Introduction
Fetal growth restriction (FGR) is a pregnancy
complication associated not only with adverse perinatal outcomes
but also with increased risk of cardiovascular diseases in adult
life of offspring (1,2). The unborn baby has an estimated fetal
weight (EFW) below the 10th percentile by gestational age as
determined by prenatal ultrasound evaluation (3).
The etiology of FGR is multifactorial. However, one
of the most common contributing factors is placental insufficiency
(4), a condition that also serves a
role in the pathogenesis of hypertensive disorders of pregnancy
(HDP), including preeclampsia (5).
In a recent study, it was demonstrated that FGR-HDP, a condition
with significant obstetric morbidity and mortality, exhibits
maternal vascular malperfusion of the placental bed, abnormal
feto-placental Doppler parameters and signs of oxidative stress of
the syncytiotrophoblast (6). Other
recent studies show that extracellular vesicles derived from
placental tissue influence endothelial cell function, explaining
the relationship between placental insufficiency and hypertensive
disorder (7,8).
There is widespread evidence for endothelial
dysregulation of the fetoplacental vascular tone in FGR, which
compensates for restricted blood flow (9-12).
In this regard, nitric oxide production by the endothelium, which
is key in maintenance of blood flow in the fetal placental bed
during normotensive pregnancy (10)
may be disordered in pregnancy with FGR and/or preeclampsia
(11,12). Nitric oxide is produced by nitric
oxide synthases, including endothelial nitric oxide synthase
isozyme whose activity is markedly increased when intracellular
Ca2+ concentration ([Ca2+]i) rises
(13). The activation of this
endothelial isozyme depends on conformational changes induced by
its interaction with the Ca2+-calmodulin complex
(14). The vascular endothelium
responds to agonists by increasing [Ca2+]i.
This response depends on different elements, including the type of
receptor activated, release of Ca2+ from intracellular
stores and store-operated Ca2+ entry (SOCE). The latter
mechanism is activated by depletion of internal Ca2+
stores (15).
Our previous studies demonstrated that bradykinin,
histamine, ATP and α-7 nicotinic acetylcholine receptors agonists
increase [Ca2+]i in endothelial cells
(16-18).
In human umbilical vein endothelial cells (HUVECs), ATP-induced
Ca2+ signal typically consists of an initial transient
phase followed by a prolonged sustained phase (18). ATP is an important autacoid and
paracrine molecule that exerts dual control of vascular tones by
being released from perivascular nerves and endothelial cells in
response to changes in blood flow (shear stress) and hypoxia
(19). Among purinergic receptors
reportedly involved in ATP-induced Ca2+ responses in
HUVECs are the metabotropic P2Y2(20) and ionotropic P2X4 receptors
(21). The P2Y2 receptor, in
addition to inducing rapid Ca2+ response, promotes
nitric oxide production and appears to be the purinergic receptor
that contributes most to the ATP-induced Ca2+ signal
(20). P2X4 receptor seems to be
overexpressed in pathological conditions and involved in production
of reactive oxygen species and pro-inflammatory activators but not
in nitric oxide synthesis (22).
The present study aimed to record clinical data of
newborns and mothers from normotensive and pathologic pregnancies
and investigated Ca2+ signals induced by ATP and SOCE in
HUVECs.
Subjects and methods
Study design and patients
The present study was a prospective cohort study of
single pregnancies complicated by FGR-HDP and healthy pregnancies
(controls). Data and samples were collected from January to
December of 2006 from Dr Gustavo Fricke Hospital, Viña Mar, Chile.
All patients (13-39 years, n=26) gave informed written consent
according to the Declaration of Helsinki.
Inclusion criteria for full-term normal pregnancies
(control) were as follows: i) No medical or obstetrical
complications during pregnancy, labor, or puerperium and ii)
pregnancies with EFW between the 10th and 90th percentiles adjusted
for gestational age within the local population (23,24)
Exclusion criteria were as follows: i) Chronic pathologies, such as
chronic hypertension or gestational diabetes; ii) patients taking
aspirin or nitric oxide donor agents; iii) consumption of alcohol
or any illicit drugs during pregnancy and iv) fetuses with
chromosomal abnormality, congenital infection or malformation.
Inclusion criteria for FGR-HDP were as follows: i)
Patients diagnosed with HDP, such as pregnancy-induced hypertension
(preeclampsia and eclampsia), chronic hypertension in the presence
or absence of superimposed preeclampsia and transient hypertension,
as outlined in the Perinatal Guide of the Ministry of Health, Chile
(24); ii) EFW and abdominal
circumference (AC) <10th percentile for their gestational age
(validated locally) (24,25), combined with Doppler-defined
intrauterine hypoxia or oligohydramnios and iii) EFW and/or AC
<5th percentile for gestational age (validated locally),
regardless of other parameters (Doppler or oligohydramnios).
Exclusion criteria, determined according to the recommendations of
the International Federation of Gynecology and Obstetrics (FIGO)
(23) were as follows: i) Anemia,
preexisting high blood pressure or maternal chronic disease; ii)
patients taking aspirin or nitric oxide donor agents; iii)
consumption of alcohol or any illicit drugs during pregnancy; iv)
fetuses with genetic disorder, structural anomalies, congenital
infections, or exposure to teratogens and v) multiple
pregnancies.
For all patients, maternal age, parity, hemoglobin
levels in the blood, mode of delivery, gestational age at delivery,
birth weight, neonate sex, Apgar score and neonatal intensive care
unit admission were recorded. The gestational age was calculated
with respect to the last menstrual period or estimated by
ultrasonography before the 12th week of pregnancy (24). Apgar score is a method for reporting
clinical status of the newborn at 1 and 5 min of life, is useful
for response to resuscitation, and considers heart rate; 2)
respiratory effort; 3) muscle tone; 4) reflex response or
irritability; 5) skin color; each of these components is given
score of 0, 1, or 2(26).
For neonates with FGR-HDP, AC, oligohydroamnios
(27,28), Doppler velocimetry (29,30),
biophysical profile score, fetal heart rate monitoring and neonatal
death were also analyzed. Doppler velocimetry reflects the
resistance to flow produced by the vascular bed (29,30).
The biophysical profile score to quantify fetal behavior uses
dynamic variables such as fetal tone, breathing movement, gross
body movement, amniotic fluid volume and fetal heart rate analysis
(31). Other profile is the
non-stress test that measures fetal heart rate in responses to
spontaneous fetal movement (32).
Endothelial cell culture and cytosolic
Ca2+ measurement
The umbilical cords were collected after delivery.
The endothelial cells were isolated as described by Jaffe et
al (33) according to the
validated methodologies developed in the Cellular and Molecular
Biochemistry laboratory of the Pharmacy Faculty at the University
of Valparaíso (17). In summary,
endothelial cells was isolated by collagenase-I (0.5 mg/ml; Gibco;
Thermo Fisher Scientific, Inc.) digestion from human umbilical
veins a 37˚C for 15 min. After this, dissociated cells were
cultured in 199 medium (cat. no. M199; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 2.5 mM L-glutamine, 14 mM HEPES
acid, 200 IU/l penicillin, 400 IU/l streptomycin, 10% fetal bovine
serum and 10% newborn calf serum (Gibco, Thermo Fisher Scientific,
Inc.), pH 7.42 at 37˚C Experiments were performed on confluent
primary cultures (80% confluence) 2-5 days after seeding.
[Ca2+]i was measured using the
fluorescent indicator Fluo-3 AM, as previously reported (17,18).
Briefly, confluent HUVECs grown in coverslips and incubated in
Locke's solution (NaCl, 135.0; KCl, 5.6;
CaCl2·2H2O, 2.5; HEPES-acid, 10.0;
MgCl2·6H2O, 1.2 and D-glucose, 5.5 mM) were
mounted in a perfusion chamber on the stage of an epifluorescence
microscope (Nikon Eclipse E600FN) implemented with 490 nm
excitation and 530 nm emission filters. The fluorescence signals
were measured using a photomultiplier (Hamamatsu Photonics K.K.),
digitalized at 3 Hz using an analogue converter board (Data
Translation) and collected using Axotape software (version 2.0;
Axon Instruments). The amplitude of the fluorescent signal was
expressed as
ΔFt/Fb=(Ft-Fb)/Fb,
where Ft is the fluorescence at time t and Fb
is basal fluorescence (34).
Cytosolic Ca2+ response to ATP was
evaluated in HUVECs, as described in a previous study (18). In the present study, the parameters
analyzed were time to initial peak (tP), amplitude of
initial peak, amplitude of sustained phase, and return to the
baseline [Ca2+]i.
To isolate the initial phase (Ca2+
release from ternal stores) without contributing P2X4 receptors and
SOCE, HUVECs were stimulated with 100 µM ATP for 3 min in a
Ca2+-free Locke's solution (0 Ca2+). When
fluorescence returned to levels close to the baseline (~50 sec),
cells were perfused with 10 mM Ca2+ in Locke's solution
for 180 sec. This latter [Ca2+]i rise
corresponds to the sustained phase associated with SOCE (35). After that, HUVECs were returned to
the 0 Ca2+ Locke's solution, and fluorescence declined.
These experiments were performed in HUVECs from 13 healthy and nine
FGR-HDP umbilical cords. HUVEC cultures from two healthy and two
FGR-HDP umbilical cords were not included because of technical
problems. The pathological umbilical cords from neonates with FGR
of gestational ages of 31 and 33 weeks with severe pre-eclampsia
were not evaluated.
Statistical analysis
Clinical data are expressed as mean ± standard
deviation for continuous numerical variables and median and ranges
for discrete numerical variables. [Ca2+]i
signal data are expressed as means ± standard error of 1-3
experimental replicates. The Kolmogorov-Smirnov test was used to
verify normality of the distribution of numerical variables.
Results were compared using unpaired Student's t test for data with
normal distribution and Mann Whitney U-test for non-normal
distribution. Differences between proportions of nominal variables
were compared using Fisher's exact or Yates' χ2 test.
P<0.05 was considered to indicate a statistically significant
difference. The data were analyzed using the software Stata/SE 18.0
(Universidad de Valparaíso).
Results
Clinical parameters of patients
A total of 26 patients were enrolled (11 with
FGR-HDP and 15 controls; Table I).
No significant differences were found in age and parity between
patients and healthy controls, but hemoglobin levels of FGR-HDP
patients were significantly higher than healthy controls. There was
a significant difference in delivery mode between the groups due to
fetal compromise and clinical decision (24). Gestational age and birth weight of
the FGR-HDP group were significantly lower than in the healthy
group (24). There was no
significant difference in Apgar score at 5 min between both groups.
However, 82% FGR-HDP neonates were admitted to the neonatal
intensive care unit, while no healthy control neonates were
admitted.
 | Table IClinical characteristics of pregnant
patients and newborns. |
Table I
Clinical characteristics of pregnant
patients and newborns.
| Characteristic | Healthy control
(n=15) | FGR-HDP (n=11) | P-value |
|---|
| Mean age (range),
years | 22.0±4.0
(13.0-28.0) | 26.0±7.9
(17.0-39.0) | 0.145a |
| First pregnancy,
% | 53.3 | 72.7 | 0.428b |
| Hemoglobin,
g/dl | 11.6±0.8 | 13.1±1.1 |
<0.001c |
| Delivery,
cesarean/vaginal | 0/15 | 11/0 |
<0.001d |
| Mean gestational
age (range), weeks | 39.0±0.8
(38.0-40.5) | 32.1±4.1
(24.5-38.2) |
<0.001a |
| Mean birth weight
(range), g | 3,320±336
(2,800-3,940) | 1,260±646
(560-2,520) |
<0.001a |
| Female, % | 73.3 | 54.5 | 0.419b |
| Apgar score <7
at 5 min | 0 | 1 | 0.428d |
| NICU admission | 0 | 9 |
<0.001a |
The FGR-HDP neonate group exhibited 82% AC <3rd
percentile and a 73% EFW ≤5th percentile for gestational age, which
was confirmed in all cases at birth (Table II). The highest gestational age in
this group was 38 weeks; this neonate had EFW in the 2nd percentile
for gestational age without alteration in biophysical profile
score, fetal heart rate monitoring, and UA Doppler; and without
oligohydroamnios. On the other hand, one of the three neonates with
EFW >5th percentile (considered low severity), had EFW at the
10th percentile and a gestational age of 24 weeks. This neonate
experienced severe asphyxia and died on the second day after
birth.
 | Table IIObstetric and neonatal
characteristics from pregnancies with fetal growth restriction
associated with HDP. |
Table II
Obstetric and neonatal
characteristics from pregnancies with fetal growth restriction
associated with HDP.
| |
Patient
no. |
|---|
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|
| HDP | SPE | SPE | MPE | SPE | CrHT | SPE | SPE | CrHT + PE | SPE | SPE | HDPw |
| GA at birth,
weeks | 24 | 27 | 28 | 31 | 31 | 32 | 33 | 34 | 34 | 37 | 38 |
| EFW,
percentile | 10 | 2-5 | 5 | 2 | 2-5 | 5-10 | 5 | 2 | 10 | 2-5 | 2 |
| AC <3rd
percentile | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| OHA | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| UA Doppler | A-AEDF | A-AEDF | A-AEDF | N | A | N | A | A-AEDF | A | N | N |
| BPS | ND | N | N | N | N | N | N | A | N | N | N |
| FHRM | ND | ND | R | R | NR | NR | ND | NR | NR | R | R |
| NICU admission,
days | 2 | 88 | >60 | 63 | 50 | >25 | 38 | 31 | 21 | 0 | 0 |
| Neonatal death | Yes | No | No | No | No | No | No | No | No | No | No |
Abnormal (pulsatility index >95th percentile)
umbilical artery (UA) Doppler was found in 64% of neonates with
FGR. Abnormal UA Doppler, especially if end-diastolic flow
velocities are absent, is a predictor of fetal compromise (29,30). A
total of five patients showed absent end-diastolic flow (Table II). In addition, four neonates did
not react during the basal recording of the non-stress test (low or
absent accelerations of heart rate responses to spontaneous fetal
movement) (32). Only a neonate
from the pathological group, who had abnormal UA Doppler as well as
oligohydroamnios, exhibited an abnormal biophysical profile
score.
Calcium signals in HUVECs from healthy
and FGR-HDP pregnancies
Our previous study demonstrated that
[Ca2+]i rise induced by ATP is time- and
concentration-dependent, with a biphasic process typically
consisting of an initial transient phase (initial peak) followed by
a sustained phase (18). To obtain
a maximum response and observe both phases, Fluo-3-loaded HUVECs in
confluent primary cultures were treated with 100 µM ATP in Locke's
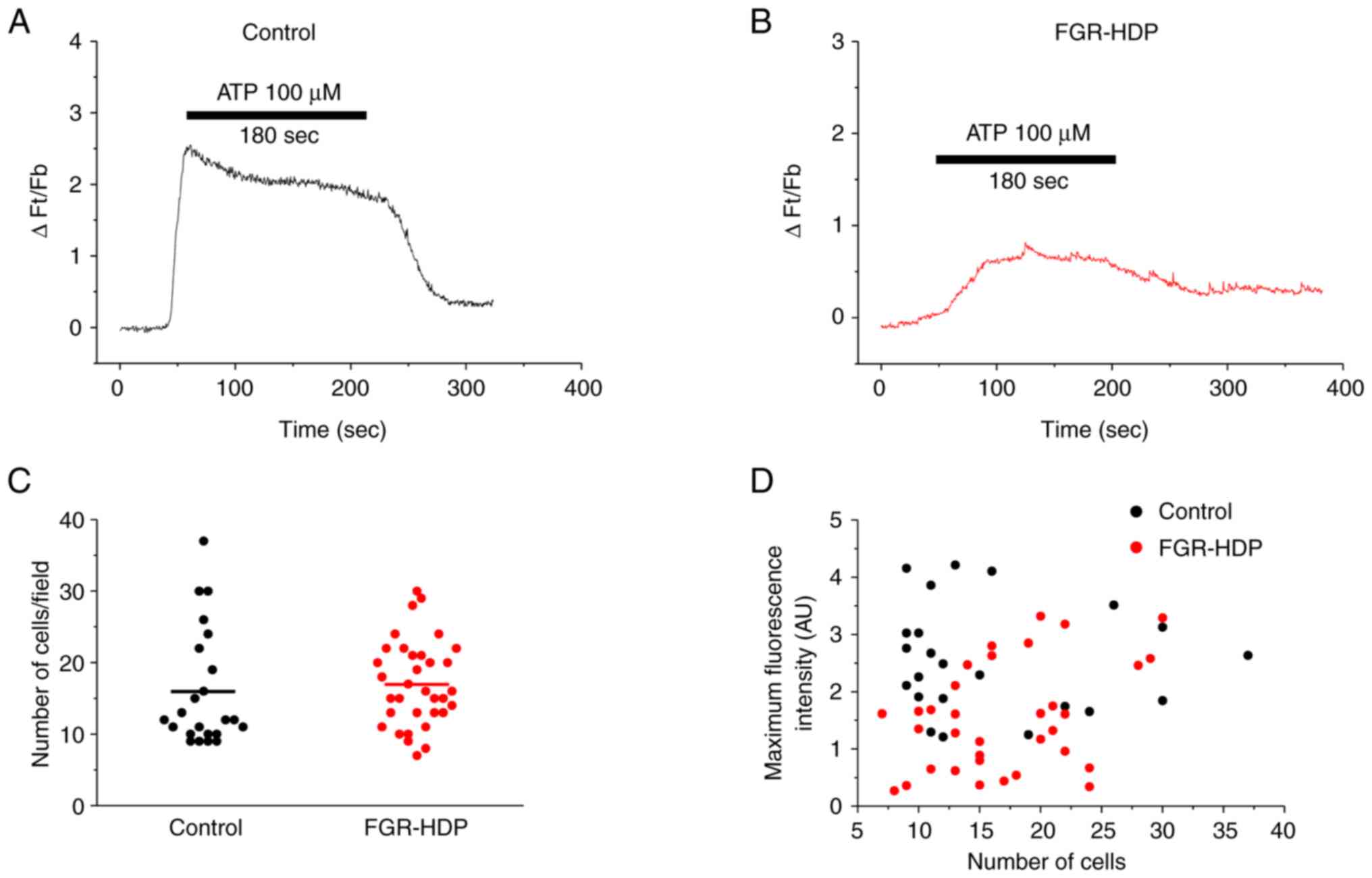
solution for 3 min. Fig. 1A and
B show representative ATP-induced
[Ca2+]i signals in control and FGR-HDP
HUVECs, respectively. Similar numbers of cells/field were recorded
for each group, with 16±2 cells in 23 coverslips from control and
17±1 cells in 34 coverslips from FGR-HDP groups (Fig. 1C). The scatter plot displays the
association between ATP-induced maximum fluorescence and the number
of cells of each coverslip (Fig.
1D). Mean maximum fluorescence intensity (measured as ∆Ft/Fb)
was 2.60±0.20 and 1.50±0.16 for control and FGR-HDP HUVECs
(P<0.0001), suggesting altered [Ca2+]i
responses in the pathological condition.
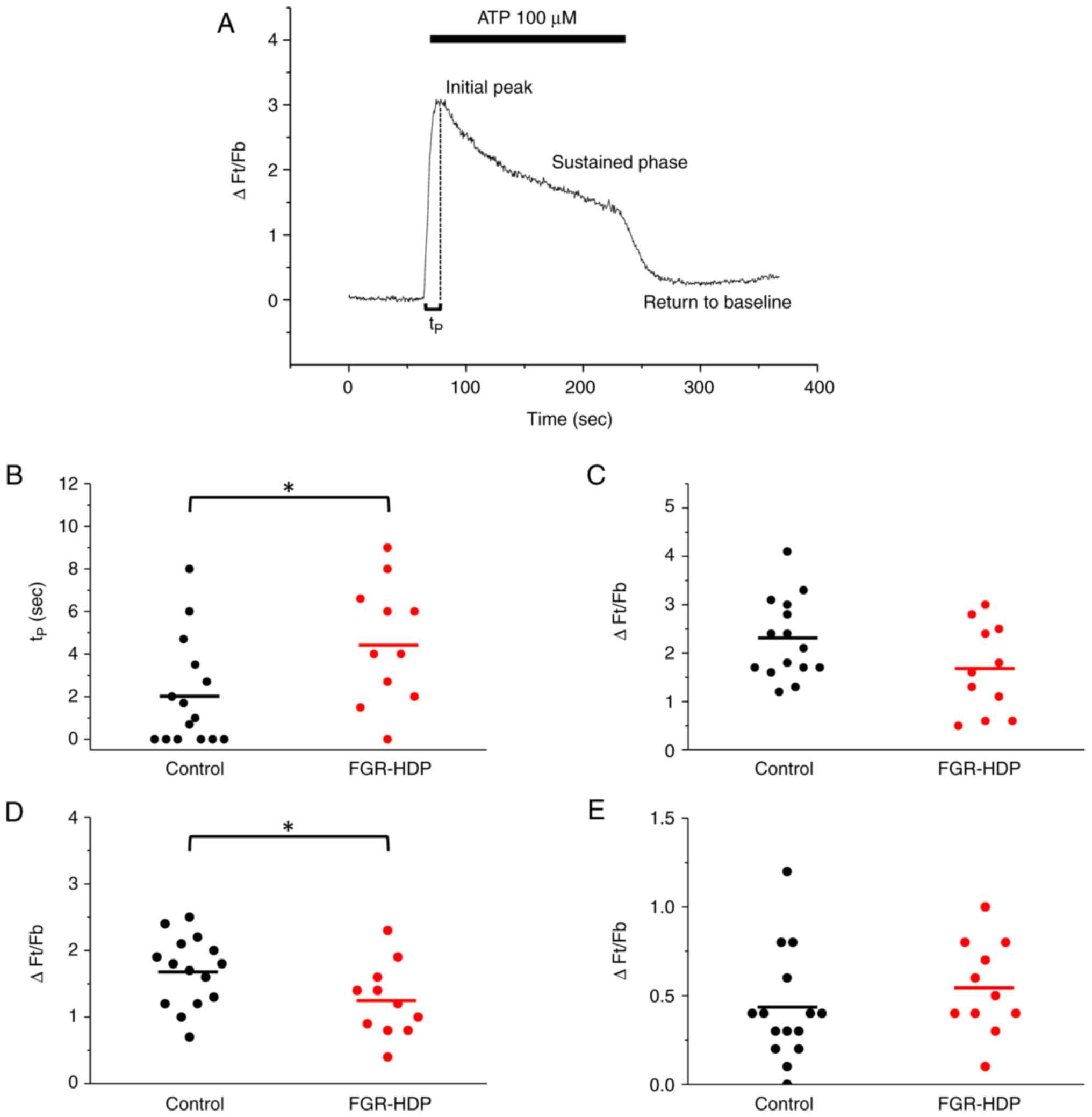
Parameters of the ATP-induced
[Ca2+]i signals were analyzed (Fig. 2A). Time to peak (tP) was
significantly increased in HUVECs from FGR-HDP (Fig. 2B); tP values were 2.0±0.7
and 4.5±0.9 sec (P<0.05) for control and FGR-HDP HUVECs,
respectively. On the other hand, no statically significant
difference was found between the control and FGR-HDP groups in
amplitude of the initial peak (∆Ft/Fb,
2.3±0.2 and 1.7±0.3, respectively; P>0.05; Fig. 2C), but delayed phase of the
[Ca2+]i signal was significantly lower in
FGR-HDP compared with control, with ∆Ft/Fb of
1.7±0.1 and 1.3±0.2 for control and FGR-HDP cells, respectively
(P<0.05; Fig. 2D). Finally, no
significant difference was found between the control and FGR-HDP
groups in the return to the baseline [Ca2+]i
following termination of the stimulus with ATP
(∆Ft/Fb, 0.40±0.08 and 0.50±0.08 for control
and FGR-HDP cells, respectively; P>0.05; Fig. 2E).
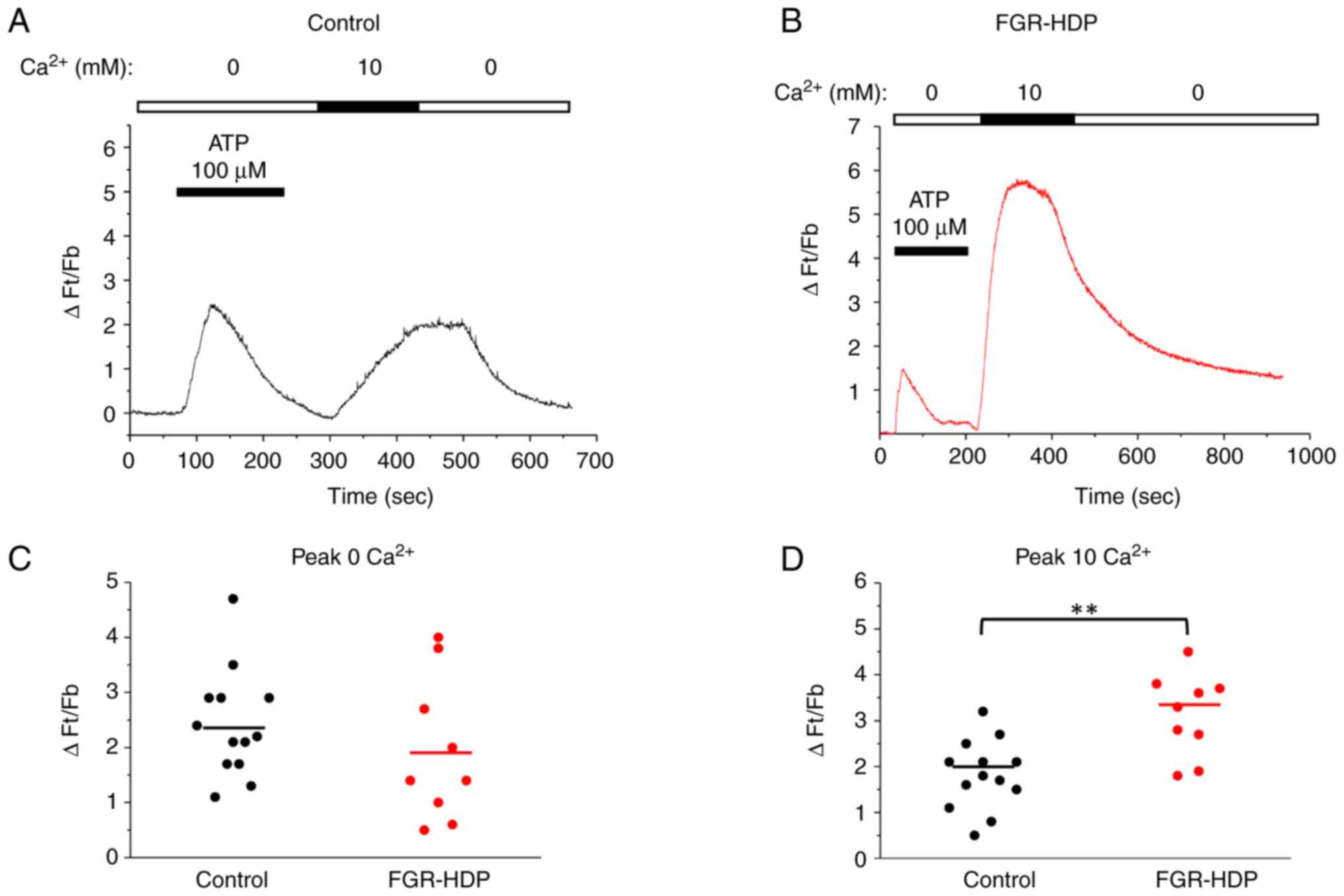
Fig. 3A and B show [Ca2+]i
signals induced in HUVECs from control and FGR-HDP groups,
respectively. The maximum amplitudes of the ATP-induced
Ca2+ responses in the absence and presence of
extracellular Ca2+ are shown in Fig. 3C and D. The analysis of the maximum amplitude of
ATP-induced Ca2+ signals in the absence of extracellular
Ca2+ showed no significant difference with ∆Ft/Fb values
of 2.4±0.3 and 1.9±0.4 (P>0.05), control and FGR-HDP
respectively. Conversely, the amplitude of Ca2+
responses to 10 mM Ca2+ were significantly higher in
HUVECs from the FGR-HDP group, with ∆Ft/Fb values of 1.8±0.2 in
control and 3.1±0.3 in FGR-HDP cells (P<0.005), respectively.
These results suggested that SOCE-mediated Ca2+ influx,
but not ATP-induced Ca2+ release from internal stores,
was altered in FGR-HDP HUVECs.
Discussion
During gestation, there is an active metabolic
exchange between the fetus and mother, a process in which the
efficiency depends on adequate development of the fetoplacental
unit. Alterations in the efficacy of this exchange trigger hypoxia,
which is associated with fetal distress, perinatal mortality and a
potential risk of cardiovascular diseases in offspring (1,2,36).
Both FGR and HDP are associated with placental insufficiency and
are aggravated by placental ischemia, a condition accompanied by
oxidative stress, wherein nitric oxide is unable to compensate for
this impairment (37). Recent
studies show that during hypoxia, placenta releases extracellular
vesicles, which carry cytokines and microRNA that alter the
function of endothelial cells (7,8,38).
This phenomenon worsens pregnancy pathologies such as FGR and HDP
(39).
The present study analyzed clinical characteristics
of 11 single pregnant patients with FGR-HDP, a complication that
has the highest rates of obstetric morbidity and mortality and
elevated incidences of low gestational age at delivery, cesarean
section and neonatal death compared with other types of pregnancy
disorder (6). Despite attending to
~3,000 pregnant patients annually at Gustavo Fricke Hospital, only
11 met the inclusion criteria FGR-HDP and consented to participate.
Given the small sample size, it is difficult to generalize the
present findings to the broader population. Future research should
employ larger and more representative samples to ensure
generalizability of results.
The present study excluded cases of multiple
pregnancies as this constitutes an independent risk factor for HDP
(40), regardless of chorionicity
or zygosity (41-43).
In terms of fetal outcomes, moderately elevated blood pressure in
multiple pregnancies increases blood flow to the placenta,
contributing to decreased risk of preterm birth and low birth
weight (43). Multiple pregnancies
have other risks, the major one being prematurity, which is
associated with adverse outcomes such as respiratory morbidity,
intraventricular hemorrhage, necrotizing enterocolitis, and
metabolic disorders (23,44). However, the risk of future
cardiovascular disease is not increased (45), which contrasts with single
pregnancies, where HDP is a risk factor for future cardiovascular
disease (23,46,47).
In addition, other maternal, placental or fetal risk
factors for abnormal placentation may result in placenta-mediated
FGR. Therefore, in the present study these other co-existing
factors were excluded due to their potential interference with
normal fetal growth and effect on outcomes (23). To the best of our knowledge,
however, there is no evidence that the combination of risk factors
predicts the presence FGR (48,49).
FIGO does not recommend using multiparameter algorithms (combining
ultrasound and biochemical markers) for universal screening. This
recommendation is based on the lack of sufficient validation of the
effectiveness of these models in predicting FGR (23).
Here, gestational age and birth weight were
significantly lower and neonatal intensive care admission was
higher in the FGR-HDP group compared with the healthy group. The
death of a newborn in the FGR-HDP group with an EFW in the 10th
percentile and a 5-min Apgar score of 3 suggests that factors
beyond these metrics play a critical role in determining the risk
of neonatal mortality (50). This
case highlights the greater prognostic value of the 5-min Apgar
score compared to the 1-min score in predicting neonatal morbidity
and mortality (26).
A total of 64% (7/11) of neonates from FGR-HDP
pregnancies had abnormal UA Doppler, with 57% (4/7) exhibiting
absent end diastolic flow; of these 75% (3/4) had oligohydroamnios.
The use of Doppler velocimetry evaluation, especially of the UA,
has been studied and reviewed in cases of FGR (30,31,51). A
recent study showed that compared with other pregnancy
complications, FGR-HDP has higher values of UA Doppler velocimetry,
and maternal vascular malperfusion (6). A progressively increasing pulsatility
index in UA corresponds to increased fetal artery resistance, which
generates a progressive decrease of the placental area available
for gas and nutrient exchange (52,53).
FGR is associated with a dysfunction of the feto-placental
vasculature involving endothelial cells. Compensatory upregulation
of the nitric oxide system in feto-placental endothelial cells has
been observed in FGR (12).
In accordance with the recommendations outlined by
FIGO (23) for managing FGR,
pregnant patients affected by FGR should be monitored using
biophysical assessments and cardiovascular tests, to determine the
timing of delivery. Many studies have focused on the prevention and
treatment of HDP and/or FGR (23,54).
To the best of our knowledge, however, there is currently no
effective treatment to reverse the course of FGR and improve fetal
growth (54). For future
pregnancies, patients with a history of FGR should be counseled on
risk of recurrence, considering the timing of onset, severity of
FGR and placental histopathological findings (23). If the placenta is available,
histopathological examination may provide valuable insights for
counseling in future pregnancies (23).
HUVECs are used as a model to study cardiovascular
diseases (55). They are also
considered as potential predictors of cardiovascular risk in
offspring of pregnancies involving preeclampsia (56). In this regard, HUVECs from
preeclampsia pregnancies reportedly display impaired functional
capacity, such as migration and tubule formation (57,58).
As [Ca2+]i signals are involved in these
processes, the present study investigated how they were altered in
FGR-HDP.
ATP-induced [Ca2+]i signals
were altered in HUVECs from the FGR-HDP group, including slower
tp and lower sustained phase. The initial phase of the
ATP-[Ca2+]i signal is primarily mediated by
P2Y2 receptors (20), whose
activation generates inositol triphosphate (IP3) and
Ca2+ release from Ca2+ stores, whereas the
sustained phase of the [Ca2+]i signals is
determined by SOCE (59). The
present result suggest that the kinetics of the
IP3-induced Ca2+ release and SOCE-mediated
Ca2+ influx are diminished in FGR-HDP cells. The
sustained phase of histamine-induced [Ca2+]i
signal is decreased in preeclampsia HUVECs (60,61),
which agrees with the present results in FGR-HDP HUVECs, where the
delayed phase of the ATP-induced [Ca2+]i
signal was also diminished, suggesting that HDP influences
endothelial dysfunction.
To understand how Ca2+ dynamics is
altered by FGR-HDP, components of the ATP-induced Ca2+
signals were separated (18). There
were no significant changes in the Ca2+ signal induced
by ATP in the absence of extracellular Ca2+, suggesting
that the P2Y2 receptor mediated response was not affected in HUVECs
from FGR-HDP group. However, the Ca2+ signal induced in
presence of 10 mM Ca2+ was significantly higher in
HUVECs from FGR-HDP compared with control HUVECs. This
Ca2+ signal is associated with SOCE, a mechanism that
depends on the Ca2+ sensor stromal interaction
molecule-1, which senses Ca2+ depletion in the
endoplasmic reticulum and activates Ca2+
release-activated Ca2+ channel protein 1 at the plasma
membrane (15). This mechanism
involves transient receptor potential and connexin channels and
mitochondria (59,62). A recent study demonstrate that
mitochondrial Ca2+ uniporter regulates SOCE at different
levels, including Ca2+ store replenishment and cytosolic
Ca2+ buffering systems, and that deletion of the
mitochondrial uniporter increases SOCE-mediated
[Ca2+]i signals (63). Mitochondrial Ca2+
uniporter is impaired in hypertension and cardiovascular disease
generating high cytosolic [Ca2+]i levels
(64,65).
An unexpected finding of the present study was that
whereas the sustained phase of ATP-induced
[Ca2+]i signals was diminished in HUVECs from
the FGR-HDP group, Ca2+ peak amplitude induced with 10
mM Ca2+ following depletion of internal Ca2+
stores was significantly higher. This result was also different
from that observed by Steinert et al (60) using a similar protocol but applying
a lower extracellular Ca2+ concentration (1 mM) to
induce the second peak. High extracellular Ca2+
concentration (10 mM) may saturate the Ca2+ buffering
mechanisms and induce dysfunction. In physiological conditions, the
sustained phase of the response induced by agonists such ATP or
histamine stimulates the synthesis of nitric oxide, which favors
vasodilation (66). However,
dysfunction of Ca2+ buffering mechanisms might cause
overload of cytosolic Ca2+ levels, resulting in
deleterious cellular effects such as oxidative stress, which
contribute to FGR (67).
In conclusion, the present study found that FGR-HDP
resulted in impaired UA resistance and altered Ca2+
responses to ATP regulated by SOCE in HUVECs. The present results
provide better understanding of the mechanisms that regulate
[Ca2+]i dynamics in fetal endothelial cells
and how they are altered in FGR-HDP. As dysfunction of HUVECs is a
potential predictor of cardiovascular risk in offspring (56), the present study also provides an
in vitro model to assess novel therapeutic approaches for
decreasing or preventing cardiovascular disease in adulthood.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by ANID (Chile; grant
no. FONDECYT 1220825).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MPC and AMC conceived the study and wrote the
manuscript. MPC, and CA performed the experiments and wrote the
manuscript. LMS and KVC performed statistical analysis and confirm
the authenticity of all the raw data. RV and TFBC interpreted data.
RV, KVC, LMS and TFBC critically revised and edited the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate.
The present study was approved (approval no.
9395.06) by the Scientific Ethics Committee of Dr. Gustavo Fricke
Hospital (Viña Mar, Chile). All patients provided written informed
consent prior to data and umbilical cord collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lees CC, Stampalija T, Baschat A, da Silva
Costa F, Ferrazzi E, Figueras F, Hecher K, Kingdom J, Poon LC,
Salomon LJ and Unterscheider J: ISUOG Practice Guidelines:
Diagnosis and management of small-for-gestational-age fetus and
fetal growth restriction. Ultrasound Obstet Gynecol. 56:298–312.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dudink I, Hüppi PS, Sizonenko SV,
Castillo-Melendez M, Sutherland AE, Allison BJ and Miller SL:
Altered trajectory of neurodevelopment associated with fetal growth
restriction. Exp Neurol. 347(113885)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Baschat A: Planning management and
delivery of the growth-restricted fetus. Best Pract Res Clin Obstet
Gynaecol. 49:53–65. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zur RL, Kingdom JC, Parks WT and Hobson
SR: The placental basis of fetal growth restriction. Obstet Gynecol
Clinics North Am. 47:81–98. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wardinger JE and Ambati S: Placental
insufficiency. In: StatPearls. StatPearls Publishing, Treasure
Island, FL, 2024.
|
|
6
|
Di Martino DD, Avagliano L, Ferrazzi E,
Fusè F, Sterpi V, Parasiliti M, Stampalija T, Zullino S, Farina A,
Bulfamante GP, et al: Hypertensive disorders of pregnancy and fetal
growth restriction: clinical characteristics and placental lesions
and possible preventive nutritional targets. Nutrients.
14(3276)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gu M, Zhang F, Jiang X, Chen P, Wan S, Lv
Q, Lu Y, Zhou Q, Wang Y and Li L: Influence of placental exosomes
from early onset preeclampsia women umbilical cord plasma on human
umbilical vein endothelial cells. Front Cardiovasc Med.
9(1061340)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aharon A, Rebibo-Sabbah A, Ahmad RS,
Dangot A, Bar-Lev TH, Brenner B, Cohen AH, David CB, Weiner Z and
Solt I: Associations of maternal and placental extracellular
vesicle miRNA with preeclampsia. Front Cell Dev Biol.
11(1080419)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morley LC, Debant M, Walker JJ, Beech DJ
and Simpson NAB: Placental blood flow sensing and regulation in
fetal growth restriction. Placenta. 113:23–28. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zullino S, Buzzella F and Simoncini T:
Nitric oxide and the biology of pregnancy. Vascul Pharmacol.
110:71–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tashie W, Fondjo LA, Owiredu WKBA, Ephraim
RKD, Asare L, Adu-Gyamfi EA and Seidu L: Altered bioavailability of
nitric oxide and L-arginine is a key determinant of endothelial
dysfunction in preeclampsia. Biomed Res Int.
2020(3251956)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pisaneschi S, Strigini FA, Sanchez AM,
Begliuomini S, Casarosa E, Ripoli A, Ghirri P, Boldrini A, Fink B,
Genazzani AR, et al: Compensatory feto-placental upregulation of
the nitric oxide system during fetal growth restriction. PLoS One.
7(e45294)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J.
33:829–837.837a-837d. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He Y, Haque MM, Stuehr DJ and Lu HP:
Conformational states and fluctuations in endothelial nitric oxide
synthase under calmodulin regulation. Biophys J. 120:5196–5206.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lu T, Zhang Y, Su Y, Zhou D and Xu Q: Role
of store-operated Ca2+ entry in cardiovascular disease. Cell Commun
Signal. 20(33)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vinet R, Cortés MP, Álvarez R and Delpiano
MA: Bradykinin and histamine-induced cytosolic calcium increase in
capillary endothelial cells of bovine adrenal medulla. Cell Biol
Int. 38:1023–1031. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cortés MP, Alvarez R, Sepulveda E,
Jimenez-Aspee F, Astudillo L, Vallejos G and Gutierrez M: A new
isoxazolic compound acts as alpha7 nicotinic receptor agonist in
human umbilical vein endothelial cells. Z Naturforsch C J Biosci.
69:291–299. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cortés MP, Becerra JP, Vinet R, Álvarez R
and Quintana I: Inhibition of ATP-induced calcium influx by
homocysteine in human umbilical vein endothelial cells. Cell Biol
Int. 37:600–607. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Burnstock G: Dual control of vascular tone
and remodelling by ATP released from nerves and endothelial cells.
Pharmacol Rep. 60:12–20. 2008.PubMed/NCBI
|
|
20
|
Raqeeb A, Sheng J, Ao N and Braun AP:
Purinergic P2Y2 receptors mediate rapid Ca2+ mobilization, membrane
hyperpolarization and nitric oxide production in human vascular
endothelial cells. Cell Calcium. 49:240–248. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yamamoto K, Korenaga R, Kamiya A and Ando
J: Fluid shear stress activates Ca+2 influx into human endothelial
cells via P2X4 purinoceptors. Circ Res. 87:385–391. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lv Q, Xue Y, Li G, Zou L, Zhang X, Ying M,
Wang S, Guo L, Gao Y, Li G, et al: Beneficial effects of evodiamine
on P2X(4)-mediated inflammatory injury of human umbilical vein
endothelial cells due to high glucose. Int Immunopharmacol.
28:1044–1049. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Melamed N, Baschat A, Yinon Y,
Athanasiadis A, Mecacci F, Figueras F, Berghella V, Nazareth A,
Tahlak M, McIntyre HD, et al: FIGO (International Federation of
Gynecology and obstetrics) initiative on fetal growth: Best
practice advice for screening, diagnosis, and management of fetal
growth restriction. Int J Gynaecol Obstet. 152 (Suppl 1):S3–S57.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ministry of Health Government of Chile:
Perinatal Guide, 2015. Available online: https://www.minsal.cl/sites/default/files/files/GUIA%20PERINATAL_2015_%20PARA%20PUBLICAR.pdf.
|
|
25
|
Hadlock FP, Harrist RB, Sharman RS, Deter
RL and Park SK: Estimation of fetal weight with the use of head,
body, and femur measurements-A prospective study. Am J Obstet
Gynecol. 151:333–337. 1985.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li F, Wu T, Lei X, Zhang H, Mao M and
Zhang J: The apgar score and infant mortality. PLoS One.
8(e69072)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Moore TR and Cayle JE: The amniotic fluid
index in normal human pregnancy. Am J Obstet Gynecol.
162:1168–1173. 1990.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rabie N, Magann E, Steelman S and
Ounpraseuth S: Oligohydramnios in complicated and uncomplicated
pregnancy: A systematic review and meta-analysis. Ultrasound Obstet
Gynecol. 49:442–449. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dall'Asta A, Stampalija T, Mecacci F,
Minopoli M, Schera GBL, Cagninelli G, Ottaviani C, Fantasia I,
Barbieri M, Lisi F, et al: Ultrasound prediction of adverse
perinatal outcome at diagnosis of late-onset fetal growth
restriction. Ultrasound Obstet Gynecol. 59:342–349. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Levytska K, Higgins M, Keating S, Melamed
N, Walker M, Sebire NJ and Kingdom JCP: Placental pathology in
relation to uterine artery doppler findings in pregnancies with
severe intrauterine growth restriction and abnormal umbilical
artery doppler changes. Am J Perinatol. 34:451–457. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Baschat AA, Galan HL, Bhide A, Berg C,
Kush ML, Oepkes D, Thilaganathan B, Gembruch U and Harman CR:
Doppler and biophysical assessment in growth restricted fetuses:
Distribution of test results. Ultrasound Obstet Gynecol. 27:41–47.
2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Street P, Dawes GS, Moulden M and Redman
CW: Short-term variation in abnormal antenatal fetal heart rate
records. Am J Obstet Gynecol. 165:515–523. 1991.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Takahashi A, Camacho P, Lechleiter JD and
Herman B: Measurement of intracellular calcium. Physiol Rev.
79:1089–1125. 1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Parekh AB and Putney JW Jr: Store-operated
calcium channels. Physiol Rev. 85:757–810. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim SM, Lee SM, Kim SJ, Kim BJ, Shin S,
Kim JR and Cho KH: Cord and maternal sera from small neonates share
dysfunctional lipoproteins with proatherogenic properties: Evidence
for Barker's hypothesis. J Clin Lipidol. 11:1318–1328.e3.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hempstock J, Jauniaux E, Greenwold N and
Burton GJ: The contribution of placental oxidative stress to early
pregnancy failure. Hum Pathol. 34:1265–1275. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
León J, Acurio J, Bergman L, López J,
Karin Wikström A, Torres-Vergara P, Troncoso F, Castro FO, Vatish M
and Escudero C: Disruption of the blood-brain barrier by
extracellular vesicles from preeclampsia plasma and hypoxic
placentae: Attenuation by magnesium sulfate. Hypertension.
78:1423–1433. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Condrat CE, Varlas VN, Duică F, Antoniadis
P, Danila CA, Cretoiu D, Suciu N, Crețoiu SM and Voinea SC:
Pregnancy-Related extracellular vesicles revisited. Int J Mol Sci.
9(3904)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Duckitt K and Harrington D: Risk factors
for pre-eclampsia at antenatal booking: Systematic review of
controlled studies. BMJ. 330(565)2005.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bartnik P, Kosinska-Kaczynska K,
Kacperczyk J, Ananicz W, Sierocí Nska A, Wielgos M and Szymusik I:
Twin Chorionicity and the Risk of Hypertensive Disorders:
Gestational Hypertension and Pre-eclampsia. Twin Res Hum Genet.
19(2016)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kuleva M, Youssef A, Maroni E, Contro E,
Pilu G, Rizzo N, Pelusi G and Ghi T: Maternal cardiac function in
normal twin pregnancy: A longitudinal study. Ultrasound Obstet
Gynecol. 38:575–580. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu T, Gao R, Liu Y, Zhao K, Su X, Wong
HC, Li L, Xie B, Huang Y, Qiu C, et al: Hypertensive disorders of
pregnancy and neonatal outcomes in twin vs. singleton pregnancies
after assisted reproductive technology. Front Pediatr.
10(839882)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Montgomery KS, Cubera S, Belcher C,
Patrick D, Funderburk H, Melton C and Fastenau M: Childbirth
education for multiple pregnancy part 2: Intrapartum and postpartum
considerations. J Perinat Educ. 14:33–38. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bergman L, Nordlöf-Callbo P, Wikström AK,
Snowden JM, Hesselman S, Edstedt Bonamy AK and Sandström A:
Multi-Fetal pregnancy, preeclampsia, and long-term cardiovascular
disease. Hypertension. 76:167–175. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
ACOG Practice Bulletin No. 202.
Gestational hypertension and preeclampsia. Obstet Gynecol.
133(1)2019.
|
|
47
|
Proctor LK, Kfouri J, Hiersch L, Aviram A,
Zaltz A, Kingdom J, Barrett J and Melamed N: Association between
hypertensive disorders and fetal growth restriction in twin
compared with singleton gestations. Am J Obstet Gynecol.
221:251.e1–251.e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
McCowan LM, Thompson JM, Taylor RS, Baker
PN, North RA, Poston L, Roberts CT, Simpson NA, Walker JJ, Myers J,
et al: Prediction of small for gestational age infants in healthy
nulliparous women using clinical and ultrasound risk factors
combined with early pregnancy biomarkers. PLoS One.
12(e0169311)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Crovetto F, Triunfo S, Crispi F,
Rodriguez-Sureda V, Dominguez C, Figueras F and Gratacos E:
Differential performance of first-trimester screening in predicting
small-for-gestational-age neonate or fetal growth restriction.
Ultrasound Obstet Gynecol. 49:349–356. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lees C, Marlow N, Arabin B, Bilardo CM,
Brezinka C, Derks JB, Duvekot J, Frusca T, Diemert A, Ferrazzi E,
et al: Perinatal morbidity and mortality in early-onset fetal
growth restriction: Cohort outcomes of the trial of randomized
umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet
Gynecol. 42:400–408. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Martins JG, Biggio JR and Abuhamad A:
Society for Maternal-Fetal Medicine (SMFM): Consult Series #52:
Diagnosis and management of fetal growth restriction. Am J Obstet
Gynecol. 223:B2–B17. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Burton GJ, Woods AW, Jauniaux E and
Kingdom JC: Rheological and physiological consequences of
conversion of the maternal spiral arteries for uteroplacental blood
flow during human pregnancy. Placenta. 30:473–482. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
James JL, Saghian R, Perwick R and Clark
AR: Trophoblast plugs: Impact on utero-placental haemodynamics and
spiral artery remodelling. Hum Reprod. 33:1430–1441.
2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Groom KM and David AL: The role of
aspirin, heparin, and other interventions in the prevention and
treatment of fetal growth restriction. Am J Obstet Gynecol.
218:S829–S840. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Medina-Leyte DJ, Domínguez-Pérez M,
Mercado I, Villarreal-Molina MT and Jacobo-Albavera L: Use of human
umbilical vein endothelial cells (HUVEC) as a model to study
cardiovascular disease: A review. Appl Sci. 10(938)2020.
|
|
56
|
Reckelhoff JF, LaMarca B, Garovic VD and
Alexander BT: Human umbilical venous endothelial cells: Early
predictors of cardiovascular risk in offspring? Hypertension.
74:32–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhou C, Yan Q, Zou QY, Zhong XQ, Tyler CT,
Magness RR, Bird IM and Zheng J: Sexual dimorphisms of
preeclampsia-dysregulated transcriptomic profiles and cell function
in fetal endothelial cells. Hypertension. 74:154–163.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Brodowski L, Burlakov J, Hass S, Von
Kaisenberg C and Von Versen-Hö F: Impaired functional capacity of
fetal endothelial cells in preeclampsia. PLoS One.
12(e0178340)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Nan J, Li J, Lin Y, Saif Ur Rahman M, Li Z
and Zhu L: The interplay between mitochondria and store-operated
Ca2+ entry: Emerging insights into cardiac diseases. J Cell Mol
Med. 25:9496–9512. 2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Steinert JR, Wyatt AW, Poston L, Jacob R
and Mann GE: Preeclampsia is associated with altered Ca2+
regulation and NO production in human fetal venous endothelial
cells. FASEB J. 16:721–723. 2002.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gifford SM, Yi FX and Bird IM:
Pregnancy-enhanced store-operated Ca2+ channel function in uterine
artery endothelial cells is associated with enhanced
agonist-specific transient receptor potential channel 3-inositol
1,4,5-trisphosphate receptor 2 interaction. J Endocrinol.
190:385–395. 2006.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Rozas-Villanueva MF, Casanello P and
Retamal MA: Role of ROS/RNS in preeclampsia: Are connexins the
missing piece? Int J Mol Sci. 21(4698)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Yoast RE, Emrich SM, Zhang X, Xin P, Arige
V, Pathak T, Benson JC, Johnson MT, Abdelnaby AE, Lakomski N, et
al: The mitochondrial Ca2+ uniporter is a central regulator of
interorganellar Ca2+ transfer and NFAT activation. J Biol Chem.
297(101174)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Bick AG, Wakimoto H, Kamer KJ, Sancak Y,
Goldberger O, Axelsson A, DeLaughter DM, Gorham JM, Mootha VK,
Seidman JG and Seidman CE: Cardiovascular homeostasis dependence on
MICU2, a regulatory subunit of the mitochondrial calcium uniporter.
Proc Natl Acad Sci USA. 114:E9096–E9104. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Balderas E, Eberhardt DR, Lee S, Pleinis
JM, Sommakia S, Balynas AM, Yin X, Parker MC, Maguire CT, Cho S, et
al: Mitochondrial calcium uniporter stabilization preserves
energetic homeostasis during complex I impairment. Nat Commun.
13(2769)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Yi FX, Boeldt DS, Gifford SM, Sullivan JA,
Grummer MA, Magness RR and Bird IM: Pregnancy enhances sustained
Ca2+ bursts and endothelial nitric oxide synthase activation in
ovine uterine artery endothelial cells through increased connexin
43 function. Biol Reprod. 82:66–75. 2010.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zhang Z, Zhao L and Zhou X, Meng X and
Zhou X: Role of inflammation, immunity, and oxidative stress in
hypertension: New insights and potential therapeutic targets. Front
Immunol. 13(1098725)2023.PubMed/NCBI View Article : Google Scholar
|