Introduction
In 2020, ~115,949 new cases of gallbladder cancer
(GBC) were reported globally (1).
GBC is a rare, aggressive and complex disease that mostly
originates from the organ's epithelial cells (2,3). The
highest incidence rates were observed among indigenous populations
in South America, northern India and East Asia, as well as
individuals aged 70-74 years (1-3).
GBC, while uncommon overall, ranks as the 23rd most prevalent
cancer globally. Among men, it holds the same position, while among
women, it occupies the 20th space (2). Matsuda and Saika (4) report that in Japan, the incidence of
GBC and biliary tract cancers (classified under the International
Classification of Diseases version 10 category C23-C24) is 11,641
cases in males and 10,699 in females. The age-standardized
incidence rates for these cancers are 5.9 per 100,000 male and 3.6
per 100,000 female individuals. Furthermore, these rates vary with
age and geographical region, with the highest incidence being
observed in individuals in the seventh and eighth decades of life,
as well as those living in the northern regions of the country
(5).
The etiology of GBC involves a complex interplay of
numerous genetic, epigenetic and environmental risk factors,
including gallstones, chronic inflammation, congenital anomalies,
obesity and genetic/molecular alterations (6). Comprehensive diagnosis and management
of GBC includes thorough clinical evaluation, imaging,
histopathological examination, staging and development of
individualized treatment strategies that adopt a multidisciplinary
approach (7). Despite significant
advancements in treatment, the overall survival rates of GBC remain
relatively low, predominantly due to delayed diagnosis, as
early-stage lesions that are confined to the gall bladder and have
not invaded adjacent structures or metastasized are typically
associated with better prognosis (8).
Metastatic tumors in the oral region account for ~1%
of all oral malignancies (9).
However, while several studies have reported metastasis of primary
breast, lung and kidney tumors to this region, there is limited
evidence of the spread of GBC (10). Metastasis typically involves a
complex series of steps wherein the cancer cells first invade the
blood and/or lymphatic vessels before spreading to distant sites,
such as the mandible (11).
Numb chin syndrome (NCS) is a rare, sensory
neuropathy that affects the mental nerve, leading to unilateral or
bilateral altered sensations, decreased sensitivity or absence of
pain in the chin and surrounding regions. Perez et al
(12) recently reported that NCS
was associated with various local and systemic etiological factors
(summarized in Table I), including
primary mandibular tumors or distant metastases of other cancers
(13-33).
It is commonly observed in association with metastasized breast
cancer (32%), lymphoma and leukemia (24%), or prostate cancer (9%),
and less frequently observed in association with lung cancer,
myeloma, bone cancer, soft-tissue cancer, colon cancer, kidney
cancer, adenoid cystic carcinoma, melanoma, skin cancer and
digestive cancers such as GBC (13-19).
The exact mechanisms underlying NCS remain unclear, although
certain studies hypothesized nerve compression or damage as a
consequence of tumor infiltration and invasion into the mandibular
canal and bone (13,34). Accurate diagnosis of the cause of
NCS can be challenging, highlighting the importance of prompt
confirmatory testing and timely treatment.
 | Table IEtiological factors of numb chin
syndrome. |
Table I
Etiological factors of numb chin
syndrome.
| Category | Condition | (Refs.) |
|---|
| Malignancy | Common: Breast
cancer, lymphoma and leukemia, prostate cancer Uncommon: Lung
cancer, myeloma, bone cancer, soft-tissue cancer, colon cancer,
kidney cancer, adenoid cystic carcinoma, melanoma and other skin
cancers, other digestive tract cancers (e.g.,gall bladder
carcinoma) | (13-19) |
| Non-malignancy | Iatrogenic injuries
during dental extractions, orthognathic surgery, trauma involving
mandibular fractures, radicular dentigerous cysts, infections
(e.g., chronic apical periodontitis, dental abscess), osteomyelitis
of the jaw, medication-related osteonecrosis of the jaw, cysts and
other benign tumors of dental origin, dental anesthetic
administration, dental implants | (13,20-26) |
| Systemic | Syphilis
arachnoiditis, connective tissue diseases, acromegaly, Paget's
disease, van Buchem syndrome, diabetes mellitus, systemic lupus
erythematosus, multiple myeloma, sickle cell disease, amyloidosis
and sarcoidosis, brain stem infarcts, carotid aneurysms, temporal
arteritis, multiple sclerosis, Sjögren's syndrome, infections
(e.g., herpes and the human immunodeficiency virus) | (13,27-32) |
| Ageing | Mandibular
atrophy | (33) |
The current study focuses on a rare case of GBC
metastasis to the mandible accompanied by NCS. This combination
posed significant diagnostic challenges due to its similarity with
other mandibular pathologies, and the current case report aims to
emphasize the critical significance of timely confirmatory testing
to ensure accurate diagnosis and effective management of this
complex condition.
Materials and methods
Literature search strategy
Multiple databases, including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase
(https://www.embase.com/) and Web of Science
(https://www.webofscience.com/), were
extensively searched for studies examining patients with metastatic
GBC or extra biliary tract cancer. The search terms used included
‘gallbladder cancer’, ‘carcinoma of the gallbladder’, ‘metastatic
gallbladder cancer’, ‘carcinoma to the mandible’, ‘metastatic
gallbladder cancer to the oral and maxillofacial region’, ‘head and
neck metastatic gallbladder cancer’ and ‘numb chin syndrome’. The
subsequent literature review primarily focused on studies published
in English, although those published in other languages were also
evaluated for relevance and new information. No restrictions
regarding the publication date were applied, with the earliest
study being published in 1961, and the findings of the literature
search were summarized in Table II
(35-43).
 | Table IIPrevious reports of metastasis of
gall bladder carcinoma to the mandible. |
Table II
Previous reports of metastasis of
gall bladder carcinoma to the mandible.
| Case no. | Author, year of
publication | Gender | Age, years | Diagnosis | TNM stage | Presence of
NCS | Treatment | Prognosis/survival
status | (Refs.) |
|---|
| 1 | Rominger, 1961 | Female | 68 | GBC | NI | Not clear |
Radiation/chemotherapy | Deceased | (35) |
| 2 | Kim, 1990 | Female | 38 | GBC | NI | Not clear | Chemotherapy | Poor
prognosis/unknown survival status | (36) |
| 3 | Suzuki, 1995 | Female | 61 | GBC | IV | Hypoaesthesia of
lower lip | Surgery | Deceased | (37) |
| 4 | Chang, 2002 | Female | 62 | GBC | IV | Not clear | Chemotherapy | Deceased | (38) |
| 5 | Tanaka, 2010 | Male | 78 | GBC | IV | Not clear | Palliative
care | Deceased | (39) |
| 6 | Chen, 2010 | Female | 63 | GBC | IV | Not clear | Palliative
care | Deceased | (40) |
| 7 | Marin, 2013 | Female | 66 | GBC | NI | Not clear | Aesthetic
surgery | Poor prognosis/5
months' survival after diagnosis | (41) |
| 8 | Savithri, 2018 | Female | 64 | GBC? | IV | Paresthesia of the
lower lip and chin | Palliative
radiotherapy | Poor
prognosis/survived for over 10 months after diagnosis | (42) |
| 9 | Dall'Magro,
2022 | Female | 33 | EBC | IV | Not clear | Palliative
radiotherapy/chemotherapy and care | Deceased | (43) |
Case report
In April 2015, a 69-year-old Japanese female patient
presented at the Oral and Maxillofacial Surgery Department of the
Red Cross Hospital (Naha, Japan) with the chief complaint of a
tingling (numbness) sensation on the right side of the chin and
lower lip and mild pain during molar occlusion since February 2015.
The patient had been referred by a dentist for further
investigation and management of suspected periodontitis in the
right mandibular second premolar region and/or right mandibular
osteomyelitis. The patient had previously undergone dental
prosthetic treatment, including a partial denture for a missing
right mandibular first molar. In addition, the patient had a
history of chronic, well-controlled hypertension and type 2
diabetes mellitus and an unremarkable family history.
Upon examination, the patient was indicated to be
well-nourished and in good condition, and exhibited no signs of
pallor, jaundice, cyanosis, clubbing or localized/generalized
lymphadenopathy. Although systemic examination yielded inconclusive
results, the patient declined further exploration due to a lack of
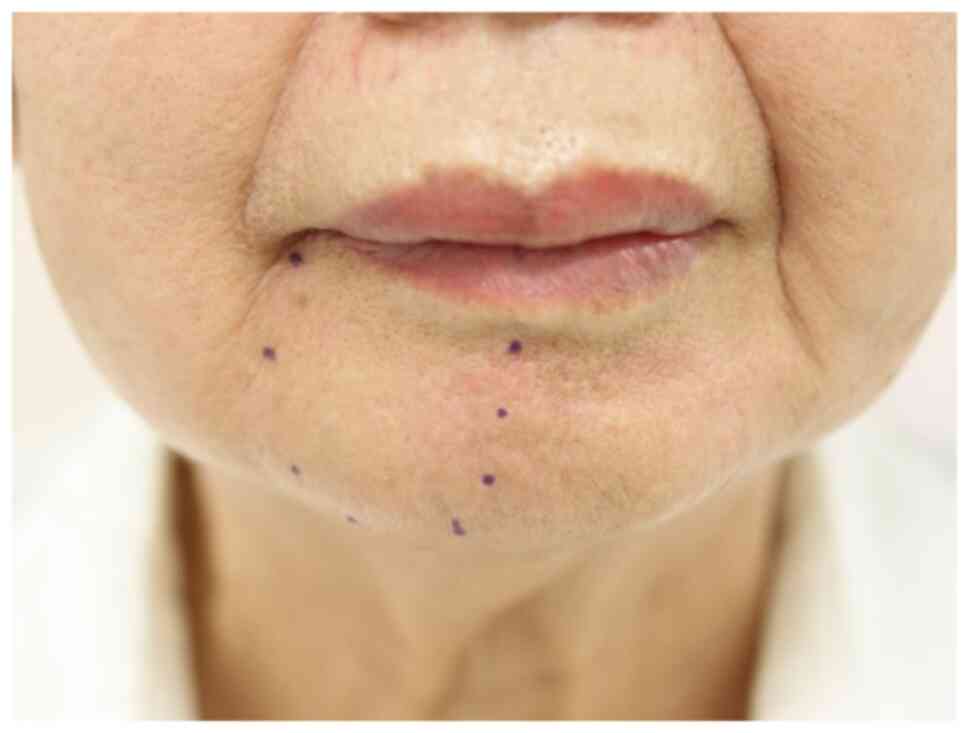
clear indications. Local examination revealed normal facial
symmetry and a lack of sensation on the right side of the mandible
extending from the labial commissure of the mouth to the midline of
the inferior labium, including the vermilion and anterior chin.
This area is innervated by the mental nerve (Fig. 1). Intraoral examination showed mild
gingival erythema extending from the right mandibular second
premolar to the right mandibular second molar. No obvious
abnormalities were observed in the right mandibular first molar
region.
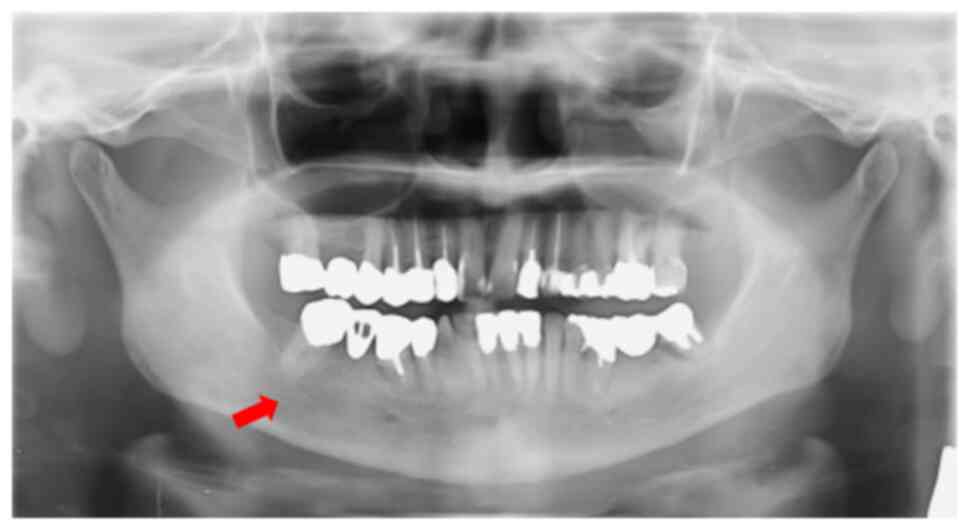
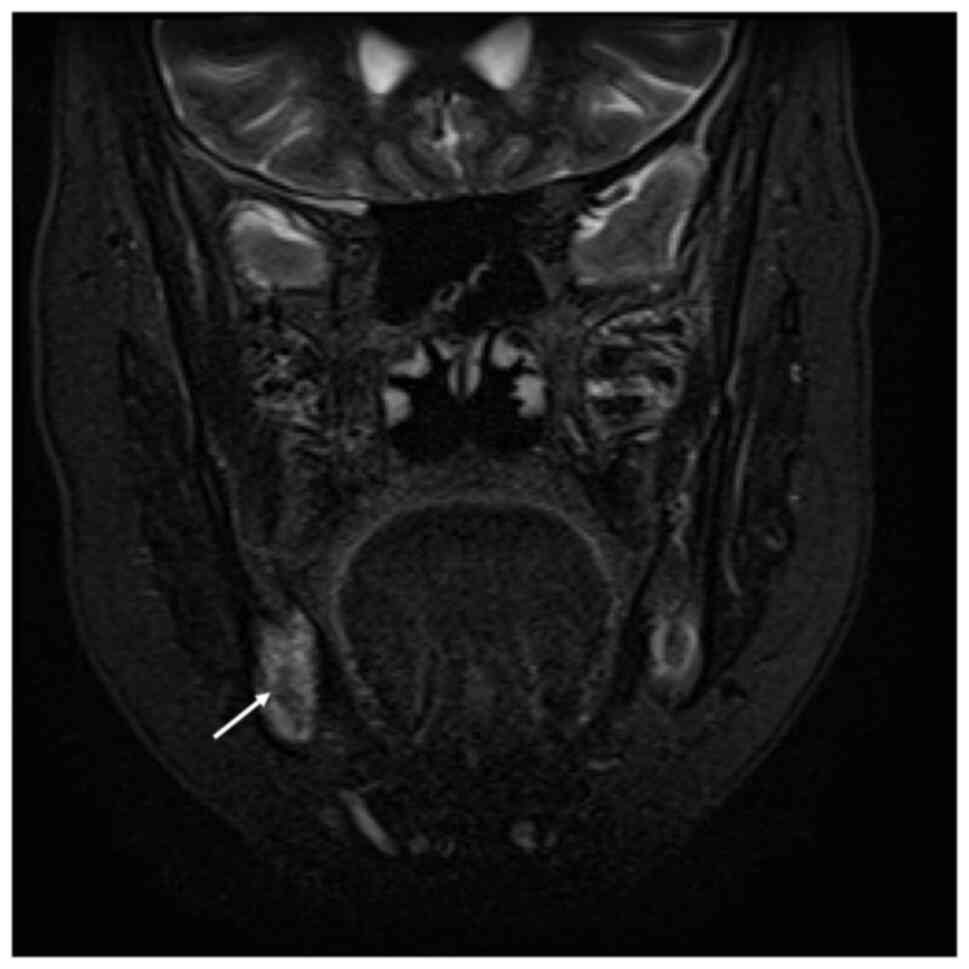
Further investigation included orthopantomogram
(OPG), contrast-enhanced computed tomography (CT),
contrast-enhanced magnetic resonance imaging (MRI) using the
short-tau inversion recovery (STIR)-PROPELLER technique, as well as
hematological, cytology and histopathological examinations. OPG and
MRI T2-weighted/STIR imaging showed alveolar bone resorption and
high signal intensity between the right mandibular second premolar
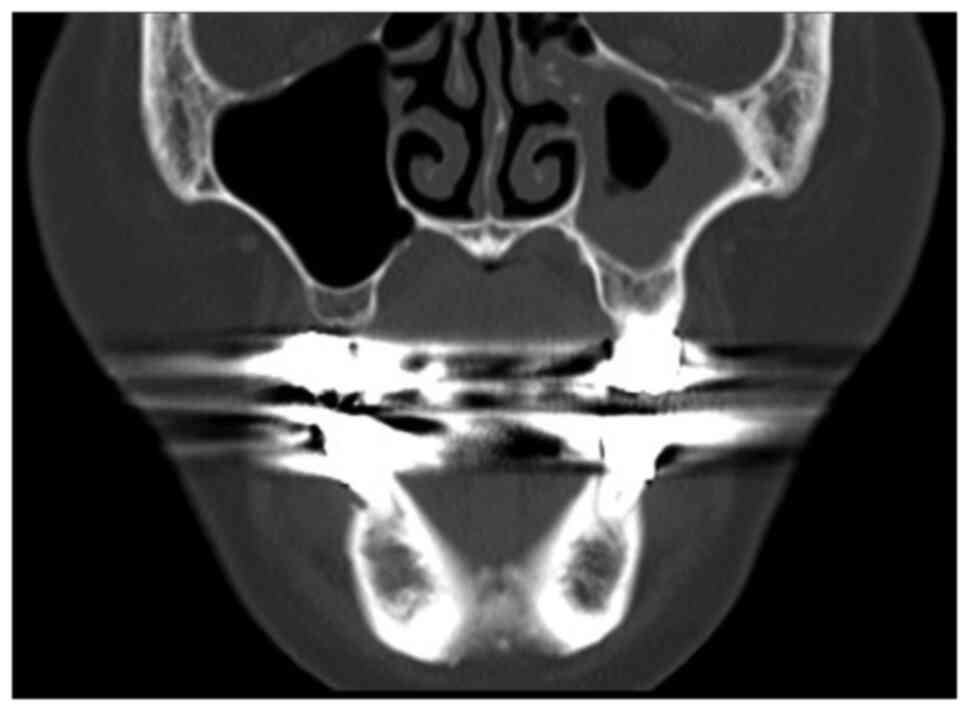
and second molar, indicating aggressive inflammation (Figs. 2 and 3). CT imaging showed mild osteolytic
changes around the root of the right mandibular second premolar
(Fig. 4), while hematological
examination showed normal complete blood count levels. However, the
C-reactive protein (CRP) and carbohydrate antigen 19-9 (CA 19-9;
sialyl-Lewisᴬ) levels were elevated at 2.71 (reference value:
0-0.5) mg/dl and 1161.99 (reference value: 0-37) U/ml,
respectively. Although non-specific, the erythrocyte sedimentation
rate test was an unremarkable at 28 mm/h (local reference range for
the 60-69-year age category, 2-40 mm/h; Westergren method). The
patient's electrolyte levels, liver and renal function, complement
titer, thyroid function, autoantibody levels, cytomegalovirus
antibody levels and Epstein-Barr virus antibody levels were all
normal. Core needle cytology examination indicated an inflammatory
process (data not shown), prompting an initial diagnosis of right
mandibular osteomyelitis with a strong suspicion of cancer. As the
patient's medical history included tooth extraction, prosthetic
treatment and absence of a prior diagnosis of cancer, the
possibility of tumorous lesions could not be ruled out based on CT
and MRI images of the head and neck only. However, the patient
declined additional comprehensive investigations including
histopathology and a full-body evaluation using positron emission
tomography (PET)-CT. The right mandibular second premolar was
subsequently extracted, and mandible biopsy cytology examination
suggested osteomyelitis (Fig. S1).
Treatment with broad-spectrum antibiotics alleviated the numbness.
However, an immediate evaluation for extraoral cancer was strongly
recommended. The patient returned with a chief complaint of
swelling and pain in the right mandible two months later and
underwent extraction of the right mandibular second molar. Biopsy
cytology examination suggested mandibular osteomyelitis again and
the patient was advised to continue with anti-inflammatory and
antibacterial treatment. However, the patient's symptoms persisted
despite ongoing treatment, and she developed difficulty in mouth
opening and further lip paresthesia 1 month later. At this point,
the patient also developed severe pain in the right upper arm, and
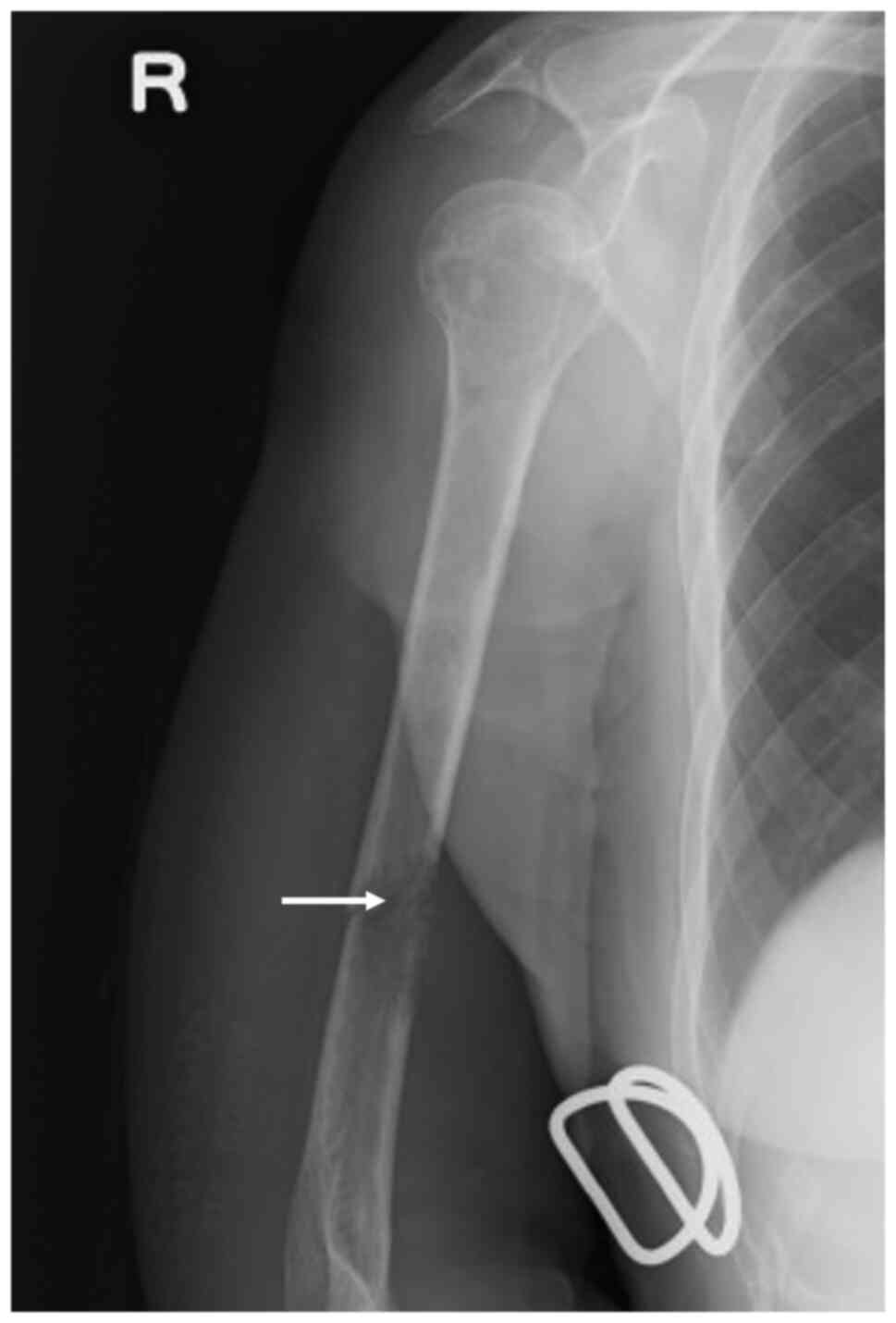
an orthopedic surgeon was consulted. Radiographic examination
showed a pathological fracture (Fig.
5) due to suspected bone metastasis. Consequently, the patient
was referred to the Department of Internal Medicine of the
University of the Ryukyus Hospital (Nishihara, Japan) for extended
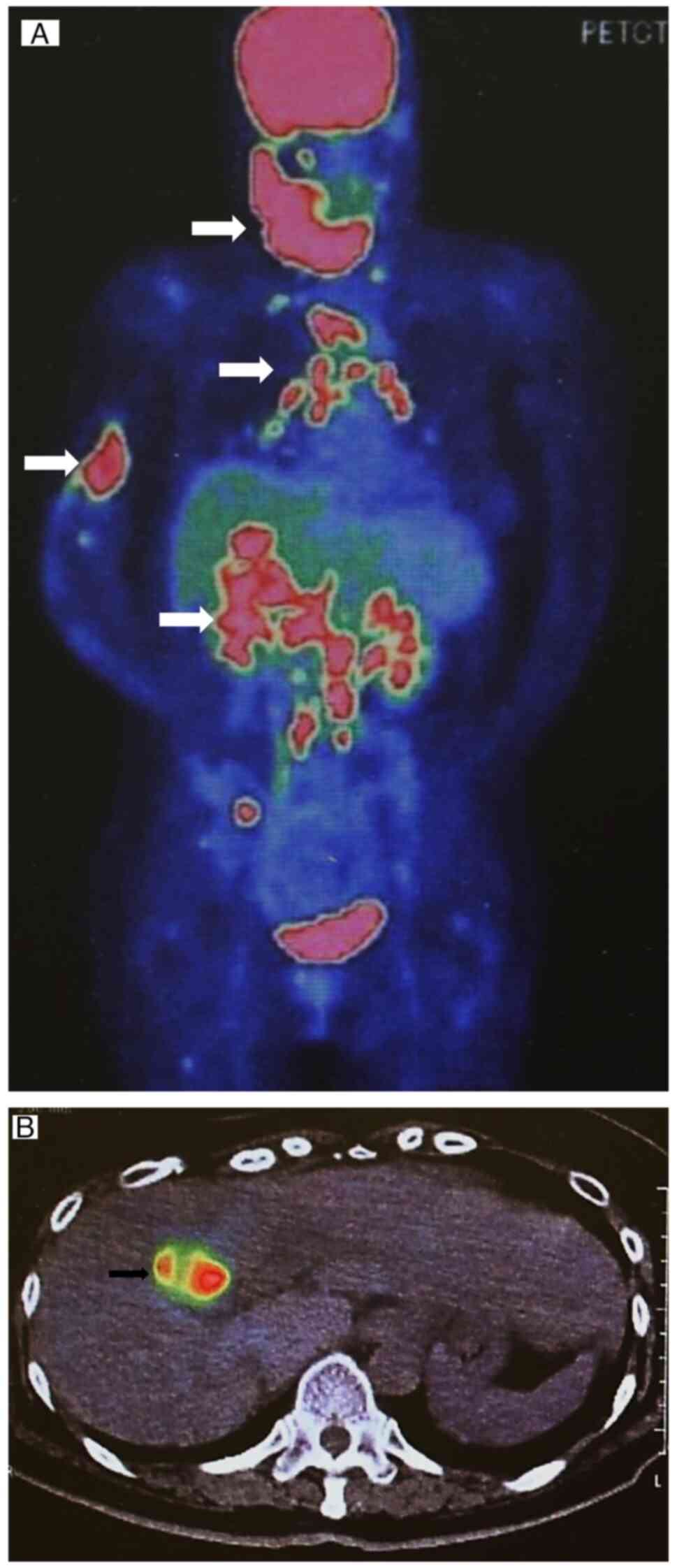
investigation of the primary tumor. Contrast-enhanced CT imaging of
the thorax and abdomen and full-body PET-CT imaging showed features
suggestive of GBC with lymph node, bone, lung and liver metastasis
(Fig. 6A and B). The lung and liver lesions were
strongly suspected to be metastatic tumors based on CT imaging and
the absence of any evidence of viral hepatitis.
Intraoral biopsy of the jaw tumor was repeated and
histopathological analysis including hematoxylin and eosin (HE)
staining and immunohistochemical (IHC) examination was carried out
by at least two pathologists at the pathology department of the
University of the Ryukyus Hospital (Nishihara, Japan). The
specimens were cut into 4-µm-thick sections, fixed with 96% ethanol
and immersed in a 10% formalin solution at 4˚C for 24 h.
Thereafter, hematoxylin staining was performed for 30 sec and the
samples were rinsed with water at 4˚C for 5 min and then
counterstained with eosin Y at 4˚C for 15 sec. The samples then
underwent dehydration with 96 and 99.8% ethanol and xylene
fixation, before being sealed with coverslips using mounting
medium. Diagnostic images were captured using a Nikon Eclipse Ci
microscope equipped with a Nikon DS-Fi3 camera with a x4 objective
(Nikon Corporation). IHC examination was conducted using the
streptavidin-peroxidase technique at room temperature. The
4-µm-thick sections were rinsed with PBS before being subjected to
10 min of pepsase (1:1,000 dilution; Sigma-Aldrich; Merck KGaA)
processing for antigen retrieval. They were then incubated in
methanol and 3% hydrogen peroxide to deactivate endogenous
peroxidases, blocked with PBS containing 0.5% Tween-20
(Sigma-Aldrich; Merck KGaA) and 3% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature, and then
incubated with primary antibodies, including rabbit
anti-cytokeratin (CK)7 (1:1,600 dilution; cat. no. ab199718; Abcam)
and rabbit anti-CK20 (1:200; cat. no. ab64090; Abcam), overnight at
4˚C. After three washes with PBS, a secondary antibody, goat
anti-rabbit IgG H&L (HRP; 1:500 dilution; cat. no. ab97051;
Abcam) was introduced along with fresh diaminobenzidine as the
substrate for 1 h at room temperature. Negative controls were
prepared using PBS instead of the primary antibody, and the Philips
IntelliSite Pathology Solution (Philips Medical Systems) was used
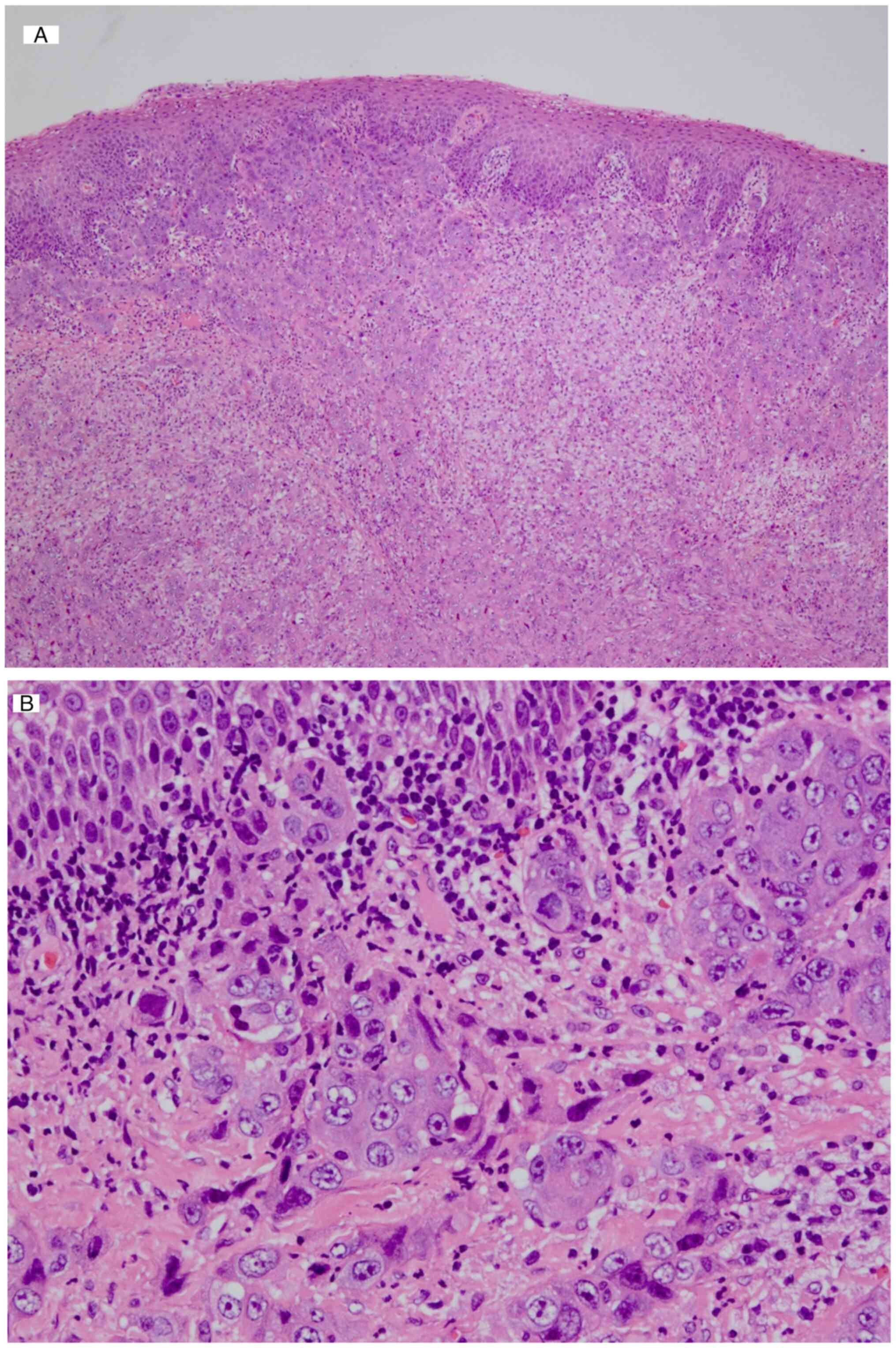
for image acquisition (data not shown). The histopathological
analysis showed the presence of tumor cells with strongly atypical
nuclei of unequal size, eosinophilic sporangia and an indistinct
cord-like structure (Fig. 7) in the
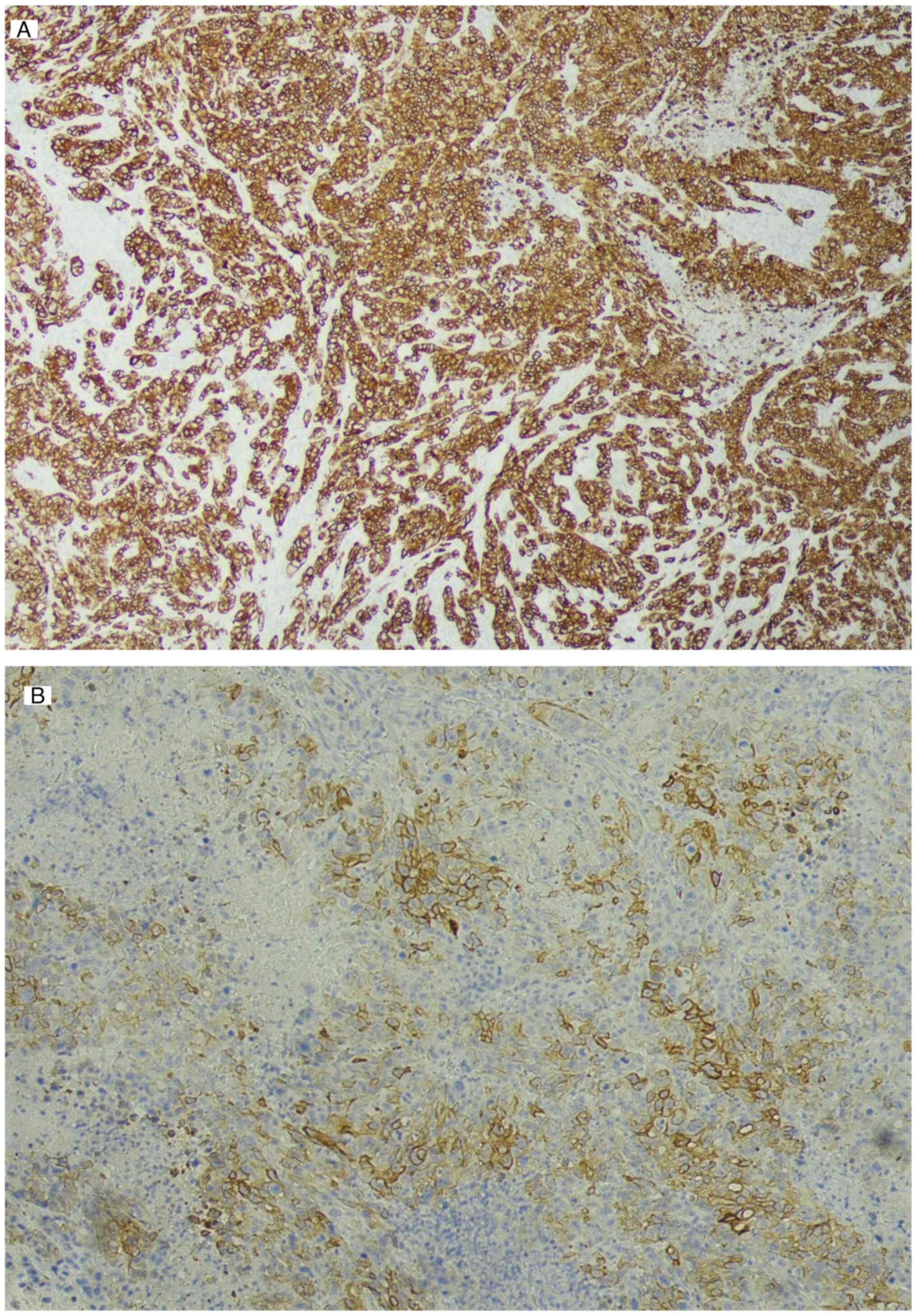
mandibular gingiva. Immunostaining revealed diffuse positivity for
CK7 (Fig. 8A), partial positivity
for CK20 (Fig. 8B) and negativity
for hepatocyte-specific antigen and p40 (results not shown). The
staining pattern was negative for hepatocellular and squamous cell
carcinomas, indicating GBC metastasis (Fig. 8).
A diagnosis of stage IVb (T4N3M1) GBC, as per the
American Joint Committee on Cancer classification (44), was made and the patient was admitted
to the Red Cross Hospital (Naha, Japan) in October 2015 for
palliative treatment due to the advanced stage of cancer and
presence of multiple metastases. Combination intravenous
chemotherapy with 1,000 mg gemcitabine (Eli Lilly and Co.) and 32.5
mg cisplatin (Teva Pharmaceutical Industries Ltd.) was administered
along with a pain control regimen consisting of a subcutaneous
injection of 12 mg denosumab (Amgen Inc.) and a combination of
narcotic drugs. Furthermore, 27 Gy irradiation was applied to the
right upper arm. Despite treatment, the patient's symptoms did not
improve significantly and she succumbed to multiple organ failure
in November 2015.
Discussion
Extraoral metastatic tumors are an exceptionally
rare occurrence, accounting for only 1% of all oral malignancies
(9,45). Primary lesions that may metastasize
to the oral cavity include lung, kidney, liver and prostate
neoplasms in men and breast, genital, kidney and colorectal
neoplasms in women (10). GBC
typically spreads through direct extension to the liver and
adjacent organs within the gastrointestinal tract before involving
the regional lymph nodes. Instances of metastatic GBC in distant
organs have been documented, but its spread to the oral cavity
remains conspicuously rare, with only a small number of cases
observed to date (Table II).
The determinants of oral cavity metastasis include
the nature of the primary tumor, lesion stage, presence of
lymphatic and vascular channel invasion, regional lymph node
involvement, tumor size, histological characteristics of the
lesion, patient's immune status, genetic factors and the
micro-environments of the tumor and distant site. Delay in
diagnosis and treatment of the primary tumor may substantially
increase the risk of metastasis, with the mandible being
particularly vulnerable in the oral region due to its rich blood
supply (46). Metastasis of GBC to
the mandible involves a complex interplay of molecular mechanisms,
such as epithelial-mesenchymal transition, angiogenesis, lymphatic
and hematogenous dissemination, extracellular matrix remodeling,
evasion of immune surveillance, adaptation to the distinct
mandibular microenvironment and concurrent genetic mutations and
alterations that may potentially influence lesion aggressiveness
and its capacity to infiltrate adjacent tissues and spread to
distant sites (47-51).
Oral cavity metastasis typically has a poor
prognosis, with most patients dying within one year of diagnosis
(35-43).
The prognosis depends on the treatment of the oral cavity lesion,
as well as adequate control of the primary tumor and metastasis
(advanced disease) (35-43,52).
In the present case, the patient was diagnosed with extensive GBC
metastasis throughout the body, and the patient's condition had
deteriorated significantly by the time of admission to the
Department of Internal Medicine of the University of the Ryukyus
Hospital (Nishihara, Japan). Consequently, the patient succumbed to
the disease 7 months after the initial diagnosis.
The present study was limited by the absence of
initial intraoral photographs and images documenting the oral
treatment process. These images could have provided additional
insight into the progression and response of the oral metastasis to
treatment, thereby enriching the visual documentation of this rare
case. However, it should be emphasized that their absence does not
diminish the clinical and diagnostic relevance of the findings
presented. The absence of negative control IHC images, excluded
from the analysis, could have potentially aided in the validation
of the staining specificity and enhanced the interpretive accuracy
for the reader. However, this potential limitation is mitigated by
the comprehensive diagnostic approach employed in the present
retrospective case report. To further uphold and enhance the
scientific integrity of the reported findings, improved access to
and inclusion of relevant investigation control data/images will be
a key focus in our future studies.
A diagnosis of metastatic tumors in the oral and
maxillofacial region may be made if the presence of a primary tumor
located outside this region has been histologically and clinically
confirmed, a clear distinction and absence of invasion between the
primary tumor and metastases is observed and the patient has no
history of a previous primary lesion in the same region (9,52). In
the present study, the patient met all of these criteria, leading
to the diagnosis of a metastatic tumor originating from GBC. GBC is
a highly aggressive cancer that often spreads to nearby organs.
Distant metastasis to the lungs, chest cavity (9.4%), skeletal
system (2.4%) and the brain has been reported, although there is
limited evidence of metastasis to the oral region (37,53-56).
NCS, first described by Calverley in 1963 and
characterized by hypoesthesia limited to the lower lip and mental
region due to mental nerve palsy (34), is often observed in association with
metastatic malignancies of the mandible or infiltration of
hematopoietic tumors such as lymphomas (13). While malignant lymphomas commonly
present with NCS as the first symptom, followed by leukemia,
gastric cancer, lung cancer and others, metastatic GBC with NCS as
the initial symptom is a rare occurrence (34). In the current study, OPG showed no
significant abnormalities, and a definitive diagnosis was made
after histopathological confirmation. OPG alone may be insufficient
during the early stages of invasion. CT and MRI scans may be used
for lesion identification, although differentiation from mandibular
osteomyelitis may be challenging. The patient's lack of relevant
surgical and/or cancer history and initial failure to fully explore
the condition due to personal reasons may have contributed to
delays in diagnosis. As the majority of patients exhibiting
malignancies with NCS as the initial symptom have a poor prognosis,
it is presumed that the cancer had already progressed significantly
at the time of onset of symptoms (10).
Distinguishing metastatic malignant tumors arising
in the jawbone from mandibular osteomyelitis may be challenging due
to the similarities in their clinical and radiological features,
with both conditions presenting with lower lip hypoesthesia and
mandibular bone resorption (57,58).
Hariya et al (57)
categorized the morphology of bone resorption into point, mass and
infiltrative fractures, and found that both malignant tumors of the
jawbone and mandibular osteomyelitis were associated with a higher
degree of bone resorption. Intra-mandibular malignant tumors with
infiltrative bone resorption typically present as a worm-eaten
pattern, while mandibular osteomyelitis presents as point-like and
massive resorption of cortical bone without infiltrative bone
resorption on CT images. Cortical bone swelling is typically
observed in intra-mandibular malignancies and is absent in patients
with mandibular osteomyelitis. Furthermore, although periosteal
reactions and osteosclerotic features are frequently seen in
patients with mandibular osteomyelitis, they have also been
reported in association with intra-mandibular malignant tumors
(57,58). In the present study, initial CT
images revealed no resorption or mass formation in the lateral
cortical bone of the mandible. However, elevated levels of CRP,
CA19-9 and ESR were observed, and a history of tooth extraction
with subsequent denture placement, coupled with pain in the region
of the right mandibular second premolar, suggested potential
mandibular osteomyelitis or malignancy. Consequently, biopsy
cytology was undertaken following the premolar extraction, leading
to a diagnosis of mandibular osteomyelitis. Diagnosis was delayed
due to several factors, including the absence of a significant
surgical or cancer history of the patient and the typically occult
presentation of GBC (1,2). Although core needle biopsy provides a
valuable tool for preliminary assessment of tumorous lesions and
treatment guidance, its limitations, particularly regarding the
potential for inadequate tissue sampling and preservation, as well
as the ability to accurately assess certain biomarkers by IHC
testing, should be recognized to avoid negatively impacting the
diagnostic process (59).
Omics analyses, including metabolomics,
transcriptomics and proteomics studies, were not conducted in this
study due to the rapid deterioration of the patient's condition and
her reluctance to undergo comprehensive assessments. Furthermore,
attempts to conduct posthumous analyses were hindered by the
absence of stored blood samples from the patient. Omics analyses,
employing liquid biopsies, chromatographic separation, mass
spectrometry and molecular biology techniques, show great promise
for cancer diagnosis and prognosis (60). However, challenges persist, as
identified by Wang et al (60), including variability in
quantification and detection depending on the metabolomics
approach-targeted or untargeted- and the choice of instruments. In
addition, the considerable heterogeneity of metabolites among
different cancer types, influenced by numerous factors, adds to
these challenges (60). Therefore,
while these analyses could have provided deeper insight into the
molecular underpinnings of the metastatic process, their absence
does not detract from the clinical and histopathological findings
that form the basis of the present case report. This highlights the
importance of considering the preservation of biological samples in
future cases for advanced molecular studies.
The current study emphasizes the critical need for
timely diagnosis and multimodal therapy in patients presenting with
initial vague symptoms to improve outcomes in aggressive metastatic
malignancies such as GBC.
In conclusion, the present case report underscores
the rarity of GBC metastasizing to the oral region and presenting
as NCS. It highlights the critical need for prompt, thorough
evaluation of neurological symptoms to detect hidden malignancies
and the importance of differentiating bony metastases from
conditions such as osteomyelitis through comprehensive diagnostic
methods. The study also emphasizes the value of multidisciplinary
collaboration for the timely diagnosis and treatment of complex
metastatic diseases. In addition, it points to the potential of
emerging technologies such as metabolomics in cancer research,
underscoring the necessity for swift confirmatory tests and
accurate diagnoses in managing metastatic cancers.
Supplementary Material
Biopsy cytology examination. The image
shows scattered necrotic bone and the presence of inflammatory
cells (HE staining; magnification, x40).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
MM and EHN: Study conception and design; MM, EHN,
HK, TG, KI, JS, NM, TK, KN, YS and HN: Data acquisition, analysis
and interpretation; MM, EHN, KN, YS and HN: Preparation of
manuscript draft and revision. MM and HN confirm the authenticity
of all the raw data. All authors have read and approved the final
version of the manuscript prior to submission.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient's next-of-kin prior to publication of their clinical
information and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gallbladder Cancer Statistics. World
Cancer Research Fund International n.d. Available from: https://www.wcrf.org/cancer-trends/gallbladder-cancer-statistics/
(accessed May 24, 2023).
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roa JC, García P, Kapoor VK, Maithel SK,
Javle M and Koshiol J: Gallbladder cancer. Nat Rev Dis Primers.
8(69)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matsuda T and Saika K: Cancer burden in
Japan based on the latest cancer statistics: Need for
evidence-based cancer control programs. Ann Cancer Epidemiol.
2(2)2018.
|
|
5
|
Hariharan D, Saied A and Kocher HM:
Analysis of mortality rates for gallbladder cancer across the
world. HPB (Oxford). 10:327–331. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rawla P, Sunkara T, Thandra KC and Barsouk
A: Epidemiology of gallbladder cancer. Clin Exp Hepatol. 5:93–102.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lamarca A, Barriuso J, McNamara MG and
Valle JW: Molecular targeted therapies: Ready for ‘prime time’ in
biliary tract cancer. J Hepatol. 73:170–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meyer I and Shklar G: Malignant tumors
metastatic to mouth and jaws. Oral Surg Oral Med Oral Pathol.
20:350–362. 1965.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hirshberg A, Shnaiderman-Shapiro A, Kaplan
I and Berger R: Metastatic tumours to the oral cavity-pathogenesis
and analysis of 673 cases. Oral Oncol. 44:743–752. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pisani P, Airoldi M, Allais A, Aluffi
Valletti P, Battista M, Benazzo M, Briatore R, Cacciola S, Cocuzza
S, Colombo A, et al: Metastatic disease in head & neck
oncology. Acta Otorhinolaryngol Ital. 40 (Suppl 1):S1–S86.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Perez C, de Leeuw R, Escala PF, Fuentealba
R and Klasser GD: Numb chin syndrome: What all Oral Health care
professionals should know. J Am Dent Assoc. 154:79–93.
2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baskaran RK, Krishnamoorthy SM and Smith
M: Numb chin syndrome-a reflection of systemic malignancy. World J
Surg Oncol. 4(52)2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Maeda K, Taniguchi JI and Matsui K: Two
cases of numb chin syndrome diagnosed as malignant disease. Oxf Med
Case Reports. 2018(omy097)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sanchis JM, Bagán JV, Murillo J, Díaz JM,
Poveda R and Jiménez Y: Mental neuropathy as a manifestation
associated with malignant processes: Its significance in relation
to patient survival. J Oral Maxillofac Surg. 66:995–998.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Evans RW, Kirby S and Purdy RA: Numb chin
syndrome. Headache. 48:1520–1524. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu SY, Huang SH and Chen YH: Numb chin
with mandibular pain or masticatory weakness as indicator for
systemic malignancy-A case series study. J Formos Med Assoc.
116:897–906. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Smith SF, Blackman G and Hopper C: Numb
chin syndrome: A nonmetastatic neurological manifestation of
malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
105:e53–e56. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tejani N, Cooper A, Rezo A, Pranavan G and
Yip D: Numb chin syndrome: A case series of a clinical syndrome
associated with malignancy. J Med Imaging Radiat Oncol. 58:700–705.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Divya KS, Moran NA and Atkin PA: Numb chin
syndrome: A case series and discussion. Br Dent J. 208:157–160.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kalladka M, Proter N, Benoliel R,
Czerninski R and Eliav E: Mental nerve neuropathy: Patient
characteristics and neurosensory changes. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 106:364–370. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shah D, Shetty S, MacBean AD and Olley SF:
Numb chin syndrome: A metastatic deposit in the mandible. Dent
Update. 37:244–246. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hirshberg A, Leibovich P and Buchner A:
Metastatic tumors to the jawbones: Analysis of 390 cases. J Oral
Pathol Med. 23:337–341. 1994.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Maillefert JF, Gazet-Maillefert MP,
Tavernier C and Farge P: Numb chin syndrome. Joint Bone Spine.
67:86–93. 2000.PubMed/NCBI
|
|
25
|
Neal CE and Kiyak HA: Patient perceptions
of pain, paresthesia, and swelling after orthognathic surgery. Int
J Adult Orthodon Orthognath Surg. 6:169–181. 1991.PubMed/NCBI
|
|
26
|
Worthington P: Injury to the inferior
alveolar nerve during implant placement: A formula for protection
of the patient and clinician. Int J Oral Maxillofac Implants.
19:731–734. 2004.PubMed/NCBI
|
|
27
|
Hogan MC, Lee A, Solberg LA and Thomé SD:
Unusual presentation of multiple myeloma with unilateral visual
loss and numb chin syndrome in a young adult. Am J Hematol.
70:55–59. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mestoudjian P, Steichen O, Stankovic K,
Lecomte I and Lionnet F: Sickle cell disease, a benign cause of
numb chin syndrome. Am J Med. 121(e1)2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
da Silva CJ, da Rocha AJ, Mendes MF, Maia
AC Jr, Braga FT and Tilbery CP: Trigeminal involvement in multiple
sclerosis: Magnetic resonance imaging findings with clinical
correlation in a series of patients. Mult Scler. 11:282–285.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mori K, Iijima M, Koike H, Hattori N,
Tanaka F, Watanabe H, Katsuno M, Fujita A, Aiba I, Ogata A, et al:
The wide spectrum of clinical manifestations in Sjögren's
syndrome-associated neuropathy. Brain. 128:2518–2534.
2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Abilleira S and Bowler JV: The numb chin
syndrome as an early manifestation of giant-cell (temporal)
arteritis: A case report. Headache. 45:1411–1413. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yura Y, Kusaka J, Yamakawa R, Bando T,
Yoshida H and Sato M: Mental nerve neuropathy as a result of
primary herpes simplex virus infection in the oral cavity. A case
report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
90:306–309. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Furukawa T: Numb chin syndrome in the
elderly. J Neurol Neurosurg Psychiatry. 53(173)1990.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Smith RM, Hassan A and Robertson CE: Numb
chin syndrome. Curr Pain Headache Rep. 19(44)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rominger CJ, Lockwood DW and Canino CW:
Carcinoma of the gallbladder with mandibular metastasis: Report of
case. J Oral Surg Anesth Hosp Dent Serv. 19:425–427.
1961.PubMed/NCBI
|
|
36
|
Kim CS, Lee JH, Ann HY, Chung SC and Choi
HS: Metastatic carcinoma of oral cavity. Maxillofac Plast Reconstr
Surg. 12:142–147. 1990.
|
|
37
|
Suzuki K, Onizawa K, Yoshida H and Fukuda
H: A case of metastatic gallbladder adenocarcinoma of the mandible.
Jpn J Oral Maxillofac Surg. 41:154–156. 1995.
|
|
38
|
Chang TS, Liaw CC, Lee KF and Wu CS:
Gingival metastasis from gallbladder cancer. Chang Gung Med J.
25:553–556. 2002.PubMed/NCBI
|
|
39
|
Tanaka A, Shigematsu H, Kikuchi K, Inoue
K, Ide F, Hasegawa A, Kusama K and Sakashita H: Metastatic tumor of
the mandible from occult gallbladder cancer: A case report. Asian J
Oral Maxillofac Surg. 22:168–171. 2010.
|
|
40
|
Chen YS, Hsu YH, Lee CF and Chou YF:
Metastatic gallbladder cancer presenting as a gingival tumor and
deep neck infection. Kaohsiung J Med Sci. 26:558–561.
2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Marin H, Bouras AF, Patenôtre P,
Boleslawski E, Zerbib P, Pruvot FR and Truant S: Cheek metastasis
from gallbladder adenocarcinoma. J Visc Surg. 150:225–226.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Savithri V, Suresh R, Janardhanan M and
Aravind T: Metastatic adenocarcinoma of mandible: In search of the
primary. BMJ Case Rep. 11(e227862)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dall'Magro AK, Dogenski LC, Bade P, Cé LC,
Dall'Magro E and De Carli JP: Mandibular metastasis of primary
extrahepatic biliary carcinoma: Case report. Int J Surg Case Rep.
98(107498)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Amin MB: AJCC Cancer Staging Manual. 8th
edition. Springer, Heidelberg, 2017.
|
|
45
|
Adewale AO, Mofoluwake LA, Olamide OT and
Yussuf SA: Two case reports on mandibular metastases. Ghana Med J.
52:168–172. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hirshberg A, Berger R, Allon I and Kaplan
I: Metastatic tumors to the jaws and mouth. Head Neck Pathol.
8:463–474. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xu S, Zhan M and Wang J:
Epithelial-to-mesenchymal transition in gallbladder cancer: From
clinical evidence to cellular regulatory networks. Cell Death
Discov. 3(17069)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Leong SP, Naxerova K, Keller L, Pantel K
and Witte M: Molecular mechanisms of cancer metastasis via the
lymphatic versus the blood vessels. Clin Exp Metastasis.
39:159–179. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fares J, Fares MY, Khachfe HH, Salhab HA
and Fares Y: Molecular principles of metastasis: A hallmark of
cancer revisited. Signal Transduct Target Ther.
5(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Andrén-Sandberg A: Molecular biology of
gallbladder cancer: Potential clinical implications. N Am J Med
Sci. 4:435–441. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kang M, Na HY, Ahn S, Kim JW, Lee S, Ahn
S, Lee JH, Youk J, Kim HT, Kim KJ, et al: Gallbladder
adenocarcinomas undergo subclonal diversification and selection
from precancerous lesions to metastatic tumors. Elife.
11(e78636)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Clausen F and Poulsen H: Metastatic
carcinoma of the jaws. Acta Pathol Microbiol Scand. 57:361–374.
1963.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Arnaud JP, Graf P, Gramfort JL and Adloff
M: Primary carcinoma of the gallbladder: Review of 25 cases. Am J
Surg. 138:403–406. 1979.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sons HU, Borchard F and Joel BS: Carcinoma
of the gallbladder: Autopsy findings in 287 cases and review of the
literature. J Surg Oncol. 28:199–206. 1985.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bosset JF, Mantion G, Gillet M, Pelissier
E, Boulenger M, Maingon P, Corbion O and Schraub S: Primary
carcinoma of the gallbladder. Adjuvant postoperative external
irradiation. Cancer. 64:1843–1847. 1989.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zachariades N: Neoplasms metastatic to the
mouth, jaws and surrounding tissues. J Craniomaxillofac Surg.
17:283–290. 1989.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Hariya Y, Yuasa K, Nakayama E, Kawazu T,
Okamura K and Kanda S: Value of computed tomography findings in
differentiating between intraosseous malignant tumors and
osteomyelitis of the mandible affecting the masticator space. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 95:503–509.
2003.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Tanaka R and Hayashi T: Computed
tomography findings of chronic osteomyelitis involving the
mandible: Correlation to histopathological findings.
Dentomaxillofac Radiol. 37:94–103. 2008.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sun C, Lu Q, Zhang X, Zhang Y, Jia S, Wang
J, Zhu H, He W and Zhang Z: Comparison between core needle biopsy
and excisional biopsy for breast neoplasm. Medicine (Baltimore).
100(e26970)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang W, Rong Z, Wang G, Hou Y, Yang F and
Qiu M: Cancer metabolites: Promising biomarkers for cancer liquid
biopsy. Biomark Res. 11(66)2023.PubMed/NCBI View Article : Google Scholar
|