Introduction
Stroke or ‘brain attack’ occurs when the blood
supply to part of the brain is suddenly interrupted by occlusion or
hemorrhage, leading to neuronal dysfunction or neuronal death. It
is recognized as a major cause of mortality in Thailand and other
developing countries (1,2). Stroke is also a common cause of
neurological problems and disability, with very high associated
medical costs (3). Recent evidence
suggests that cerebral ischemia-induced brain damage is accompanied
by increased formation of free oxygen radicals in brain tissue.
Excessive production of reactive oxygen species (ROS) such as
superoxide anions, hydroxyl radicals and hydrogen peroxide may
cause oxidative stress-induced cell injury (4). Cerebral ischemia also initiates an
inflammatory response in the brain, involving the activation of
immune cells such as microglia and astrocytes, as well as the
release of inflammatory mediators including cytokines, chemokines
and adhesion molecules (5). This
inflammatory response can exacerbate neuronal damage by promoting
additional ROS production and causing the breakdown of the
blood-brain barrier, enabling immune cells and harmful substances
to enter the brain (6,7). In addition, prolonged cerebral
ischemia can trigger apoptotic pathways in neurons, especially in
the penumbra area (8). Apoptosis,
also known as programmed cell death, is a regulated process
involving removal of damaged or unnecessary cells. However,
excessive or uncontrolled apoptosis can contribute to neuronal
death in ischemic conditions. The apoptotic pathway, which involves
Bax, caspase-3 and Bcl-xL, plays a critical role in determining
neuronal cell death during a stroke (9).
Targeting the aforementioned factors is a promising
strategy for the development of innovative therapeutic
interventions. For example, inhibition of Bax activity, inhibition
of caspase-3 activity and enhancement of Bcl-xL expression all have
neuroprotective effects in preclinical stroke studies (10-12).
Mitogen-activated protein kinase (MAPK) is another key factor
contributing to the pathogenic process and has been linked to
neuronal damage during cerebral ischemia (13). The MAPK signaling pathway plays an
important role in cerebral ischemia-induced apoptosis, especially
the p38 MAPK pathway (14).
Previous studies have shown that inhibition of p38 MAPK reduces
apoptosis and improves stroke recovery (14,15).
Reduced mitofusin (Mfn) 1 and Mfn2 levels, because they cause
excess calcium (Ca2+) to accumulate in mitochondria,
facilitate the translocation of Bax to mitochondria, and contribute
to neuronal apoptosis too (16).
Previous investigations have revealed that Mfn2 plays a protective
role in an ischemic stroke model by reducing apoptosis (16-18).
Ginger (Zingiber officinale) has been widely
used in traditional medicine, and 6-gingerol is considered one of
its key active constituents. 6-Gingerol exhibits numerous
pharmacological activities, including anti-inflammatory,
antioxidant (19,20), anticancer (21), gastroprotective and anti-diabetic
(22) effects. These properties are
attributed to its ability to modulate various molecular targets and
signaling pathways, and some research suggests 6-gingerol exerts
neuroprotective effects in stroke. A previous study by the authors
revealed that 6-gingerol reduces brain damage and infarct volume in
the right middle cerebral artery occlusion (Rt.MCAO) model, partly
via anti-inflammatory and antioxidant pathways (19). To discover if any other mechanisms
are contributing to the beneficial effects of 6-gingerol in this
model, the present study examined how 6-gingerol affects cell
morphology, antioxidant defenses, and the anti-apoptotic factors
p38 MAPK and Mfn2.
Materials and methods
Experimental compounds
The test compound, 6-gingerol
(C17H26O4; PubChem ID: 442793),
was supplied by Chengdu Biopurify Phytochemicals Ltd. (http://www.biopurify.com/about_us.html)
with a purity of 98.7%. Piracetam, which served as the positive
control, was obtained from GSK plc. The vehicle, dimethyl sulfoxide
(DMSO), was obtained from Thermo Fisher Scientific, Inc.
Animals
Male Wistar rats (8 weeks-old; weighing 250-300 g)
were obtained from the Northeastern Laboratory Animal Center at
Khon Kaen University in Khon Kaen, Thailand. The rats were housed
in groups of five within typical metal cages measuring 37.5x48x21
cm3. They were maintained under standard conditions,
following a 12/12-h light/dark cycle, with humidity levels
maintained around 30-60%, and temperature set at 23±2˚C. Adequate
water and commercial pellets were available to them at all times.
All animal-related procedures carried out in the present study
received approval (approval no. IACUC-KKU-6/65) from the
Institutional Animal Care and Use Committee at Khon Kaen
University, Thailand.
Experimental design
A total of 60 healthy male Wistar rats were randomly
allocated into six groups (10 rats per group). Group 1 (control
group): Rats underwent a placebo surgery and received no treatment.
Group 2 (Rt.MCAO + vehicle group): Animals received DMSO, which was
used as a vehicle to dissolve the test substance. Group 3 (Rt.MCAO
+ piracetam group): Animals received piracetam at a dose of 250
mg/kg of body weight (BW), serving as a positive control. Groups
4-6 [Rt. MCAO + 6-gingerol (6-Gin) groups]: Animals received
different concentrations of 6-gingerol (5, 10 and 20 mg/kg BW).
Following the induction of Rt.MCAO, all groups received their
respective treatments intraperitoneally once daily for seven
consecutive days. Hematoxylin-eosin (H&E) staining was applied
to observe morphological changes of the cortex and the hippocampus
in five rats from each group. Biochemical assays were conducted on
the cortex and hippocampus of the remaining five animals per group
to examine catalase (CAT) and glutathione peroxidase (GSH-Px)
activities. In the cortex and hippocampus of rats, the expression
levels of Bax, Bcl-xL, caspase-3, MAPK and Mfn2 were assessed. This
evaluation was performed in rats treated with doses of 6-gingerol
that resulted in optimal changes in oxidative parameters. Piracetam
and 6-gingerol doses were selected based on previous research
findings by the authors (23,24).
The Rt.MCAO model
Before performing the surgery, all animals underwent
an overnight fasting period while being provided with unrestricted
access to water. During the operation, the rats were anesthetized
with isoflurane, with 5% for induction and 1-3% for maintenance,
delivered in 100% oxygen. The focal ischemic model was produced by
permanently occluding the right middle cerebral artery using a 4-0
silicone-coated monofilament, as previously described (25). The monofilament was carefully
inserted into the internal carotid artery, typically reaching a
depth of ~17 mm or until a slight resistance was detected.
Following the procedure, the wound was sutured, and a 10% povidone
iodine solution was applied to the incision site for postoperative
antiseptic care. Later in the present study, when rat brains were
being removed following the 7-day treatments, images of the
filaments occluding each middle cerebral artery were captured to
ensure that occlusion was consistent in every animal. In the sham
operation, rats underwent the same procedure as aforementioned, but
without the insertion of the monofilament. The criteria for humane
endpoints were defined as the inability to move, wound infection
following surgery, a weight loss of >20%, dehydration, dyspnea,
progressive pain, lack of response to external stimuli and bleeding
from any orifice. The authors along with the vet responsible for
the present study, monitored the animal health and behavior every
day. No animals were lost during the experimental period. The
infarct volume was measured in all animals, and this data has
recently been published (19).
Histopathological detection with
H&E staining
H&E staining involved staining for frozen
sections with hematoxylin for 4 min at room temperature (RT),
rinsing with running water for 10 min, then staining with eosin for
1 min at RT. Sections were then dehydrated, mounted and examined by
light microscopy.
Protein quantification
At the end of the experimental period, rats were
anesthetized with thiopental sodium (80 mg/kg BW) via
intraperitoneal administration before undergoing cardiac perfusion
with a cold normal saline solution. Subsequently, the brains were
rapidly removed from the skulls and separated into the cerebral
cortex and hippocampus. The concentration of protein in the cortex
and hippocampus was determined by the method described by Lowry
et al (26). Bovine serum
albumin (MilliporeSigma) was used as a standard during the
process.
Determination of CAT activity
CAT activity was measured using the method of
Goldblith and Proctor (27). In
brief, brain tissue was homogenized using phosphate buffer on ice
to prevent any enzymatic degradation. This homogenate was then
centrifuged (10,000 x g, 10 min, 4˚C) and the supernatant
containing the CAT was collected. Next, the supernatant was
combined with phosphate buffer and hydrogen peroxide
(H2O2), and absorbance was measured at 240 nm
using a spectrophotometer. The results are expressed as units per
milligram of protein (units/mg protein).
Determination of GSH-Px activity
GSH-Px activity was measured using a GSH-Px assay
kit from MilliporeSigma (cat. no. MAK437-1KT). Following
homogenization of the rat brain tissue and centrifugation (10,000 x
g, 10 min, 4˚C) of these homogenates, supernatant containing the
GSH-Px was collected. The supernatant was then mixed with phosphate
buffer, glutathione reductase, nicotinamide adenine dinucleotide
(NADPH), and hydrogen peroxide. Reduction in NADPH absorbance at
340 nm was used as a measure of GSH-Px activity. Enzyme activity
was quantified by scrutinizing the temporal evolution of absorbance
changes. Data are expressed as units/mg protein.
Western blot analysis
Bax, Bcl-xL, caspase-3, MAPK and Mfn2 expression
levels were measured in the rat cortices and hippocampi using the
western blot method as previously described (25). Each cortex and hippocampus were
homogenized with lysis buffer (Thermo Fisher Scientific, Inc.), and
the total protein concentrations were determined using the Lowry
method (26). Equal quantities (40
µg of protein) of protein were separated by 10% SDS-polyacrylamide
gel electrophoresis and subsequently transferred onto a Hybond-P
(PVDF) membrane (GE Healthcare; Cytiva). Non-specific binding sites
on the membrane were blocked by incubating with 5% non-fat dried
milk in 0.1% Tween-20 in Tris buffered saline (TBS-T), pH 7.4 at
room temperature for 1 h. The membrane was then incubated overnight
at 4˚C with mouse monoclonal anti-Bax (1:500; cat. no. 14-6999-82;
Thermo Fisher Scientific, Inc.), rabbit monoclonal anti-Bcl-xL
(1:1,000; cat. no. ab32370; Abcam), rabbit monoclonal
anti-caspase-3 (recognizing pro-caspase 3; 1:2,000; cat. no.
ab184787; Abcam), rabbit monoclonal anti-p38 MAPK (1:500; cat. no.
A14401; Abclonal Biotech Co., Ltd.), rabbit monoclonal
anti-mitofusin 2 (1:500; cat. no. A12771; Abclonal Biotech Co.,
Ltd.) and rabbit monoclonal anti-β-actin (1:5,000; cat. no. AC026;
Abclonal Biotech Co., Ltd.) antibodies. After washing with 0.1%
Tween-20 in Tris-buffered saline, the membrane was incubated with
anti-mouse (1:2,000; cat. no. 12-349; MilliporeSigma) or
anti-rabbit (1:2,000; cat. no. AS063; Abclonal Biotech Co., Ltd.)
secondary antibodies for 1 h at room temperature. The reactivity
was visualized with chemiluminescent substrate
(SupersignalTM PLUS Chemiluminescent Substrate West
Pico; Pierce; Thermo Fisher Scientific, Inc.). A ChemiDoc™ MP
imaging system with Image Lab software (Bio-Rad Laboratories, Inc.)
was used to capture photographs of the membranes against a white
background. The density of Bax, Bcl-xL, caspase-3, MAPK and Mfn2
bands was normalized to β-actin, and protein expression levels were
quantified using Image J® software version 1.53e
(National Institutes of Health).
Statistical analysis
The data are presented as the mean ± standard error
of the mean. Statistical analysis was performed using SPSS®
software (IBM Corp.). One-way analysis of variance (ANOVA) was
conducted, followed by a Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Protective effect of 6-gingerol on
morphological alterations in the cortex and hippocampus of rats
with induced-Rt.MCAO
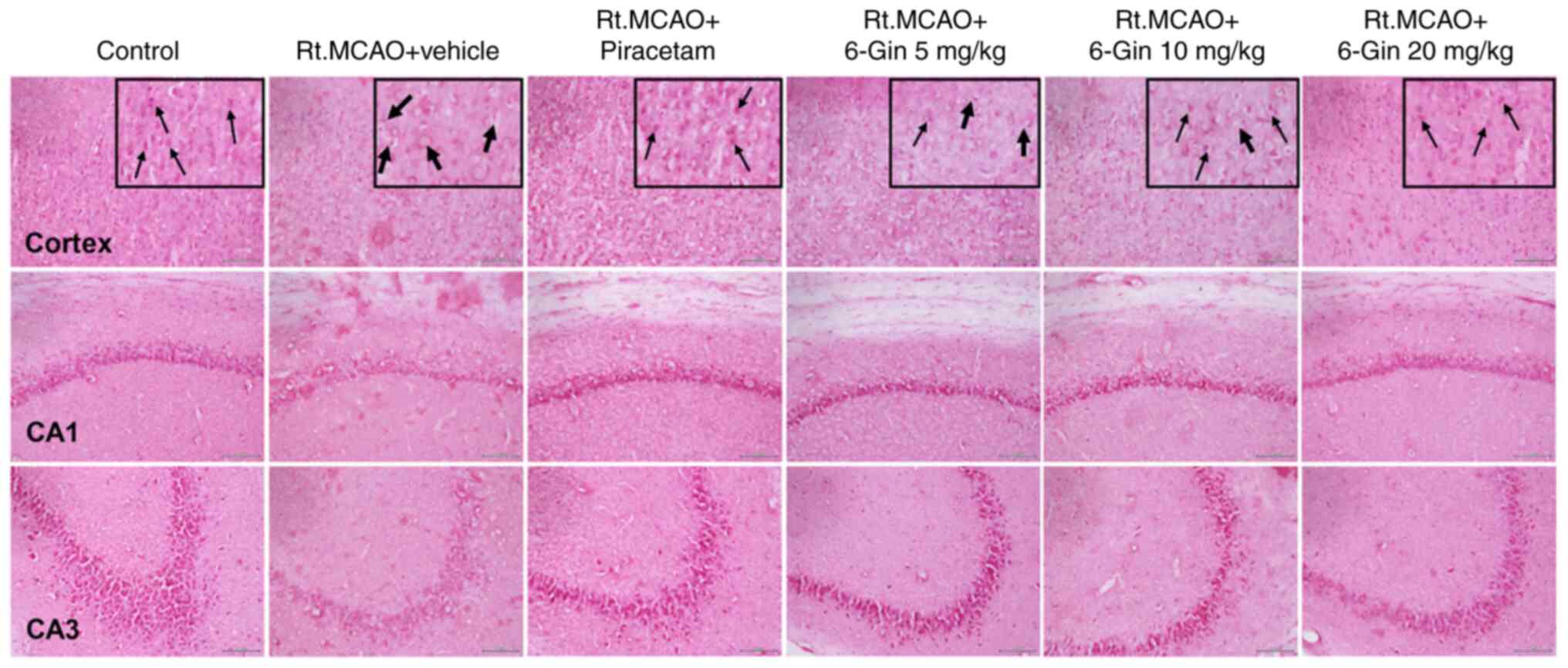
Previous work in the authors' laboratory (Faculty of
Medicine, Mahasarakham University) has quantified Nissl-positive
neurons and revealed that 6-gingerol can reverse ischemic
stroke-induced neuronal loss. Thus, the present study assessed the
potential of 6-gingerol to protect neurons against
histopathological changes following Rt. MCAO. The effect of
6-gingerol on Rt.MCAO-induced morphological alterations of the
cortex and hippocampus was evaluated qualitatively by H&E
staining (Fig. 1). H&E staining
of the control group revealed there were intact pyramidal neurons
with large nuclei. The Rt.MCAO + vehicle group, by contrast,
demonstrated evidence of cell loss, pyknotic nuclei, vacuolation
within the cytoplasm, and decreased neuronal density in the cortex
and pyramidal layer of the cerebral artery (CA)1 and CA3
hippocampus sub-regions. Treatment with various doses of 6-gingerol
reversed these alterations.
Protective effect of 6-gingerol on
antioxidant enzymes in the cortex and hippocampus of rats with
induced-Rt.MCAO
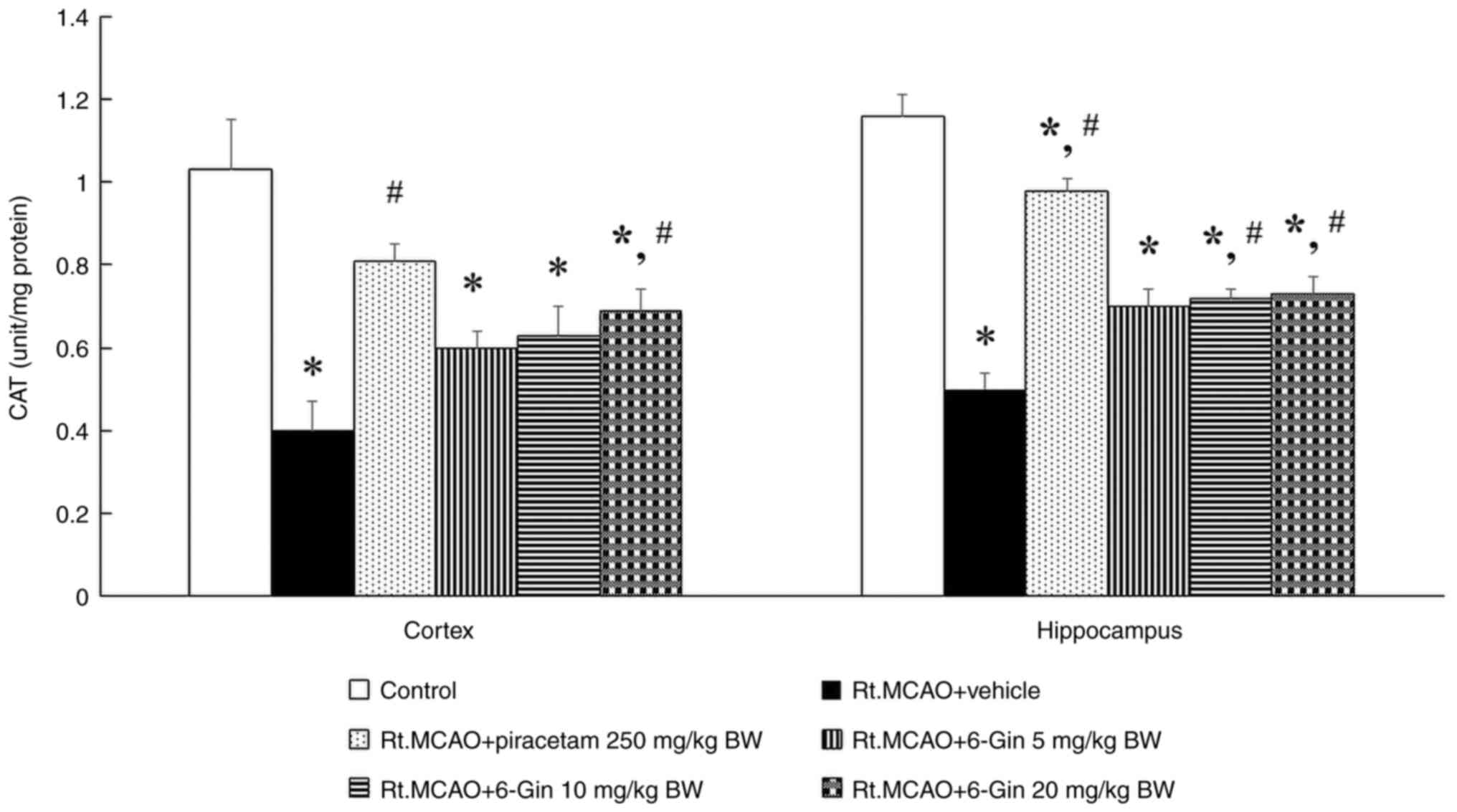
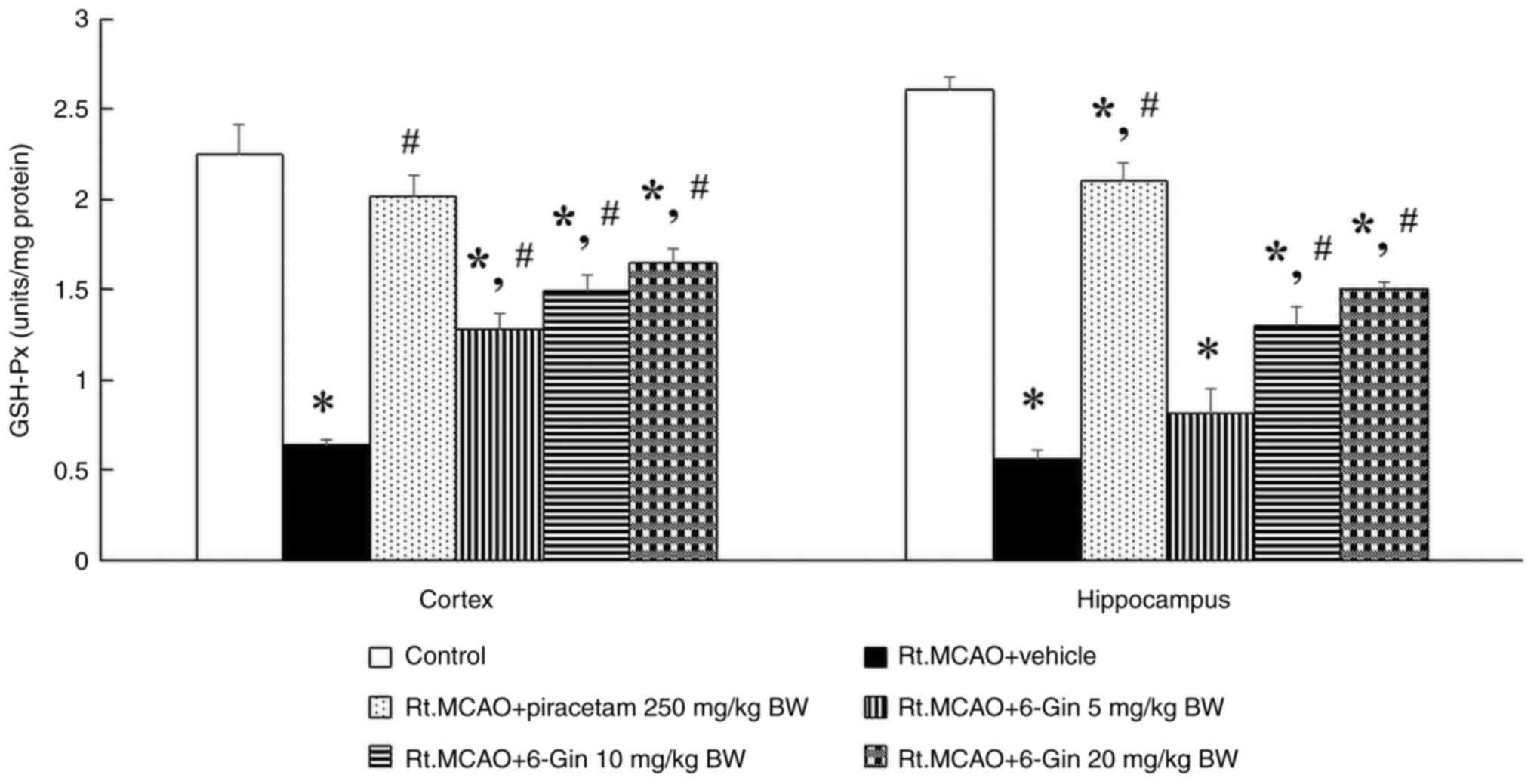
Lack of oxygen and nutrients leads to an imbalance
between the production of ROS and the brain's antioxidant defense
mechanisms. Enhancing the activity of these enzymes has been
associated with neuroprotection and improved functional recovery
following stroke. Therefore, CAT and GSH-Px activities were
assessed through biochemical assays performed on the cortex and
hippocampus. Rats administered with vehicle following Rt.MCAO
exhibited a significant decrease in CAT and GSH-Px activities
compared with the control group (P<0.05; Figs. 2 and 3). Conversely, the treatments with
piracetam or 6-gingerol at doses of 10 and 20 mg/kg BW markedly
attenuated Rt.MCAO-induced reduction of CAT and GSH-Px activities
compared with the Rt.MCAO + vehicle group (P<0.05; Figs. 2 and 3).
Ameliorative effect of 6-gingerol in
rats with induced-Rt.MCAO by activating anti-apoptotic pathway
Understanding the relationship between apoptosis and
ischemic stroke is essential if apoptosis-modulating drugs capable
of reducing brain damage are to be successfully developed. The
present study therefore measured Bax, Bcl-xL and caspase-3 protein
expression in the cerebral cortex and hippocampus by western blot
analysis. Previous experiments demonstrated that a dose of 20 mg/kg
BW 6-gingerol yielded favorable modifications in antioxidant enzyme
parameters; this dose was chosen to investigate the influence of
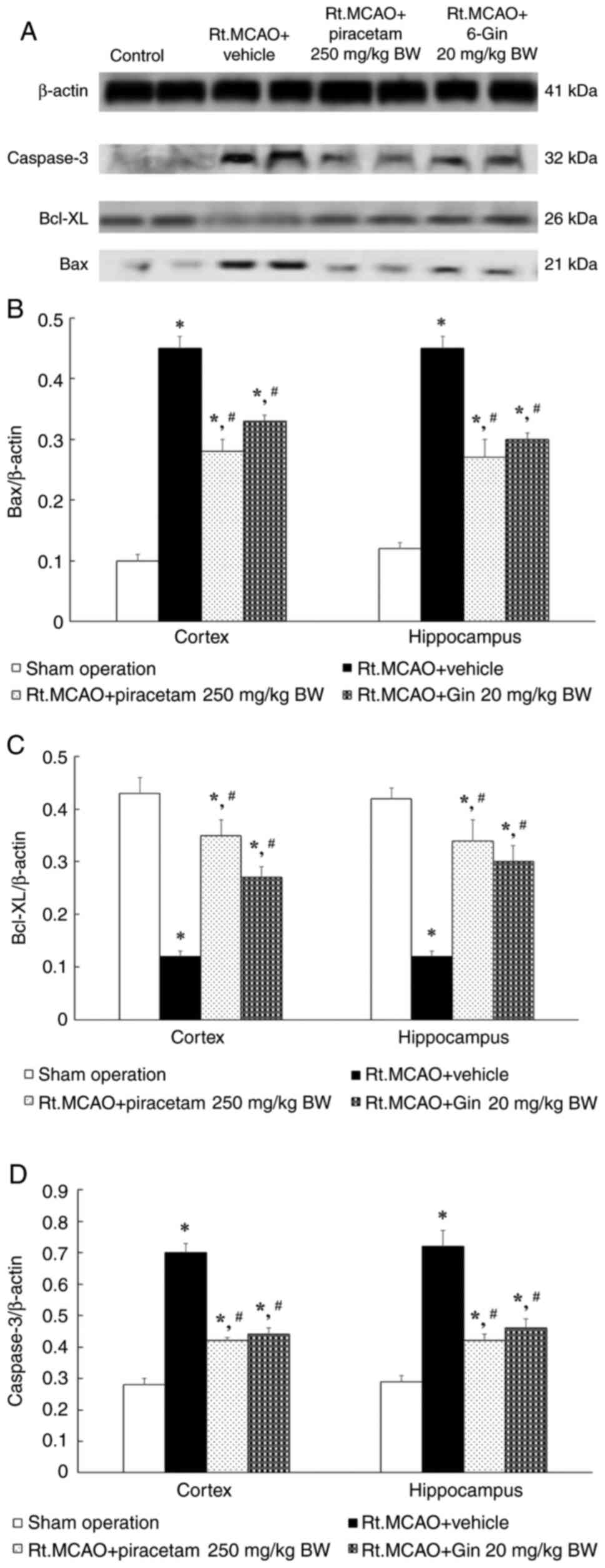
6-gingerol on Bax, Bcl-xL and caspase-3. In Fig. 4A, it can be observed that Rt.MCAO
rats treated with 250 mg/kg BW piracetam or 20 mg/kg BW 6-gingerol
for 7 days demonstrated a reduction in the density ratio of Bax and
caspase-3 to the β-actin band compared with the Rt.MCAO + vehicle
group (P<0.05; Fig. 4B and
D). By contrast, the density ratio
of Bcl-xL to the β-actin band exhibited a significant increase
compared with the Rt.MCAO + vehicle group (P<0.05; Fig. 4C).
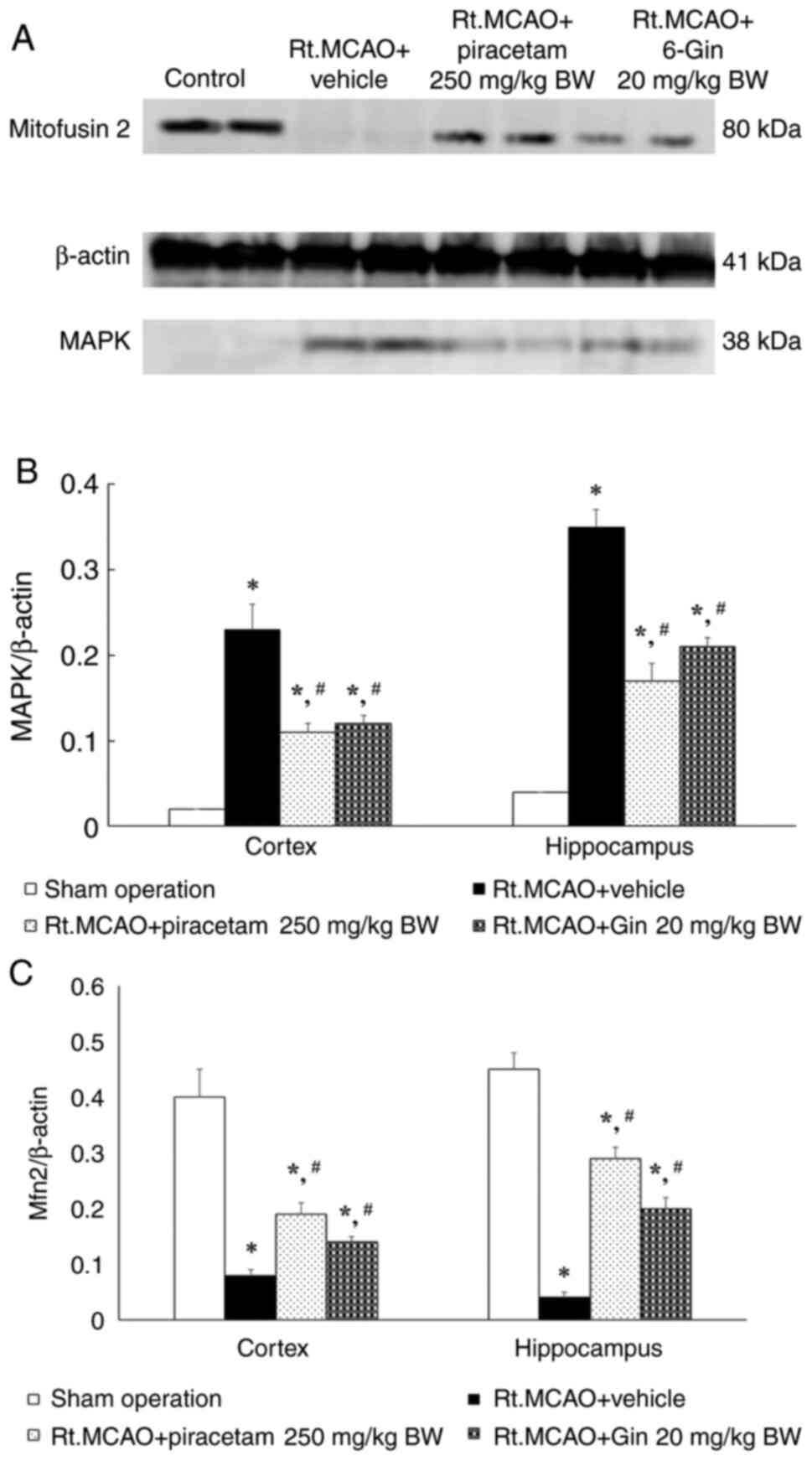
The effect of 6-gingerol on MAPK and
Mfn2 protein expression evaluated using western blot analysis
MAPK signaling and Mfn2 play significant roles in
stroke pathophysiology and could be suitable drug targets for
mitigating stroke-induced damage, restoring mitochondrial
homeostasis, and promoting neuronal survival and recovery. The
effect of 6-gingerol on MAPK and Mfn2 protein expression was
investigated using western blot analysis. The rats in the Rt.MCAO +
vehicle group exhibited a significant increase in the density ratio
of MAPK to the β-actin band; while experiencing a decrease in the
density ratio of Mfn2 to the β-actin band compared with the control
group (P<0.05). Importantly, treatment with piracetam (250 mg/kg
BW) or 6-gingerol (20 mg/kg BW) reduced the density ratio of MAPK
to the β-actin band compared with the vehicle group (P<0.05;
Fig. 5A). Additionally, the density
ratio of Mfn2 to the β-actin band exhibited significantly less
reduction compared with the rats in the Rt.MCAO + vehicle group
(P<0.05; Fig. 5B and C).
Discussion
Oxidative stress, resulting from an imbalance
between ROS production and antioxidant defenses, plays a pivotal
role in stroke pathophysiology. Antioxidants, through their ability
to scavenge ROS, may offer neuroprotective effects and mitigate the
detrimental consequences of stroke. CAT is an integral component of
the cellular antioxidant defense system. It cooperates with other
antioxidant enzymes, such as superoxide dismutase and GSH-Px, to
maintain cellular redox homeostasis and protect cells from
oxidative damage. Hence, the present study examined the influence
of 6-gingerol on CAT and GSH-Px enzymes in the cortex and
hippocampus. It was revealed that both the positive control,
piracetam, and the experimental compound, 6-gingerol, led to
increased CAT and GSH-Px activities in both the cortex and
hippocampus. These effects were observed when compared with the
group treated with vehicle only following Rt.MCAO. Numerous studies
have reported that augmenting the levels of these antioxidant
enzymes can alleviate brain damage resulting from ischemic stroke
(19,23,25,28).
Moreover, several studies have demonstrated that 6-gingerol and its
analogues have strong antioxidant activity. This is exerted via
multiple mechanisms, including the scavenging of free radicals,
oxidative stress reduction and enhancement of antioxidant enzyme
activity (19,29-32).
These properties enable 6-gingerol to protect against oxidative
damage in various tissues and organs, such as the brain, kidneys,
heart and colon (32).
Apoptosis, or programmed cell death, is a regulated
process that plays a role in removing damaged or unnecessary cells.
Prolonged cerebral ischemia has the potential to induce the
activation of apoptotic pathways in neurons, leading to neuronal
death (8). Bcl-xL expression has
been observed in both the developing embryonic and adult neurons of
the central nervous system (CNS) (33). It plays a crucial role in
safeguarding against neuronal apoptosis during brain development
and in response to various pathological triggers, such as cerebral
ischemia (12). Caspase-3 plays a
critical role as a mediator of apoptosis in acute and chronic
neurodegenerative conditions, including ischemic stroke (34,35).
Additionally, Bax serves as a pro-apoptotic protein localized in
the mitochondria, and its expression elevates during the initiation
of the intrinsic apoptotic pathway, resulting in mitochondrial
damage (36-38).
Numerous studies have revealed that apoptosis inhibitors can
effectively decrease ischemic neuronal injury (34,36).
In comparison with the Rt.MCAO + vehicle group, a significant
decrease was observed in the density ratio of Bax and caspase-3 to
the β-actin band in Rt.MCAO rats treated with 250 mg/kg BW
piracetam or 20 mg/kg BW 6-gingerol during the 7-day study period.
Conversely, the density ratio of Bcl-xL to the β-actin band
demonstrated a significant increase when compared with the Rt.MCAO
+ vehicle group in the present study. Accumulating evidence
revealed that inhibition of Bax or caspase-3 activity and
enhancement of Bcl-xL expression have neuroprotective effects in
preclinical studies (34,39-41).
The findings of the present study are consistent with a previous
cerebral ischemia study showing that gingerol administration
elevates Bcl-2 and brain-derived neurotrophic factor (BDNF) levels,
while simultaneously reducing Bax and cleaved caspase-3 levels
(42). In previous studies, it has
also been demonstrated that 6-gingerol exhibits robust
antiapoptotic and anti-inflammatory properties (43,44).
Additionally, its ability to induce autophagy has been associated
with the dissociation of the TRPV1/FAF1 complex (44). In the present study, piracetam was
used as a positive control due to its known protective effects.
Piracetam enhances cholinergic functions and reduces neuronal
inflammation, apoptosis and oxidative stress, thus highlighting its
beneficial effects as a therapeutic agent (45,46).
Previous studies have demonstrated that administration of piracetam
at doses of 250 or 500 mg/kg via the intraperitoneal (i.p.) route,
6, 9 and 22 h following ischemic insult, leads to a significant
reduction in infarct volume (47).
Furthermore, piracetam has been revealed to enhance cerebral blood
flow (48) and restore the fluidity
of the plasma membrane in the brain, thereby promoting improved
functioning of neuronal cells (49).
MAPKs are key signaling molecules that contribute to
the pathophysiology of stroke through their involvement in neuronal
injury, neuroinflammation, blood-brain barrier disruption and
oxidative stress (13-15).
Hence, the investigation conducted in the present study examined
the effect of 6-gingerol on MAPK using the rat model of focal
cerebral ischemia. It was demonstrated that rats treated with
piracetam at a dosage of 250 mg/kg BW or 6-gingerol at a dosage of
20 mg/kg BW for 7 days following Rt.MCAO, exhibit notable
reductions in the density ratios of MAPK to the β-actin band.
Bioinformatics studies have revealed that certain miRNAs play a
role in regulating MAPK, a crucial pathway involved in mitigating
inflammation and apoptosis in ischemic stroke (13,50,51).
The findings of the present study correspond well with a previous
sepsis study showing that 6-gingerol treatment diminishes
macrophage pyroptosis by inhibiting MAPK signaling pathways
(52), and a previous hypoxia study
showing that 6-gingerol effectively deactivates the p38 MAPK and
JNK pathways (53).
Mitochondrial dysfunction, characterized by impaired
oxidative phosphorylation and increased ROS production, has been
implicated in the pathogenesis of ischemic stroke (54). Mfn1 and Mfn2 are also involved in
the pathophysiology of ischemic stroke (55). In experimental work using the MCAO
rat model, it has been observed that reductions in Mfn1 and Mfn2
lead to mitochondrial Bax translocation and subsequent
Ca2+ overload, resulting in excitotoxicity and neuronal
apoptosis (16,56). Furthermore, Mfn2 has been revealed
to decrease caspase-3 activity and increase the ratio of Bcl-2/Bax,
thereby reducing cellular vulnerability to apoptosis in the context
of cerebral ischemic stroke (57).
In line with available studies, the current results demonstrated
that animals treated with piracetam (250 mg/kg BW) or 6-gingerol
(20 mg/kg BW) exhibited significantly less reduction in the density
ratio of Mfn2 to the β-actin band compared with rats in the Rt.MCAO
+ vehicle group. Supplementation with gingerol-enriched ginger has
been revealed to alleviate Mfn2 and mitochondrial inner membrane
fusion (OPA1) in diabetic rats (58). According to findings presented in a
previous study, phenolic compounds such as 6-gingerol and 6-shogaol
have pharmacological activities that support the generation of
functional mitochondria, thereby promoting mitochondrial biogenesis
(59).
DMSO is a commonly used solvent and vehicle in
biological and biochemical research. Studies have reported that the
lethal dose (LD50) of DMSO in rats, administered via
i.p. injection, is 9.9 ml/kg BW (60). Furthermore, another study revealed
that significant localized toxic impacts on the liver and kidneys
of rats were observed when plant extracts were dissolved in 10%
(v/v) DMSO (61). In the present
study, 1% (v/v) DMSO solution was selected as the vehicle. This
decision was based on literature reviews, which generally consider
DMSO safe at low concentrations (62). A total of 1% (v/v) DMSO
concentration is frequently employed in numerous biological
applications without causing significant toxicity, as supported by
previous publications made by the authors (19,25,63).
In addition to determining brain histomorphological
changes in rats after a stroke, it is useful to measure functional
outcomes such as motor, sensory, and cognitive abilities. The
present study did not assess behavioral impairments following
stroke. Therefore, a limitation of the present study is the lack of
assessment of neurological function to evaluate the degree of
damage over time.
The present study suggested that 6-gingerol can
ameliorate some of the pathological changes induced by ischemic
stroke and does so via antioxidant and anti-apoptotic pathways. In
addition to evaluating histomorphological damage to neurons, future
research is recommended to investigate the interaction between
neurons and glial cells, particularly involving neuroinflammation,
in an ischemic stroke model. Glial cells, including microglia,
astrocytes and oligodendrocytes, are the primary components of the
peri-infarct environment in the CNS and have been implicated in
immune regulation following a stroke. Previous studies have
demonstrated that glial cells regulate post-stroke
neuroinflammation. These cells can modulate signals of neuronal
damage, release cytokines, attract immune cells to the site of the
stroke, and interact with and affect the condition of other immune
cells (64,65). Assessment of the effect of
6-gingerol on neurotrophic factors such as BDNF, nerve growth
factor, and glial cell-derived neurotrophic factor will also be
necessary to fully elucidate the neuroprotective and neurogenic
effects of this compound.
In conclusion, 6-gingerol demonstrates significant
in vivo effectiveness in mitigating pathological changes
induced by cerebral ischemia. This beneficial effect is attributed,
in part, to its antioxidant and anti-apoptotic pathways. Further
investigations are warranted to explore the neurological and
biochemical effects of 6-gingerol in focal cerebral ischemia, as
well as to elucidate its complete mechanism of action.
Acknowledgements
The authors are grateful to Dr Tim Cushnie (Faculty
of Medicine, Mahasarakham University, Mahasarakham) for
language-editing assistance.
Funding
Funding: The present study was supported by Mahasarakham
University Faculty of Medicine (grant no. MED MSU 01/03/2566).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RK was involved in data analysis, wrote, reviewed
and edited the manuscript. JJ was the project's administrator,
designed and conceptualized the present study, acquired funding,
curated data, analyzed data, wrote the original draft, and wrote,
reviewed and edited the manuscript. RK and JJ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All protocols related to animal experimentation were
meticulously designed to minimize any potential suffering to the
animals involved. These protocols were conducted in strict
accordance with the approval granted by the Institutional Animal
Care and Use Committee at Khon Kaen University (Thailand), with a
designated record number of (approval no. IACUC-KKU-6/65).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Samuthpongtorn C, Jereerat T and Suwanwela
NC: Stroke risk factors, subtypes and outcome in elderly Thai
patients. BMC Neurol. 21(322)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gebreyohannes EA, Bhagavathula AS, Abebe
TB, Seid MA and Haile KT: In-hospital mortality among ischemic
stroke patients in Gondar University Hospital: A retrospective
cohort study. Stroke Res Treat. 2019(7275063)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lucas-Noll J, Clua-Espuny JL,
Lleixa-Fortuno M, Gavaldà-Espelta E, Queralt-Tomas L,
Panisello-Tafalla A and Carles-Lavila M: The costs associated with
stroke care continuum: A systematic review. Health Econ Rev.
13(32)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Checa J and Aran JM: Reactive Oxygen
Species: Drivers of Physiological and Pathological Processes. J
Inflamm Res. 13:1057–1073. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zivancevic K, Lovic D, Andjus PR and
Radenovic L: Neuroinflammation in Post-Ischemic Brain. In: Cerebral
Ischemia. Pluta R (ed). Brisbane, AU, 2021.
|
|
6
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z
and Gu L: Targeting oxidative stress and inflammation to prevent
ischemia-reperfusion injury. Front Mol Neurosci.
13(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qin C, Yang S, Chu YH, Zhang H, Pang XW,
Chen L, Zhou LQ, Chen M, Tian DS and Wang W: Signaling pathways
involved in ischemic stroke: Molecular mechanisms and therapeutic
interventions. Signal Transduct Target Ther. 7(215)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mao R, Zong N, Hu Y, Chen Y and Xu Y:
Neuronal death mechanisms and therapeutic strategy in ischemic
stroke. Neurosci Bull. 38:1229–1247. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Otsuka S, Itashiki Y, Tani A, Matsuoka T,
Takada S, Matsuzaki R, Nakanishi K, Norimatsu K, Tachibe Y,
Kitazato R, et al: Effects of different remote ischemia
perconditioning methods on cerebral infarct volume and neurological
impairment in rats. Sci Rep. 13(2158)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun Y, Xu Y and Geng L: Caspase-3
inhibitor prevents the apoptosis of brain tissue in rats with acute
cerebral infarction. Exp Ther Med. 10:133–138. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Park HA, Broman K and Jonas EA: Oxidative
stress battles neuronal Bcl-xL in a fight to the death. Neural
Regen Res. 16:12–15. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gao ZK, Shen XY, Han Y, Guo YS, Li K and
Bi X: Pre-ischemic exercise prevents inflammation and apoptosis by
inhibiting MAPK pathway in ischemic stroke. Transl Neurosci.
13:495–505. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu Z, Ge M, Li C, Yu L, Gu Y, Hu Y and
Cao Z: Effects of p38 MAPK signaling pathway on cognitive function
and recovery of neuronal function after hypoxic-ischemic brain
injury in newborn rats. J Clin Neurosci. 78:365–370.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hou K, Xiao ZC and Dai HL: p38 MAPK
Endogenous inhibition improves neurological deficits in global
cerebral ischemia/reperfusion mice. Neural Plast.
2022(3300327)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vongsfak J, Pratchayasakul W, Apaijai N,
Vaniyapong T, Chattipakorn N and Chattipakorn SC: The alterations
in mitochondrial dynamics following cerebral ischemia/reperfusion
injury. Antioxidants (Basel). 10(1384)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li J, Wu J, Zhou X, Lu Y, Ge Y and Zhang
X: Targeting neuronal mitophagy in ischemic stroke: An update.
Burns Trauma. 11(tkad018)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li C, Chen C, Qin H, Ao C, Chen J, Tan J
and Zeng L: The role of mitochondrial dynamin in stroke. Oxid Med
Cell Longev. 2022(2504798)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kongsui R and Jittiwat J: Ameliorative
effects of 6-gingerol in cerebral ischemia are mediated via the
activation of antioxidant and anti-inflammatory pathways. Biomed
Rep. 18(26)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Almatroodi SA, Alnuqaydan AM, Babiker AY,
Almogbel MA, Khan AA and Husain Rahmani A: 6-Gingerol, a bioactive
compound of ginger attenuates renal damage in
streptozotocin-induced diabetic rats by regulating the oxidative
stress and inflammation. Pharmaceutics. 13(317)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang S, Zhang C, Yang G and Yang Y:
Biological properties of 6-gingerol: A brief review. Nat Prod
Commun. 9:1027–1030. 2014.PubMed/NCBI
|
|
22
|
Mao QQ, Xu XY, Cao SY, Gan RY, Corke H,
Beta T and Li HB: Bioactive compounds and bioactivities of ginger
(Zingiber officinale Roscoe). Foods. 8(185)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jittiwat J, Suksamrarn A, Tocharus C and
Tocharus J: Dihydrocapsaicin effectively mitigates cerebral
ischemia-induced pathological changes in vivo, partly via
antioxidant and anti-apoptotic pathways. Life Sci.
283(119842)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Adetuyi BO and Farombi EO: 6-Gingerol, an
active constituent of ginger, attenuates lipopolysaccharide-induced
oxidation, inflammation, cognitive deficits, neuroplasticity, and
amyloidogenesis in rat. J Food Biochem. 45(e13660)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jittiwat J: Baihui point laser acupuncture
ameliorates cognitive impairment, motor deficit, and neuronal loss
partly via antioxidant and anti-inflammatory effects in an animal
model of focal ischemic stroke. Evid Based Complement Alternat Med.
2019(1204709)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
27
|
Goldblith SA and Proctor BE: Photometric
determination of catalase activity. J Biol Chem. 187:705–709.
1950.PubMed/NCBI
|
|
28
|
Davis SM and Pennypacker KR: Targeting
antioxidant enzyme expression as a therapeutic strategy for
ischemic stroke. Neurochem Int. 107:23–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Alharbi KS, Nadeem MS, Afzal O, Alzarea
SI, Altamimi ASA, Almalki WH, Mubeen B, Iftikhar S, Shah L and
Kazmi I: Gingerol, a natural antioxidant, attenuates hyperglycemia
and downstream complications. Metabolites. 2(1274)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ajayi BO, Adedara IA and Farombi EO:
Protective mechanisms of 6-gingerol in dextran sulfate
sodium-induced chronic ulcerative colitis in mice. Hum Exp Toxicol.
37:1054–1068. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ozkur M, Benlier N, Takan I, Vasileiou C,
Georgakilas AG, Pavlopoulou A, Cetin Z and Saygili EI: Ginger for
healthy ageing: A systematic review on current evidence of its
antioxidant, anti-inflammatory, and anticancer properties. Oxid Med
Cell Longev. 2022(4748447)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Abolaji AO, Ojo M, Afolabi TT, Arowoogun
MD, Nwawolor D and Farombi EO: Protective properties of
6-gingerol-rich fraction from Zingiber officinale (Ginger)
on chlorpyrifos-induced oxidative damage and inflammation in the
brain, ovary and uterus of rats. Chem Biol Interact. 270:15–23.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bas J, Nguyen T and Gillet G: Involvement
of Bcl-xL in neuronal function and development. Int J Mol Sci.
22(3202)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–339.
2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Glushakova OY, Glushakov AA, Wijesinghe
DS, Valadka AB, Hayes RL and Glushakov AV: Prospective clinical
biomarkers of caspase-mediated apoptosis associated with neuronal
and neurovascular damage following stroke and other severe brain
injuries: Implications for chronic neurodegeneration. Brain Circ.
3:87–108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bagheri G, Rezaee R, Tsarouhas K, Docea
AO, Shahraki J, Shahriari M, Wilks MF, Jahantigh H, Tabrizian K,
Moghadam AA, et al: Magnesium sulfate ameliorates carbon
monoxide-induced cerebral injury in male rats. Mol Med Rep.
19:1032–1039. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu G, Wang T, Wang T, Song J and Zhou Z:
Effects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on
cerebral ischemia rats. Biomed Rep. 1:861–867. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gupta R and Ghosh S: Putative roles of
mitochondrial Voltage-Dependent Anion Channel, Bcl-2 family
proteins and c-Jun N-terminal Kinases in ischemic stroke associated
apoptosis. Biochim Open. 4:47–55. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yenari MA: Pathophysiology of acute
ischemic stroke. Cleve Clin J Med. 71 (Suppl 1):S25–S27.
2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shang JL, Cheng Q, Duan SJ, Li L and Jia
LY: Cognitive improvement following ischemia/reperfusion injury
induced by voluntary running-wheel exercise is associated with
LncMALAT1-mediated apoptosis inhibition. Int J Mol Med.
41:2715–2723. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li Z, Xiao G, Wang H, He S and Zhu Y: A
preparation of Ginkgo biloba L. leaves extract inhibits the
apoptosis of hippocampal neurons in post-stroke mice via regulating
the expression of Bax/Bcl-2 and Caspase-3. J Ethnopharmacol.
280(114481)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhai Y, Liu BG, Mo XN, Zou M, Mei XP, Chen
W, Huang GD and Wu L: Gingerol ameliorates neuronal damage induced
by hypoxia-reoxygenation via the miR-210/brain-derived neurotrophic
factor axis. Kaohsiung J Med Sci. 38:367–377. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Alsahli MA, Almatroodi SA, Almatroudi A,
Khan AA, Anwar S, Almutary AG, Alrumaihi F and Rahmani AH:
6-Gingerol, a major ingredient of ginger attenuates
diethylnitrosamine-induced liver injury in rats through the
modulation of oxidative stress and anti-inflammatory activity.
Mediators Inflamm. 2021(6661937)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Luo J, Chen J, Yang C, Tan J, Zhao J,
Jiang N and Zhao Y: 6-Gingerol protects against cerebral
ischemia/reperfusion injury by inhibiting NLRP3 inflammasome and
apoptosis via TRPV1/FAF1 complex dissociation-mediated autophagy.
Int Immunopharmacol. 100(108146)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Verma DK, Gupta S, Biswas J, Joshi N,
Singh A, Gupta P, Tiwari S, Sivarama Raju K, Chaturvedi S,
Wahajuddin M and Singh S: New therapeutic activity of metabolic
enhancer piracetam in treatment of neurodegenerative disease:
Participation of caspase independent death factors, oxidative
stress, inflammatory responses and apoptosis. Biochim Biophys Acta
Mol Basis Dis. 1864:2078–2096. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mohammed AS, Al-Hassani AN, Alrawi RA and
Tawfeeq RD: The protective effect of taurine, piracetam and
vinpocetine on etoposide-induced inflammation and brain injury in
the serum of female albino rats. Ecancermedicalscience.
17(1499)2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tortiglione A, Minale M, Pignataro G,
Amoroso S, DiRenzo G and Annunziato L: The 2-oxopyrrolidinacetamide
piracetam reduces infarct brain volume induced by permanent middle
cerebral artery occlusion in male rats. Neuropharmacology.
43:427–433. 2002.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kessler J, Thiel A, Karbe H and Heiss WD:
Piracetam improves activated blood flow and facilitates
rehabilitation of poststroke aphasic patients. Stroke.
31:2112–2116. 2000.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yang Y, Feng J, Xu F and Wang J: Piracetam
inhibits ethanol (EtOH)-induced memory deficit by mediating
multiple pathways. Brain Res. 1676:83–90. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gugliandolo A, Silvestro S, Sindona C,
Bramanti P and Mazzon E: MiRNA: Involvement of the MAPK pathway in
ischemic stroke. A promising therapeutic target. Medicina (Kaunas).
57(1053)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Safa A, Abak A, Shoorei H, Taheri M and
Ghafouri-Fard S: MicroRNAs as regulators of ERK/MAPK pathway: A
comprehensive review. Biomed Pharmacother.
132(110853)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhang FL, Zhou BW, Yan ZZ, Zhao J, Zhao
BC, Liu WF, Li C and Liu KX: 6-Gingerol attenuates macrophages
pyroptosis via the inhibition of MAPK signaling pathways and
predicts a good prognosis in sepsis. Cytokine.
125(154854)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kang C, Kang M, Han Y, Zhang T, Quan W and
Gao J: 6-Gingerols (6G) reduces hypoxia-induced PC-12 cells
apoptosis and autophagy through regulation of miR-103/BNIP3. Artif
Cells Nanomed Biotechnol. 47:1653–1661. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yang JL, Mukda S and Chen SD: Diverse
roles of mitochondria in ischemic stroke. Redox Biol. 16:263–275.
2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang M, He Y, Deng S, Xiao L, Tian M, Xin
Y, Lu C, Zhao F and Gong Y: Mitochondrial quality control: A
pathophysiological mechanism and therapeutic target for stroke.
Front Mol Neurosci. 14(786099)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Martorell-Riera A, Segarra-Mondejar M,
Munoz JP, Ginet V, Olloquequi J, Pérez-Clausell J, Palacín M, Reina
M, Puyal J, Zorzano A and Soriano FX: Mfn2 downregulation in
excitotoxicity causes mitochondrial dysfunction and delayed
neuronal death. EMBO J. 33:2388–2407. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Jurcau A and Ardelean AI: Oxidative stress
in ischemia/reperfusion injuries following acute ischemic stroke.
Biomedicines. 10(574)2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang R, Santos JM, Dufour JM, Stephens ER,
Miranda JM, Washburn RL, Hibler T, Kaur G, Lin D and Shen CL:
Ginger root extract improves GI health in diabetic rats by
improving intestinal integrity and mitochondrial function.
Nutrients. 14(4384)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Razak AM, Tan JK, Mohd Said M and Makpol
S: Modulating effects of zingiberaceae phenolic compounds on
neurotrophic factors and their potential as neuroprotectants in
brain disorders and age-associated neurodegenerative disorders: A
review. Nutrients. 15(2564)2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kelava T, Javar I and Culo F: Biological
actions of drug solvents. Periodicum Biologorum. 113:311–320.
2011.
|
|
61
|
Kurdi SJA, Hamid NFS, Ranneh Y, Meng GY
and Hashim ZB: Dimethyl Sulfoxide and Their Toxicity. Int J Res
Biol Pharm. 5:9–18. 2019.
|
|
62
|
Kloverpris H, Fomsgaard A, Handley A,
Ackland J, Sullivan M and Goulder P: Dimethyl sulfoxide (DMSO)
exposure to human peripheral blood mononuclear cells (PBMCs)
abolish T cell responses only in high concentrations and following
coincubation for more than two hours. J Immunol Methods. 356:70–78.
2010.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Janyou A, Wicha P, Jittiwat J, Suksamrarn
A, Tocharus C and Tocharus J: Dihydrocapsaicin attenuates blood
brain barrier and cerebral damage in focal cerebral
ischemia/reperfusion via oxidative stress and inflammatory. Sci
Rep. 7(10556)2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Xu S, Lu J, Shao A, Zhang JH and Zhang J:
Glial cells: Role of the immune response in ischemic stroke. Front
Immunol. 11(294)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Amantea D, Micieli G, Tassorelli C,
Cuartero MI, Ballesteros I, Certo M, Moro MA, Lizasoain I and
Bagetta G: Rational modulation of the innate immune system for
neuroprotection in ischemic stroke. Front Neurosci.
9(147)2015.PubMed/NCBI View Article : Google Scholar
|