Cancer is the second most deadly disease worldwide
after cardiovascular disease and one of the major threats to human
health (1). According to recent
estimates, the global cancer burden is expected to increase
significantly in the coming decades (by 47% from 2023 to 2040)
(2,3). Due to the various limitations
regarding conventional treatment regimens, a growing number of
researchers around the world are working to discover new anticancer
agents and develop new effective modalities for cancer treatment
(4). The vast majority of currently
clinically approved chemotherapeutic agents are derived from a
variety of natural sources, including microbes and terrestrial and
marine plants (5). Commonly used
anticancer drugs such as pergolide, vincristine, paclitaxel and
podophyllotoxin are found in plants (6,7).
Inspired by the recent success in drug development, an increasing
number of research groups worldwide are working to isolate new
structural leads from different plant species and to assess their
potential anticancer activity.
Dihydromyricetin (DHM, PubChem compound ID: 161557),
also known as ampelopsin, is a polyphenolic hydroxy-dihydroflavonol
compound (8). It was first isolated
from the plant Ampelopsis meliaefolia Kudo in 1940(9); it is part of a class of active natural
compounds widely present in a variety of plant families, including
the Umbelliferae, Asteraceae, Leguminosae,
Rutaceae, Mulberry, Mucuna pruriens and
Thymus families (10). DHM
has a wide range of pharmacological activities, such as
anti-inflammatory, anticoagulant, antibacterial, antifungal,
antiviral, anticancer and antihypertensive activities (11,12).
DHM has poor water solubility and is only stable at low
temperatures and in a weakly acidic environment (pH 6.0) (8,13). To
improve the medicinal properties (for example, the solubility,
permeability and stability) of DHM, researchers have developed
numerous new formulations of DHM, such as nano-formulations
(14), microemulsions (15), gels (16) and crystals (17).
Several preclinical studies have revealed that DHM
can inhibit the growth of a variety of cancer cells by modulating
various cellular signalling pathways; for example, DHM can protect
the cardiovascular system through the PI3K/Akt, Nrf2/HO-1 and SIRT3
signalling pathways (18-20)
and can prevent or inhibit the growth of a variety of cancer cells
through the ERK/Akt, AMPK/MAPK/XAF1, Akt-mTOR, ROS/NF-κB and
mitochondrial apoptosis signalling pathways to prevent or inhibit
hepatocellular carcinoma, lung cancer, colorectal cancer, breast
cancer, leukaemia and melanoma (21-23).
DHM can also prevent or inhibit neurodegenerative diseases through
the TRL4/NF-κB and p53/p21 signalling pathways (24,25).
In addition, DHM has anti-inflammatory, antibacterial, antiviral
and skin-protective effects, thus it has great potential for the
treatment of tumours (10).
Previous studies have revealed that DHM can inhibit the
proliferation of various types of tumour cells, induce apoptosis,
and inhibit migration, invasion, and metastasis (12). In addition, treatment of malignant
tumour cells with DHM has been shown to suppress drug resistance
and increase the response to standard anticancer drugs such as
adriamycin or doxorubicin (DOX), opening new avenues for cancer
treatment (18,26). Therefore, in the present review, the
multiple molecular and cellular mechanisms underlying the
tumour-suppressive effects of DHM will be described in depth to
provide ideas for the research and development of clinical
treatments involving DHM.
Given that DHM has drawn widespread concern in
recent years, its anticancer effects have been widely described
(9,12,27),
while there are few reviews that summarize the antitumour effects
of DHM from the molecular mechanism level, and the reviews about
the role of DHM in conventional antitumour drugs are less
understood. In 2018 Zhang et al (28) suggested that DHM may be associated
with several different molecules involved in cellular apoptosis,
oxidative stress and inflammation. However, further research on DHM
discovered that DHM is also associated with tumor cell
proliferation (29), invasion and
metastasis (30), and autophagy
(31). To improve understanding of
the antitumour mechanism of DHM, in the present review, four types
of antitumour effects of DHM were summarized: Inhibition of tumour
cell proliferation, promotion of apoptosis, inhibition of tumour
cell invasion and migration, scavenging of tumour cell reactive
oxygen species (ROS), and induction of autophagy. Relevant articles
were screened from recent years and the results related to the
molecular and cellular mechanisms were summarized. The present
review also summarized the potential application of DHM in
combination with conventional antitumour drugs, as well as the
advantages and disadvantages of DHM as an antitumour drug.
The process of the cell cycle is divided into
interphase (G1 phase, S phase, G2 phase), division phase (M phase),
and stationary phase (G0 phase), and the normal progression of the
cell cycle is regulated by various mechanisms of action to ensure
orderly cell division (32); if any
of the related factors are dysregulated, the cell cycle is
terminated (32,33). The central cell cycle regulation
mechanism is the Cyclin-CDK-CDI mechanism, and CDK inhibitor (CKI)
genes have positive regulators such as cyclin and p16 and negative
regulators such as p53 and p21 (34,35).
The overexpression of positive regulators or the absence of
negative regulators results in the downregulation of the cell cycle
threshold and weakening of cell cycle function, resulting in
reduced sensitivity of cells to exogenous regulatory signals
(32,35). CDC genes (cell division cycle genes)
are a class of genes whose expression is cell cycle-dependent or
directly involved in cell cycle regulation; these genes mainly
include Cyclin genes, CDK genes and CKI genes (36,37).
In addition, DNA polymerase and DNA ligase genes related to DNA
replication are also part of the CDC gene family (38).
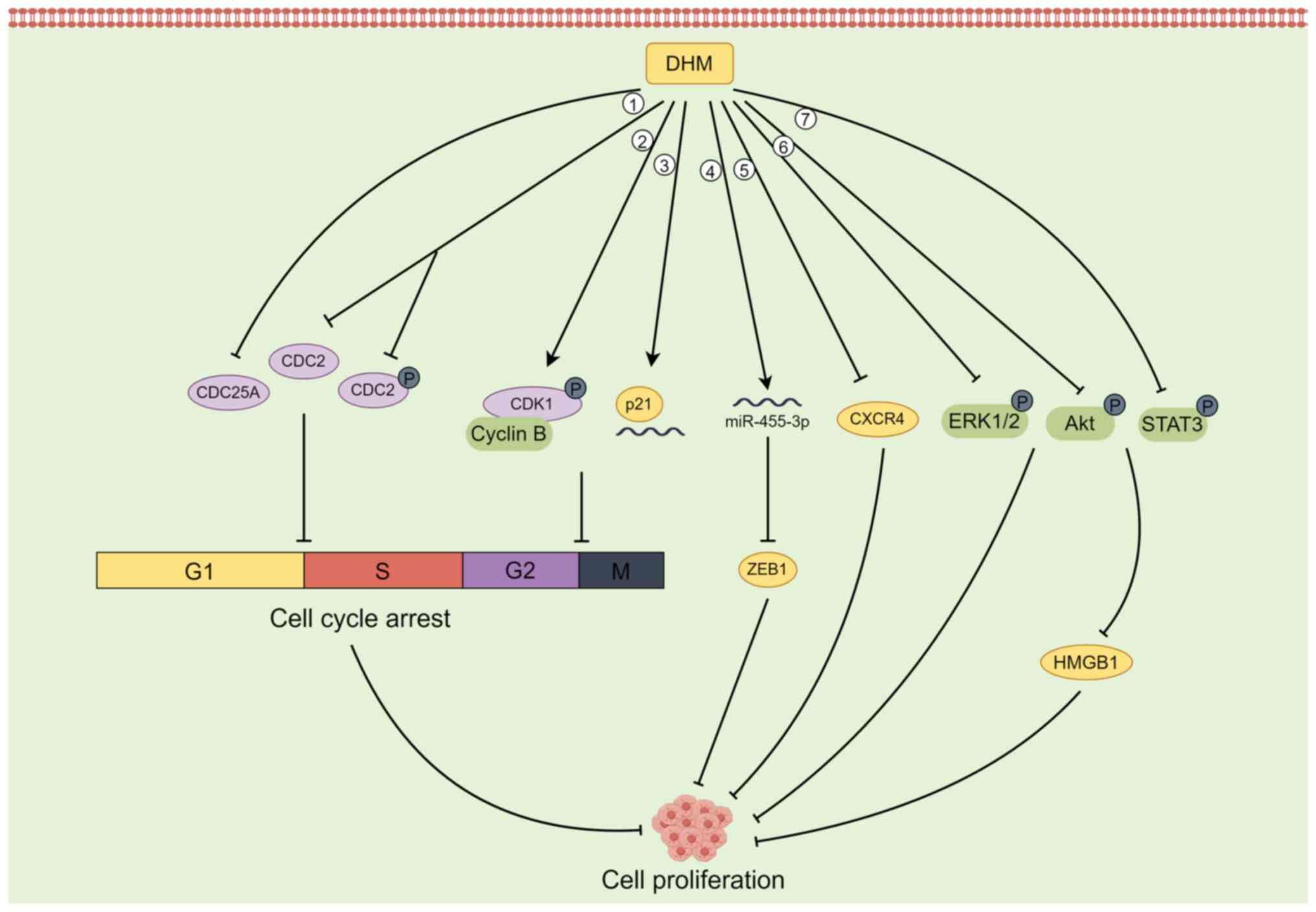
Several studies have reported that in some tumour
cells, DHM can inhibit cell proliferation by blocking the G2/M
phase and G1/S phase of the cell cycle (29,39,40).
For example, DHM can inactivate the CDK1/cell cycle protein B1
complex by phosphorylating CDK1, thus inducing G2/M phase cell
cycle arrest and inhibiting the growth of hepatocellular carcinoma
cells (29). In SK-MEL-28 melanoma
cells, DHM downregulates the expression of the CDC25A, CDC2, and
phosphorylated (p-)CDC2 proteins to induce cell cycle arrest in the
G1/S phase (39). DHM also induces
G2-M arrest of the U2OS cell cycle in osteosarcoma cells by
increasing the levels of p21 protein and RNA and inhibiting cell
proliferation (40).
In addition to inhibiting the proliferation of
tumour cells by blocking the cell cycle, DHM can also target the
activation of ERK1/2 and Akt to inhibit the proliferative potential
of fibroblasts in lung cancer cells (41) and upregulate the expression of p53
to inhibit the proliferation of hepatocellular carcinoma cells
(42). In animal studies using an
in situ prostate tumour model, Ni et al (43) reported that DHM inhibited the
proliferation of PC-3 tumours in a dose-dependent manner, which was
associated with a decrease in the CXCR4 protein. In BGC-823 gastric
cancer cells, DHM induced a decrease in the expression of Cyclin
D1, Cyclin E1 and N-cadherin; increased the expression of
E-cadherin; inhibited the phosphorylation of Akt and STAT3; and
downregulated the expression of HMGB1 in cells. Therefore, DHM
inhibits BGC-823 cell proliferation and migration by regulating the
activation of the Akt/STAT3 signalling pathway and the expression
of HMGB1(44). In
cholangiocarcinoma (CAA) cells, DHM upregulated the expression of
miR-455-3p, which in turn inhibited the expression of its
downstream target ZEB1, thereby suppressing the proliferation of
CAA cells (45). ZEB1 is a
transcription factor that promotes metastasis and stem cell
characteristics, and it is aberrantly expressed in CAA cells
(46) (Fig. 1).
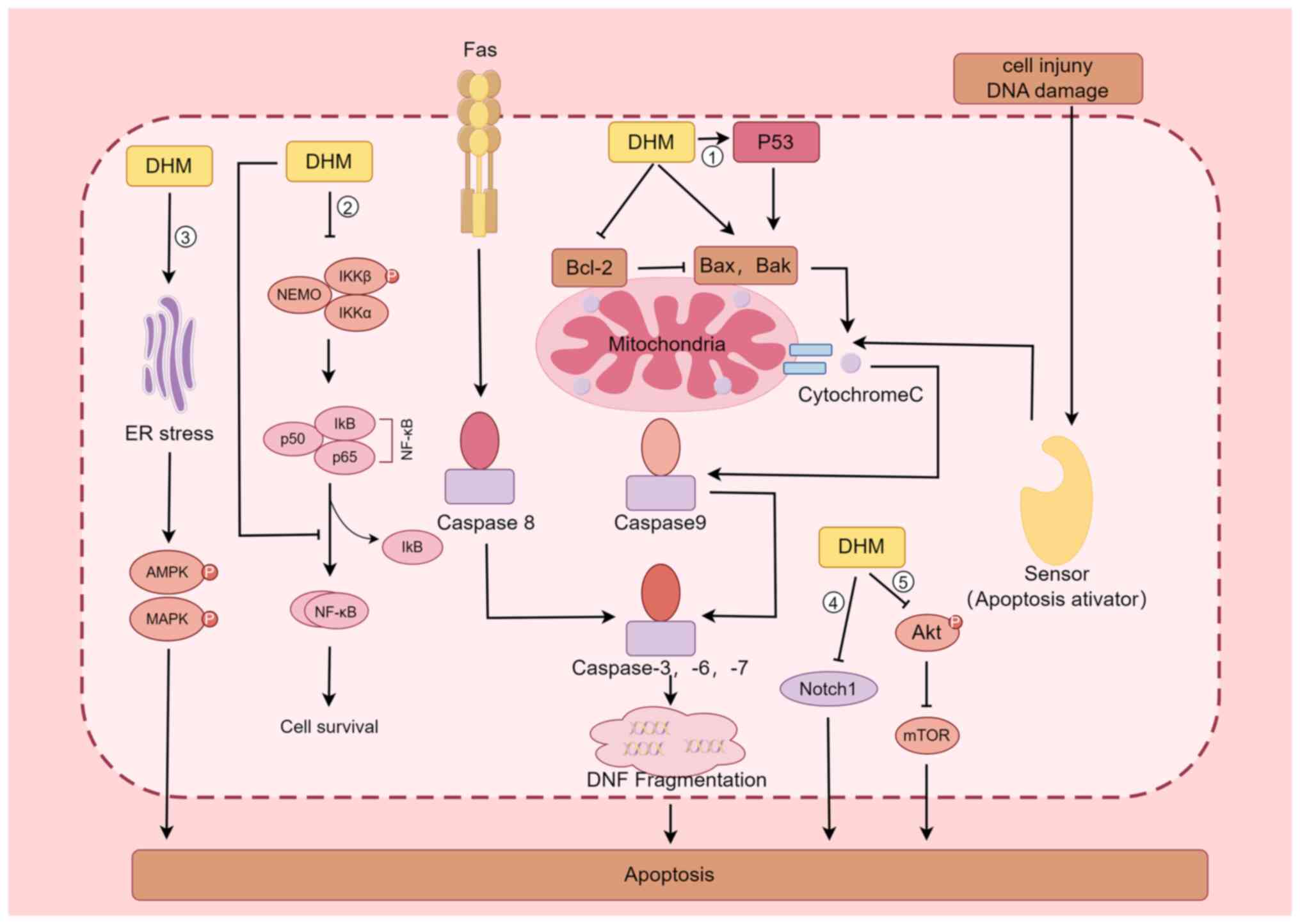
The apoptotic pathway includes both exogenous and
endogenous pathways, the former being mediated by receptors such as
TNF-α and FAS-L, while the latter uses mitochondria as the main
site of apoptosis induction by regulating the permeability of their
outer membrane (47). When various
stress responses, DNA damage and abnormal cell signalling occur,
Bax is activated, and under the regulation of this protein, the
permeability of the mitochondrial outer membrane increases, and
Cytochrome C is released into the cytoplasm, where it binds to
Apaf-1 in the presence of dATP, causing Apaf-1 to expose its Card
structural domain and bind to the Card of Procaspase-9 to form an
apoptotic complex; this complex in turn activates Caspase-9, which
further activates Caspase-3, thus causing apoptosis (48-50).
By contrast, Bcl-2 plays the opposite role as Bax in inhibiting
Cytochrome C release, thus inhibiting the process of apoptosis;
therefore, regulation of the expression of Bcl-2 has an important
impact on the apoptosis of tumour cells (51). Because of the inverse effects of
Bcl-2 and Bax, when cells are stimulated, the ratio of the two
determines cell survival (52). Liu
et al (42) discovered that
DHM significantly increases p53 protein expression in four types of
hepatocellular carcinoma cells (HepG2, QGY7701, Hepal-6, and
MHcc97L), which activated Bax and Bak, and inhibited Bcl-2
expression, which in turn activated Caspase-3 and eventually led to
the apoptosis of tumour cells; the regulatory effect was
dose-dependent. Ji et al (53) revealed that DHM significantly
(P<0.05) inhibited AGS cell proliferation and induced cell
cytotoxicity in a dose- and time-dependent manner; DHM also
regulated the expression of apoptosis-related genes such as p53 and
Bcl-2 in a dose- and time-dependent manner in AGS cells treated
with DHM, as determined by western blotting (53).
In addition to inducing the apoptosis in tumour
cells through the p53-mediated signalling pathway, DHM is
associated with the Nuclear Factor Kappa B Subunit 1(NF-κB)
signalling pathway (22,23,54).
Han et al (54) reported
that in the leukemic cell lines HL60 and K562, DHM induced
apoptosis through nuclear condensation, induced loss of
mitochondrial membrane potential, increased ROS production,
activated Caspase-9, Caspase-3 and poly ADP-ribose polymerase, and
regulated the expression of Bcl-2 family members; these authors
also reported that DHM induced apoptosis in the leukaemia cell
lines HL60 and K562, possibly through the NF-κB signalling pathway.
Li et al (22) examined the
expression of phosphorylated nuclear factor kappa B kinase subunit
β (p-IKKβ), phosphorylated nuclear factor kappa B kinase subunit α
(p-IKKα), nuclear factor kappa Bα (IκB-α) inhibitor, and NF-κB/p65
in a nasopharyngeal carcinoma CNE-2 cell line via western blot
analysis and confocal laser scanning microscopy; the observed
nuclear translocation of NF-κB/p65 indicated that DHM promotes the
inactivation of p-IKKβ and p-IKKα and blocks the nuclear
translocation of the NF-κB subunit p65, promoting the apoptosis of
CNE-2 cells in nasopharyngeal carcinoma. Guo et al (23) treated a rat model of fatty liver
disease with DHM and found that DHM inhibited the protein
expression of NF-κB, p53 and Bax, acting as hepatoprotective
agent.
In addition, DHM promotes the apoptosis of tumour
cells through several other pathways (55-59).
For example, DHM inhibits the activation of Akt, which in turn
inhibits the formation of the mTOR complex and promotes apoptosis
in breast cancer cells (55). DHM
is a biologically active natural chemopreventive agent and a potent
mTOR inhibitor that may be a useful chemotherapeutic agent for
breast cancer treatment (55). Lu
et al (56) revealed that
Notch1 is involved in the development of HCC and that DHM inhibits
cell proliferation and promotes apoptosis by downregulating Notch1
expression. A study by Ye et al (57) demonstrated that in
cisplatin-resistant nasopharyngeal carcinoma cell lines (Hone1/Cis
and CNE1/Cis), cotreatment with DHM increased the growth-inhibitory
effect of cisplatin by blocking the Wnt/β-catenin signalling
pathway to increase the antitumour activity of cisplatin in
nasopharyngeal carcinoma. In a mouse model of cerebral
ischaemia-reperfusion injury, DHM inhibited oxidative stress and
apoptosis in mouse hippocampal neuronal HT22 cells by activating
the Nrf2/HO-1 signalling pathway (58). Treatment of colon cancer cells with
DHM resulted in dose- and time-dependent apoptosis through the
activation of endoplasmic reticulum (ER) stress, 5'-adenosine
monophosphate-activated protein kinase (AMPK) and JNK/p38 MAPKs,
and AMPK/MAPK/XAF1 signalling initiated by DHM through the ER
stress pathway, which induces apoptosis in colon cancer cells
(59) (Fig. 2).
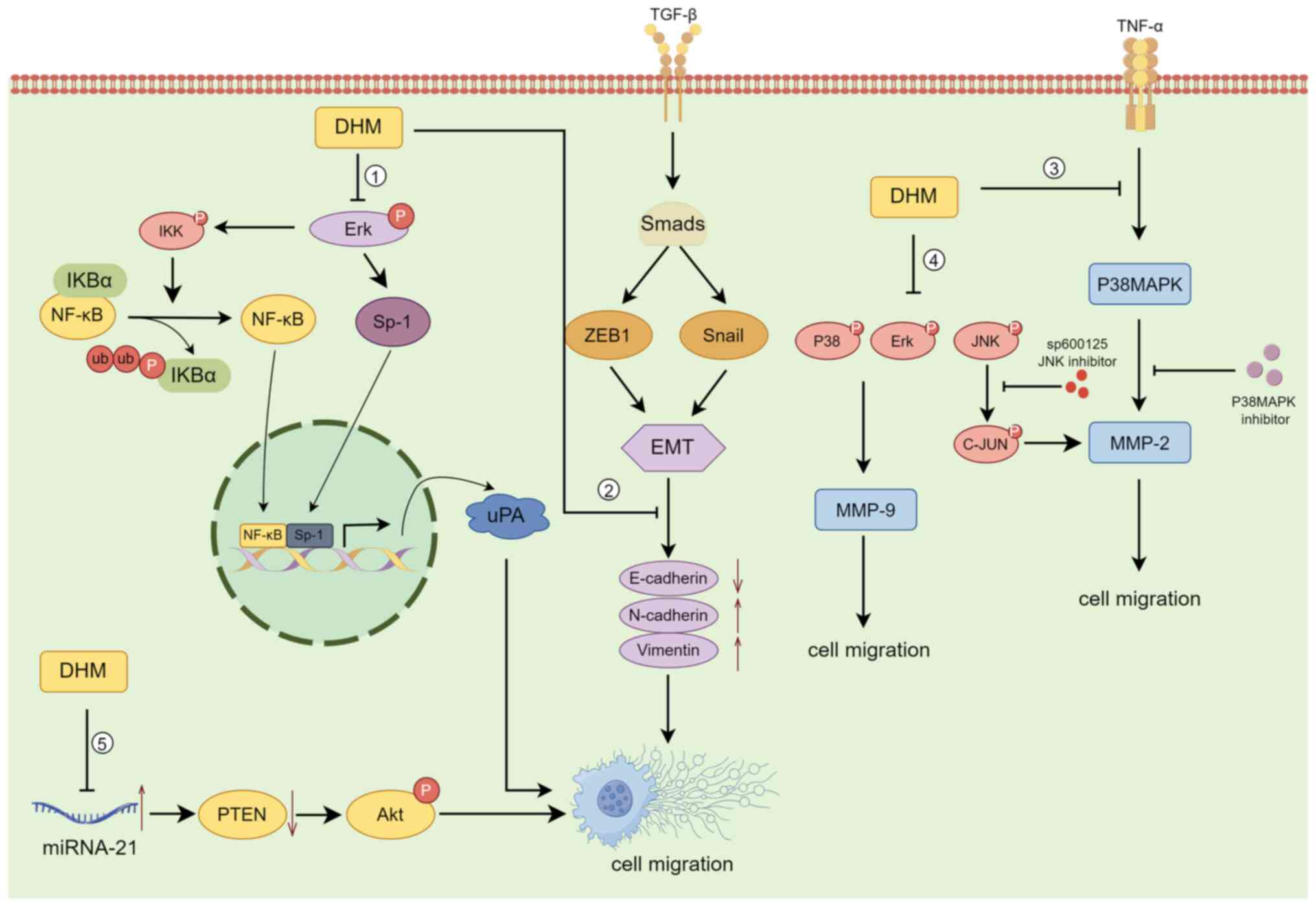
The invasion-migration cascade is a complex
biological process that includes the following major events: (i)
Cell migration and local invasion of the basement membrane, (ii)
invasion of the vasculature and/or lymphatic system, (iii) survival
in the circulation, (iv) arrest and extravasation at distant organ
sites, and (v) colonization at metastatic sites (60,61).
According to previous case reports, >80% of cancer patients
succumb to tumour invasion/metastasis, making it one of the major
causes of death in cancer patients. The decrease in 5-year survival
upon metastasis is particularly severe in patients with
osteosarcoma (62). DHM can
suppress the invasion and metastasis of osteosarcoma cells by
blocking the TNF-α/p38MAPK/MMP-2 signalling pathway (30). Moreover, Chou et al (63) reported that DHM regulates
osteosarcoma metastasis through the ERK pathway; it also inhibits
metastasis by suppressing the expression of the downstream
urokinase plasminogen activator through the inhibition of SP-1 and
NF-κB.
DHM inhibits the invasion and migration of human
retinal pigment epithelial cells (ARPE-19) by decreasing the
expression of MMP-2(64). By
contrast, Zhang et al (65)
reported that DHM significantly inhibited the migration and
invasion of SK-Hep-1 and MHCC97L hepatocellular carcinoma cells by
decreasing MMP-9 protein expression, while downregulation of MMP-9
protein expression was closely associated with increased PKC-δ
protein levels and decreased phosphorylation of p38, ERK1/2 and JNK
in SK-Hep-1 and MHCC97L cells. In the ovarian cancer cell line
A2780, DHM upregulated E-cadherin and downregulated N-cadherin and
Vimentin in the Snail signalling pathway in a concentration- and
time-dependent manner, inhibiting the nuclear translocation of
NF-κB; these results suggested that DHM inhibits
epithelial-mesenchymal transition (EMT) via the NF-κB/Snail pathway
and suppresses ovarian cancer cell invasion (66). DHM also was also demonstrated to
inhibit the migration and invasion of human gastric cancer MKN45
cells and reverse EMT through the downregulation of MMP-2
expression via the JNK signalling pathway (67). In an in vivo experiment on
mice transplanted with B16 melanoma cells, Zheng et al
(68) reported that mice given DHM
at doses of 150, 200 and 250 mg/kg exhibited a significant
reduction in the number of metastatic tumours compared with those
in the control group, demonstrating the anti-invasive and
anti-metastatic effects of DHM on B16 melanoma. Moreover, DHM
inhibits the migration and invasion of nasopharyngeal carcinoma
cells by suppressing the ERK1/2 signalling pathway and suppressing
MMP-2 expression (69); it also
inhibits the proliferation, migration and invasion of CAA HCCC9810
and TFK-1 cells by regulating miR-21 and promoting apoptosis
(70) (Fig. 3).
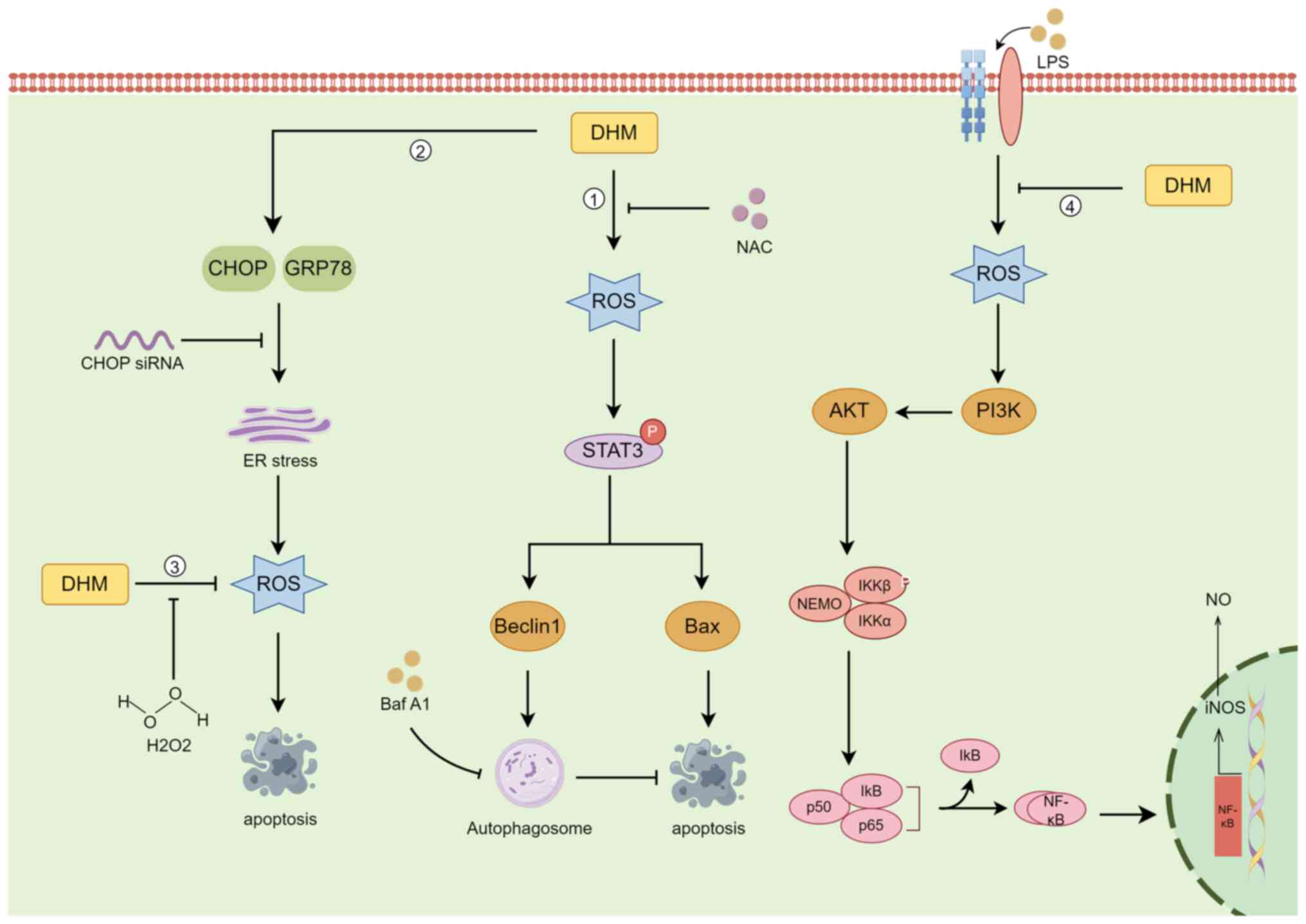
ROS are highly reactive substances containing oxygen
radicals, and ROS are produced in various biochemical reactions in
cellular organelles, such as the ER, mitochondria and peroxisomes,
as a by-product of normal oxygen metabolism (71). ROS are cytotoxic molecules that
stimulate apoptosis, but high levels of ROS can induce
tumorigenesis, leading to uncontrolled cancer cell proliferation
(72). Autophagy is an
evolutionarily conserved catabolic mechanism by which eukaryotic
cells recycle or degrade internal components through a membrane
transport pathway (73). The
process of autophagy is divided into four key steps: Initiation,
nucleation, maturation and degradation (74). In cancer, autophagy plays a
dichotomous role, that is, it inhibits tumorigenesis and supports
tumour development (75).
DHM induces autophagy in head and neck squamous cell
carcinoma (HNSCC) through the phosphorylation and activation of the
STAT3 transcription factor (31).
In HNSCC cells, the increase in ROS levels was proportional to the
increase in DHM concentration, and DHM activated p-STAT3-dependent
autophagy through ROS production in HNSCC (31). Zhou et al (76) demonstrated that in the human breast
cancer cell lines MCF-7 and MDA-MB-231, DHM had antitumour effects
through ROS production, and the ER stress pathway had antitumour
effects on breast cancer cells. However, in human melanoma cells,
autophagy has a protective role in DHM-induced apoptosis, and
pharmacological inhibition or genetic blockade of autophagy
increases DHM-induced cell death and apoptosis (77). Liu et al (78) evaluated the effects of DHM on the
induction of ROS accumulation and activation of mitochondrial
signalling pathways in human hepatocellular carcinoma HepG2 cells.
DHM reduced the accumulation of ROS in a concentration-dependent
manner, while the expression of proteins involved in the apoptotic
program increased in a concentration-dependent manner. This
suggests that ROS can act as redox signalling messengers that
regulate DHM-induced apoptosis (78). In a pharmacobiochemical study of the
effect of DHM on inflammation in a RAW264.7 macrophage model, DHM
was discovered to inhibit the accumulation of ROS, suppress the
release of NO and the proinflammatory cytokines IL-1β, IL-6 and
TNF-α, and suppress lipopolysaccharide-induced inducible nitric
oxide synthase by inhibiting nuclear factor-κB (NF-κB) activation
(79). In cutaneous squamous cell
carcinoma (CSCC), DHM induced TFEB (Ser142) dephosphorylation,
activated TFEB nuclear translocation, increased TFEB reporter
activity, decreased lncRNA MALAT1 expression, and induced CSCC cell
death by inducing excessive autophagy via the MALAT1-TFEB pathway
(80) (Fig. 4).
Ovarian cancer is one of the leading causes of
cancer-related death in gynaecologic malignancies, and resistance
to chemotherapeutic agents remains a major challenge in ovarian
cancer treatment (90). Xu et
al (90) reported that DHM
significantly increased the sensitivity of ovarian cancer cells to
paclitaxel and DOX by inhibiting the expression of survivin, a
member of the IAP family of apoptosis-inhibitory proteins. One of
the most common treatments for gastric cancer is chemotherapy, but
multidrug resistance often leads to the failure of anticancer
therapy, and the combination of DHM and mitomycin increases the
inhibitory effect of mitomycin on the proliferation of gastric
cancer cells (91).
The advantages of DHM as an antitumour drug for
combination with chemotherapy are as follows: It has fewer toxic
side effects than chemotherapy, it has multiple antitumour
mechanisms, and it has a lower risk of drug resistance than
chemotherapy alone (81-84).
Several studies confirmed that DHM was not cytotoxic to the
immortalized normal human hepatocyte line LO-2(92), the normal prostate epithelial cell
line PrEC (43), or the normal
mammary epithelial cell line MCF-10A (76). Moreover, Dong et al (93) reported that DHM pre-treatment
regulated APAP metabolism by regulating the expression of
UDP-glucuronosyltransferase 1 and cytochrome P4502E1 to ameliorate
APAP-associated hepatocyte necrosis and stimulate liver
regeneration. DHM can be used in combination with a variety of
known chemotherapeutic agents, such as DHM in combination with NDP,
to regulate the balance of Bcl-2/Bax and Bcl-2/Bak ratios through
the p53/Bcl-2 signalling pathway and inhibit NDP-induced ROS
production, thereby increasing the chemosensitivity of
hepatocellular carcinoma cells to NDP (85). DHM increases the antitumour activity
of adriamycin (ADR) and prevents ADR-induced DIC in a p53-dependent
manner by inhibiting MDM2-mediated degradation of ARC via
ubiquitination (90).
Although DHM is a promising cancer treatment, its
chemical instability and low bioavailability hinder its application
(9). The phenolic hydroxyl
structure of DHM makes it unstable (93). In particular, when DHM is exposed to
light, pH buffers, pepsin and trypsin, it undergoes various
chemical reactions, such as oxidation, hydrolysis, cleavage,
reduction and decomposition, to produce metabolites (93). Pharmacokinetic studies have also
shown that DHM is not readily absorbed into the bloodstream and is
unstable in the intestinal environment (94). Therefore, the combination of DHM
with other chemotherapeutic drugs requires consideration of
differences in the physicochemical properties, absorption sites,
pharmacokinetic behaviours, and effective doses of different drugs
(95).
The present review mainly summarized the molecular
mechanism underlying the inhibitory effect of DHM on tumours. A
total of four effects of DHM were described: It inhibits tumour
cell proliferation, promotes apoptosis, inhibits invasion and
migration, clears ROS, and induces autophagy. The effects of DHM
were also summarized in combination with several traditional
antitumour drugs, as well as the advantages and disadvantages of
DHM as an antitumour drug. Currently, there are two reported
clinical trials related to DHM: In a double-blind clinical trial,
Chen et al (96) conducted a
three-month follow-up observation on 60 adult patients with
non-alcoholic fatty liver disease and it was identified that the
serum levels of alanine, aspartate aminotransferase, γ-glutamyl
transpeptidase, glucose, low-density lipoprotein-cholesterol and
apolipoprotein B, and the homeostasis model assessment of insulin
resistance index were significantly decreased in the DHM group
compared with the placebo group, and DHM supplementation improves
glucose and lipid metabolism as well as various biochemical
parameters in patients with non-alcoholic fatty liver disease. Ran
et al (97) conducted a
follow-up experiment on 80 participants with type 2 diabetes
mellitus (T2DM) and it was revealed that compared with the placebo
group, the levels of fasting blood glucose, glycosylated albumin,
cystatin C and retinol binding protein-4 in the DHM group
significantly decreased, and DHM can effectively improve the blood
sugar control in patients with T2DM. However, there are no studies
reporting that DHM has been tested in any clinical trials for
treating cancer. The antitumour mechanisms of DHM are diverse and
not limited to these four aspects, and the antitumour effects of
DHM on different tumour cells may be the result of a combination of
mechanisms.
With advances in diagnostic and treatment
technology, the mortality rate of cancer patients is gradually
decreasing. However, innovative and more effective drugs are needed
to further suppress the progression of cancer. Natural herbal
medicines are attracting increasing attention because they offer
clear advantages in terms of research and development and medical
costs. DHM, a flavonoid extracted from the stem and leaves of
buttercups, has promising anticancer effects. In the present
review, different molecular and cellular mechanisms by which DHM
induces antitumour effects were described. DHM has been
demonstrated to be highly effective and to have low toxicity and
few side effects. The combination of DHM with other existing
anticancer drugs could increase the inhibitory effects on tumour
cells. Thus, the present review provided a comprehensive reference
for the development of DHM as an anticancer drug.
Not applicable.
Funding: No funding was received.
Not applicable.
RZ and TX designed the study. TX collected the
information and wrote the manuscript. RZ was responsible for
handling the revisions. All authors contributed to the article and
approved the submitted version. Data authentication is not
applicable. All the authors have read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Mullard A: Addressing cancer's grand
challenges. Nat Rev Drug Discov. 19:825–826. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kashyap D, Tuli HS, Yerer MB, Sharma A,
Sak K, Srivastava S, Pandey A, Garg VK, Sethi G and Bishayee A:
Natural product-based nanoformulations for cancer therapy:
Opportunities and challenges. Semin Cancer Biol. 69:5–23.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chang L, Ruiz P, Ito T and Sellers WR:
Targeting pan-essential genes in cancer: Challenges and
opportunities. Cancer Cell. 39:466–479. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shafabakhsh R and Asemi Z: Quercetin: A
natural compound for ovarian cancer treatment. J Ovarian Res.
12(55)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim C and Kim B: Anti-Cancer natural
products and their bioactive compounds inducing ER stress-mediated
apoptosis: A review. Nutrients. 10(1021)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Demain AL and Vaishnav P: Natural products
for cancer chemotherapy. Microb Biotechnol. 4:687–699.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zuo W and Kwok HF: Development of
marine-derived compounds for cancer therapy. Mar Drugs.
19(342)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun CC, Li Y, Yin ZP and Zhang QF:
Physicochemical properties of dihydromyricetin and the effects of
ascorbic acid on its stability and bioavailability. J Sci Food
Agric. 101:3862–3869. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu J, Xiao Z, Li H, Zhu N, Gu J, Wang W,
Liu C, Wang W and Qin L: Present status, challenges, and prospects
of dihydromyricetin in the battle against cancer. Cancers (Basel).
14(3487)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun Y, Liu S, Yang S, Chen C, Yang Y, Lin
M, Liu C, Wang W, Zhou X, Ai Q, et al: Mechanism of
dihydromyricetin on inflammatory diseases. Front Pharmacol.
12(794563)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheng P, Gui C, Huang J, Xia Y, Fang Y, Da
G and Zhang X: Molecular mechanisms of ampelopsin from Ampelopsis
megalophylla induces apoptosis in HeLa cells. Oncol Lett.
14:2691–2698. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen L, Shi M, Lv C, Song Y, Wu Y, Liu S,
Zheng Z, Lu X and Qin S: Dihydromyricetin acts as a potential redox
balance mediator in cancer chemoprevention. Mediators Inflamm.
2021(6692579)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun J, Wang Y, Tang W and Gong J:
Enantioselectivity of chiral dihydromyricetin in multicomponent
solid solutions regulated by subtle structural mutation. IUCrJ.
10(Pt 2):164–176. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Geng S, Yuan Y, Jiang X, Zhang R, Ma H,
Liang G and Liu B: An investigation on pickering nano-emulsions
stabilized by dihydromyricetin/high-amylose corn starch composite
particles: Preparation conditions and carrier properties. Curr Res
Food Sci. 6(100458)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lyu Q, Chen L, Lin S, Cao H and Teng H: A
designed self-microemulsion delivery system for dihydromyricetin
and its dietary intervention effect on high-fat-diet fed mice. Food
Chem. 390(132954)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ye J, Bao S, Zhao S, Zhu Y, Ren Q, Li R,
Xu X and Zhang Q: Self-Assembled micelles improve the oral
bioavailability of dihydromyricetin and anti-acute alcoholism
activity. AAPS PharmSciTech. 22(111)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu L, Li Y, Zhang M, Zhang Y and Lou B: A
Drug-Drug cocrystal of dihydromyricetin and pentoxifylline. J Pharm
Sci. 111:82–87. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun Z, Lu W, Lin N, Lin H, Zhang J, Ni T,
Meng L, Zhang C and Guo H: Dihydromyricetin alleviates
doxorubicin-induced cardiotoxicity by inhibiting NLRP3 inflammasome
through activation of SIRT1. Biochem Pharmacol.
175(113888)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wei C, Chen X, Chen D, Yu B, Zheng P, He
J, Chen H, Yan H, Luo Y and Huang Z: Dihydromyricetin enhances
intestinal antioxidant capacity of growing-finishing pigs by
activating ERK/Nrf2/HO-1 signaling pathway. Antioxidants (Basel).
11(704)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou J, Hou P, Yao Y, Yue J, Zhang Q, Yi L
and Mi M: Dihydromyricetin improves high-fat diet-induced
hyperglycemia through ILC3 Activation via a SIRT3-Dependent
Mechanism. Mol Nutr Food Res. 66(e2101093)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang ZY, Liu C, Wang PX, Han YW, Zhang
YW, Hao ML, Song ZX and Zhang XY: Dihydromyricetin Alleviates H9C2
cell apoptosis and autophagy by regulating CircHIPK3 Expression and
PI3K/AKT/mTOR pathway. Chin J Integr Med. 29:434–440.
2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li CH, Ding H, Shi JL, Huang B, Ding H,
Lin HG, Zeng JC, Zhao Y and Luo GQ: Dihydromyricetin promotes
apoptosis, suppresses proliferation and tumor necrosis
factor-α-mediated nuclear factor kappa-B activation in
nasopharyngeal carcinoma CNE-2 cell. J Tradit Chin Med. 41:367–375.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo L, Zhang H and Yan X: Protective
effect of dihydromyricetin revents fatty liver through nuclear
factor-κB/p53/B-cell lymphoma 2-associated X protein signaling
pathways in a rat model. Mol Med Rep. 19:1638–1644. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jing N and Li X: Retraction on
‘Dihydromyricetin attenuates inflammation through TLR4/NF-kappa B
pathway’. Open Med (Wars). 16(1082)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu M, Guo H, Li Z, Zhang C, Zhang X, Cui
Q and Tian J: Molecular level insight into the benefit of myricetin
and dihydromyricetin uptake in patients with Alzheimer's diseases.
Front Aging Neurosci. 12(601603)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu H, Luo P, Fu Y, Wang J, Dai J, Shao J,
Yang X, Chang L, Weng Q, Yang B and He Q: Dihydromyricetin prevents
cardiotoxicity and enhances anticancer activity induced by
adriamycin. Oncotarget. 6:3254–3267. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang Y, Wang J, Xiang H, Ding P, Wu T and
Ji G: Recent update on application of dihydromyricetin in metabolic
related diseases. Biomed Pharmacother. 148(112771)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang J, Chen Y, Luo H, Sun L, Xu M, Yu J,
Zhou Q, Meng G and Yang S: Recent update on the pharmacological
effects and mechanisms of dihydromyricetin. Front Pharmacol.
9(1204)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang H, Hu M, Zhao R, Li P and Li M:
Dihydromyricetin suppresses the proliferation of hepatocellular
carcinoma cells by inducing G2/M arrest through the
Chk1/Chk2/Cdc25C pathway. Oncol Rep. 30:2467–2475. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu C, Zhao P, Yang Y, Xu X, Wang L and Li
B: Ampelopsin suppresses TNF-α-induced migration and invasion of
U2OS osteosarcoma cells. Mol Med Rep. 13:4729–4736. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fan TF, Wu TF, Bu LL, Ma SR, Li YC, Mao L,
Sun ZJ and Zhang WF: Dihydromyricetin promotes autophagy and
apoptosis through ROS-STAT3 signaling in head and neck squamous
cell carcinoma. Oncotarget. 7:59691–59703. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Martínez-Alonso D and Malumbres M:
Mammalian cell cycle cyclins. Semin Cell Dev Biol. 107:28–35.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tokunaga Y, Otsuyama KI and Hayashida N:
Cell cycle regulation by heat shock transcription factors. Cells.
11(203)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gonzales AJ, Goldsworthy TL and Fox TR:
Chemical transformation of mouse liver cells results in altered
cyclin D-CDK protein complexes. Carcinogenesis. 19:1093–1102.
1998.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang M, Zhang L, Hei R, Li X, Cai H, Wu
X, Zheng Q and Cai C: CDK inhibitors in cancer therapy, an overview
of recent development. Am J Cancer Res. 11:1913–1935.
2021.PubMed/NCBI
|
|
36
|
Wood DJ and Endicott JA: Structural
insights into the functional diversity of the CDK-cyclin family.
Open Biol. 8(180112)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Riba A, Oravecz A, Durik M, Jiménez S,
Alunni V, Cerciat M, Jung M, Keime C, Keyes WM and Molina N: Cell
cycle gene regulation dynamics revealed by RNA velocity and
deep-learning. Nat Commun. 13(2865)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee JH and Berger JM: Cell Cycle-Dependent
control and roles of DNA Topoisomerase II. Genes (Basel).
10(859)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zeng G, Liu J, Chen H, Liu B, Zhang Q, Li
M and Zhu R: Dihydromyricetin induces cell cycle arrest and
apoptosis in melanoma SK-MEL-28 cells. Oncol Rep. 31:2713–2719.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao Z, Yin JQ, Wu MS, Song G, Xie XB, Zou
C, Tang Q, Wu Y, Lu J, Wang Y, et al: Dihydromyricetin activates
AMP-activated protein kinase and P38(MAPK) exerting antitumor
potential in osteosarcoma. Cancer Prev Res (Phila). 7:927–938.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fan KJ, Yang B, Liu Y, Tian XD and Wang B:
Inhibition of human lung cancer proliferation through targeting
stromal fibroblasts by dihydromyricetin. Mol Med Rep. 16:9758–9762.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu J, Shu Y, Zhang Q, Liu B, Xia J, Qiu
M, Miao H, Li M and Zhu R: Dihydromyricetin induces apoptosis and
inhibits proliferation in hepatocellular carcinoma cells. Oncol
Lett. 8:1645–1651. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ni F, Gong Y, Li L, Abdolmaleky HM and
Zhou JR: Flavonoid ampelopsin inhibits the growth and metastasis of
prostate cancer in vitro and in mice. PLoS One.
7(e38802)2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang S, Ge F, Cai T, Qi S and Qi Z:
Dihydromyricetin inhibits proliferation and migration of gastric
cancer cells through regulating Akt/STAT3 signaling pathways and
HMGB1 expression. Nan Fang Yi Ke Da Xue Xue Bao. 41:87–92.
2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
45
|
Li X, Yang ZS, Cai WW, Deng Y, Chen L and
Tan SL: Dihydromyricetin inhibits tumor growth and
epithelial-mesenchymal transition through regulating miR-455-3p in
Cholangiocarcinoma. J Cancer. 12:6058–6070. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lobe C, Vallette M, Arbelaiz A,
Gonzalez-Sanchez E, Izquierdo L, Pellat A, Guedj N, Louis C,
Paradis V, Banales JM, et al: Zinc Finger E-Box Binding Homeobox 1
promotes cholangiocarcinoma progression through tumor
dedifferentiation and tumor-stroma paracrine signaling. Hepatology.
74:3194–3212. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xu X, Lai Y and Hua ZC: Apoptosis and
apoptotic body: Disease message and therapeutic target potentials.
Biosci Rep. 39(BSR20180992)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Morana O, Wood W and Gregory CD: The
apoptosis paradox in cancer. Int J Mol Sci. 23(1328)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cheng X and Ferrell JE Jr: Apoptosis
propagates through the cytoplasm as trigger waves. Science.
361:607–612. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rahmani M, Nkwocha J, Hawkins E, Pei X,
Parker RE, Kmieciak M, Leverson JD, Sampath D, Ferreira-Gonzalez A
and Grant S: Cotargeting BCL-2 and PI3K Induces BAX-Dependent
mitochondrial apoptosis in AML cells. Cancer Res. 78:3075–3086.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zuo Y, Xu Q, Lu Y, Sun D, Wang K, Lei Y,
Liang X and Li Y: Dihydromyricetin induces apoptosis in a human
choriocarcinoma cell line. Oncol Lett. 16:4229–4234.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chipuk JE, McStay GP, Bharti A, Kuwana T,
Clarke CJ, Siskind LJ, Obeid LM and Green DR: Sphingolipid
metabolism cooperates with BAK and BAX to promote the mitochondrial
pathway of apoptosis. Cell. 148:988–1000. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ji FJ, Tian XF, Liu XW, Fu LB, Wu YY, Fang
XD and Jin HY: Dihydromyricetin induces cell apoptosis via a
p53-related pathway in AGS human gastric cancer cells. Genet Mol
Res. 14:15564–15571. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Han JM, Kim HL and Jung HJ: Ampelopsin
inhibits cell proliferation and induces apoptosis in HL60 and K562
leukemia cells by downregulating AKT and NF-κB signaling pathways.
Int J Mol Sci. 22(4265)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chang H, Peng X, Bai Q, Zhou Y, Yu X,
Zhang Q, Zhu J and Mi M: Ampelopsin suppresses breast
carcinogenesis by inhibiting the mTOR signalling pathway.
Carcinogenesis. 35:1847–1854. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lu CJ, He YF, Yuan WZ, Xiang LJ, Zhang J,
Liang YR, Duan J, He YH and Li MY: Dihydromyricetin-mediated
inhibition of the Notch1 pathway induces apoptosis in QGY7701 and
HepG2 hepatoma cells. World J Gastroenterol. 23:6242–6251.
2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ye L, Yin G, Jiang M, Tu B, Li Z and Wang
Y: Dihydromyricetin exhibits antitumor activity in nasopharyngeal
cancer cell through antagonizing Wnt/β-catenin signaling. Integr
Cancer Ther. 20(1534735421991217)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhang Q, Wang J, Zhang H and Zeng T:
Dihydromyricetin inhibits oxidative stress and apoptosis in oxygen
and glucose deprivation/reoxygenation-induced HT22 cells by
activating the Nrf2/HO-1 pathway. Mol Med Rep.
23(397)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Park GB, Jeong JY and Kim D:
Ampelopsin-induced reactive oxygen species enhance the apoptosis of
colon cancer cells by activating endoplasmic reticulum
stress-mediated AMPK/MAPK/XAF1 signaling. Oncol Lett. 14:7947–7956.
2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Trepat X, Chen Z and Jacobson K: Cell
migration. Compr Physiol. 2:2369–2392. 2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zanotelli MR, Zhang J and Reinhart-King
CA: Mechanoresponsive metabolism in cancer cell migration and
metastasis. Cell Metab. 33:1307–1321. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chen C, Xie L, Ren T, Huang Y, Xu J and
Guo W: Immunotherapy for osteosarcoma: Fundamental mechanism,
rationale, and recent breakthroughs. Cancer Lett. 500:1–10.
2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chou CH, Lu KH, Yang JS, Hsieh YH, Lin CW
and Yang SF: Dihydromyricetin suppresses cell metastasis in human
osteosarcoma through SP-1- and NF-κB-modulated urokinase
plasminogen activator inhibition. Phytomedicine.
90(153642)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wang K, Yang SF, Hsieh YH, Chang YY, Yu
NY, Lin HW and Lin HY: Effects of dihydromyricetin on ARPE-19 cell
migration through regulating matrix metalloproteinase-2 expression.
Environ Toxicol. 33:1298–1303. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhang QY, Li R, Zeng GF, Liu B, Liu J, Shu
Y, Liu ZK, Qiu ZD, Wang DJ, Miao HL, et al: Dihydromyricetin
inhibits migration and invasion of hepatoma cells through
regulation of MMP-9 expression. World J Gastroenterol.
20:10082–10093. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Liu T, Liu P, Ding F, Yu N, Li S, Wang S,
Zhang X, Sun X, Chen Y, Wang F, et al: Ampelopsin reduces the
migration and invasion of ovarian cancer cells via inhibition of
epithelial-to-mesenchymal transition. Oncol Rep. 33:861–867.
2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wang FJ, Zong XY, DU JL, Wang WS, Yuan DP
and Chen XB: [Effects of dihydromyricetin on the migration and
invasion of human gastric cancer MKN45 cells and its mechanism].
Zhongguo Ying Yong Sheng Li Xue Za Zhi. 35:428–432. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
68
|
Zheng HQ and Liu DY: Anti-invasive and
anti-metastatic effect of ampelopsin on melanoma. Ai Zheng.
22:363–367. 2003.PubMed/NCBIIn Chinese.
|
|
69
|
Huang CC, Su CW, Wang PH, Lu YT, Ho YT,
Yang SF, Hsin CH and Lin CW: Dihydromyricetin inhibits cancer cell
migration and matrix metalloproteinases-2 expression in human
nasopharyngeal carcinoma through extracellular signal-regulated
kinase signaling pathway. Environ Toxicol. 37:1244–1253.
2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chen L, Yang ZS, Zhou YZ, Deng Y, Jiang P
and Tan SL: Dihydromyricetin inhibits cell proliferation,
migration, invasion and promotes apoptosis via regulating miR-21 in
human cholangiocarcinoma cells. J Cancer. 11:5689–5699.
2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Sahoo BM, Banik BK, Borah P and Jain A:
Reactive oxygen species (ROS): Key components in cancer therapies.
Anticancer Agents Med Chem. 22:215–222. 2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Villalpando-Rodriguez GE and Gibson SB:
Reactive oxygen species (ROS) Regulates different types of cell
death by acting as a rheostat. Oxid Med Cell Longev.
2021(9912436)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kocaturk NM, Akkoc Y, Kig C, Bayraktar O,
Gozuacik D and Kutlu O: Autophagy as a molecular target for cancer
treatment. Eur J Pharm Sci. 134:116–137. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Feng Y, He D, Yao Z and Klionsky DJ: The
machinery of macroautophagy. Cell Res. 24:24–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Onorati AV, Dyczynski M, Ojha R and
Amaravadi RK: Targeting autophagy in cancer. Cancer. 124:3307–3318.
2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhou Y, Shu F, Liang X, Chang H, Shi L,
Peng X, Zhu J and Mi M: Ampelopsin induces cell growth inhibition
and apoptosis in breast cancer cells through ROS generation and
endoplasmic reticulum stress pathway. PLoS One.
9(e89021)2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zhou DZ, Sun HY, Yue JQ, Peng Y, Chen YM
and Zhong ZJ: Dihydromyricetin induces apoptosis and cytoprotective
autophagy through ROS-NF-κB signalling in human melanoma cells.
Free Radic Res. 51:517–528. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Liu B, Tan X, Liang J, Wu S, Liu J, Zhang
Q and Zhu R: A reduction in reactive oxygen species contributes to
dihydromyricetin-induced apoptosis in human hepatocellular
carcinoma cells. Sci Rep. 4(7041)2014.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Qi S, Xin Y, Guo Y, Diao Y, Kou X, Luo L
and Yin Z: Ampelopsin reduces endotoxic inflammation via repressing
ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int
Immunopharmacol. 12:278–287. 2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Tan M, Jiang B, Wang H, Ouyang W, Chen X,
Wang T, Dong D, Yi S, Yi J, Huang Y, et al: Dihydromyricetin
induced lncRNA MALAT1-TFEB-dependent autophagic cell death in
cutaneous squamous cell carcinoma. J Cancer. 10:4245–4255.
2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Tuli HS, Mistry H, Kaur G, Aggarwal D,
Garg VK, Mittal S, Yerer MB, Sak K and Khan MA: Gallic Acid: A
dietary polyphenol that exhibits anti-neoplastic activities by
modulating multiple oncogenic targets. Anticancer Agents Med Chem.
22:499–514. 2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Tuli HS, Kashyap D, Sharma AK and Sandhu
SS: Molecular aspects of melatonin (MLT)-mediated therapeutic
effects. Life Sci. 135:147–157. 2015.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Manu KA, Shanmugam MK, Ramachandran L, Li
F, Siveen KS, Chinnathambi A, Zayed ME, Alharbi SA, Arfuso F, Kumar
AP, et al: Isorhamnetin augments the anti-tumor effect of
capecitabine through the negative regulation of NF-κB signaling
cascade in gastric cancer. Cancer Lett. 363:28–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Manu KA, Shanmugam MK, Li F, Chen L,
Siveen KS, Ahn KS, Kumar AP and Sethi G: Simvastatin sensitizes
human gastric cancer xenograft in nude mice to capecitabine by
suppressing nuclear factor-kappa B-regulated gene products. J Mol
Med (Berl). 92:267–276. 2014.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Jiang L, Zhang Q, Ren H, Ma S, Lu C, Liu
B, Liu J, Liang J, Li M and Zhu R: Dihydromyricetin enhances the
chemo-sensitivity of nedaplatin via regulation of the p53/Bcl-2
pathway in hepatocellular carcinoma cells. PLoS One.
10(e0124994)2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
He MH, Zhang Q, Shu G, Lin JC, Zhao L,
Liang XX, Yin L, Shi F, Fu HL and Yuan ZX: Dihydromyricetin
sensitizes human acute myeloid leukemia cells to retinoic
acid-induced myeloid differentiation by activating STAT1. Biochem
Biophys Res Commun. 495:1702–1707. 2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wang Z, Sun X, Feng Y, Liu X, Zhou L, Sui
H, Ji Q, E Q, Chen J, Wu L and Li Q: Dihydromyricetin reverses
MRP2-mediated MDR and enhances anticancer activity induced by
oxaliplatin in colorectal cancer cells. Anticancer Drugs.
28:281–288. 2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wang Z, Sun X, Feng Y, Wang Y, Zhang L,
Wang Y, Fang Z, Azami NLB, Sun M and Li Q: Dihydromyricetin
reverses MRP2-induced multidrug resistance by preventing NF-κB-Nrf2
signaling in colorectal cancer cell. Phytomedicine.
82(153414)2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Zhu XH, Lang HD, Wang XL, Hui SC, Zhou M,
Kang C, Yi L, Mi MT and Zhang Y: Synergy between dihydromyricetin
intervention and irinotecan chemotherapy delays the progression of
colon cancer in mouse models. Food Funct. 10:2040–2049.
2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Xu Y, Wang S, Chan HF, Lu H, Lin Z, He C
and Chen M: Dihydromyricetin induces apoptosis and reverses drug
resistance in ovarian cancer cells by p53-mediated Downregulation
of Survivin. Sci Rep. 7(46060)2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Wu M, Jiang M, Dong T, Xu L, Lv J, Xue M
and Huang M: Reversal effect of dihydromyricetin on multiple drug
resistance in SGC7901/5-FU cells. Asian Pac J Cancer Prev.
21:1269–1274. 2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Dong S, Ji J, Hu L and Wang H:
Dihydromyricetin alleviates acetaminophen-induced liver injury via
the regulation of transformation, lipid homeostasis, cell death and
regeneration. Life Sci. 227:20–29. 2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Xiang D, Wang CG, Wang WQ, Shi CY, Xiong
W, Wang MD and Fang JG: Gastrointestinal stability of
dihydromyricetin, myricetin, and myricitrin: An in vitro
investigation. Int J Food Sci Nutr. 68:704–711. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Tong Q, Hou X, Fang J, Wang W, Xiong W,
Liu X, Xie X and Shi C: Determination of dihydromyricetin in rat
plasma by LC-MS/MS and its application to a pharmacokinetic study.
J Pharm Biomed Anal. 114:455–461. 2015.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zhang R, Zhang H, Shi H, Zhang D, Zhang Z
and Liu H: Strategic developments in the drug delivery of natural
product dihydromyricetin: Applications, prospects, and challenges.
Drug Deliv. 29:3052–3070. 2022.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Chen S, Zhao X, Wan J, Ran L, Qin Y, Wang
X, Gao Y, Shu F, Zhang Y, Liu P, et al: Dihydromyricetin improves
glucose and lipid metabolism and exerts anti-inflammatory effects
in nonalcoholic fatty liver disease: A randomized controlled trial.
Pharmacol Res. 99:74–81. 2015.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Ran L, Wang X, Lang H, Xu J, Wang J, Liu
H, Mi M and Qin Y: Ampelopsis grossedentata supplementation
effectively ameliorates the glycemic control in patients with type
2 diabetes mellitus. Eur J Clin Nutr. 73:776–782. 2019.PubMed/NCBI View Article : Google Scholar
|