, Iwaya C, Kashiwagi Y,
Yamada S, Hosokawa Y, Mariya Y, Nakamura T and Wojcik A: Serum
miR-375-3p increase in mice exposed to a high dose of ionizing
radiation. Sci Rep. 8(1302)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chiba M, Kimura M and Asari S: Exosomes
secreted from human colorectal cancer cell lines contain mRNAs,
microRNAs and natural antisense RNAs, that can transfer into the
human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep.
28:1551–1558. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chiba M, Kubota S, Sato K and Monzen S:
Exosomes released from pancreatic cancer cells enhance angiogenic
activities via dynamin-dependent endocytosis in endothelial cells
in vitro. Sci Rep. 8(11972)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li X: MiR-375, a microRNA related to
diabetes. Gene. 533:1–4. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kaviani M, Azarpira N, Karimi MH and
Al-Abdullah I: The role of microRNAs in islet β-cell development.
Cell Biol Int. 40:1248–1255. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Eliasson L: The small RNA miR-375-a
pancreatic islet abundant miRNA with multiple roles in endocrine
beta cell function. Mol Cell Endocrinol. 456:95–101.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Esguerra JLS, Nagao M, Ofori JK, Wendt A
and Eliasson L: MicroRNAs in islet hormone secretion. Diabetes Obes
Metab. 20 (Suppl 2):S11–S19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fendler W, Malachowska B, Meghani K,
Konstantinopoulos PA, Guha C, Singh VK and Chowdhury D:
Evolutionarily conserved serum microRNAs predict radiation-induced
fatality in nonhuman primates. Sci Transl Med.
9(eaal2408)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Larsen A, Hovdenak N, Karlsdottir A,
Wentzel-Larsen T, Dahl O and Fagerhol MK: Faecal calprotectin and
lactoferrin as markers of acute radiation proctitis: a pilot study
of eight stool markers. Scand J Gastroenterol. 39:1113–1118.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yamaguchi S, Takeuchi Y, Arai K, Fukuda K,
Kuroki Y, Asonuma K, Takahashi H, Saruta M and Yoshida H: Fecal
calprotectin is a clinically relevant biomarker of mucosal healing
in patients with quiescent ulcerative colitis. J Gastroenterol
Hepatol. 31:93–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Manceau H, Chicha-Cattoir V, Puy H and
Peoc'h K: Fecal calprotectin in inflammatory bowel diseases: update
and perspectives. Clin Chem Lab Med. 55:474–483. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Urushikubo J, Yanai S, Nakamura S,
Kawasaki K, Akasaka R, Sato K, Toya Y, Asakura K, Gonai T, Sugai T
and Matsumoto T: Practical fecal calprotectin cut-off value for
Japanese patients with ulcerative colitis. World J Gastroenterol.
24:4384–4392. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hille A, Schmidt-Giese E, Hermann RM,
Herrmann MK, Rave-Fränk M, Schirmer M, Christiansen H, Hess CF and
Ramadori G: A prospective study of faecal calprotectin and
lactoferrin in the monitoring of acute radiation proctitis in
prostate cancer treatment. Scand J Gastroenterol. 43:52–58.
2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hille A, Rave-Fränk M, Christiansen H,
Herrmann MK, Kertesz T, Hermann RM, Wolff HA, Schirmer M, Hess CF
and Ramadori G: Faecal calprotectin and lactoferrin values during
irradiation of prostate cancer correlate with chronic radiation
proctitis: Results of a prospective study. Scand J Gastroenterol.
44:939–946. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang K, Zhang S, Weber J, Baxter D and
Galas DJ: Export of microRNAs and microRNA-protective protein by
mammalian cells. Nucleic Acids Res. 38:7248–7259. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Tanigawa K: Case review of severe acute
radiation syndrome from whole body exposure: Concepts of
radiation-induced multi-organ dysfunction and failure. J Radiat
Res. 62 (Suppl 1):i15–i20. 2021.PubMed/NCBI View Article : Google Scholar
|