Introduction
An inflammatory pseudotumor (IPT) is a benign, rare
chronic inflammatory process that most frequently affects the lung
and orbits as an extrapulmonary site but can occur in almost any
organ in the body such as pelvic organs (1). Its occurrence in the pelvis is rare
with only two cases being reported (2,3). IPTs
typically follow a benign clinical course, with 25% rate of local
recurrence (4). While IPT may occur
in any age group, more than half of the cases occur in patients
aged 30-49 years with peak age of 38.9 years and female
predominance (5).
The origin of IPT is not known, but various
mechanisms have been suggested including immune-autoimmune, trauma
and surgical inflammation (6),
furthermore, it may resemble low-grade fibrosarcoma with
inflammatory cells (6).
IPT may develop as the result of an infection linked
to different pathogens at different body sites, such as
mycoplasmata and Nocardiae in the lungs, actinomycetes in the
liver, Epstein-Barr virus in the spleen and mycobacteria in spindle
cell tumors (1). The diagnosis of
IPT is difficult as it is typically made by exclusion based on the
histopathological findings (1). For
the majority of IPT, the preferred treatment is surgical resection
(7).
Case report
A 39-year-old healthy female patient with
hypothyroidism and no previous surgical operations or sexual
activity presented to Dr Ismaiel Abu Mahfouz gynaecology clinic in
The Specialty Hospital (Amman, Jordan) in January 2023 with mild
lower abdominal pain. Ultrasound examination showed a complex right
sided ovarian cyst measuring 6.0x6.5 cm. There was no evidence of
free fluid in the pelvis, and the left ovary and uterus were
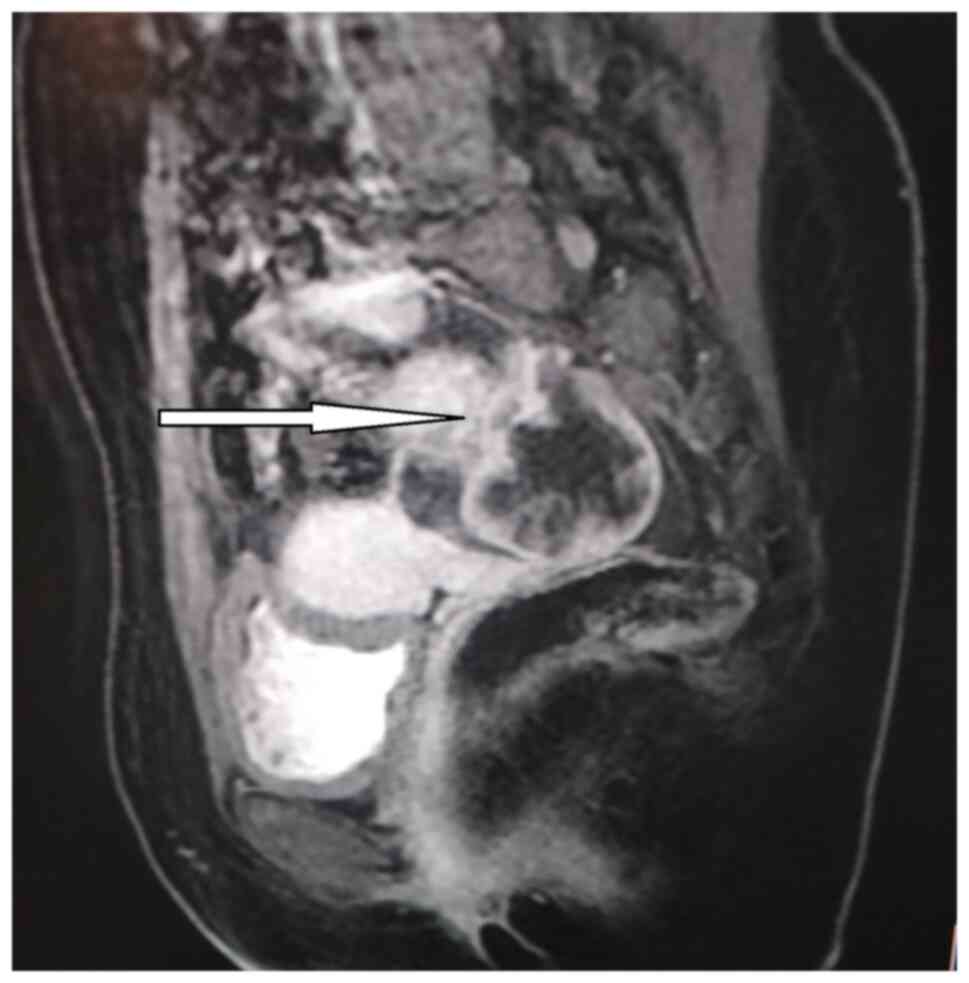
unremarkable. Pelvic magnetic resonance imaging (MRI) and ovarian
tumor marker assessment (cancer antigen 125 and 19-9,
carcinoembryonic antigen, and beta subunit of human chorionic
gonadotropin) was requested to rule out malignancy. MRI confirmed
the presence of a complex cystic lesion mostly arising from the
right ovary and extending posteriorly, measuring ~6.5x5.6 cm and
having a thick nodular enhancing wall with thin enhancing
septations. The uterus was of normal size with no focal lesion; no
other solid masses could be seen in the pelvis and there was a
small amount of free fluid seen in the pelvis and no recognized
pelvic adenopathy (Fig. 1).
Tumor markers showed CA-125 was 30 (normal range:
0-35 units/ml), cancer antigen 19-9 was 6 (normal range 0-37
units/ml), carcinoembryonic antigen was 0.9 (normal range 0-2.5
ng/ml), beta subunit of human chorionic gonadotropin was negative
and lactate Dehydrogenase was 27 (normal range 105-333 IU/L)
(8).
Considering the complex nature of the cyst and after
counselling, a low transverse laparotomy was performed in January
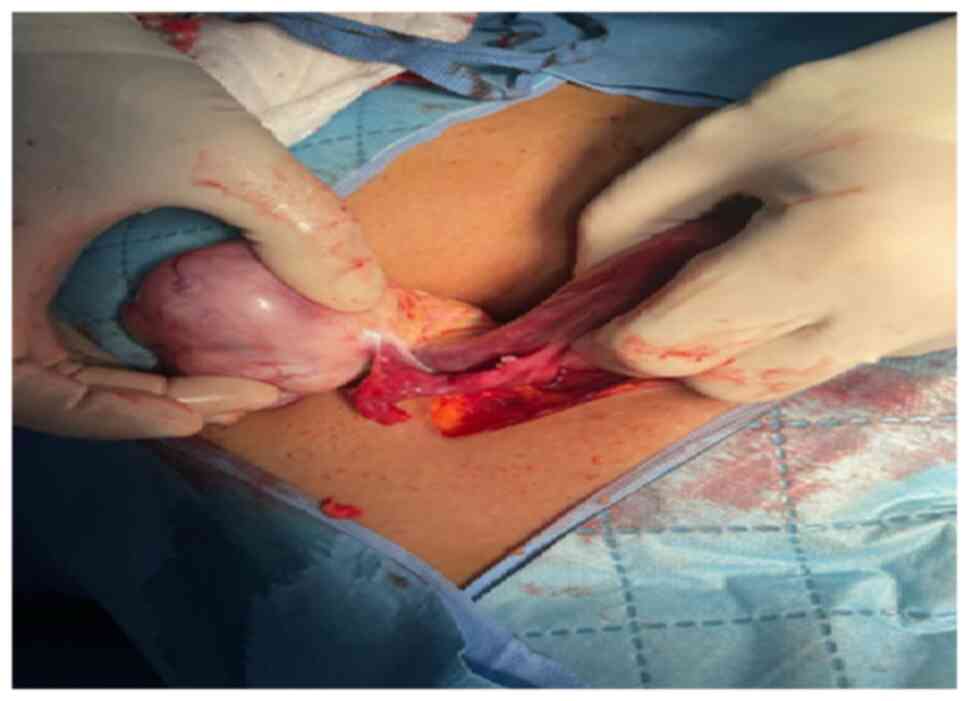
2023 at The Specialty Hospital, Amman Jordan. The findings during
laparotomy showed a right ovarian mass measuring ~7x8 cm (Fig. 2). The uterus and left ovary looked
normal and there was a small amount of serous free fluid in the
pelvis. The peritoneal fluid was aspirated, and a right ovarian
cystectomy was performed along with left ovarian, peritoneal and
omental biopsies.
The histopathology techniques were as follows:
Dehydration by ethanol from 70 to 100%, Clearing by xylene,
Impregnation using wax 52-56˚C. Paraffin embedding was at 65˚C for
16 h A section Thickness was 5 micron. BenchMark GX, VENTANA) with
ready-to-use made antibodies was used for antibody dilution. For
detection and counter-stain hematoxylin was used and viewed under a
light microscope.
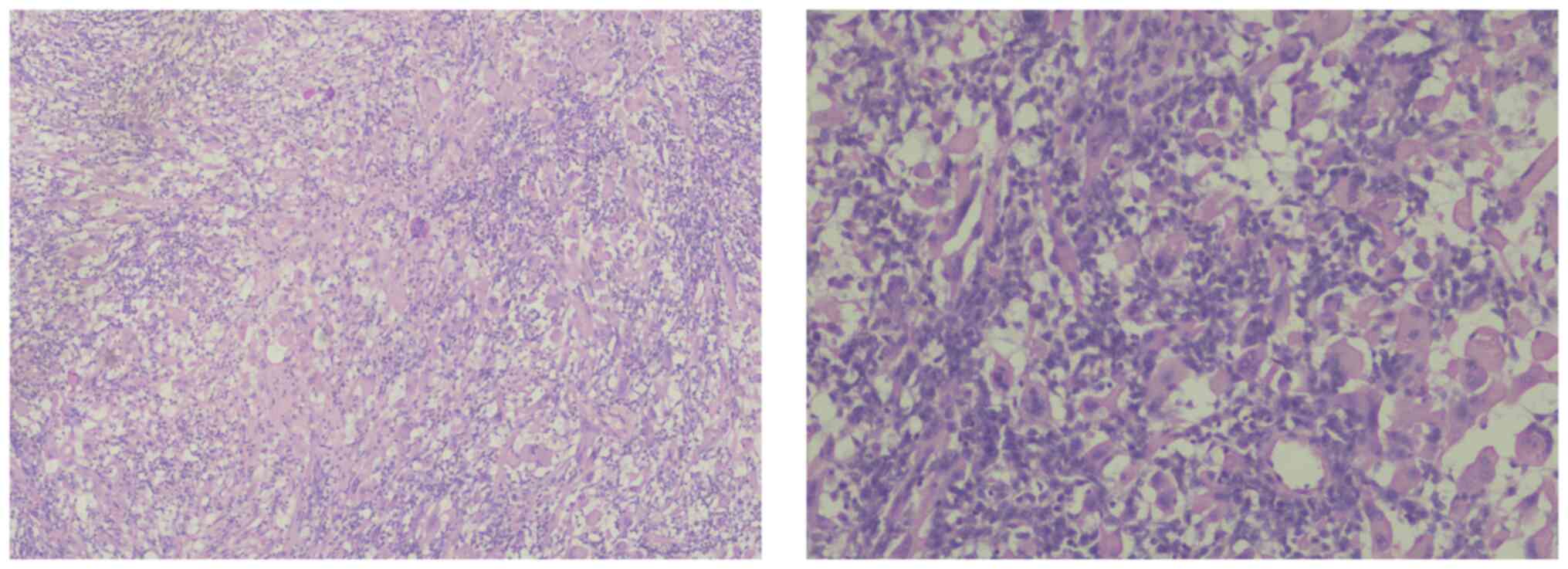
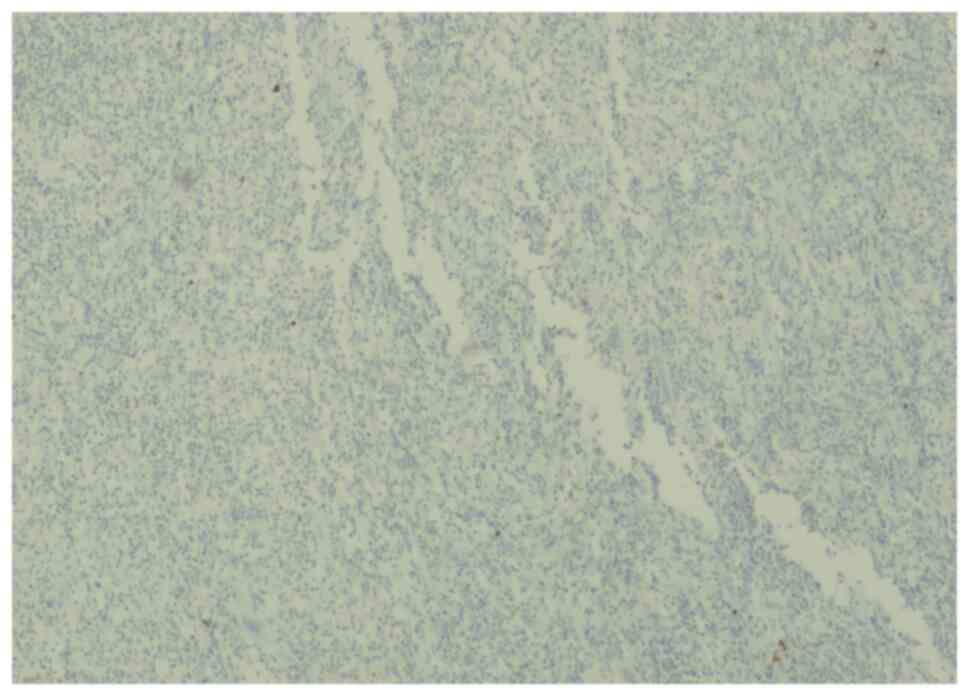
The histopathology of the right ovarian cyst showed
fibrovascular core (hemangiopericytoma-like) infiltrate by
mononuclear chronic inflammatory cells with histiocytes and
numerous multinucleated giant cells. The infiltrate was in a
diffuse pattern with perivascular cuffing, myxoid degeneration,
fibrinoid material and hyalinized fibrosis (Fig. 3).
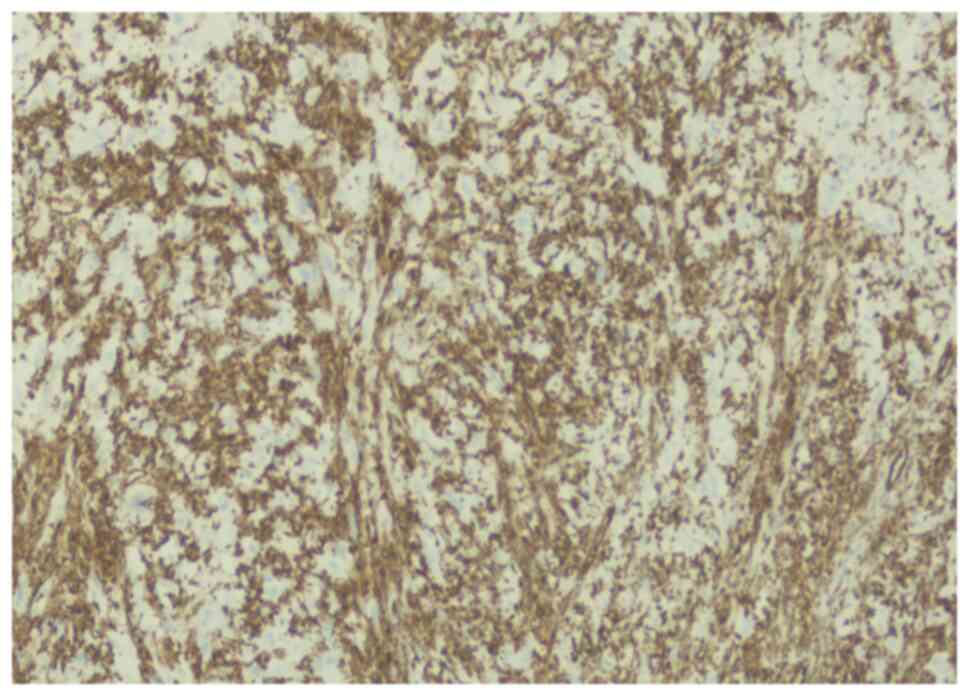
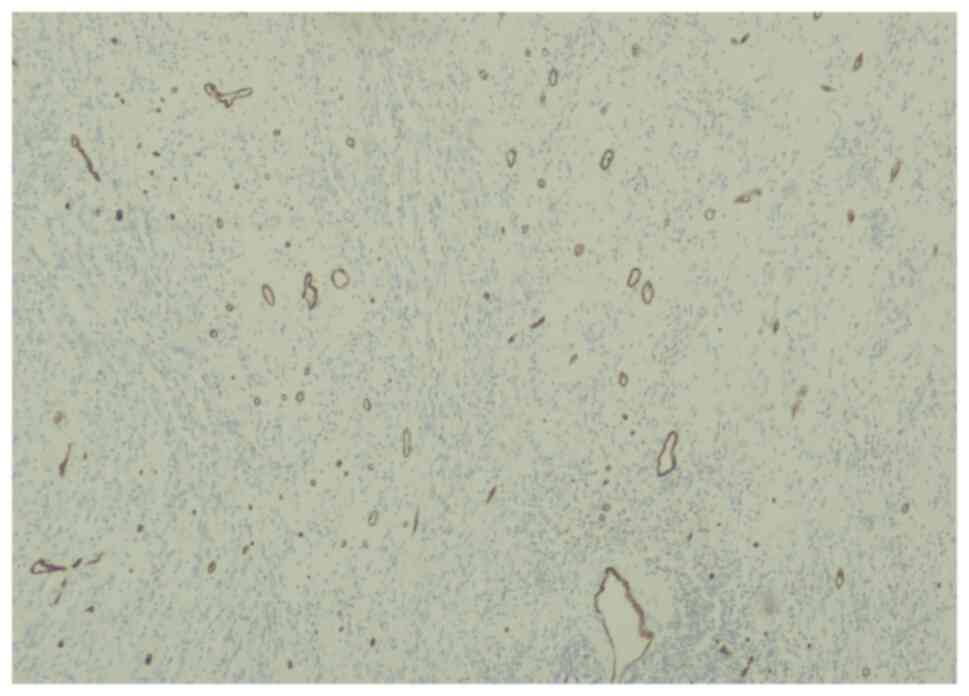
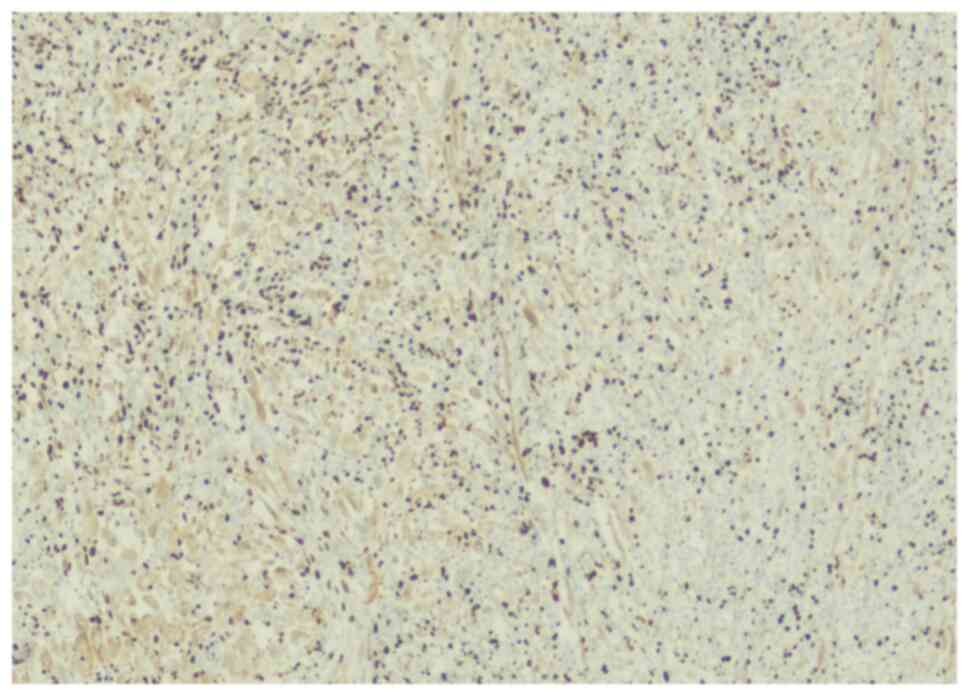
The lesion was immunoreactive for vimentin (Fig. 4) but not reactive for actin
(Fig. 5), positively stained for
S100 (Fig. 6), CD34 (Fig. 7) and CD68 (Fig. 8), highlighting the histiocytes.
Ki-67 proliferating index was high >20% (Fig. 9). The overall pathology and immune
results indicated IPT. While the results of peritoneal fluid
cytology was negative for malignant cells; there were hyperplastic
mesothelial cells and macrophages with rare inflammatory exudate.
Additionally, peritoneal and omental biopsies were normal. The
patient was uneventfully followed up till September 2023.
Discussion
IPT is rarely seen in the pelvis. To the best of our
knowledge, there are few reported cases of IPT affecting the female
reproductive system (2,9). The patient in our case report was
found to have ovarian inflammatory pseudotumor.
Here, the patient presented with mild lower
abdominal pain and was diagnosed with IPT. Patients in these cases
may present with symptoms associated with mass effect or with
non-specific symptoms and signs of inflammation such as fever,
weight loss, and abdominal pain (1).
There are no specific characteristic features for
IPT on imaging studies (1). The
present MRI findings showed features of a complex cystic ovarian
tumor with a thick wall and enhancing septation, which were
non-specific (1,7). The lack of specific radiological
characteristics may be related to the degree of fibrosis and
cellular infiltration, where lesions may seem hypoechoic or
hyperechoic with ill-defined or well-circumscribed boundaries on
ultrasound images. These lesions frequently have more vascularity
in the Doppler test (1).
A homogenous or heterogeneous lesion may be visible
on contrast-enhanced MRI scan, and in the presence of fibrosis,
more enhancement may be demonstrated with delayed MRI images
(1). Diagnosis of IPT depends on
histopathological studies, which may show myofibroblasts and mixed
inflammatory and spindle-shaped cells (plasma cells, lymphocytes
and, sporadically, histiocytes) (10). Furthermore, IPT may be misdiagnosed
as low-grade fibrosarcoma. Histopathological features
differentiating these are seen in low grade fibrosarcoma and
include the presence of atypia (3,11).
Here, immunostaining for vimentin was positive and negative for
actin and the Ki 67 proliferation index was 20% (11). The IPT was surgically removed for
both diagnosis and treatment. The prognosis of the disease is still
unknown and there is risk of recurrence (2,12).
In conclusion, IPT can arise from the ovary, with no
pathognomonic features on the imaging. In addition, it is rare and
may not be easily diagnosed on histopathological studies as it may
resemble low grade fibrosarcoma. Surgical resection is the most
common therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
BS, SS, AAB, RI, AA and IAM performed clinical
management and surgery. AAB and RI collected clinical data, edited
the manuscript and constructed figures. AA performed
histopathological experiments and wrote the manuscript. IAM and AA
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Patnana M, Sevrukov AB, Elsayes KM,
Viswanathan C, Lubner M and Menias CO: Inflammatory pseudotumor:
The great mimicker. AJR Am J Roentgenol. 198:W217–W227.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stolnicu S and Soslow RA: Inflammatory
pseudotumor presenting as a mesosalpingeal mass. Int J Gynecol
Pathol. 37:473–476. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sun TT, Cheng NH, Cao DY and Peng P:
Ovarian fibrosarcoma: A single-institution experience and a review
of the literature. J Ovarian Res. 13(142)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gude D, Rayudu R and Bansal D: How pseudo
is an inflammatory pseudotumor? Indian J Med Paediatr Oncol.
32:204–206. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ajani MA, Fatunla EO, Onakpoma FA and
Salami AA: Inflammatory Pseudotumor: A 20-year single institutional
experience. Adv Biomed Res. 9(68)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fayad FT, Bezerra MCT, da Rosa MRP and
Pinheiro TN: Diagnosis, treatment, and rehabilitation of a patient
with inflammatory pseudotumor. Eur J Dent. 12:454–458.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sedlic T, Scali EP, Lee WK, Verma S and
Chang SD: Inflammatory Pseudotumours in the Abdomen and Pelvis: A
pictorial essay. Can Assoc Radiol J. 65:52–59. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Perkins GL, Slater ED, Sanders GK and
Prichard JG: Serum tumor markers. Am Fam Physician. 68:1075–1082.
2003.PubMed/NCBI
|
|
9
|
Gücer F, Altaner S, Mülayim N and Yapicier
O: Invasive inflammatory pseudotumor of uterine cervix: A case
report. Gynecol Oncol. 98:325–328. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ntinas A, Kardassis D, Miliaras D,
Tsinoglou K, Dimitriades A and Vrochides D: Inflammatory
pseudotumor of the liver: A case report and review of the
literature. J Med Case Rep. 5(196)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gultekin M, Dursun P, Ozyuncu O, Usubutun
A, Yuce K and Ayhan A: Primary ovarian fibrosarcoma: A case report
and review of the literature. Int J Gynecol Cancer. 15:1142–1147.
2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goto T, Akanabe K, Maeshima A and Kato R:
Surgery for recurrent inflammatory pseudotumor of the lung. World J
Surg Oncol. 9(133)2011.PubMed/NCBI View Article : Google Scholar
|