1. Introduction
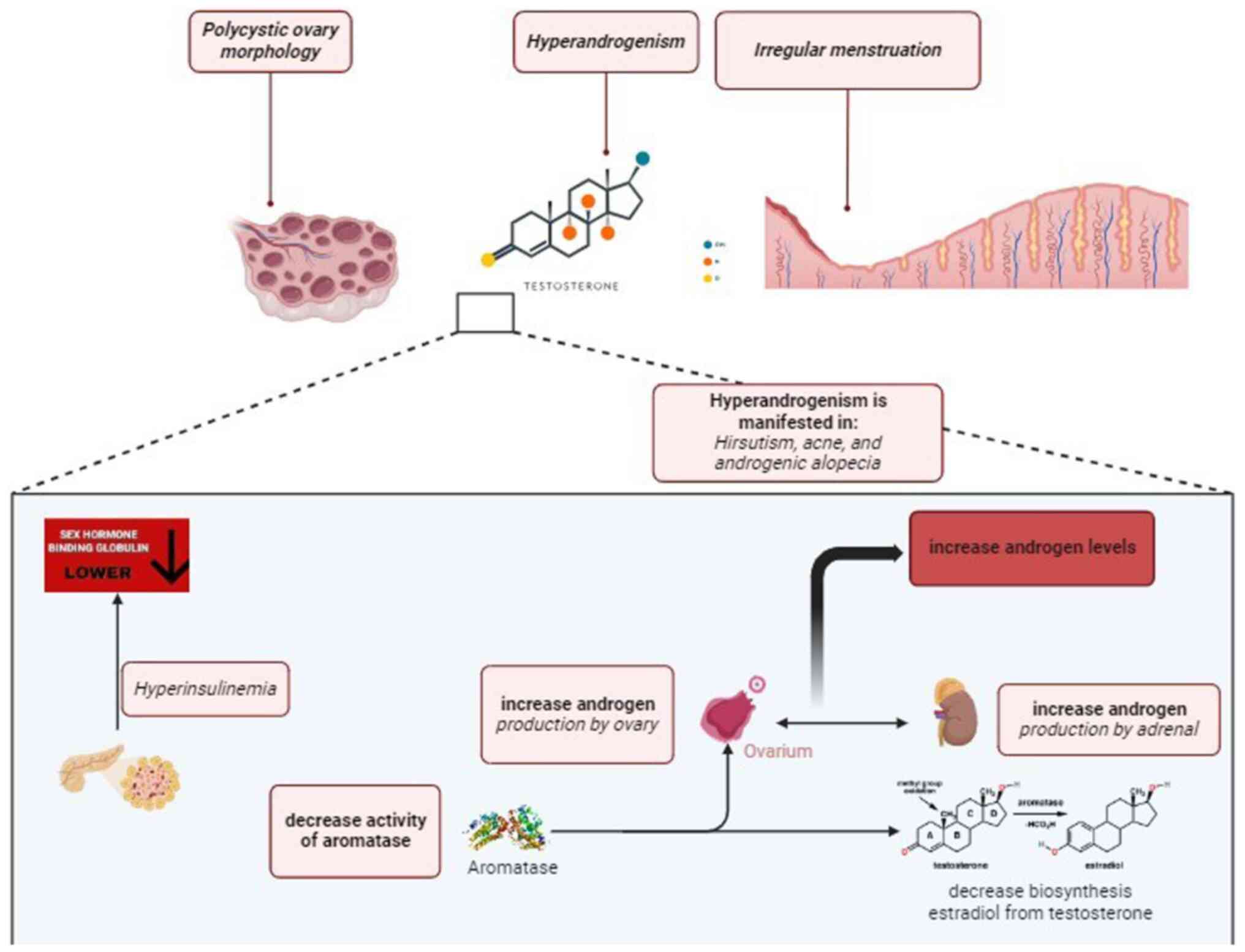
The most widespread hormonal and metabolic disorder
affecting women of reproductive age is polycystic ovarian syndrome
(PCOS), which manifests clinically as irregular menstruation,
hyperandrogenism and polycystic ovaries (1). These females also have metabolic
issues such as obesity, insulin resistance and dyslipidemia
(2,3). Increased levels of androgen and lower
levels of sex hormone-binding globulin are associated with
irregular menstruation (4).
Patients with PCOS have hyperandrogenism, which may include
hirsutism, acne, androgenic alopecia, as well as high testosterone
levels. Hyperandrogenism is caused by an increase in androgen
production by the ovaries and adrenals (5). The Rotterdam criteria for PCOS define
the polycystic ovary morphology as a follicle count per ovary 12
and/or an ovarian volume >10 cc in ≥1 ovary (6). Insulin resistance may influence
various elements of ovarian shape by boosting theca cell
proliferation, according to morphological anomalies in PCOS
(7). The progression of PCOS
symptoms over time is depicted in Fig.
1.
Combined oral contraceptives (COCs) contain two
hormones, estrogen and progesterone that inhibit hypothalamic,
pituitary and ovarian steroidogenesis; they consist the first-line
treatment for monthly abnormalities of the menstrual cycle in
patients with PCOS. Medication for PCOS-related hirsutism, acne and
androgenic alopecia is just as successful as the COC treatment for
monthly abnormalities. In addition, the metabolic characteristics
of PCOS are aided by the anti-androgenic chemicals in
third-generation combined oral contraceptive tablets. While this
may be the case, oral contraceptives are used by women who are not
actively trying to conceive (4,8).
Metformin, spironolactone, clomiphene, thiazolidinediones and
insulin-sensitizing agents are just a few of the synthetic
medications used to treat PCOS and reduce insulin resistance,
testosterone levels, inflammatory symptoms and menstrual cycle
abnormalities. Congenital heart defects, nausea, vomiting and
diarrhea are only some of the possible adverse reactions to these
medications (9,10).
Several medicinal herbs, including Cinnamomum
verum, Trigonella foenum-graecum and Vitex
agnus-castus, were suggested as adjunctive treatments for PCOS
(11,12). A varied effect of soy on PCOS was
discovered, making it one of the medicinal plants with contentious
results (12). Soy isoflavones may
be useful in the treatment of PCOS as they enhance both metabolic
conditions and reproductive hormones. The simple glucosides
daidzin, genistin and glycitein, as well as the acylated glucosides
genistin, glycitein and glycidyl isoflavone, are all soybean
isoflavones (13,14). Isoflavones bind to the estrogen
receptor (ER)-α and ER-β because of their chemical structure, which
is comparable to that of the estrogen-like substance 17β-estradiol
(15-17).
Either an estrogenic or an anti-estrogenic effect follows. This
effect is useful in the treatment of PCOS and other
estrogen-related disorders (18,19).
Previous research on the role of isoflavones in PCOS produced both
positive and negative results (12). The present review aimed to analyze
in vivo and clinical trial studies related to the activities
of isoflavone in treating PCOS symptoms.
2. Model of in vivo and clinical
studies of PCOS symptoms
The gold standard for in vivo PCOS research
is the letrozole-induced PCOS rat model, in which female
Sprague-Dawley rats (4-6 weeks old) received letrozole (1 mg/kg PO
once daily) for 21 days (3,20,21).
Letrozole, an aromatase inhibitor, boosts androgen levels in PCOS
by preventing the conversion of androstenedione to estrone, and
testosterone to estradiol (22,23).
Female Wistar rats (8-10 weeks old) may receive subcutaneous
injections of 2 mg/kg (24) or 4
mg/kg (25) estradiol valerate for
60 consecutive days to establish a PCOS model. This PCOS model,
which is similar to PCOS in humans and can be generated in rats by
a single dose of estradiol valerate, is characterized by
infertility, irregular reproductive cycles and polycystic ovaries
with a high number of atretic follicles and cysts (26).
A randomized, double-blind, placebo-controlled trial
design is typically used when conducting a clinical experiment. To
participate in a clinical trial, participants should have two of
the following characteristics and match the Rotterdam criteria
(27) for PCOS: i) Individuals must
have irregular menstruation, hyperandrogenism or polycystic ovarian
changes; ii) individuals who have not taken oral contraceptives
within the last 3 months; and iii) individuals who have not taken
antioxidant-related drugs, medications affecting reproductive
endocrine functions or the metabolism of glucose and lipids, with
high prolactin levels, thyroid disorders, endocrine diseases,
gastrointestinal issues, diseases that cause elevated testosterone
levels and those who have contracted any of these conditions
(28). Tables I and II summarize clinical trials and in
vivo studies looking at isoflavone therapy for PCOS.
 | Table IEffects of isoflavone administration
in patients with polycystic ovary syndrome. |
Table I
Effects of isoflavone administration
in patients with polycystic ovary syndrome.
| First author/s,
year | Isoflavone
source | Subjects, n | Dose and
duration | Effects | (Refs.) |
|---|
| Li et al,
2021 | Pueraria
lobate (Willd.) Ohwi Soy | 36 | 150 mg/day for 3
months | Increased SOD and
SHBG, and decreased TC, T and SBP | (28) |
| Jamilian and Asemi,
2016 | | 35 | 50 mg/day for 3
months | Decreased levels of
lipids, FAI, insulin, HOMA-IR, HOMA-B, T and MDA in the blood, and
increased QUICKI, SHBG and GSH levels | (41) |
| Karamali et
al, 2018 | Soy | 30 | 0.28 g/kg for 2
months | Decreased body
weight, HOMA-IR, FPG, T, TG, VLDL and MDA levels, and increased
QUICKI, NO and GSH | (42) |
| Khani et al,
2011 | Genistein | 69 | 18 mg twice a day
for 3 months | Reduced LDL | (47) |
| Jamilian and
Sahebkashaf, 2017 | Soy isoflavone
supplements | 35 | 50 mg for 3
months | Reduced T and MDA
levels, and increased plasma GSH | (43) |
 | Table IIEffects of isoflavone treatment in
PCOS rats. |
Table II
Effects of isoflavone treatment in
PCOS rats.
| A, Isoflavone from
soy |
|---|
| First author/s,
year | Induction | Doses and duration
of treatment | Effective dose | Effects | (Refs.) |
|---|
| Ma et al,
2021 | Letrozole | 20 mg/200 g for 4
weeks | - | Notable reductions
in body weight, number of atretic follicles in ovarian tissue,
serum T, LH, LH/FSH ratio, MDA, TNF-α, IL-1β and IL-6 levels
Enhanced estrus cycle, granulosa cell layer in the ovary and
elevated levels of FSH and estradiol Ovarian corpus luteum and
healthy follicles. Decreased p-p65/p65 and p-IκBα/IκBα ratios in
ovarian tissues | (3) |
| Manzar et
al, 2021 | Estradiol
valerate | 0.02/200, 0.03/200
and 0.04/200 g for 12 weeks | 0.04/200 g | Higher level of
progesterone and HDL, and lower FSH/LH ratio Decreased
triglycerides, cholesterol, LDL and body weight | (14) |
| Rajan et al,
2017 | Letrozole | 50 and 100 mg/kg
for 2 weeks | 100 mg/kg | Reduced body
weight, diestrus phase %, T levels, 3β and 17β-HSD enzyme activity
and oxidative stress (LPO and NO) Increased antioxidant activity in
the ovary (SOD, CAT, GPx and GSH) Rat ovary with well-formed antral
follicles, a typical granulosa cell layer and a distinct theca
layer | (20) |
| Rajaei et
al, 2019 | Estradiol
valerate | 1 mg/kg for 2
weeks | - | Decreased plasma
and ovarian MDA levels, body weight, and the overall population of
ovarian atretic follicles Increased plasma and ovarian TAC, SOD,
and GPx activities Well-developed antral follicles, a normal
granulosa cell layer, a distinct theca layer and few corpora
lutea | (24) |
| Alivandi Farkhad
and Khazali, 2019 | Estradiol
valerate | 50 and 100 mg/kg
for 3 weeks | 100 mg/kg | Fully-grown antral
follicles, healthy granulosa and theca cells in the ovary,
normalized theca follicle thickness and enhanced granulosa layer
thickness Reduced TNF-α, IL-6 and overall oxidative state levels
Increased capacity of all antioxidants | (25) |
| Amanat et
al, 2021 | Letrozole | 20 mg/kg for 6
weeks | - | Decreased body
mass, LDL, MDA and cyst count Increased TAC and SOD Wholesome
corpora lutea and follicles | (45) |
| Teixeira et
al, 2019 | - | 150 mg/kg daily for
8 weeks | - | Low number of
growing follicles, and corpora lutea and atretic follicles
predominate Reduced percentage of BCL-2 and cleaved
caspase-3+ cells Lowered lipid peroxidation and reactive
oxygen species Increased capacity of all antioxidants | (55) |
| Geethika and Okada,
2018 | Letrozole | 0.05% for 2
weeks | - | Decreased number of
cysts Enhanced the estrous cycle Lower number of gut microbiota
granulicatella | (58) |
| Isoflavone from red
clover (Trifolium pratense) |
| Abbasian et
al, 2020 | Letrozole | 100 mg/200 g or 150
mg/200 g) for 4 weeks | 150 mg/200 g | Decreased levels of
LDL, MDA, NO and T Elevated levels of CAT, GSH, SOD, HDL and
estrogen Increased number of atretic follicles and decreased number
of antral follicles | (21) |
| B, Isoflavone from
chickpea (Cicer arietinum) |
| First author/s,
year | Induction | Doses and duration
of treatment | Effective dose | Effects | (Refs.) |
| Ali et al,
2021 | Letrozole | 50 mg/200 g and 100
mg/200 g for 4 weeks | 100 mg/200 g | Both doses reduced
the levels of MDA, triglycerides, LDL, glucose, testosterone and
ovarian cysts Both doses increased GSH and granulosa cell
thickness | (57) |
3. Effect of isoflavone on body weight and
testosterone level
Abdominal obesity is linked to high plasma androgen
levels in patients with PCOS. Obesity is associated with high
plasma levels of total or free testosterone (29). Increased testosterone levels are
linked to abdominal obesity and fat accumulation because of its
anti-lipolytic effect on abdominal subcutaneous preadipocytes due
to the selective inhibition of catecholamine-induced lipolysis,
which in turn can decrease the expression of β2-adrenergic receptor
and hormone-sensitive lipase protein (30,31).
PCOS may decrease lipolytic activity, which would increase the
lipid content of fat cells and result in obesity.
Isoflavone treatment led to weight loss in
PCOS-induced rats (3,20,24).
Isoflavone was shown to reduce peripheral blood testosterone
levels, which in turn reduces fatty production, hence leading to
weight loss (20,24). The chemical structure of
isoflavones, which contains a phenolic B ring in the third position
of the pyran ring, may impede the action of steroidogenic enzymes
like 3β-hydroxysteroid dehydrogenase (HSD) and/or 17β-HSD, hence
lowering testosterone levels (20).
Androgens are synthesized using both enzymes (32,33).
4. Effect of isoflavone as an
antioxidant
The chemical structures of isoflavones are
essentially linked to their ability to reduce oxidative stress and
increase antioxidative levels (20). Advanced glycosylated end products
(AGEs), nitric oxide (NO), malondialdehyde (MDA) and xanthine
oxidase (XO) are markers of oxidative stress and some studies
(34-37)
indicated that their levels are higher in patients with PCOS than
in women without the condition. Nonetheless, another study showed
that patients with PCOS had reduced levels of the antioxidants
glutathione (GSH), and the vitamins C and E (38). The level of reactive oxygen species
is higher in the ovarian follicle of patients with PCOS than in
normal subjects, which results in increased tissue damage (39). Moreover, MDA-induced polyunsaturated
lipid peroxidation acts as a recognizable hallmark of the wounded
tissue. MDA may also result from excessive androgen production,
which affects insulin resistance and hyperglycaemia in patients
with PCOS (40). Through a variety
of mechanisms, antioxidants serve a key role in preventing,
treating and eradicating oxidative damage. The free radicals formed
by superoxide anion radicals and hydrogen peroxide are neutralized
by the antioxidant enzymes superoxide dismutase (SOD), GSH
peroxidase (GPx) and catalase (CAT) (21,24).
Human study
Regarding antioxidant status, isoflavone therapy was
linked to an increase in SOD and GSH, while MDA was decreased
(28,41-43).
Karamali et al (42)
reported that the level of NO was notably increased after 8 weeks
of treatment with soy in patients with PCOS likely due to the
L-arginine found in soy that can act as a NO precursor.
Consequently, an increase in the NO level was observed in that
study. NO in PCOS could play a role in determining polycystic
characteristics (38) and further
research on soy intake in patients with PCOS is required.
Animal study
Ma et al (3)
discovered that soy isoflavones have an antioxidant effect on rats
by increasing GPx and SOD. According to previous research results,
the antioxidant effect of isoflavone via MDA also decreased
(21,24,41,42).
Isoflavone also affects the reduction of NO levels reported by
Abbasian et al (21), who
evaluated the treatment of isoflavone from red clover.
Total antioxidant capacity (TAC) levels in patients
with PCOS were observed to be inconsistently higher or lower
compared with those in controls. An elevation in TAC in PCOS is
thought to act as a defence mechanism against the negative
consequence of increased oxidative stress in immature ovum
destruction (40). Further study on
the effects of isoflavone on TAC and the relationship between TAC
and PCOS is required.
5. Effect of isoflavone as an
anti-inflammatory agent
The adipose tissue of patients with PCOS and obesity
produces proinflammatory cytokines such as TNF-α and IL-6 in
response to hypoxia (44).
In vivo study on rats with PCOS treated with
soybean isoflavone-aglycone fraction demonstrated decreased IL-6
and TNF-α levels in ovarian tissue (25). Those findings were comparable with
those of Amanat et al (45),
who identified reduced levels of TNF-α in PCOS rat model compared
with those in healthy rats. Theca cells, which make androgens, had
increased proliferation due to increased TNF-α levels, which also
contributed to hyperandrogenism (25). The ability of isoflavone to reduce
inflammation by decreasing TNF-α also makes it an anti-androgenic
and antioxidant agent.
Moreover, soy isoflavone treatment was reported to
reduce the expression levels of NF-κB p65, phosphorylated (p)-NF-κB
p65 and p-IκBα in ovarian tissues (46), suggesting a decrease in TNF-α, IL-1β
and IL-6(3).
6. Effect of isoflavone in the improvement
of metabolic profile and reproductive hormone
Human study
Jamilian and Asemi (41) discovered that in patients with PCOS,
blood levels of insulin, triglycerides and very low-density
lipoprotein (VLDL) cholesterol were considerably reduced throughout
the course of a 12-week soy isoflavone therapy compared with those
in the placebo group. The homeostasis model of assessment (HOMA)
for β cell function and that for insulin resistance (HOMA-IR) were
shown to be lower in that study, and the quantitative insulin
sensitivity check index (QUICKI) was also shown to be higher. In a
3-month clinical trial of the drug genistein, patients with PCOS
demonstrated a notable reduction in LDL and triglycerides compared
with that in the placebo group (47). Over the course of 8 weeks, patients
with PCOS who had a soy diet high in unsaturated fat and low in
saturated fat had decreased insulin levels, HOMA-IR, triglycerides
and VLDL. Moreover, the soy diet improved QUICKI scores compared
with a control diet (42). Clinical
trial outcomes demonstrated that 3 months of soy isoflavone
(genistein) treatment notably decreased luteinizing hormone (LH)
and testosterone levels in patients with PCOS compared with the
placebo group (47). These findings
imply that genistein may directly influence the
hypothalamic-pituitary-gonadal or hypothalamic-pituitary-adrenal
(HPA) axis, improving the production pattern function of
gonadotrophin-releasing hormone (GnRH). To demonstrate that these
systems do function, further studies are required.
Animal study
In an in vivo study, red clover isoflavone
was shown to be more efficient than the PCOS group in reducing LDL
while increasing high-density lipoprotein levels (21). The soy isoflavone genistein
treatment was demonstrated to considerably lower the LDL level in
the PCOS group and improve insulin resistance and dysregulated
glucose haemostasis (45). However,
plasma glucose and cholesterol levels were not substantially
different between PCOS vehicle-treated rats and soy
isoflavone-treated rats (20).
The hormonal disturbance observed in PCOS is caused
by modifications to the hypothalamic-pituitary-ovarian or HPA axis,
which originates from an abnormality in the GnRH production
pattern. As a result, LH levels increased, follicle-stimulating
hormone (FSH) levels decreased and thereby the LH/FSH ratio
increased (48). The unfavourable
feedback of oestradiol influences the generation of FSH as well
(49). The activation of androgen
synthesis by LH release in endometrial cells results in an increase
in androgen levels in the body. Contrary to the PCOS group, rats
treated with soy isoflavones exhibited considerably higher levels
of FSH and oestradiol, and notably lower levels of LH, testosterone
and LH/FSH ratio (3). Treatment
with 50 and 100 mg/kg of soy isoflavones did not markedly raise
estradiol levels (20).
7. Effect of isoflavone in normalizing the
oestrous cycle
Similar to the menstrual cycle (ovarian and uterine
cycles) in humans, rodents such as rats have a reproductive cycle
known as the oestrous cycle (50).
Dioestrus, proestrus, oestrus and metestrus are the four stages of
the cycle, and each of them lasts 4-5 days (51). The cycle of letrozole-induced PCOS
rats was studied for 42 days. Between day 9 and 30, the cycle was
in the dioestrus phase. On days 31 and 32, it entered the proestrus
phase. Subsequently, it went back into the dioestrus phase for
another 10 days (52). Compared
with the group demonstrating a protracted dioestrus phase due to
letrozole-induced PCOS, rats that were given soy isoflavone
progressively returned to a normal oestrous cycle. Changes in the
levels of sex hormones and gonadotrophins such as testosterone, LH,
FSH and oestradiol in the bloodstream may be responsible for that
result (3,20).
Nonetheless, the menstrual cycle in humans consists
of three distinct stages: Luteal, follicular and menstrual
(53). From day 1 of one period to
day 1 of the next, this cycle typically lasts ~28 days (51). To the best of our knowledge, there
has been no clinical investigation of the influence of isoflavone
on the menstrual cycle of patients with PCOS. Further research into
the therapeutic effects of isoflavone in controlling the menstrual
cycle of patients with PCOS is warranted.
8. Effect of isoflavone on restoring the
ovarian morphology
Letrozole induction stimulated the development of
cysts while suppressing oocyte, granulosa and theca layer
hyperplasia, as well as follicular atresia (54). In the ovary histology of soy
isoflavone-treated letrozole-induced PCOS rats, distinct theca
layer, well-developed antral follicles and a normal granulosa cell
layer were present (20). In a
study by Ma et al (3), soy
isoflavone was found to increase the granulosa cell layer,
encourage healthy follicle and corpus luteum development, and
reduce the number of atretic follicles compared with the control
group. Teixeira et al (55)
also discovered that the soy isoflavone group had fewer atretic
follicles compared with the placebo group. According to a study by
Amanat et al (45),
genistein treatment in PCOS rats was reduced the number of atretic
follicles; however, this number was markedly higher than that in
the control group. There were fewer cysts in the follicles and
corpora lutea of the healthy group than in the PCOS group (45).
In vivo research of isoflavone from red
clover extract, compared with study of soy isoflavone (3,45,55),
found a rise in atretic follicles and a reduction in antral
follicles (21). Full-grown antral
follicles, a few corpora lutea, normally functioning granulosa
cells and theca cells were observed in the ovaries of soybean
isoflavone-aglycone fraction-treated PCOS rats. These findings were
in agreement with those predicted by the action of genistein, the
primary component of the isoflavone-aglycone (24,56).
Contrary to the PCOS group, chickpea (Cicer
arietinum) isoflavones were shown to decrease the prevalence of
fluid-filled sac in the ovary, restore granulosa cell diameter and
enhance the existence of corpus luteum (57).
9. Conclusions
Isoflavones were shown to effectively treat PCOS
syndrome symptoms by targeting several interconnected pathways. The
primary benefit of isoflavones in treating PCOS symptoms is the
reduction of androgen levels, which are the main symptoms of PCOS.
This is achieved by inhibiting the activities of 3β-HSD and/or
17β-HSD enzymes, reducing oxidative stress levels (MDA, NO, AGEs
and XO), and increasing antioxidative levels (SOD, GPx, TAC and
CAT) and anti-inflammatory activity (TNF-α, IL-6 and NF-κB).
Additionally, several other symptoms of PCOS such as obesity,
insulin resistance, metabolic dysfunction, impaired
folliculogenesis, low-grade chronic inflammation, reproductive
hormone imbalance, irregular monthly cyclicity, aberrant gut flora
and ovarian characteristics can be treated effectively with
isoflavones. The recommended dosage of isoflavone supplementation
for patients with PCOS is 50 mg/day, which has shown promising
results (41,43).
Acknowledgements
Not applicable.
Funding
Funding: The present review was supported by the Bandung
Institute of Technology and the Asahi Glass Foundation (grant no.
1422/IT1.B07.1/TA.00/2022).
Availability of data and materials
Not applicable.
Authors' contributions
CR collected raw data for analysis, performed
critical analysis of the literature and drafted the manuscript. SS
and NFK organized the framework of this paper, supervised the work
and revised the manuscript. All authors have read and approved the
final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barbosa G, de Sá L, Rocha D and Arbex A:
Polycystic ovary syndrome (PCOS) and infertility. Open J Endocr
Metab Dis. 6:58–65. 2016.
|
|
2
|
Lentscher JA and Decherney AH: Clinical
presentation and diagnosis of polycystic ovarian syndrome. Clin
Obstet Gynecol. 64:3–11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ma X, Li X, Ma L, Chen Y and He S: Soy
isoflavones alleviate polycystic ovary syndrome in rats by
regulating NF-κB signaling pathway. Bioengineered. 12:7204–7212.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Harris HR, Titus LJ, Cramer DW and Terry
KL: Long and irregular menstrual cycles, polycystic ovary syndrome,
and ovarian cancer risk in a population-based case-control study.
Int J Cancer. 140:285–291. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ashraf S, Nabi M, Rasool SUA, Rashid F and
Amin S: Hyperandrogenism in polycystic ovarian syndrome and role of
CYP gene variants: A review. Egypt J Med Hum Genet. 20(25)2019.
|
|
6
|
Smet ME and McLennan A: Rotterdam
criteria, the end. Australas J Ultrasound Med. 21:59–60.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Reid SP, Kao CN, Pasch L, Shinkai K,
Cedars MI and Huddleston HG: Ovarian morphology is associated with
insulin resistance in women with polycystic ovary syndrome: A cross
sectional study. Fertil Res Pract. 3(8)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rodriguez Paris V and Bertoldo MJ: The
mechanism of androgen actions in PCOS etiology. Med Sci (Basel).
7(89)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kamboj A, Verma D, Sharma D, Pant K, Pant
B and Kumar V: A molecular docking study towards finding herbal
treatment against polycystic ovary syndrome (PCOS). Int J Recent
Technol Eng. 8:38–41. 2019.
|
|
10
|
Gayathri K, Supriya V and Palaniappan N:
Nutritional composition and sensory evaluation of
phytoestrogen-rich supplement for women with PCOS. Int J Nutr
Pharmacol Neurol Dis. 11:303–308. 2021.
|
|
11
|
Shehab NG, Anbar HS, Alrouby NM,
Abouelalamin A, Lutfi L, Allo IT and Elayoty SM: New alternative
herbal remedies for treatment of letrozole-induced polycystic ovary
syndrome in rats. J Appl Pharm Sci. 13:163–179. 2023.
|
|
12
|
Moini Jazani A, Nasimi Doost Azgomi H,
Nasimi Doost Azgomi A and Nasimi Doost Azgomi R: A comprehensive
review of clinical studies with herbal medicine on polycystic ovary
syndrome (PCOS). DARU. 27:863–877. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jang HH, Lee YM, Choe JS and Kwon O:
Validation of soy isoflavone intake and its health effects: A
review of the development of exposure biomarkers. Nutr Res Pract.
15:1–11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Manzar N, Khan AS, Fatima N, Nisa M, Ahmad
MH, Afzal MI, Saeed HFU, Imran M, Anjum FM and Arshad MS: Exploring
the prophylactic role of soy isoflavones against polycystic ovarian
syndrome. Food Sci Nutr. 9:4738–4744. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gómez-Zorita S, González-Arceo M,
Fernández-Quintela A, Eseberri I, Trepiana J and Portillo MP:
Scientific evidence supporting the beneficial effects of
isoflavones on human health. Nutrients. 12(3853)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Křížová L, Dadáková K, Kašparovská J and
Kašparovský T: Isoflavones. Molecules. 24(1076)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leonard LM, Choi MS and Cross TL:
Maximizing the estrogenic potential of soy isoflavones through the
gut microbiome: Implication for cardiometabolic health in
postmenopausal women. Nutrients. 14(553)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Desmawati D and Sulastri D: Phytoestrogens
and their health effect. Open Access Maced J Med Sci. 7:495–499.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu XL, Deng SL, Lian ZX and Yu K: Estrogen
receptors in polycystic ovary syndrome. Cells.
10(459)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rajan RK, Kumar MSS and Balaji B: Soy
isoflavones exert beneficial effects on letrozole-induced rat
polycystic ovary syndrome (PCOS) model through anti-androgenic
mechanism. Pharm Biol. 55:242–251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Abbasian Z, Barmak MJ, Barazesh F,
Ghavamizadeh M and Mirzaei A: Therapeutic efficacy of Trifolium
pratense L. on letrozole induced polycystic ovary syndrome in rats.
Plant Sci Today. 7:501–507. 2020.
|
|
22
|
Xu J, Dun J, Yang J, Zhang J, Lin Q, Huang
M, Ji F, Huang L, You X and Lin Y: Letrozole rat model mimics human
polycystic ovarian syndrome and changes in insulin signal pathways.
Med Sci Monit. 26(e923073)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang AM, Cui N, Sun YF and Hao GM:
Letrozole for female infertility. Front Endocrinol (Lausanne).
12(676133)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rajaei S, Alihemmati A and Abedelahi A:
Antioxidant effect of genistein on ovarian tissue morphology,
oxidant and antioxidant activity in rats with induced polycystic
ovary syndrome. Int J Reprod Biomed. 17:11–22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Alivandi Farkhad S and Khazali H:
Therapeutic effects of isoflavone-aglycone fraction from soybean
(Glycine max L. Merrill) in rats with estradiol valerate-induced
polycystic ovary syndrome as an inflammatory state. Gynecol
Endocrinol. 35:1078–1083. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ramadoss M, Vijayaraman M and Subbiah AJ:
Estradiol valerate dose determination for PCOS induction. Int J
Pharm Biol Sci. 9:1131–1136. 2019.
|
|
27
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
consensus workshop group. Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome (PCOS). Hum Reprod. 19:41–47. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li W, Hu H, Zou G, Ma Z, Liu J and Li F:
Therapeutic effects of puerarin on polycystic ovary syndrome: A
randomized trial in Chinese women. Medicine (Baltimore).
100(e26049)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Blouin K, Boivin A and Tchernof A:
Androgens and body fat distribution. J Steroid Biochem Mol Biol.
108:272–280. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Homburg R: Androgen circle of polycystic
ovary syndrome. Hum Reprod. 24:1548–1555. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dumesic DA, Abbott DH, Sanchita S and
Chazenbalk GD: Endocrine-metabolic dysfunction in polycystic ovary
syndrome: An evolutionary perspective. Curr Opin Endocr Metab Res.
12:41–48. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rasmussen MK, Ekstrand B and Zamaratskaia
G: Regulation of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase: A
review. Int J Mol Sci. 14:17926–17942. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kater CE, Giorgi RB and Costa-Barbosa FA:
Classic and current concepts in adrenal steroidogenesis: A
reappraisal. Arch Endocrinol Metab. 66:77–87. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Murri M, Luque-Ramı´rez M, Insenser M,
Ojeda-Ojeda M and Escobar-Morreale HF: Circulating markers of
oxidative stress and polycystic ovary syndrome (PCOS): A systematic
review and meta-analysis. Hum Reprod Update. 19:268–288.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Karabulut AB, Cakmak M, Kiran RT and Sahin
I: Oxidative stress status, metabolic profile and cardiovascular
risk factors in patients with polycystic ovary syndrome. Med
Science. 1:27–34. 2012.
|
|
36
|
Diamanti-Kandarakis E, Piperi C,
Kalofoutis A and Creatsas G: Increased levels of serum advanced
glycation end-products in women with polycystic ovary syndrome.
Clin Endocrinol (Oxf). 62:37–43. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Isık H, Aynıoglu O, Tımur H, Sahbaz A,
Harma M, Can M, Guven B, Alptekin H and Kokturk F: Is Xanthine
oxidase activity in polycystic ovary syndrome associated with
inflammatory and cardiovascular risk factors? J Reprod Immunol.
116:98–103. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mohammadi M: Oxidative stress and
polycystic ovary syndrome: A brief review. Int J Prev Med.
10(86)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Enechukwu CI, Onuegbu AJ, Olisekodiaka MJ,
Eleje GU, Ikechebelu JI, Ugboaja JO, Amah UK, Okwara JE and Igwegbe
AO: Oxidative stress markers and lipid profiles of patients with
polycystic ovary syndrome in a Nigerian tertiary hospital. Obstet
Gynecol Sci. 62:335–343. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fathi FH: Biomarkers of oxidative stress
in polycystic ovary disorder. Ann Coll Med Mosul. 41:112–116.
2019.
|
|
41
|
Jamilian M and Asemi Z: The effects of soy
isoflavones on metabolic status of patients with polycystic ovary
syndrome. J Clin Endocrinol Metab. 101:3386–3394. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Karamali M, Kashanian M, Alaeinasab S and
Asemi Z: The effect of dietary soy intake on weight loss, glycaemic
control, lipid profiles and biomarkers of inflammation and
oxidative stress in women with polycystic ovary syndrome: A
randomised clinical trial. J Hum Nutr Diet. 31:533–543.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jamilian M and Sahebkashaf R: Metabolic
response to soy supplementation in women with polycystic ovary
syndrome. J Arak Uni Med Sci. 20:14–23. 2017.
|
|
44
|
Herman R, Sever MJ, Janež A and Vita D:
Interplay between oxidative stress and chronic inflammation in
PCOS: the role of genetic variability in PCOS risk and treatment
responses. IntechOpen. Ch 8:2020.
|
|
45
|
Amanat S, Ashkar F, Eftekhari MH, Tanideh
N, Doaei S, Gholamalizadeh M, Koohpeyma F and Mokhtari M: The
effect of genistein on insulin resistance, inflammatory factors,
lipid profile, and histopathologic indices in rats with polycystic
ovary syndrome. Clin Exp Reprod Med. 48:236–244. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yu J, Bi X, Yu B and Chen D: Isoflavones:
Anti-inflammatory benefit and possible caveats. Nutrients.
8(361)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Khani B, Mehrabian F, Khalesi E and
Eshraghid A: Effect of soy phytoestrogen on metabolic and hormonal
disturbance of women with polycystic ovary syndrome. J Res Med Sci.
16:297–302. 2011.PubMed/NCBI
|
|
48
|
Saadia Z: Follicle stimulating hormone
(LH: FSH) ratio in polycystic ovary syndrome (PCOS)-obese vs.
non-obese women. Med Arch. 74:289–293. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Madnani N, Khan K, Chauhan P and Parmar G:
Polycystic ovarian syndrome. Indian J Dermatol Venereol Leprol.
79:310–321. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ekambaram G, Sampath Kumar SK and Joseph
LD: Comparative study on the estimation of estrous cycle in mice by
visual and vaginal lavage method. J Clin Diagnostic Res.
11:AC05–AC07. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ajayi AF and Akhigbe RE: Staging of the
estrous cycle and induction of estrus in experimental rodents: An
update. Fertil Res Pract. 6(5)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nallathambi A and Bhargavan R: Regulation
of estrous cycle by Cynodon dactylon in letrozole induced
polycystic ovarian syndrome in Wistars albino rats. Anat Cell Biol.
52:511–517. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shilpa N, Itagi V and Rani R: A study of
hemoglobin concentration in different phases of menstrual cycle.
Int J Physiol. 6:90–94. 2018.
|
|
54
|
Jahan S, Abid A, Khalid S, Afsar T,
Qurat-Ul-Ain Shaheen G, Almajwal A and Razak S: Therapeutic
potentials of quercetin in management of polycystic ovarian
syndrome using letrozole induced rat model: A histological and a
biochemical study. J Ovarian Res. 11(26)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Teixeira CP, Florencio-Silva R, Sasso GRS,
Carbonel AAF, Simões RS and Simões MJ: Soy isoflavones protect
against oxidative stress and diminish apoptosis in ovary of
middle-aged female rats. Gynecol Endocrinol. 35:586–590.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Khezri S, Alihemmati A and Abedelahi A:
Genistein blunted detrimental effects of polycystic ovary syndrome
on the ovarian tissue of rats by improving follicular development
and gonadotropin secretion. JBRA Assist Reprod. 26:379–386.
2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ali SE, El Badawy SA, Elmosalamy SH, Emam
SR, Azouz AA, Galal MK, Abd-Elsalam RM, Issa MY and Hassan BB:
Novel promising reproductive and metabolic effects of Cicer
arietinum L. extract on letrozole induced polycystic ovary
syndrome in rat model. J Ethnopharmacol. 278(114318)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Geethika L and Okada S: Investigating the
effect of soy isoflavones on improving polycystic ovary syndrome
hallmarks liyanage. Soy Protein Research. 21:90–94. 2018.
|