|
1
|
Dini V, Iannone M, Michelucci A, Manzo
Margiotta F, Granieri G, Salvia G, Oranges T, Janowska A, Morganti
R and Romanelli M: Ultra-high frequency ultrasound (UHFUS)
assessment of barrier function in moderate-to-severe atopic
dermatitis during dupilumab treatment. Diagnostics (Basel).
13(2721)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Silverberg JI, Gelfand JM, Margolis DJ,
Boguniewicz M, Fonacier L, Grayson MH, Simpson EL, Ong PY and
Chiesa Fuxench ZC: Patient burden and quality of life in atopic
dermatitis in US adults: A population-based cross-sectional study.
Ann Allergy Asthma Immunol. 121:340–347. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vilsbøll AW, Anderson P, Piercy J,
Milligan G and Kragh N: extent and impact of inadequate disease
control in US adults with a history of moderate to severe atopic
dermatitis following introduction of new treatments. Dermatol Ther
(Heidelb). 11:475–486. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rerknimitr P, Otsuka A, Nakashima C and
Kabashima K: The etiopathogenesis of atopic dermatitis: Barrier
disruption, immunological derangement, and pruritus. Infamm Regen.
37(14)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Williams H, Robertson C, Stewart A,
Aït-Khaled N, Anabwani G, Anderson R, Asher I, Beasley R, Björkstén
B, Burr M, et al: Worldwide variations in the prevalence of
symptoms of atopic eczema in the international study of asthma and
allergies in childhood. J Allergy Clin Immunol. 103:125–138.
1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Odhiambo JA, Williams HC, Clayton TO,
Robertson CF and Asher MI: ISAAC Phase Three Study Group. Global
variations in prevalence of eczema symptoms in children from ISAAC
phase three. J Allergy Clin Immunol. 124:1251–1258.e23.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mandlik DS and Mandlik SK: Atopic
dermatitis: New insight into the etiology, pathogenesis, diagnosis
and novel treatment strategies. Immunopharmacol Immunotoxicol.
43:105–125. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dubin C, Del Duca E and Guttman-Yassky E:
The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev
Clin Immunol. 17:835–852. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Thyssen JP, Halling AS,
Schmid-Grendelmeier P, Guttman-Yassky E and Silverberg JI:
Comorbidities of atopic dermatitis-what does the evidence say? J
Allergy Clin Immunol. 151:1155–1162. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weidinger S and Novak N: Atopic
dermatitis. Lancet. 387:1109–1122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Paolino A, Alexander H, Broderick C and
Flohr C: Non-biologic systemic treatments for atopic dermatitis:
Current state of the art and future directions. Clin Exp Allergy.
53:495–510. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Drucker AM, Morra DE, Prieto-Merino D,
Ellis AG, Yiu ZZN, Rochwerg B, Di Giorgio S, Arents BWM, Burton T,
Spuls PI, et al: Systemic immunomodulatory treatments for atopic
Dermatitis: Update of a living systematic review and network
meta-analysis. JAMA Dermatol. 158:523–532. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Blauvelt A, Teixeira HD, Simpson EL,
Costanzo A, De Bruin-Weller M, Barbarot S, Prajapati VH, Lio P, Hu
X, Wu T, et al: Efficacy and safety of upadacitinib vs dupilumab in
adults with moderate-to-severe atopic dermatitis: A randomized
clinical trial. JAMA Dermatol. 157:1047–1055. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Buhl T, Rosmarin D, Serra-Baldrich E,
Fernandez-Peñas P, Igarashi A, Konstantinou MP, Chen S, Lu N,
Pierce E and Casillas M: Itch and sleep improvements with
baricitinib in patients with atopic dermatitis: A post hoc analysis
of 3 phase 3 studies. Dermatol Ther (Heidelb). 11:971–182.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Blauvelt A, Ardern-Jones MR, Bieber T,
Hong HCH, Chu CY, Liu M, Yang Y, Ladizinski B, Teixeira H, Calimlim
BM and Thyssen JP: 28032 Rapid itch improvement with upadacitinib
with or without concomitant topical corticosteroids (TCS) in
moderate-to-severe atopic dermatitis (AD): Results from 3 phase 3
studies (Measure Up1, Measure Up 2, and AD Up). J Am Acad Dermatol.
85(AB171)2021.
|
|
16
|
Silverberg JI, Yosipovitch G, Simpson EL,
Kim BS, Wu JJ, Eckert L, Guillemin I, Chen Z, Ardeleanu M, Bansal
A, et al: Dupilumab treatment results in early and sustained
improvements in itch in adolescents and adults with moderate to
severe atopic dermatitis: Analysis of the randomized phase 3
studies SOLO 1 and SOLO 2, AD ADOL, and CHRONOS. J Am Acad
Dermatol. 82:1328–1336. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Simpson E, Wollenberg A, Soong W, Mark T,
Kuznetsova A, Steffensen LA and Silverberg J: Rapid and sustained
improvements in itch and sleep with tralokinumab treatment in
patients with moderate-to-severe Atopic Dermatitis, a post hoc
analysis of pooled data from ECZTRA 1 and 2. SKIN J Cutan Med.
5(S61)2021.
|
|
18
|
Reich K, Silverberg JI, Papp KA, Deleuran
M, Katoh N, Strober B, Beck LA, de Bruin-Weller M, Werfel T, Zhang
F, et al: Abrocitinib efficacy and safety in patients with
moderate-to-severe atopic dermatitis: Results from phase 3 studies,
including the long-term extension JADE EXTEND study. J Eur Acad
Dermatol Venereol. 37:2056–2066. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Simpson EL, Silverberg JI, Thyssen JP,
Viguier M, Thaçi D, de Bruin-Weller M, Weidinger S, Chan G,
DiBonaventura M, Biswas P, et al: Efficacy and safety of
abrocitinib in patients with severe and/or difficult-to-treat
atopic dermatitis: A post hoc analysis of the randomized phase 3
JADE COMPARE trial. Am J Clin Dermatol. 24:609–621. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Berthe P, Scailteux LM, Lescoat A,
Staumont D, Coiffier G, Guéret P, Dupuy A, Oger E and Droitcourt C:
Oral Janus kinase inhibitors and venous thromboembolic events in
atopic dermatitis: Protocols for a case-time control study and a
nested case-control study based on the French national health
insurance (SNDS) cohort. BMJ Open. 12(e059979)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tong Z, Zhang Y, Zhou K, Zou Y, Wu Z, Chen
J, Zhuang Z, Zhao Y, Gong T and Ji C: An observational study of
abrocitinib in adults with moderate-to-severe atopic dermatitis
after switching from dupilumab. J Am Acad Dermatol. 89:826–828.
2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Blauvelt A, de Bruin-Weller M, Gooderham
M, Cather JC, Weisman J, Pariser D, Simpson EL, Papp KA, Hong HC,
Rubel D, et al: Long-term management of moderate-to-severe atopic
dermatitis with dupilumab and concomitant topical corticosteroids
(LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded,
placebo-controlled, phase 3 trial. Lancet. 389:2287–2303.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Banerjee S, Biehl A, Gadina M, Hasni S and
Schwartz DM: JAK-STAT signaling as a target for inflammatory and
autoimmune diseases: Current and future prospects. Drugs.
77:521–546. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Blauvelt A, Silverberg JI, Lynde CW,
Bieber T, Eisman S, Zdybski J, Gubelin W, Simpson EL, Valenzuela F,
Criado PR, et al: Abrocitinib induction, randomized withdrawal, and
retreatment in patients with moderate-to-severe atopic dermatitis:
Results from the JAK1 atopic dermatitis efficacy and safety (JADE)
REGIMEN phase 3 trial. J Am Acad Dermatol. 86:104–112.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Clarke B, Yates M, Adas M, Bechman K and
Galloway J: The safety of JAK-1 inhibitors. Rheumatology (Oxford).
60 (Suppl 2):ii24–ii30. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Romero Jiménez RM, Herranz Pinto P, Campos
Domínguez M, Aceituno Mata S, Bellmunt A, Prades M, Arumi D,
Hernández-Martín I, Herrera-Lasso V, Llevat N, et al:
Cost-effectiveness analysis of abrocitinib compared with other
systemic treatments for severe atopic dermatitis in spain.
Pharmacoecon Open. 8:291–302. 2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Edwards SJ, Karner C, Jhita T, Barton S,
Marceniuk G, Yiu ZZN and Wittmann M: Abrocitinib, tralokinumab and
upadacitinib for treating moderate-to-severe atopic dermatitis.
Health Technol Assess. 28:1–113. 2024.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Lugović-Mihić L, Meštrović-Štefekov J,
Potočnjak I, Cindrić T, Ilić I, Lovrić I, Skalicki L, Bešlić I and
Pondeljak N: Atopic dermatitis: Disease features, therapeutic
options, and a multidisciplinary approach. Life (Basel).
13(1419)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lipsky BA, Senneville É, Abbas ZG,
Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M,
van Asten SA, et al: Guidelines on the diagnosis and treatment of
foot infection in persons with diabetes (IWGDF 2019 update).
Diabetes Metab Res Rev. 36 (Suppl 1)(e3280)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gu H, Chen XS, Chen K, Yan Y and Jin H:
Evaluation of diagnostic criteria for atopic dermatitis. Chin J
Dermatology. 33:222–226. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Langley RGB, Feldman SR, Nyirady J, van de
Kerkhof P and Papavassilis C: The 5-point investigator's global
assessment (IGA) scale: A modified tool for evaluating plaque
psoriasis severity in clinical trials. J Dermatolog Treat.
26:23–31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hanifin JM, Baghoomian W, Grinich E,
Leshem YA, Jacobson M and Simpson EL: The eczema area and severity
index-a practical guide. Dermatitis. 33:187–192. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Napolitano M, Ferrucci SM, Foggia L,
Hansel K, Pezzolo E, Stingeni L, Antonelli E, Picone V and Patruno
C: Comparison of long-term effectiveness and safety of upadacitinib
for atopic dermatitis between dupilumab-exposed and dupilumab-naïve
patients. Clin Drug Investig. 44:71–77. 2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
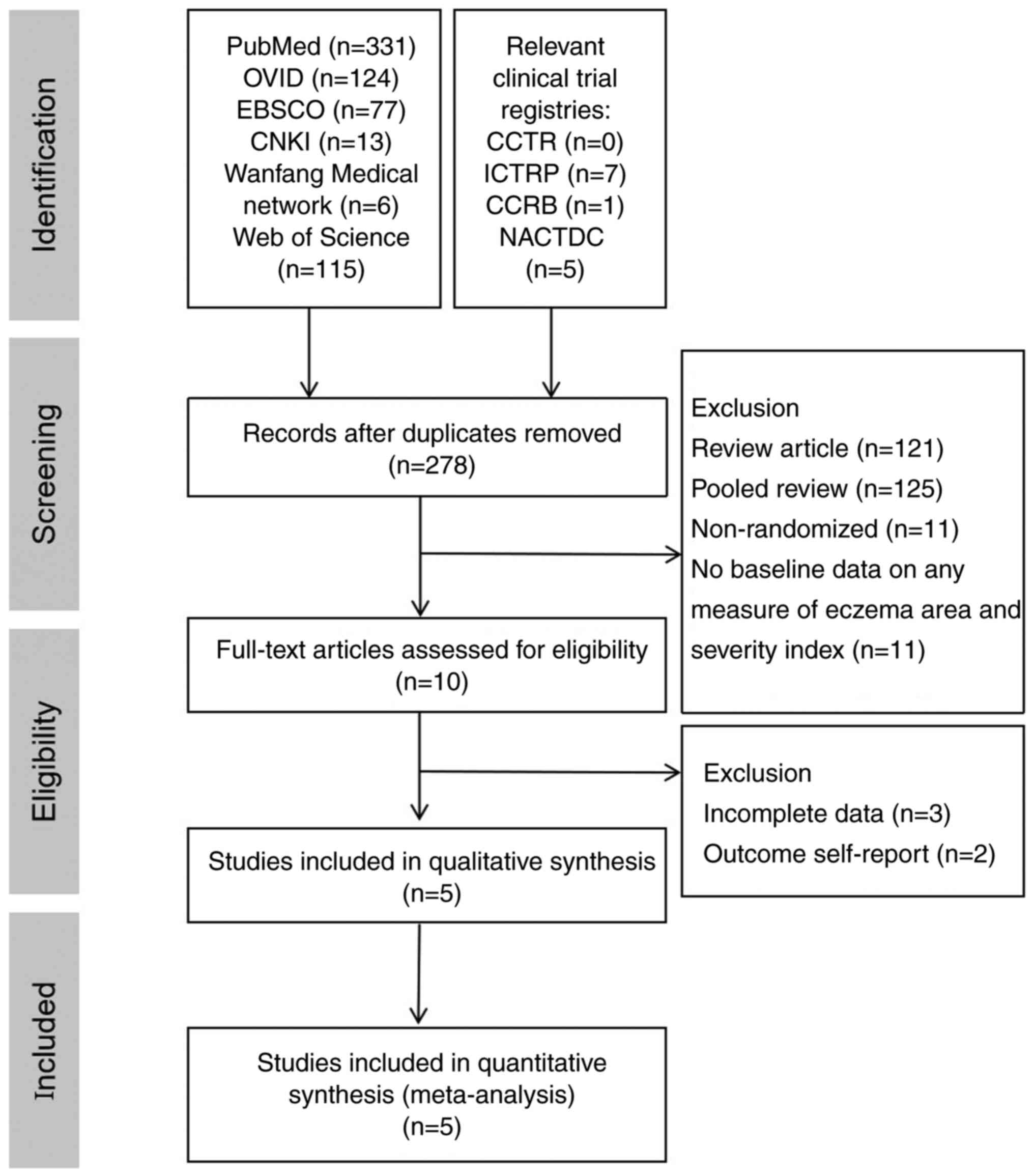
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ.
339(b2700)2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Veazie S, Peterson K, Bourne D, Anderson
J, Damschroder L and Gunnar W: Implementing high-reliability
organization principles into practice: A rapid evidence review. J
Patient Saf. 18:e320–e328. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Higgins JPT and Green S: Cochrane handbook
for systematic reviews of interventions. Version 5.1.0. The
Cochrane Collaboration, 2011. www.cochranehandbook.org.
|
|
37
|
Atkins D, Briss PA, Eccles M, Flottorp S,
Guyatt GH, Harbour RT, Hill S, Jaeschke R, Liberati A, Magrini N,
et al: Systems for grading the quality of evidence and the strength
of recommendations II: Pilot study of a new system. BMC Health Serv
Res. 5(25)2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
van Tulder M, Furlan A, Bombardier C and
Bouter L: Editorial Board of the Cochrane Collaboration Back Review
Group. Updated method guidelines for systematic reviews in the
cochrane collaboration back review group. Spine (Phila Pa 1976).
28:1290–1299. 2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
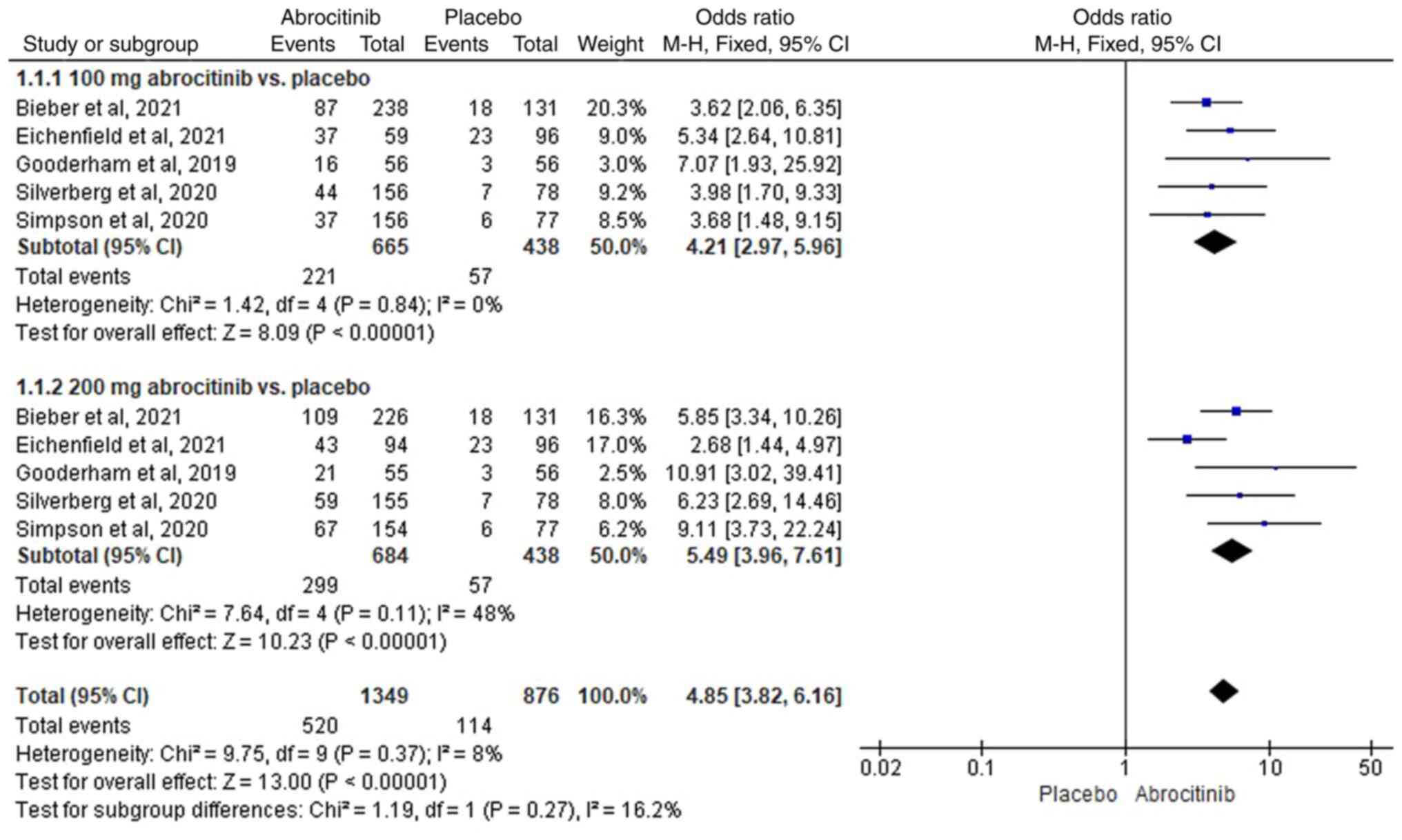
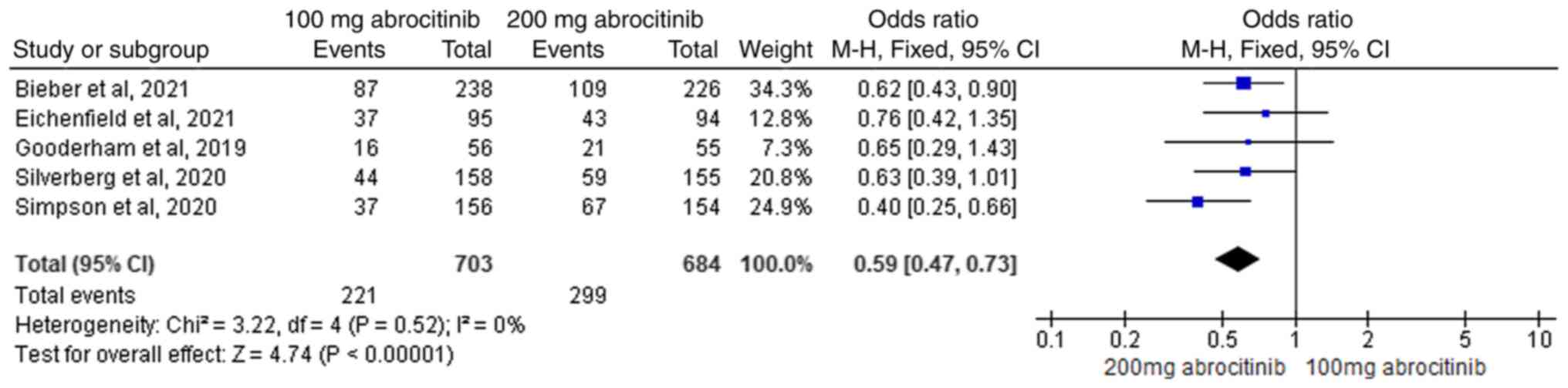
Bieber T, Simpson EL, Silverberg JI, Thaçi
D, Paul C, Pink AE, Kataoka Y, Chu CY, DiBonaventura M, Rojo R, et
al: Abrocitinib versus placebo or dupilumab for atopic dermatitis.
N Engl J Med. 384:1101–1112. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Eichenfield LF, Flohr C, Sidbury R,
Siegfried E, Szalai Z, Galus R, Yao Z, Takahashi H, Barbarot S,
Feeney C, et al: Efficacy and safety of abrocitinib in combination
with topical therapy in adolescents with moderate-to-severe atopic
dermatitis: The JADE TEEN randomized clinical trial. JAMA Dermatol.
157:1165–1173. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gooderham MJ, Forman SB, Bissonnette R,
Beebe JS, Zhang W, Banfield C, Zhu L, Papacharalambous J, Vincent
MS and Peeva E: Efficacy and safety of oral Janus kinase 1
inhibitor abrocitinib for patients with atopic dermatitis: A phase
2 randomized clinical trial. JAMA Dermatol. 155:1371–1379.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Silverberg JI, Simpson EL, Thyssen JP,
Gooderham M, Chan G, Feeney C, Biswas P, Valdez H, DiBonaventura M,
Nduaka C and Rojo R: Efficacy and safety of abrocitinib in patients
with moderate-to-severe atopic dermatitis: A randomized clinical
trial. JAMA Dermatol. 156:863–873. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Simpson EL, Sinclair R, Forman S,
Wollenberg A, Aschoff R, Cork M, Bieber T, Thyssen JP, Yosipovitch
G, Flohr C, et al: Efficacy and safety of abrocitinib in adults and
adolescents with moderate-to-severe atopic dermatitis (JADE
MONO-1): A multicentre, double-blind, randomised,
placebo-controlled, phase 3 trial. Lancet. 396:255–266.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
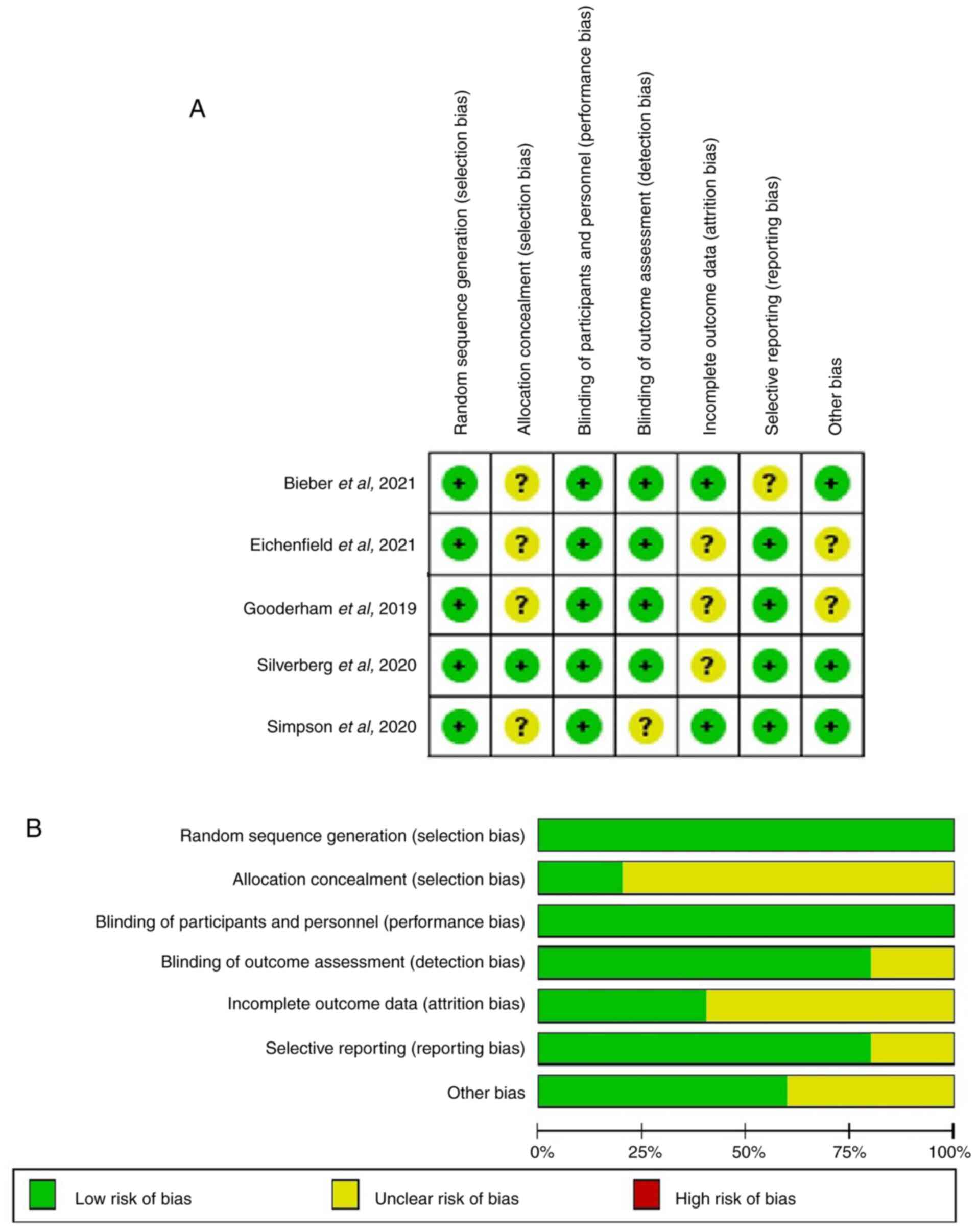
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L and Sterne JA:
, et al: The cochrane collaboration's tool for assessing
risk of bias in randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Boguniewicz M, Fonacier L, Guttman-Yassky
E, Ong PY, Silverberg J and Farrar JR: Atopic dermatitis yardstick:
Practical recommendations for an evolving therapeutic landscape.
Ann Allergy Asthma Immunol. 120:10–22.e2. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Reich K, Thyssen JP, Blauvelt A, Eyerich
K, Soong W, Rice ZP, Hong HC, Katoh N, Valenzuela F, DiBonaventura
M, et al: Efficacy and safety of abrocitinib versus dupilumab in
adults with moderate-to-severe atopic dermatitis: A randomised,
double-blind, multicentre phase 3 trial. Lancet. 400:273–282.
2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Boeri M, Sutphin J, Hauber B, Cappelleri
JC, Romero W and Di Bonaventura M: Quantifying patient preferences
for systemic atopic dermatitis treatments using a discrete-choice
experiment. J Dermatolog Treat. 33:1449–1458. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Boytsov NN, Gorritz M, Wang X,
Malatestinic WN, Wade RL and Goldblum OM: The current treatment
landscape in adult atopic dermatitis in the United States: Results
from a cross-sectional real-world study. J Dermatolog Treat.
33:1707–1717. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sterne JA, Egger M and Smith GD:
Systematic reviews in health care: Investigating and dealing with
publication and other biases in meta-analysis. BMJ. 323:101–105.
2001.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Howell MD, Kuo FI and Smith PA: Targeting
the Janus kinase family in autoimmune skin diseases. Front Immunol.
10(2342)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sroka-Tomaszewska J and Trzeciak M:
Molecular mecha-nisms of atopic dermatitis pathogenesis. Int J Mol
Sci. 22(4130)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gooderham MJ, Pink AE, Simpson EL,
Silverberg JI, Güler E and Watkins M: Abrocitinib 100 mg once daily
for moderate-to-severe atopic dermatitis: A review of efficacy and
safety, and expert opinion on use in clinical practice. Dermatol
Ther (Heidelb). 13:1893–1907. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Simpson EL, Silverberg JI, Nosbaum A,
Winthrop KL, Guttman-Yassky E, Hoffmeister KM, Egeberg A, Valdez H,
Zhang M, Farooqui SA, et al: Integrated safety analysis of
abrocitinib for the treatment of moderate-to-severe atopic
dermatitis from the phase II and phase III clinical trial program.
Am J Clin Dermatol. 22:693–707. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lee AJ and Ashkar AA: The dual nature of
type I and type II interferons. Front Immunol.
9(2061)2018.PubMed/NCBI View Article : Google Scholar
|