Introduction
Atopic dermatitis (AD) is a chronic, recurrent
inflammatory skin disease with pruritus and eczema as its primary
symptoms, and the etiology is associated with genetic
predisposition, environmental factors, immune abnormalities and
comorbidities (1). Itching is
typically the most serious symptom of AD (2,3) and
can worsen skin lesions by disrupting the skin barrier and
exacerbating discomfort (4). In
developed countries, especially those in Europe, AD affects nearly
20% of the population and is the most common type of inflammatory
skin disease. By contrast, this figure is higher in low-income
countries, such as those in Africa, Oceania and the Asian Pacific
(28-34% (5,6). Due to ineffective local treatment,
patients with moderate-to-severe AD frequently interrupt or abandon
treatment and develop other systemic diseases, such as immune
abnormalities, infection, osteoporosis and cardiovascular
dysfunction (7-9).
Therefore, the quality of life of patients with moderate-to-severe
AD is greatly reduced.
AD management aims to decrease symptoms and severity
and improve long-term disease control (10). Classical therapies for AD include
emollients, topical corticosteroids and calcineurin inhibitors and
phototherapy, which have been the primary treatment options for
decades (11). Abrocitinib is a
once-daily oral Janus kinase 1 (JAK1) inhibitor for long-term
treatment of patients with moderate-to-severe AD (12) that rapidly relieves itching symptoms
(13-17)
and was first approved by the UK Medicines and Healthcare Products
Regulatory Agency in September 2021. Due to good compliance, it was
subsequently approved in Japan, South Korea, the European Union and
the United States (18-20).
On April 11, 2022, the China Food and Drug Administration approved
abrocitinib for marketing in China for adult patients with
refractory, moderate-to-severe AD who do not respond well to other
systemic therapies, such as hormones or biological agents (21,22).
JAK is an intracellular enzyme that mediates signal
transduction generated by the interaction of cytokines with growth
factor receptors on the cell membrane, thereby regulating cell
hematopoietic function and immune cell function (23). Abrocitinib reversibly and
selectively inhibits JAK1 by blocking ATP binding sites. This drug
therefore provides a novel treatment option for patients with AD
(24). However, due to differences
between abrocitinib dosage forms and specifications, studies on
follow-up, follow-up and post-marketing reevaluation of abrocitinib
after long-term use are still preliminary. Information on the
safety comes from the randomized controlled trials (RCTs), with no
post-marketing pharmacovigilance study data available yet (25). There remains a lack of
decision-making modalities for guiding the optimal treatment
strategy for patients, resulting in large differences in the
benefit:risks ratio and in the evaluation results from drug
economics studies (26-28).
Therefore, the present study aimed to establish a
stringent literature quality screening and meta-analysis method for
systematically evaluating the efficacy and safety of 100 and 200 mg
abrocitinib for the treatment of moderate-to-severe AD, with
placebo groups also being compared. The aim was to provide an
evidence-based reference for the formulation of 100 and 200 mg
standard abrocitinib clinical application program and
pharmacoeconomic decision-making process.
Materials and methods
Inclusion criteria
According to the systematic review plan, inclusion
criteria were determined in strict accordance with the patients,
interventions, comparisons, outcomes and study design principles
(29). Inclusion criteria were as
follows: i) Patients with clinical diagnosis of moderate-to-severe
AD (30); ii) patients aged ≥18
years; iii) test group received 100 and/or 200 mg abrocitinib
(orally, once daily), whilst the control group received placebo;
iv) investigator's global assessment (IGA) score was used as the
efficacy indicator (31); v) 50, 75
and 90% response rates of eczema area and severity index (EASI)
(32,33) and the pruritus numerical rating
scale (P-NRS) were used (31); vi)
adverse reaction symptoms with an event rate of >2 were used as
the evaluation indices for safety; vii) double-blind RCT and viii)
written in English or Chinese. Example of adverse events include
gastrointestinal dysfunction, nausea, infection and infestation,
upper respiratory tract infection, upper respiratory tract viral
infection, dizziness, headache, skin and subcutaneous disease,
elevated creatine phosphokinase, thrombocytopenia and serious
adverse events (such as asthma, exacerbation of dermatitis,
malignant melanoma and pulmonary embolism).
Exclusion criteria
Exclusion criteria were as follows: i) unable to
provide valid data for analysis; ii) inappropriate statistical
methods; iii) cohort study; iv) review and clinical reviews of the
literature.
Search strategy
Pubmed (pubmed.ncbi.nlm.nih.gov/), Ovid Technologies;
ovidsp.ovid.com/), E.B.Stephens Company
(embase.com), China National Knowledge Infrastructure
(cnki.net/), Wanfang Medical network (wanfangdata.com.cn/), Web of Science (webofscience.com) and relevant clinical trial
registries, such as China Clinical Trial Registry (chictr.org.cn/), International Clinical Trial
Registration Platform (trialsearch.who.int/), Hong Kong Clinical Trials
Registry (ccrb.cuhk.edu.hk/web/) and North American Clinical
Trial Data Center (clinicaltrials.gov/) were searched for clinical RCTs
of abrocitinib compared with placebo in the treatment of
moderate-to-severe AD. The retrieval period set was between the
establishment of the database and November 2023 and the
meta-analysis was performed according to the PRISMA guidelines
(34). Default database expansion
retrieval was performed supplemented with manual retrieval. The
following terms were searched: ‘atopic dermatitis’ AND ‘moderate to
severe AD’ AND ‘abrocitinib’ OR ‘PF-04965842’ OR ‘Cibinqo’ AND
‘placebo’ AND ‘100 mg’ OR (AND) ‘200 mg’ AND ‘parallel control’ OR
‘cross-comparison’ AND ‘randomized clinical trial’ OR‘ RCT’.
Literature screening and data
extraction
To minimize selection bias, two researchers (XX and
JZ) independently reviewed the literature according to the
inclusion and exclusion criteria, screened the included literature
and extracted the data. If there was any discrepancy, a third
researcher (FH) analyzed and resolved it. The quality priority
principle was adopted for the inclusion of multiple literature with
the same data (35).
Literature quality evaluation
The RCT bias risk assessment tool in Cochrane
Handbook of Systematic Reviews 5.1.0 was adopted (36,37).
Specifically, a three-level risk assessment (low, unclear and high
bias risk) was conducted in the included literature, including
randomization, degree of blinded implementation, assignment
concealment (unblinded), outcome data integrity (such as exit/loss
rate) and selective reporting bias risk. Low bias risk indicates
high reliability of the literature data.
Statistical analysis
Meta-analysis of each effect indicator was performed
using RevMan 5.3 software recommended by the Cochrane Handbook of
Systematic Reviews (38,39). Odds ratio (OR), risk ratio (RR) and
95%CI were used as efficacy and safety statistical effect sizes. OR
and 95%CI were used as effect values for efficacy indicators and RR
and 95%CI for safety indicators. Q-test was used to evaluate the
heterogeneity of the literature. Studies with I2≤50%
were considered to be homogenous, whereby all studies could be
combined for meta-analysis using the fixed-effects model (FEM) with
Mantel-Haenszel (M-H) test. Otherwise, the random-effects model
(REM) was used for meta-analysis. Sensitivity analysis was used to
verify the results of systematic evaluation. Funnel plot was used
to evaluate risk of publication bias of associated indicators.
P<0.05 was considered to indicate a statistically significant
difference.
Results
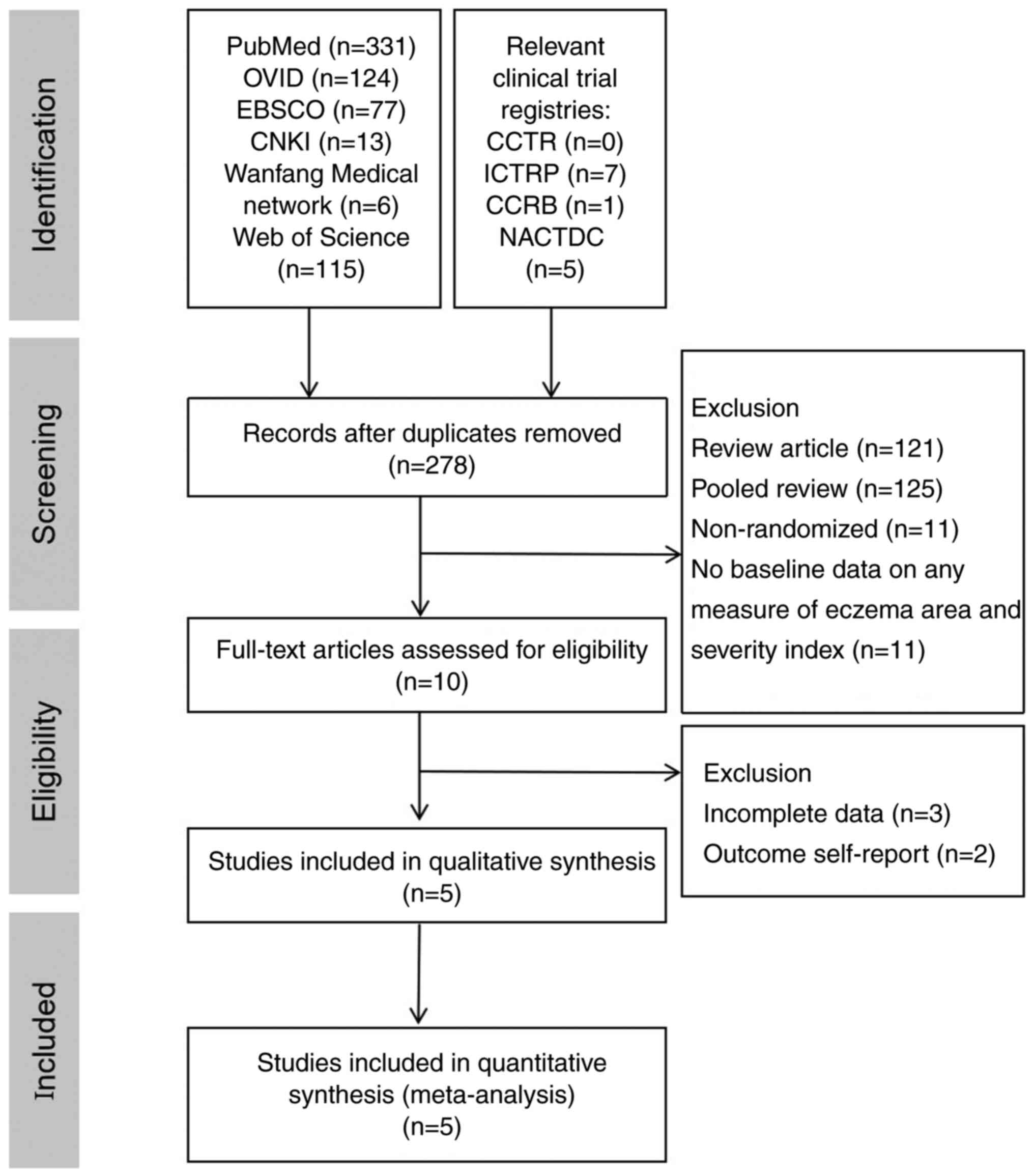
Literature search results
According to the search strategy, 679 publications
were obtained during the preliminary search. Of these, 401 were
excluded based on duplicate studies, 246 were excluded after
reading the title and abstract, 241 were excluded after careful
reading of the full text according to the exclusion criteria. In
total, five English publications were included for meta-analysis
(40-44)
(Fig. 1).
Literature features. Basic
information
The basic data of studies were complete, where the
baseline levels of age, sex, duration of disease, blinded course of
treatment and EASI score were balanced, with no statistical
significance, indicating comparability (Table I).
 | Table IBasic information of the enrolled
patients. |
Table I
Basic information of the enrolled
patients.
| | Mean IGA | |
|---|
| First author,
year | Interventions | Patients | Mean age,
years | Sex F/M | Mean duration of
illness, years | Trial duration,
weeks | Mean EASI
score | Moderate | Severe | SCORAD Index
score | NRS | (Refs.) |
|---|
| Bieber et
al, 2021 | Abr 100 mg | 238 | 37.3±14.8 | 118/120 | 22.7±16.3 | 12 | 30.3±13.5 | 153.0±64.3 | 85.0±35.7 | 66.8±13.8 | 7.1±1.7 | (40) |
| | Abr 200 mg | 226 | 38.8±14.5 | 112/114 | 23.4±15.6 | | 32.1±13.1 | 138.0±61.1 | 88.0±38.9 | 69.3±12.7 | 7.6±1.5 | |
| | placebo | 131 | 37.4±15.2 | 54/77 | 21.4±14.4 | | 31.0±12.6 | 88.0±67.2 | 43.0±32.8 | 67.9±12.0 | 7.1±1.8 | |
| Eichenfield et
al, 2021 | Abr 100 mg | 95 | 16.0±7.8 | 50/45 | 9.8±5.4 | 12 | 31.0±12.8 | 57.0±60.0 | 38.0±40.0 | 67.6±13.5 | 7.0±1.8 | (41) |
| | Abr 200 mg | 94 | 15.0±6.4 | 38/56 | 9.7±5.3 | | 29.5±12.2 | 61.0±64.9 | 33.0±35.1 | 66.2±13.3 | 6.8±2.0 | |
| | placebo | 96 | 14.0±9.3 | 52/44 | 10.5±4.8 | | 29.2±12.7 | 57.0±59.4 | 39.0±40.6 | 68.5±13.4 | 7.2±1.7 | |
| Gooderham et
al, 2019 | Abr 100 mg | 56 | 41.1±15.6 | 31/25 | 23.8±14.3 | 12 | 26.7±11.8 | 29.0±52.7 | 26.0±47.3 | 65.4±13.7 | 7.4±2.2 | (42) |
| | Abr 200 mg | 55 | 38.7±17.6 | 28/27 | 19.6±12.8 | | 24.6±13.5 | 34.0±63.0 | 20.0±37.0 | 62.7±13.7 | 6.9±2.7 | |
| | placebo | 56 | 42.6±15.1 | 21/35 | 25.6±15.2 | | 25.4±12.9 | 34.0±61.8 | 21.0±38.2 | 65.0±12.1 | 7.6±1.8 | |
| Silverberg et
al, 2020 | Abr 100 mg | 158 | 37.4±15.8 | 94/64 | 21.1±14.8 | 12 | 28.4±11.2 | 107±67.7 | 51±32.3 | 63.8±11.4 | 7.1±1.6 | (43) |
| | Abr 200 mg | 155 | 33.5±14.7 | 88/67 | 20.5±14.8 | | 29.0±12.4 | 106.0±68.4 | 49.0±31.6 | 64.1±13.1 | 7.0±1.6 | |
| | placebo | 78 | 33.4±13.8 | 47/31 | 21.7±14.3 | | 28.0±10.2 | 52.0±66.7 | 26.0±33.3 | 64.3±12.4 | 6.7±1.9 | |
| Simpson et
al, 2020 | Abr 100 mg | 156 | 32.6±15.4 | 66/90 | 24.9±16.1 | 12 | 31.3±13.6 | 92.0±58.7 | 64.0±41.2 | 67.1±13.7 | 6.9±2.0 | (44) |
| | Abr 200 mg | 154 | 33.0±17.4 | 73/81 | 22.7±14.5 | | 30.6±14.1 | 91.0±58.5 | 63.0±41.3 | 64.3±13.1 | 7.1±1.9 | |
| | placebo | 77 | 31.5±14.4 | 28/49 | 22.5±14.4 | | 28.7±12.5 | 46.0±59.6 | 31.0±40.2 | 64.5±13.2 | 7.0±1.8 | |
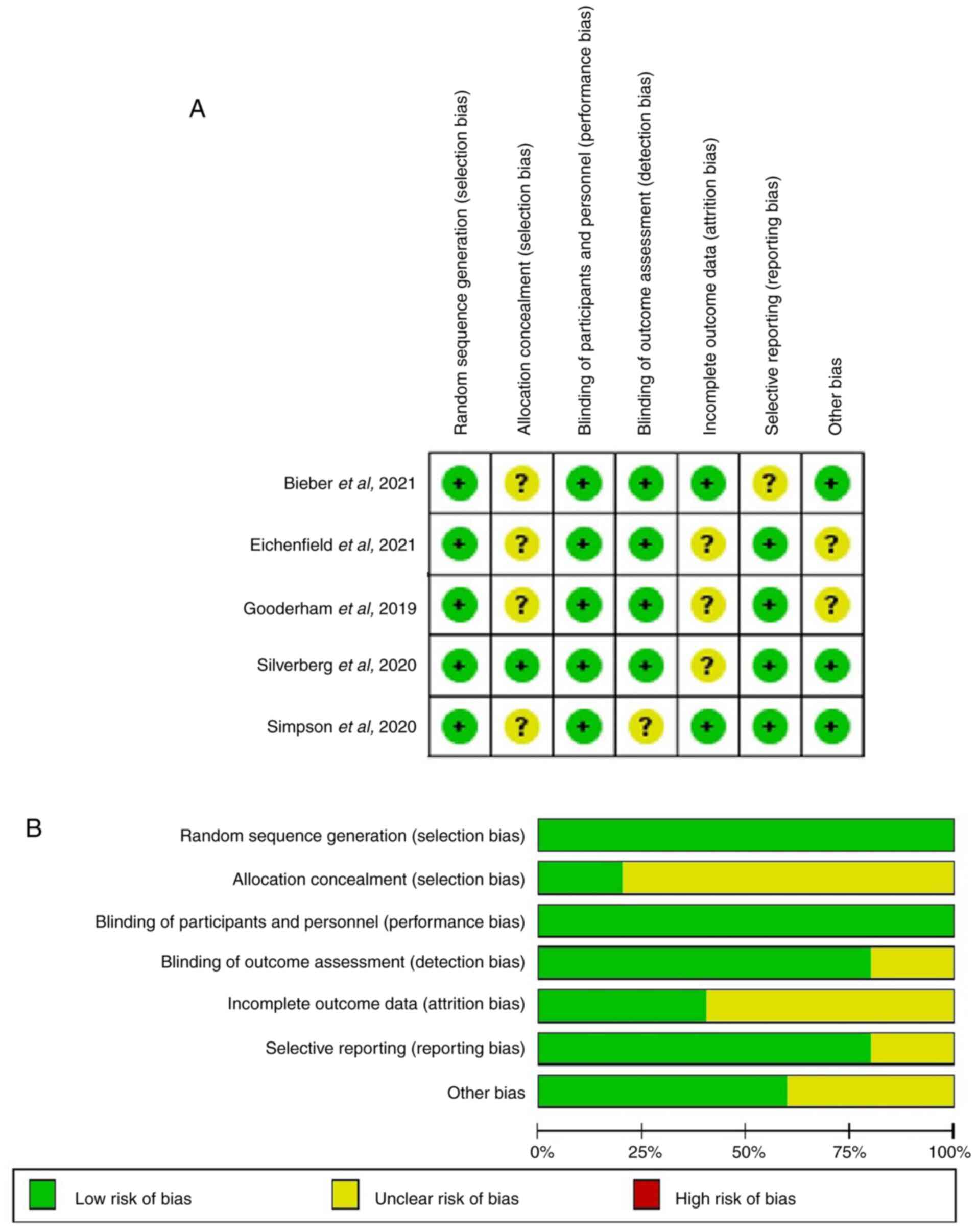
Methodological quality
characteristics
In the five RCTs (40-44)
evaluated for each item in the Cochrane Handbook of Systematic
Reviews 5.1.0, no item with high bias risk was found (Fig. 2).
Groups compared
Because there were two doses of abrocitinib compared
with placebo, 100 and 200 mg, subgroup analysis was used for the
analysis of both efficacy and safety indicators.
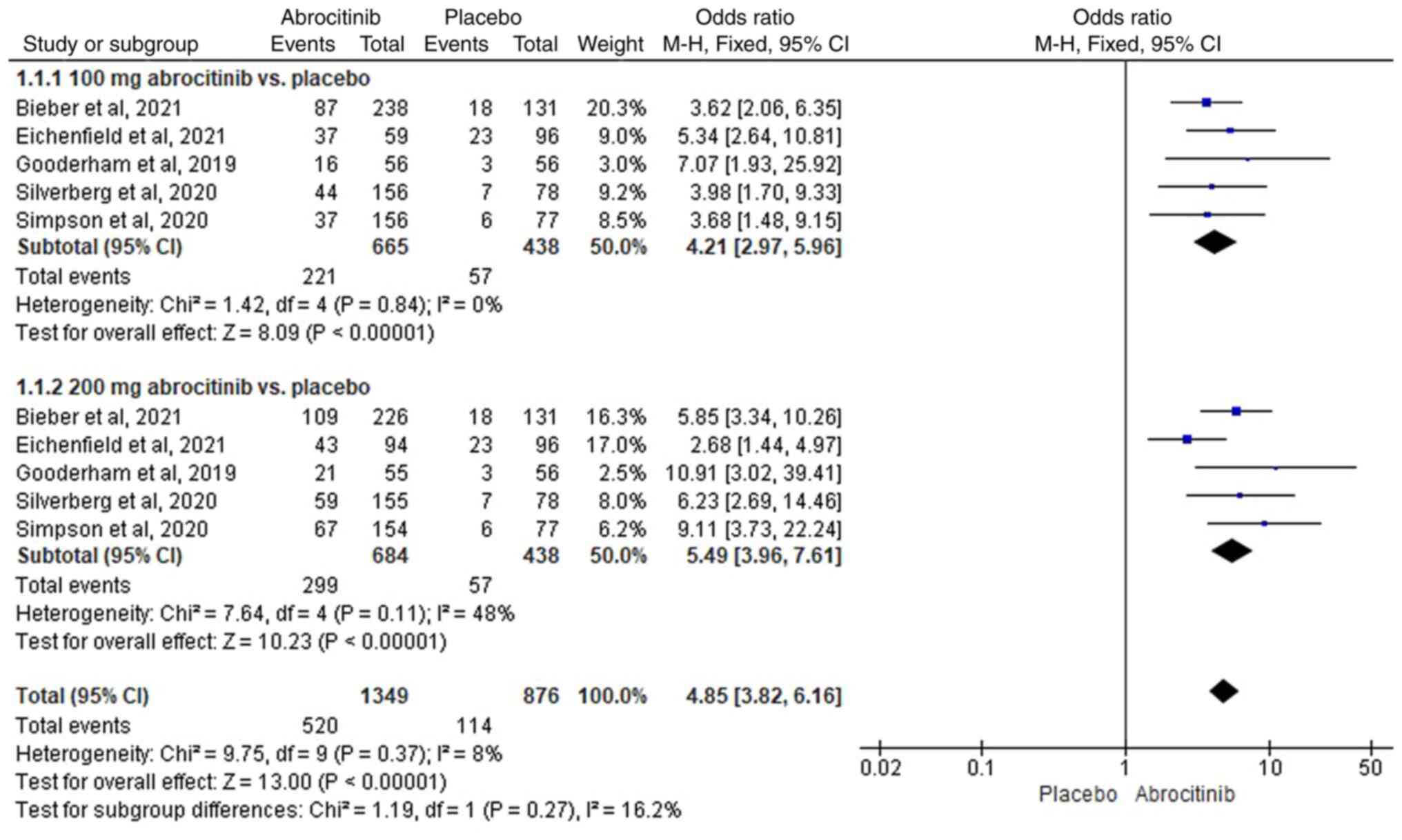
Evaluation of efficacy. IGA
improvements
For the five RCTs (40-44)
were included, results from the heterogeneity test
(I2<50%) indicated homogeneity. FEM analysis revealed
that, compared with placebo, 100 (M-H OR, 4.21; 95% CI, 2.97-5.96)
and 200 mg (M-H OR, 5.49; 95% CI, 3.96-7.61) significantly improved
the IGA score (Fig. 3). FEM
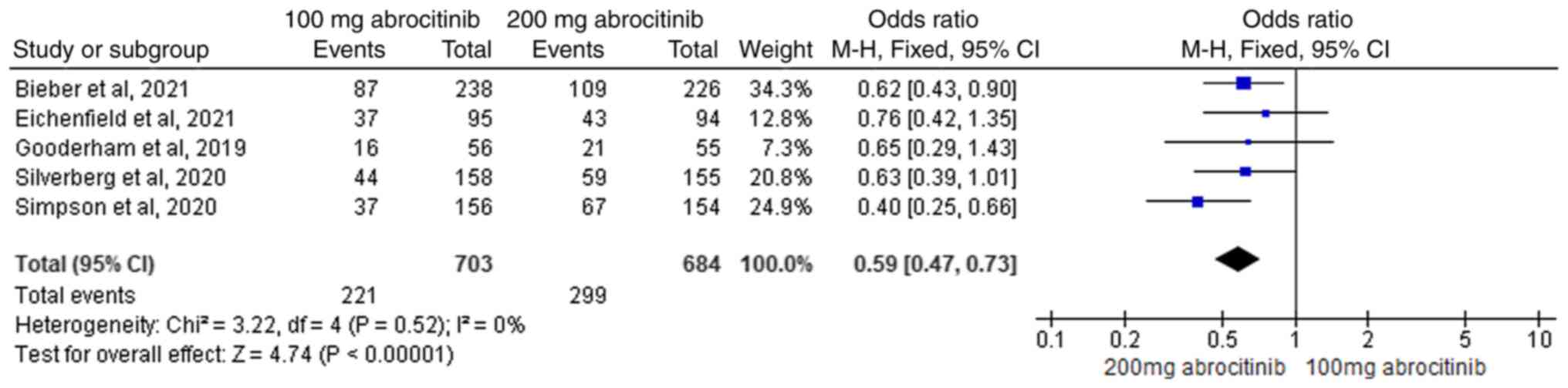
analysis showed that, the difference between 100 and 200 mg was
significant, with the 200 mg abrocitinib group yielding superior
improvements compared with the 100 mg group (M-H OR, 0.59; 95% CI,
0.47-0.73), This suggests that 200 mg dosage was superior at
improving the IGA (Fig. 4).
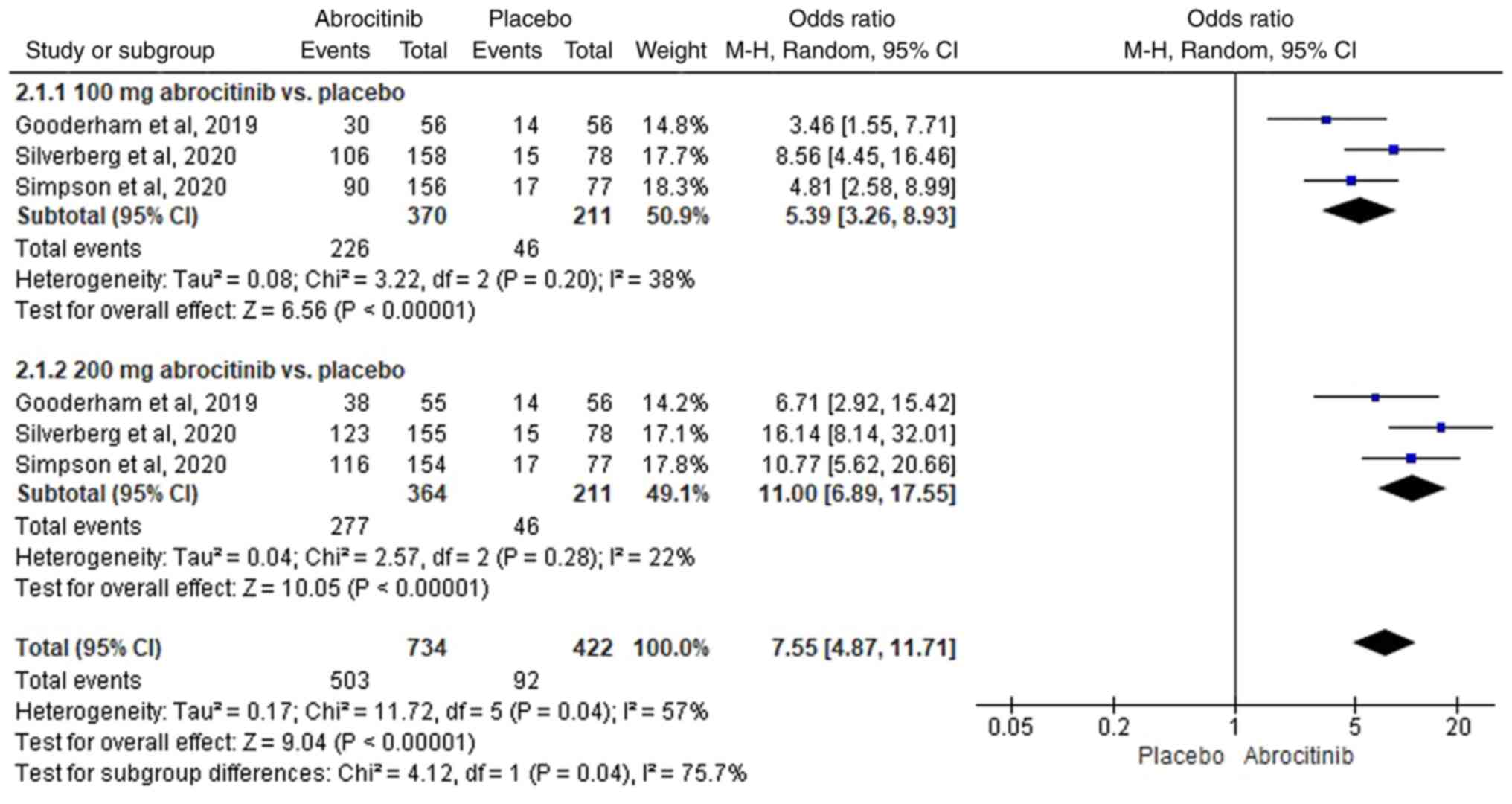
EASI-50 response rate
In total, three RCTs (42-44)
were included. Heterogeneity was detected (I2>50%),
therefore REM analysis was used. Compared with those in the placebo
group, 100 (M-H OR, 5.39; 95% CI, 3.26-8.93) and 200 mg (M-H OR,
11.00; 95%CI, 6.89-17.55) abrocitinib significantly improved
EASI-50 response rate (Fig. 5). For
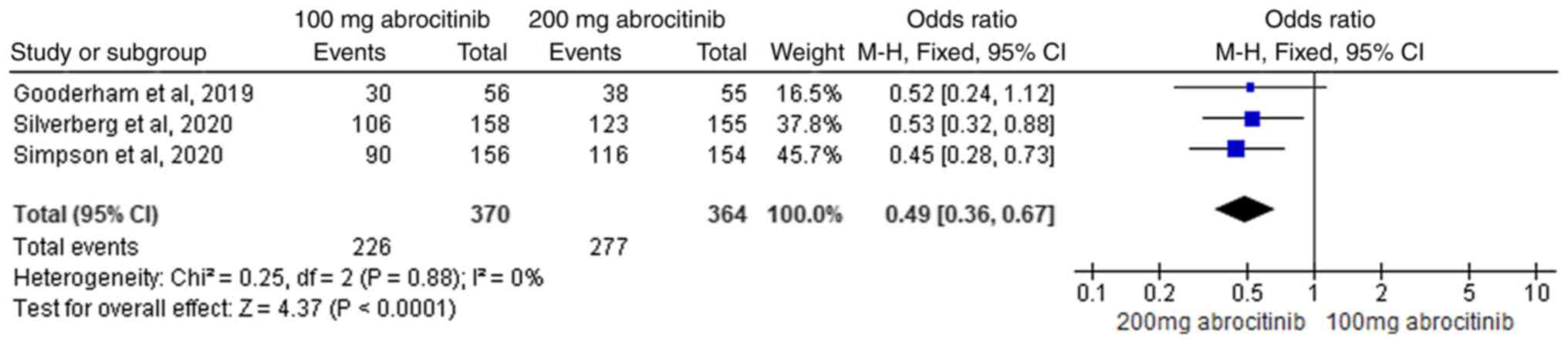
analysis of 100 and 200 mg abrocitinib, the heterogeneity test
(I2=0%) suggested homogeneity. FEM analysis showed the
difference between the two groups was statistically significant,
where 200 mg abrocitinib delivered greater EASI-50 response rate
improvement compared with 100 mg group (M-H OR, 0.49; 95% CI,
0.36-0.67; Fig. 6).
EASI-75 response rate
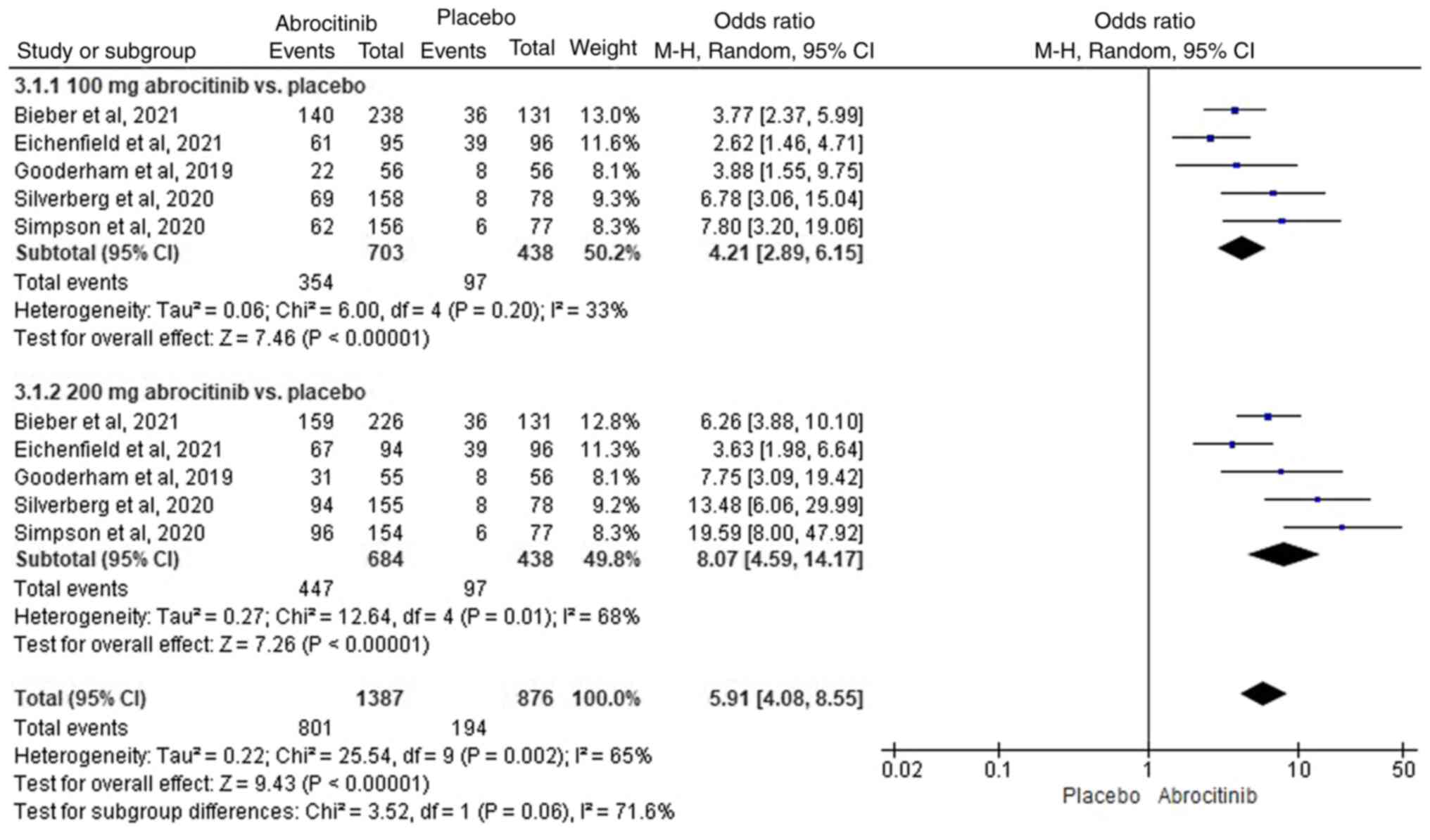
In total, five RCTs (40-44)
were included, where heterogeneity was detected
(I2>50%). Compared with those in the placebo group,
100 (M-H OR, 4.21; 95% CI, 2.89-6.15) and 200 mg (M-H OR, 8.07; 95%
CI, 4.59-14.17) abrocitinib significantly improved the EASI-75
response rate (Fig. 7). A total of
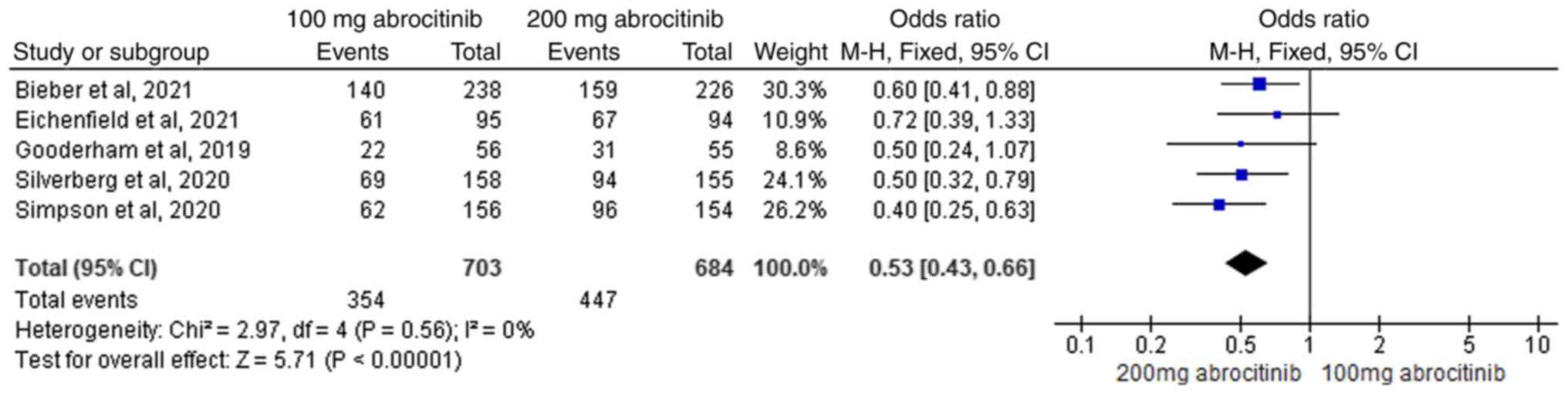
five RCTs (40-44)
were included in comparative analysis of 100 and 200 mg
abrocitinib, where the heterogeneity test (I2=0%)
suggested homogeneity. FEM analysis showed 200 mg abrocitinib
yielded significantly greater improvement compared with 100 mg (M-H
OR, 0.53; 95% CI, 0.43-0.66; Fig.
8).
EASI-90 response rate
In total, three RCTs (42-44)
were included, where heterogeneity test (I2<50%)
suggested homogeneity. FEM analysis showed that compared with
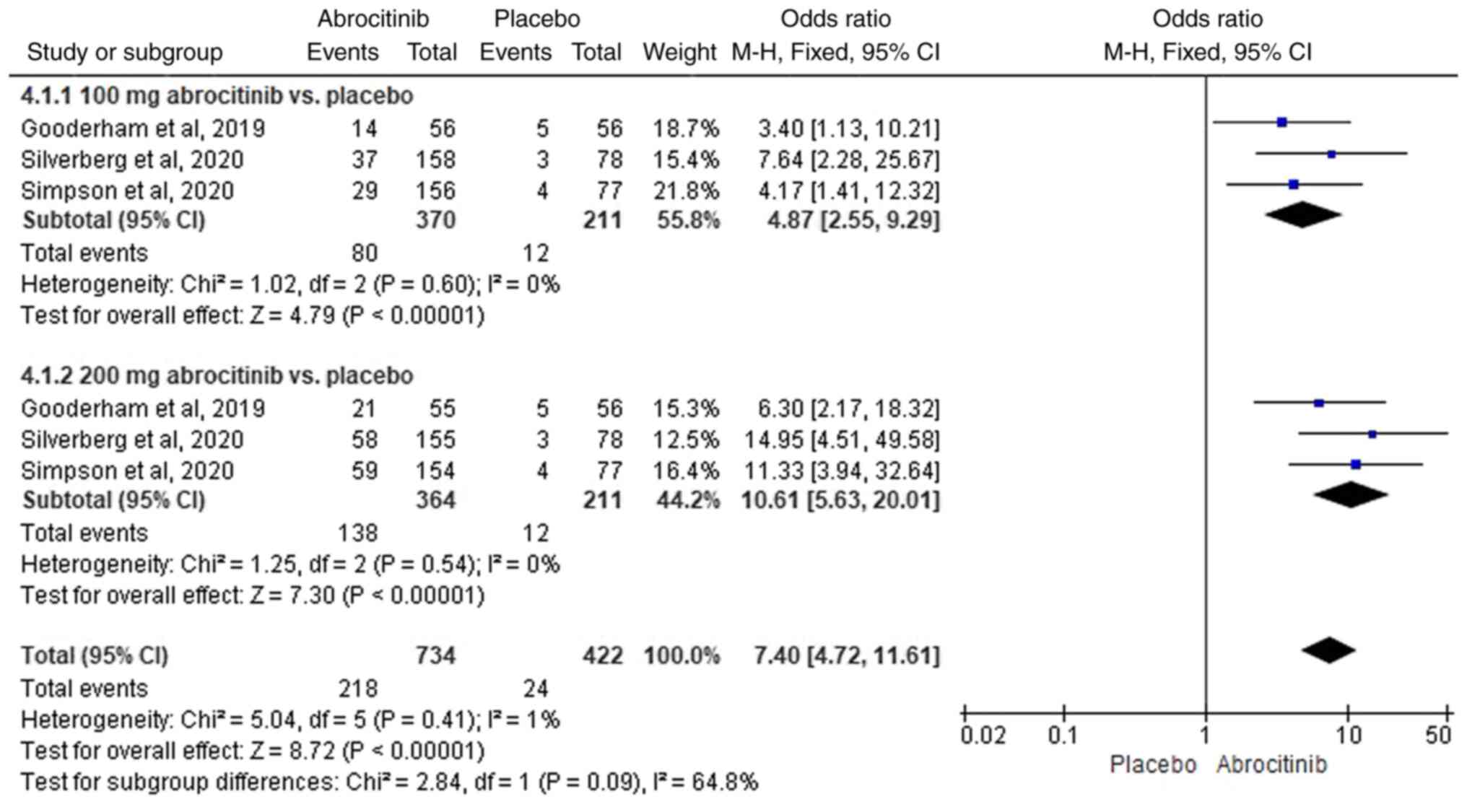
placebo, 100 (M-H OR, 4.87; 95% CI, 2.55-9.29) and 200 mg (M-H OR,
10.61; 95% CI, 5.63-20.01) significantly improved EASI-90 response
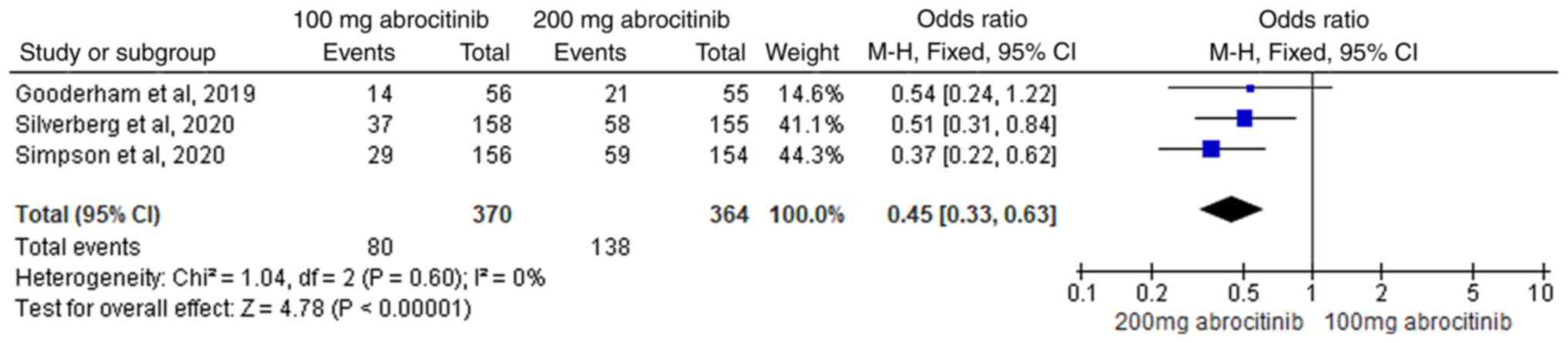
rate (Fig. 9). In total, three RCTs
(42-44)
were included in subsequent comparative analysis of 100 and 200 mg
abrocitinib, where homogeneity was suggested (I2=0%) FEM
analysis revealed that the difference between the two groups was
significant, with the 200 mg abrocitinib group yielding superior
EASI-90 response rates compared with those in 100 mg group (M-H OR,
0.45; 95% CI, 0.33-0.63; Fig.
10).
P-NRS improvement rate
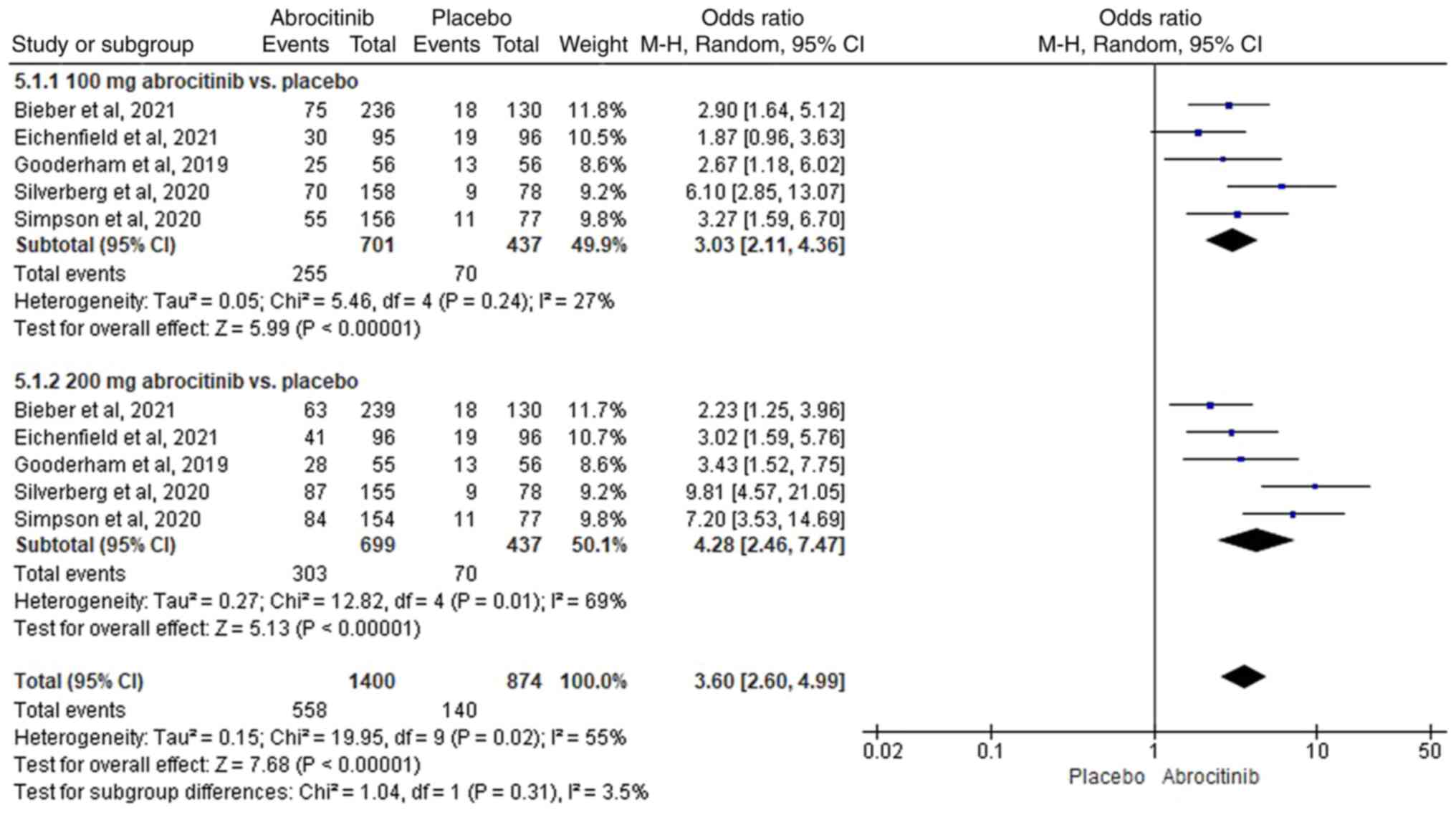
In total, five RCTs (40-44)
were included, where heterogeneity was suggested
(I2>50%). Compared with placebo, 100 (M-H OR, 3.03;
95% CI, 2.11-4.36) and 200 mg (M-H OR, 4.28; 95% CI, 2.46-7.47)
significantly improved the P-NRS (Fig.
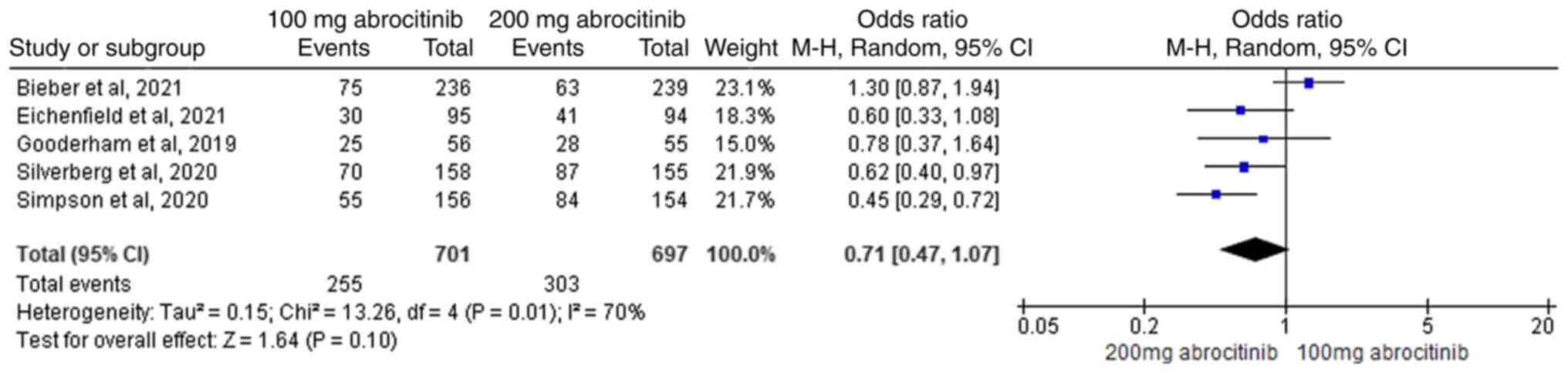
11). In the pairwise cross-control analysis of 100 and 200 mg
abrocitinib, five RCTs (40-44)
were included where heterogeneity was found (I2>50%).
No significant difference between the two groups were found in
terms of P-NRS (Fig. 12).
Safety outcomes
A combined meta-analysis of the incidence of adverse
drug reaction (ADR) in 100 and 200 mg abrocitinib and placebo
groups was next conducted. A total of 11 symptoms were observed.
Nausea (M-H RR, 3.25; 95% CI, 1.55-6.85), infection and infestation
(M-H RR, 1.45, 95% CI, 1.06-1.98) and upper respiratory tract viral
infection (M-H RR, 2.26; 95% CI, 1.11-4.60) had significantly
higher incidence in the 100 mg abrocitinib compared with the
placebo group. However, there was no significant difference in the
incidence of gastrointestinal disorder, upper respiratory tract
infection, dizziness, headache, skin and subcutaneous disorders,
blood creatine phosphokinase increased, thrombocytopenia and severe
adverse event between the two groups. In the 200 mg abrocitinib
compared with the placebo group, there were significantly higher
incidences of gastrointestinal dysfunction (RR, 5.35, 95%CI,
2.28-12.57), nausea (M-H RR, 8.03, 95% CI, 3.98-16.20), dizziness
(M-H RR, 6.46; 95% CI, 1.17-35.62) and headache (M-H RR, 1.89, 95%
CI, 1.11-3.19), but there were no significant differences in the
incidence of other ADRs. In the 100 mg abrocitinib compared with
the 200 mg abrocitinib, there were significant differences in the
incidence of gastrointestinal disorder (M-H RR, 0.39; 95% CI,
0.22-0.69), nausea (M-H RR, 0.42; 95% CI, 0.30-0.58) and dizziness
(M-H RR, 0.10; 95% CI, 0.01-0.76), but no significant differences
in the incidence of other ADRs (Table
II).
 | Table IISafety profile in each treatment
group. |
Table II
Safety profile in each treatment
group.
| | 100 mg abrocitinib
vs. placebo | 200 mg abrocitinib
vs. placebo | 100 vs. 200 mg
abrocitinib |
|---|
| Adverse drug
reaction | Number of
trials | I2,
% | RR, 95% CI | I2,
% | RR, 95% CI | I2,
% | RR, 95% CI |
|---|
| Gastrointestinal
disorder | 3 | 0 | 2.16
(0.85-5.46) | 0 | 5.35
(2.28-12.57)a | 0 | 0.39
(0.22-0.69)a |
| Nausea | 5 | 0 | 3.25
(1.55-6.85)a | 0 | 8.03
(3.98-16.20)a | 0 | 0.42
(0.30-0.58)a |
| Infection and
infestation | 5 | 0 | 1.45
(1.06-1.98)a | 0 | 1.20
(0.87-1.65) | 0 | 1.24
(0.96-1.60) |
| Upper respiratory
tract infection | 5 | 0 | 1.16
(0.75-1.80) | 0 | 0.95
(0.61-1.50) | 0 | 1.26
(0.85-1.87) |
| Upper respiratory
tract viral infection | 4 | 0 | 2.26
(1.11-4.60)a | 21 | 1.75
(0.82-3.73) | 0 | 1.24
(0.73-2.09) |
| Dizziness | 2 | 0 | 0.34
(0.04-3.18) | 0 | 6.46
(1.17-35.62)a | 0 | 0.10
(0.01-0.76)a |
| Headache | 5 | 0 | 1.41
(0.81-2.43) | 0 | 1.89
(1.11-3.19)a | 0 | 0.74
(0.50-1.10) |
| Skin and
subcutaneous disorder | 5 | 76 | 1.11
(0.49-2.49) | 68 | 0.89
(0.42-1.91) | 74 | 1.11
(0.57-2.17) |
| Increased blood
creatine phosphokinase | 2 | 56 | 2.05
(0.55-7.62) | 32 | 2.50
(0.68-9.21) | 0 | 0.77
(0.29-2.02) |
|
Thrombocytopenia | 2 | 0 | 1.57
(0.16-15.01) | 0 | 4.27
(0.53-34.20) | 0 | 0.19
(0.03-1.12) |
| Serious adverse
events | 5 | 0 | 0.88
(0.44-1.77) | 0 | 0.59
(0.27-1.2) | 0 | 1.52
(0.75-3.07) |
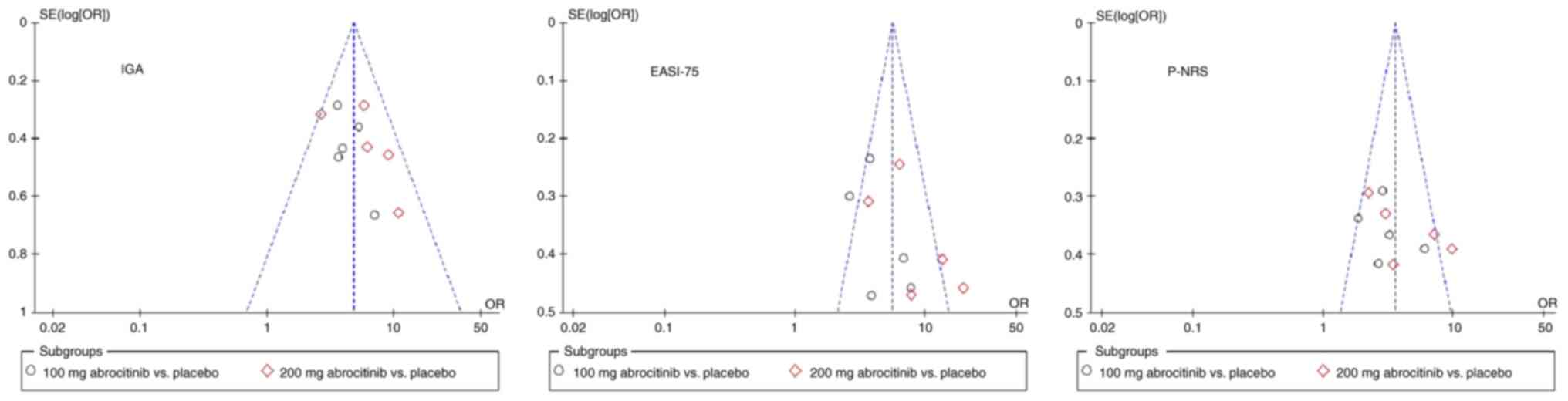
Publication offset evaluation
In a pairwise cross-control analysis of 100 and 200
mg of abrocitinib compared with placebo, funnel plots were
constructed using IGA, EASI-75 response rate and P-NRS as the
efficacy indicators. The results showed that asymmetry of the
EASI-75 scattered points along the center line; scattered points of
the IGA and P-NRS 2 evaluation indices were symmetrical along the
center line, However, all of the aforementioned indices were
scattered and stratified unevenly, suggesting that these three
evaluation indices may have the risk of publication bias. These
findings suggest that results should be judged with caution
(Fig. 13).
Sensitivity analysis
Sensitivity correction was performed on results of
the REM, before analysis was performed again after removing one
research item with large differences in the weight ratio of each
effect index at a time (45). The
results showed that the effect value of P-NRS improvements in the
control analysis of 100 and 200 mg abrocitinib showed instability.
There were no changes in the statistical values of other effect
indicators, suggesting that the results were stable (Table III).
 | Table IIISensitivity analysis of effect
indicators with large differences in weight ratio. |
Table III
Sensitivity analysis of effect
indicators with large differences in weight ratio.
| A, 100 mg
abrocitinib vs. placebo |
|---|
| | Before
exclusion | After
exclusion | |
|---|
| Effect index | RR | 95%CI | P-value | RR | 95%CI | P-value | Stability |
|---|
| Skin and
subcutaneous disorder | 1.11 | 0.49-2.49 | 0.81 | 0.96 | 0.43-2.16 | 0.93 | Yes |
| Increased blood
creatine phosphokinase | 2.05 | 0.55-7.62 | 0.28 | 9.09 | 0.50-166.61 | 0.14 | Yes |
| B, 200 mg
abrocitinib vs. placebo |
| | Before
exclusion | After
exclusion | |
| Effect index | RR | 95%CI | P-value | RR | 95%CI | P-value | Stability |
| EASI-75 | 8.07 | 4.59-14.17 | <0.01 | 8.39 | 5.79-12.17 | <0.01 | Yes |
| P-NRS | 4.28 | 2.46-7.47 | <0.01 | 5.29 | 3.71-7.55 | <0.01 | Yes |
| Skin and
subcutaneous disorder | 0.89 | 0.42-1.91 | 0.77 | 0.73 | 0.40-1.32 | 0.30 | Yes |
| C, 100 vs. 200 mg
abrocitinib |
| | Before
exclusion | After
exclusion | |
| Effect index | RR | 95%CI | P-value | RR | 95%CI | P-value | Stability |
| P-NRS | 0.71 | 0.47-1.07 | 0.10 | 0.57 | 0.44-0.74 | <0.01 | No |
| Skin and
subcutaneous disorder | 1.11 | 0.57-2.17 | 0.77 | 0.99 | 0.43-2.31 | 0.98 | Yes |
Discussion
Pruritus is the most common clinical symptom in AD.
Therefore, alleviating skin eczema symptoms and eliminating
pruritus is key to the treatment of AD (46). Systemic treatment options for
patients with moderate-to-severe AD are frequently based on those
applied for patients with refractory AD who have failed local
treatment or those who has not been cured for a long period time.
Patients typically have unrealistically high expectations of the
effect of these therapies (47).
Based on five randomized, double-blind, parallel-controlled trials,
the present study conducted a pairwise cross-control analysis of
1,825 patients with moderate-to-severe AD who received 200 and/or
100 mg abrocitinib orally or placebo once a day. The results showed
that compared the placebo group, 100 and 200 mg abrocitinib
significantly improved the therapeutic indices of IGA, EASI-50, -75
and -90 and P-NRS in patients. In particular, IGA score, skin
eczema and pruritis symptoms of patients were more significantly
improved when the dose of abrocitinib was increased to 200 mg. The
results of these trials are consistent with the prominent role of
JAK1 signaling in the development of pruritus and skin inflammation
in AD, demonstrating the potential therapeutic value of JAK1
inhibitors in patients with AD (48,49).
AD has a complex pathophysiological mechanism that remains to be
fully elucidated. However, it has been reported that immune
disorders lead to the destruction of epidermal barrier function and
aggravate symptoms of AD (50). At
present, it is hypothesized that JAK inhibition alters the
signaling mechanism of several immune and epidermal cell-derived
cytokines involved in the pathogenesis of AD, such as thymus
stromal lymphopoietin, IL-4, IL-13, IL-22 and IL-31(51).
The reliability of the results of in a systematic
review is dependent on quality of the original literature included,
whereas the quantity of high-quality literature will determine the
stability of the results (52). The
five publications included in the present study were randomized and
double-blinded studies, with 1,825 patients enrolled. The basic
information of the publication was complete; research indicators
were homogenous and data were complete. The risk of deviation from
the experimental design by the authors of the original publication
was low. In addition, high risk of deviation was not found in the
evaluation results of the methodology quality in each study and the
overall quality of the included literature was high.
In the present study, through the association
analysis of the results of the main therapeutic indicators, it was
concluded that the symptoms of pruritus and eczema in patients with
moderate-to-severe AD could be relieved within 12 weeks after the
oral administration of 100 mg abrocitinib. Specifically, the
symptoms of pruritus and eczema could be rapidly relieved, which
was then effectively consolidated with the extension of the
treatment course and increases in dosage. This finding is
consistent with the results of a previous retrospective study of
the effects of 100 mg abrocitinib once daily for the treatment of
moderate-to-severe AD by Gooderham et al (53), which found that at week 12 after the
commencement of treatment, the IGA score and EASI-75 improved
significantly from the baseline. In the present study, significant
improvements in IGA and EASI scores were observed compared with
those receiving 100 mg when the dose was increased to 200 mg. To
some extent, the efficacy of abrocitinib 200 mg appears to be more
potent, in terms of both speed and intensity of action, suggesting
that this dose may be the preferred dose for the majority of
patients, which was consistent with the results of Simpson et
al (54). However, for patients
with a higher risk of adverse reactions or those who do not
tolerated abrocitinib, a dose of 100 mg may be a more appropriate
starting dose. In addition, although 200 mg abrocitinib is
generally well-tolerated by the majority of patients ≥65 years of
age, dose-associated side effects, such as hematological changes
and shingles, are particularly common in this age group (55). Therefore, appropriate dose selection
in the adapted population before initiation is recommended to
minimize the risks associated with abrocitinib. Due to the
heterogeneity risk in the P-NRS score after 100 and 200 mg
abrocitinib treatment and the high sensitivity of data variation
found in the present study, there is likely to be a bias risk in
the association analysis of P-NRS results in the 200 mg abrocitinib
group, meaning these results should be interpreted with caution. In
a pairwise cross-control safety comparison of 100 and 200 mg
abrocitinib compared with placebo, both 100 and 200 mg abrocitinib
exhibited different incidences of gastrointestinal and central
nervous system symptoms, among which nausea, infection and
infestation, upper respiratory tract viral infection were the main
symptoms in the 100 mg abrocitinib group. By contrast,
gastrointestinal dysfunction, nausea, dizziness and headache were
the main symptoms in the 200 mg abrocitinib group. Reich et
al (18) found that serious
adverse reactions, such as inflammatory bowel disease,
peritonsillitis, dehydration and asthma occurred after oral
treatments with abrocitinib 200 mg, whereas one case of pneumonia
was also found during follow-up. Serious adverse reactions, such as
retinal detachment, acute pancreatitis, appendicitis, dizziness and
epilepsy, were associated with 100 mg abrocitinib oral treatment
(18), similar to the results of
the present study. Although comparison between 100 and 200 mg
abrocitinib found that incidence of gastrointestinal function,
nausea and headache was more pronounced in the 200 mg group,
incidence of infection and infestation (14.94 vs. 12.13%), upper
respiratory tract infection (7.54 vs. 5.99%) and viral upper
respiratory tract viral infection (5.32 vs. 4.35%) decreased as
abrocitinib dosage increased. The association between the infection
risk of patients and the dose of abrocitinib warrants further
study.
To ensure the integrity of the included data and
avoid missing literature or insufficient literature retrieval, the
present study searched the universally used, recognized,
authoritative and complete data collection databases. However, due
to the limited number of included studies and tested patients, the
results of each analysis are not strong and should be interpreted
with caution. In terms of literature screening, data extraction,
and quality evaluation, subjective selection bias or risk of
omitting relevant literature may persist due to different
researchers. The included analysis data may be confounded by
uncertain factors, such as missing literature, differences in
subjective judgments by each author and language restrictions.
Furthermore, results of the present meta-analysis may contain
volatility, necessitating further validation in future studies. The
present systematic review focused on comparing 100 and 200 mg
abrocitinib with the placebo group. The results serve as a basis
for treatment decision-making at these doses, whilst excluding
other major therapeutic drugs from the scope of this review.
Despite the limitations in the present study, the present
meta-analysis provides a systematic evaluation pathway for
subsequent multi-arm studies on 100 mg abrocitinib and single-arm
studies exploring the efficacy of its combined application.
Additionally, it offers an avenue for further investigating the
association between abrocitinib dosage and infection risk. Despite
these limitations, the controversial effects of abrocitinib between
100 and 200 mg on pruritus, eczema symptoms and tolerance in
patients with moderate-to-severe AD were mentioned.
Both 100 and 200 mg doses of abrocitinib can rapidly
alleviate the clinical symptoms of pruritus and eczema in patients
with moderate-to-severe AD, which are generally well tolerated
compared with patients as demonstrated by the acceptable adverse
reactions profile. However, there is risk of infection is different
in patients treated with different doses of abrocitinib, rendering
it necessary to perform additional benefit-risk assessments of
patients. Due to the objective factors of the systematic review,
the results of the present study are mainly for reference and
require multi-center, strictly designed, high-quality and
large-scale clinical trials for validation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Plan Fund from Yaan Science and Technology Bureau (grant
no. 22KJJH0039); Beijing Medical Award Foundation ‘Rui Ying IV
phase’ scientific research project (grant no.
YXJL-2023-0866-0316).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XX and LF conceived and designed the study. XX
performed the literature search, extracted and analyzed the data
and wrote the manuscript. JZ and FH extracted and analyzed data and
reviewed the manuscript. LF revised the manuscript. XX and JZ
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dini V, Iannone M, Michelucci A, Manzo
Margiotta F, Granieri G, Salvia G, Oranges T, Janowska A, Morganti
R and Romanelli M: Ultra-high frequency ultrasound (UHFUS)
assessment of barrier function in moderate-to-severe atopic
dermatitis during dupilumab treatment. Diagnostics (Basel).
13(2721)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Silverberg JI, Gelfand JM, Margolis DJ,
Boguniewicz M, Fonacier L, Grayson MH, Simpson EL, Ong PY and
Chiesa Fuxench ZC: Patient burden and quality of life in atopic
dermatitis in US adults: A population-based cross-sectional study.
Ann Allergy Asthma Immunol. 121:340–347. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vilsbøll AW, Anderson P, Piercy J,
Milligan G and Kragh N: extent and impact of inadequate disease
control in US adults with a history of moderate to severe atopic
dermatitis following introduction of new treatments. Dermatol Ther
(Heidelb). 11:475–486. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rerknimitr P, Otsuka A, Nakashima C and
Kabashima K: The etiopathogenesis of atopic dermatitis: Barrier
disruption, immunological derangement, and pruritus. Infamm Regen.
37(14)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Williams H, Robertson C, Stewart A,
Aït-Khaled N, Anabwani G, Anderson R, Asher I, Beasley R, Björkstén
B, Burr M, et al: Worldwide variations in the prevalence of
symptoms of atopic eczema in the international study of asthma and
allergies in childhood. J Allergy Clin Immunol. 103:125–138.
1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Odhiambo JA, Williams HC, Clayton TO,
Robertson CF and Asher MI: ISAAC Phase Three Study Group. Global
variations in prevalence of eczema symptoms in children from ISAAC
phase three. J Allergy Clin Immunol. 124:1251–1258.e23.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mandlik DS and Mandlik SK: Atopic
dermatitis: New insight into the etiology, pathogenesis, diagnosis
and novel treatment strategies. Immunopharmacol Immunotoxicol.
43:105–125. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dubin C, Del Duca E and Guttman-Yassky E:
The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev
Clin Immunol. 17:835–852. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Thyssen JP, Halling AS,
Schmid-Grendelmeier P, Guttman-Yassky E and Silverberg JI:
Comorbidities of atopic dermatitis-what does the evidence say? J
Allergy Clin Immunol. 151:1155–1162. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weidinger S and Novak N: Atopic
dermatitis. Lancet. 387:1109–1122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Paolino A, Alexander H, Broderick C and
Flohr C: Non-biologic systemic treatments for atopic dermatitis:
Current state of the art and future directions. Clin Exp Allergy.
53:495–510. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Drucker AM, Morra DE, Prieto-Merino D,
Ellis AG, Yiu ZZN, Rochwerg B, Di Giorgio S, Arents BWM, Burton T,
Spuls PI, et al: Systemic immunomodulatory treatments for atopic
Dermatitis: Update of a living systematic review and network
meta-analysis. JAMA Dermatol. 158:523–532. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Blauvelt A, Teixeira HD, Simpson EL,
Costanzo A, De Bruin-Weller M, Barbarot S, Prajapati VH, Lio P, Hu
X, Wu T, et al: Efficacy and safety of upadacitinib vs dupilumab in
adults with moderate-to-severe atopic dermatitis: A randomized
clinical trial. JAMA Dermatol. 157:1047–1055. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Buhl T, Rosmarin D, Serra-Baldrich E,
Fernandez-Peñas P, Igarashi A, Konstantinou MP, Chen S, Lu N,
Pierce E and Casillas M: Itch and sleep improvements with
baricitinib in patients with atopic dermatitis: A post hoc analysis
of 3 phase 3 studies. Dermatol Ther (Heidelb). 11:971–182.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Blauvelt A, Ardern-Jones MR, Bieber T,
Hong HCH, Chu CY, Liu M, Yang Y, Ladizinski B, Teixeira H, Calimlim
BM and Thyssen JP: 28032 Rapid itch improvement with upadacitinib
with or without concomitant topical corticosteroids (TCS) in
moderate-to-severe atopic dermatitis (AD): Results from 3 phase 3
studies (Measure Up1, Measure Up 2, and AD Up). J Am Acad Dermatol.
85(AB171)2021.
|
|
16
|
Silverberg JI, Yosipovitch G, Simpson EL,
Kim BS, Wu JJ, Eckert L, Guillemin I, Chen Z, Ardeleanu M, Bansal
A, et al: Dupilumab treatment results in early and sustained
improvements in itch in adolescents and adults with moderate to
severe atopic dermatitis: Analysis of the randomized phase 3
studies SOLO 1 and SOLO 2, AD ADOL, and CHRONOS. J Am Acad
Dermatol. 82:1328–1336. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Simpson E, Wollenberg A, Soong W, Mark T,
Kuznetsova A, Steffensen LA and Silverberg J: Rapid and sustained
improvements in itch and sleep with tralokinumab treatment in
patients with moderate-to-severe Atopic Dermatitis, a post hoc
analysis of pooled data from ECZTRA 1 and 2. SKIN J Cutan Med.
5(S61)2021.
|
|
18
|
Reich K, Silverberg JI, Papp KA, Deleuran
M, Katoh N, Strober B, Beck LA, de Bruin-Weller M, Werfel T, Zhang
F, et al: Abrocitinib efficacy and safety in patients with
moderate-to-severe atopic dermatitis: Results from phase 3 studies,
including the long-term extension JADE EXTEND study. J Eur Acad
Dermatol Venereol. 37:2056–2066. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Simpson EL, Silverberg JI, Thyssen JP,
Viguier M, Thaçi D, de Bruin-Weller M, Weidinger S, Chan G,
DiBonaventura M, Biswas P, et al: Efficacy and safety of
abrocitinib in patients with severe and/or difficult-to-treat
atopic dermatitis: A post hoc analysis of the randomized phase 3
JADE COMPARE trial. Am J Clin Dermatol. 24:609–621. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Berthe P, Scailteux LM, Lescoat A,
Staumont D, Coiffier G, Guéret P, Dupuy A, Oger E and Droitcourt C:
Oral Janus kinase inhibitors and venous thromboembolic events in
atopic dermatitis: Protocols for a case-time control study and a
nested case-control study based on the French national health
insurance (SNDS) cohort. BMJ Open. 12(e059979)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tong Z, Zhang Y, Zhou K, Zou Y, Wu Z, Chen
J, Zhuang Z, Zhao Y, Gong T and Ji C: An observational study of
abrocitinib in adults with moderate-to-severe atopic dermatitis
after switching from dupilumab. J Am Acad Dermatol. 89:826–828.
2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Blauvelt A, de Bruin-Weller M, Gooderham
M, Cather JC, Weisman J, Pariser D, Simpson EL, Papp KA, Hong HC,
Rubel D, et al: Long-term management of moderate-to-severe atopic
dermatitis with dupilumab and concomitant topical corticosteroids
(LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded,
placebo-controlled, phase 3 trial. Lancet. 389:2287–2303.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Banerjee S, Biehl A, Gadina M, Hasni S and
Schwartz DM: JAK-STAT signaling as a target for inflammatory and
autoimmune diseases: Current and future prospects. Drugs.
77:521–546. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Blauvelt A, Silverberg JI, Lynde CW,
Bieber T, Eisman S, Zdybski J, Gubelin W, Simpson EL, Valenzuela F,
Criado PR, et al: Abrocitinib induction, randomized withdrawal, and
retreatment in patients with moderate-to-severe atopic dermatitis:
Results from the JAK1 atopic dermatitis efficacy and safety (JADE)
REGIMEN phase 3 trial. J Am Acad Dermatol. 86:104–112.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Clarke B, Yates M, Adas M, Bechman K and
Galloway J: The safety of JAK-1 inhibitors. Rheumatology (Oxford).
60 (Suppl 2):ii24–ii30. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Romero Jiménez RM, Herranz Pinto P, Campos
Domínguez M, Aceituno Mata S, Bellmunt A, Prades M, Arumi D,
Hernández-Martín I, Herrera-Lasso V, Llevat N, et al:
Cost-effectiveness analysis of abrocitinib compared with other
systemic treatments for severe atopic dermatitis in spain.
Pharmacoecon Open. 8:291–302. 2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Edwards SJ, Karner C, Jhita T, Barton S,
Marceniuk G, Yiu ZZN and Wittmann M: Abrocitinib, tralokinumab and
upadacitinib for treating moderate-to-severe atopic dermatitis.
Health Technol Assess. 28:1–113. 2024.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Lugović-Mihić L, Meštrović-Štefekov J,
Potočnjak I, Cindrić T, Ilić I, Lovrić I, Skalicki L, Bešlić I and
Pondeljak N: Atopic dermatitis: Disease features, therapeutic
options, and a multidisciplinary approach. Life (Basel).
13(1419)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lipsky BA, Senneville É, Abbas ZG,
Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M,
van Asten SA, et al: Guidelines on the diagnosis and treatment of
foot infection in persons with diabetes (IWGDF 2019 update).
Diabetes Metab Res Rev. 36 (Suppl 1)(e3280)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gu H, Chen XS, Chen K, Yan Y and Jin H:
Evaluation of diagnostic criteria for atopic dermatitis. Chin J
Dermatology. 33:222–226. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Langley RGB, Feldman SR, Nyirady J, van de
Kerkhof P and Papavassilis C: The 5-point investigator's global
assessment (IGA) scale: A modified tool for evaluating plaque
psoriasis severity in clinical trials. J Dermatolog Treat.
26:23–31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hanifin JM, Baghoomian W, Grinich E,
Leshem YA, Jacobson M and Simpson EL: The eczema area and severity
index-a practical guide. Dermatitis. 33:187–192. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Napolitano M, Ferrucci SM, Foggia L,
Hansel K, Pezzolo E, Stingeni L, Antonelli E, Picone V and Patruno
C: Comparison of long-term effectiveness and safety of upadacitinib
for atopic dermatitis between dupilumab-exposed and dupilumab-naïve
patients. Clin Drug Investig. 44:71–77. 2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ.
339(b2700)2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Veazie S, Peterson K, Bourne D, Anderson
J, Damschroder L and Gunnar W: Implementing high-reliability
organization principles into practice: A rapid evidence review. J
Patient Saf. 18:e320–e328. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Higgins JPT and Green S: Cochrane handbook
for systematic reviews of interventions. Version 5.1.0. The
Cochrane Collaboration, 2011. www.cochranehandbook.org.
|
|
37
|
Atkins D, Briss PA, Eccles M, Flottorp S,
Guyatt GH, Harbour RT, Hill S, Jaeschke R, Liberati A, Magrini N,
et al: Systems for grading the quality of evidence and the strength
of recommendations II: Pilot study of a new system. BMC Health Serv
Res. 5(25)2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
van Tulder M, Furlan A, Bombardier C and
Bouter L: Editorial Board of the Cochrane Collaboration Back Review
Group. Updated method guidelines for systematic reviews in the
cochrane collaboration back review group. Spine (Phila Pa 1976).
28:1290–1299. 2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bieber T, Simpson EL, Silverberg JI, Thaçi
D, Paul C, Pink AE, Kataoka Y, Chu CY, DiBonaventura M, Rojo R, et
al: Abrocitinib versus placebo or dupilumab for atopic dermatitis.
N Engl J Med. 384:1101–1112. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Eichenfield LF, Flohr C, Sidbury R,
Siegfried E, Szalai Z, Galus R, Yao Z, Takahashi H, Barbarot S,
Feeney C, et al: Efficacy and safety of abrocitinib in combination
with topical therapy in adolescents with moderate-to-severe atopic
dermatitis: The JADE TEEN randomized clinical trial. JAMA Dermatol.
157:1165–1173. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gooderham MJ, Forman SB, Bissonnette R,
Beebe JS, Zhang W, Banfield C, Zhu L, Papacharalambous J, Vincent
MS and Peeva E: Efficacy and safety of oral Janus kinase 1
inhibitor abrocitinib for patients with atopic dermatitis: A phase
2 randomized clinical trial. JAMA Dermatol. 155:1371–1379.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Silverberg JI, Simpson EL, Thyssen JP,
Gooderham M, Chan G, Feeney C, Biswas P, Valdez H, DiBonaventura M,
Nduaka C and Rojo R: Efficacy and safety of abrocitinib in patients
with moderate-to-severe atopic dermatitis: A randomized clinical
trial. JAMA Dermatol. 156:863–873. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Simpson EL, Sinclair R, Forman S,
Wollenberg A, Aschoff R, Cork M, Bieber T, Thyssen JP, Yosipovitch
G, Flohr C, et al: Efficacy and safety of abrocitinib in adults and
adolescents with moderate-to-severe atopic dermatitis (JADE
MONO-1): A multicentre, double-blind, randomised,
placebo-controlled, phase 3 trial. Lancet. 396:255–266.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L and Sterne JA:
, et al: The cochrane collaboration's tool for assessing
risk of bias in randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Boguniewicz M, Fonacier L, Guttman-Yassky
E, Ong PY, Silverberg J and Farrar JR: Atopic dermatitis yardstick:
Practical recommendations for an evolving therapeutic landscape.
Ann Allergy Asthma Immunol. 120:10–22.e2. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Reich K, Thyssen JP, Blauvelt A, Eyerich
K, Soong W, Rice ZP, Hong HC, Katoh N, Valenzuela F, DiBonaventura
M, et al: Efficacy and safety of abrocitinib versus dupilumab in
adults with moderate-to-severe atopic dermatitis: A randomised,
double-blind, multicentre phase 3 trial. Lancet. 400:273–282.
2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Boeri M, Sutphin J, Hauber B, Cappelleri
JC, Romero W and Di Bonaventura M: Quantifying patient preferences
for systemic atopic dermatitis treatments using a discrete-choice
experiment. J Dermatolog Treat. 33:1449–1458. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Boytsov NN, Gorritz M, Wang X,
Malatestinic WN, Wade RL and Goldblum OM: The current treatment
landscape in adult atopic dermatitis in the United States: Results
from a cross-sectional real-world study. J Dermatolog Treat.
33:1707–1717. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sterne JA, Egger M and Smith GD:
Systematic reviews in health care: Investigating and dealing with
publication and other biases in meta-analysis. BMJ. 323:101–105.
2001.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Howell MD, Kuo FI and Smith PA: Targeting
the Janus kinase family in autoimmune skin diseases. Front Immunol.
10(2342)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sroka-Tomaszewska J and Trzeciak M:
Molecular mecha-nisms of atopic dermatitis pathogenesis. Int J Mol
Sci. 22(4130)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gooderham MJ, Pink AE, Simpson EL,
Silverberg JI, Güler E and Watkins M: Abrocitinib 100 mg once daily
for moderate-to-severe atopic dermatitis: A review of efficacy and
safety, and expert opinion on use in clinical practice. Dermatol
Ther (Heidelb). 13:1893–1907. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Simpson EL, Silverberg JI, Nosbaum A,
Winthrop KL, Guttman-Yassky E, Hoffmeister KM, Egeberg A, Valdez H,
Zhang M, Farooqui SA, et al: Integrated safety analysis of
abrocitinib for the treatment of moderate-to-severe atopic
dermatitis from the phase II and phase III clinical trial program.
Am J Clin Dermatol. 22:693–707. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lee AJ and Ashkar AA: The dual nature of
type I and type II interferons. Front Immunol.
9(2061)2018.PubMed/NCBI View Article : Google Scholar
|