Introduction
Human immunodeficiency virus (HIV) and
Mycobacterium tuberculosis (MTB) are key pathogens
contributing to patient morbidity and mortality in developing
countries, particularly in Southeast Asia (1-3).
TB is one of the leading causes of mortality among patients with
HIV infection, accounting for ~187,000 global fatalities in
2021(4). TB-associated mortality is
3-fold higher in patients with HIV compared with that in
HIV-negative individuals. In particular, this risk is more
pronounced when untreated latent TB (LTB) reactivates into
clinically active TB (ATB) (5). The
mandatory Bacillus Calmette-Guérin (BCG) vaccination program for
children aged 0-2 months is a national program for vaccination
against TB implemented by The Ministry of Health of the Republic of
Indonesia (6). However, the
capacity of T cells to expand and differentiate in response to BCG
stimulation tends to wane with increasing age, increasing risk of
TB infection later in adulthood (7).
The balance between cytokines produced by T helper
(Th)1, 2 and 17 lymphocytes serves an essential role in regulating
response of the immune system to specific pathogens. HIV and MTB
function synergistically in causing an imbalance in cytokine
production, leading to a dysregulated immune system. This
disruption is key to understanding immunological dynamics of
HIV/MTB co-infection (8-11).
The increased expression of pro-inflammatory
cytokines produced by Th1 cells, including IFN-γ, TNF and IL-2,
coupled with IL-17A produced by Th17 cells, serves an important
role in the defense against MTB infection (12-16).
By contrast, a shift towards anti-inflammatory cytokines released
by Th2 cells, such as IL-4, IL-10, and IL-6, can lead to the
deterioration of the host immune system by HIV. This triggers the
reactivation of LTB to ATB (17,18).
Acquisition of the immune activation status caused by MTB notably
accelerates the progression of HIV infection by enhancing viral
replication (5,19-21).
This suggests that determination and analysis of the cytokine
expression profile during HIV/MTB co-infection may be essential in
understanding the mechansim underlying HIV progression.
Although several attempts have been made to evaluate
the pattern of cytokine expression in individuals with HIV either
with or without MTB co-infection (22-27),
further investigations are required to elucidate its association
with TB, especially in HIV- and TB-endemic countries. Therefore,
the present study aimed to analyze the expression profile of
cytokines, including IL-17A, IFN-γ, TNF, IL-2, IL-10, IL-6, and
IL-4, in individuals with HIV with or without MTB co-infection. The
aim of the present study was to faciliate the future development of
potential biomarkers for predicting disease progression in patients
with HIV and HIV-MTB-co-infection.
Materials and methods
Study design and participants
The present comparative study was approved by The
Health Research Ethics Committee, Faculty of Medicine, Universitas
Padjadjaran (approval no. 834/UN6.KEP/EC/2018; Bandung, Indonesia)
and conducted in accordance with the Declaration of Helsinki. The
recruitment of study participants was conducted at Hasan Sadikin
Central Hospital, Bandung between August 2018 and August 2019. A
total of 70 participants were recruited with mean age of 35±8
years; 56 males and 14 females. Written informed consent was
obtained from all study participants.
Patients aged ≥18 years with a positive diagnosis of
HIV according to the national algorithm testing sequence by The
Ministry of Health of the Republic of Indonesia were included as
participants (28). Patients with
pregnancy, malignancy and/or autoimmune conditions were excluded.
The serum of participants was collected and stored at -80˚C before
analysis at The Immunology Research Unit, Faculty of Medicine,
Universitas Padjadjaran.
TB status was determined based on the presence of
classical clinical symptoms of TB (chronic cough, weight loss, and
night sweats) as well as the result of the acid-fast staining test
by Ziehl-Neelsen method, MTB culture by using Lowenstein-Jensen
medium and IFN-γ release assay (IGRA). LTB was defined as a
positive IGRA result without clinical or radiographic findings of
ATB. Subsequently, 70 participants were divided into three
categories: i) HIV-ATB (n=19), ii) HIV-LTB n=21) and iii) HIV-alone
(n=30).
Acid-fast staining test by
Ziehl-Neelsen method
Samples were stained with 0.3% Carbol-fuchsin for 5
min. The stain was washed off with running water. Next, the slide
was covered with 3% acid alcohol for 1 min and rinsed with running
water. For counterstaining step, the slide was covered with 0.1%
methylene blue stain for 20 sec followed by washing with running
water and air-drying. Finally, the slide was examined
microscopically under a high power objective. A positive result
from the acid-fast staining test was determined if a minimum of one
acid-fast bacili in 100 fields was found.
MTB culture
The sputum sample was mixed with an equal volume of
4% NaOH solution in a centrifuge tube, vortexed, and incubated for
15 min at room temperature. After adding 14 ml of sterile
phosphate-buffered saline (PBS) at pH 6.8, the mixture was
centrifuged at 3,000 g/min for 15 min at room temperature. To
re-suspend the sediment, the supernatant was discarded, followed by
adding 1 ml of PBS (pH 6.8). The sediment was inoculated into vials
containing Lowenstein-Jensen (L-J) medium and incubated at 37˚C for
8 weeks and inspected weekly. A positive result from MTB culture
was determined if at least one colony was detected.
QuantiFERON-TB Gold Plus ELISA
(QFT-Plus) testing
IGRA using QFT-Plus (Qiagen GmbH) was performed
according to the manufacturer's protocols as previously described
(29). In brief, 1-ml-heparinized
blood samples were incubated at room temperature (17-25˚C) before
transfer to four QFT-Plus Blood Collection Tubes as follows: Nil,
containing heparin; TB Antigen Tube 1 (TB1), comprising early
secreted antigenic target (ESAT)-6 and culture filtrate protein
(CFP)-10 peptide; TB2, containing ESAT-6 and 10 kDa CFP-10 peptide
and mitogen, contained phytohemagglutinin, a non-specific stimulant
of T-cells. The aliquoted tubes were inverted to mix 10 times
before 16-24 h incubation at 37˚C, followed by centrifugation at
3,000 x g for 15 min at room temperature. Furthermore, 50 µl
working strength conjugate, 50 µl plasma samples and 50 µl
standards were added to the appropriate ELISA well plate. The ELISA
plate was incubated at room temperature for 120 min, followed by
washing using wash buffer and incubation at room temperature for 30
min. A total of 50 µl enzyme-stopping solution was added to each
well. The optical density was measured using the BioTek ELx800™
Absorbance Microplate Reader (BioTek® Instruments, Inc.) at 450 and
650 nm and analyzed using the QFT Plus analysis software (ver.2.71;
Qiagen GmbH). A positive test for IGRA was defined as concentration
of TB1 and/or TB2 minus Nil ≥0.35 IU/ml or ≥25% of the Nil
value.
Cytokine determination by cytometric
bead array (CBA)
IL-17A, IFN-γ, TNF, IL-10, IL-6, IL-4 and IL-2
levels were quantified in plasma samples using the BD CBA Human
Th1/Th2/Th17 Cytokine kit (BD Biosciences) based on the
manufacturer's protocols. Briefly, 50 µl each mixed captured beads,
standard solutions, unknown samples and detection reagent were
added to each assay tube, followed by 3 h incubation at room
temperature with protection from light. The samples were washed
with 1 ml wash buffer and centrifuged at 200 x g for 5 min at room
temperature. The bead pellet of each assay tube was resuspended
with 300 µl wash buffer after aspirating the supernatant. The
cytokine concentration in each sample was measured using the BD
FACSLyric™ Flow Cytometry System (BD Biosciences) and analyzed
using the FCAP Array™ Software (ver.3.0; BD Biosciences). The
theoretical detection limits were 18.9, 3.7, 3.8, 4.5, 2.4, 4.9 and
2.6 pg/ml for IL-17A, IFN-γ, TNF, IL-10, IL-6, IL-4 and IL-2,
respectively.
CD4 analysis
CD4 cell count was performed using the PIMA™ CD4
assay (Abbott Pharmaceutical Co. Ltd.). Briefly, ~25 µl venous
blood sample was added to the PIMA™ CD4 cartridge. In the case of
finger prick, the sample was directly collected onto the cartridge.
PIMA™ CD4 cartridge was inserted immediately into the Alere PIMA
CD4 analyzer (Alere, Inc.) and CD4+ cell count was
automatically calculated.
Statistical analysis
Kolmogorov-Smirnov normality test was performed
using GraphPad Prism (ver.9.5.1; Dotmatics). Kruskall-Wallis and
Dunn's post hoc analyses were used to compare the cytokine levels
between >2 groups. Data are presented as the mean ± standard
deviation. The result of cytokine analyses were presented as median
(range). P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline characteristics
The characteristics of the study participants are
presented in Table I. Among 70
participants in the present study, 56 (80%) were male and 14 (20%)
were female. The mean age of participants was 35 (25-43) years. The
total participants with HIV-ATB, HIV-LTB, and HIV-alone that had a
CD4 count <350 cells/µl were 15 (78.9%), 5 (23.8%) and 11
(36.7%), respectively.
 | Table IBaseline characteristics of the study
population. |
Table I
Baseline characteristics of the study
population.
| Variable | Total (n=70) | HIV-active
tuberculosis (n=19) | HIV-latent
tuberculosis (n=21) | HIV (n=30) |
|---|
| Mean age, years | 35±8 | 39±8 | 33±8 | 36±7 |
| Sex (%) | | | | |
|
Male | 56 (80.0) | 16 (84.2) | 14 (66.7) | 26 (86.7) |
|
Female | 14 (20.0) | 3 (15.8) | 7 (33.3) | 4 (13.3) |
| Viral load, copy/ml
(%) | | | | |
|
Not
detected | 58 (82.9) | 15 (78.9) | 17 (80.9) | 26 (86.7) |
|
<1,000 | 9 (12.8) | 3 (15.8) | 3 (14.3) | 3 (10.0) |
|
≥1,000 | 3 (4.3) | 1 (5.3) | 1 (4.8) | 1 (3.3) |
| CD4 count, cells/µl
(%) | | | | |
|
<350 | 31 (44.3) | 15 (78.9) | 5 (23.8) | 11 (36.7) |
|
≥350 | 39 (55.7) | 4 (21.1) | 16 (76.2) | 19 (63.3) |
Cytokine measurement
The comparison of IL-17A, IFN-γ, TNF, IL-10, IL-6,
IL-4 and IL-2 levels in patients with HIV-ATB, HIV-LTB and HIV is
presented in Table II. In the
HIV-ATB group, the median level of all cytokines was higher
compared with that in the HIV-LTB and HIV groups. Furthermore, a
significantly higher median expression of IFN-γ, TNF, IL-10, IL-6
and IL-2 was observed in the HIV-ATB compared to HIV-LTB and HIV
groups.
 | Table IIConcentration of cytokine
production. |
Table II
Concentration of cytokine
production.
| Cytokine,
pg/ml | HIV-ATB (n=19) | HIV-LTB (n=21) | HIV (n=30) | P-value |
|---|
| IL-17A | 3.26
(0.00-120.46) | 1.74
(0.00-15.5) | 3.07
(0.00-52.52) | 0.281 |
| IFN-γ | 0.94
(0.00-17.12) | 0.00
(0.00-1.25) | 0.09
(0.00-3.23) | <0.001 |
| TNF | 0.35
(0.00-11.99) | 0.07
(0.00-5.08) | 0.24
(0.00-55.14) | 0.018 |
| IL-10 | 3.24
(0.77-27.46) | 0.73
(0.24-7.17) | 1.06
(0.48-10.18) | <0.001 |
| IL-6 | 3.93
(0.94-21.92) | 1.13
(0.1-15.63) | 1.12
(0.54-11.44) | <0.001 |
| IL-4 | 0.59
(0.00-43.81) | 0.35
(0.00-1.49) | 0.39
(0.00-3.91) | 0.210 |
| IL-2 | 0.16
(0.00-4.69) | 0.02
(0.00-1.87) | 0.02
(0.00-2.07) | 0.006 |
IFN-γ, TNF, IL-10, IL-6 and IL-2 were significantly
higher in patients with HIV-ATB compared with those with HIV-LTB.
(Fig. 1). Significant difference in
IFN-γ, IL-10, IL-6 and IL-2 levels between patients in the HIV-ATB
group and those in the HIV group was observed.
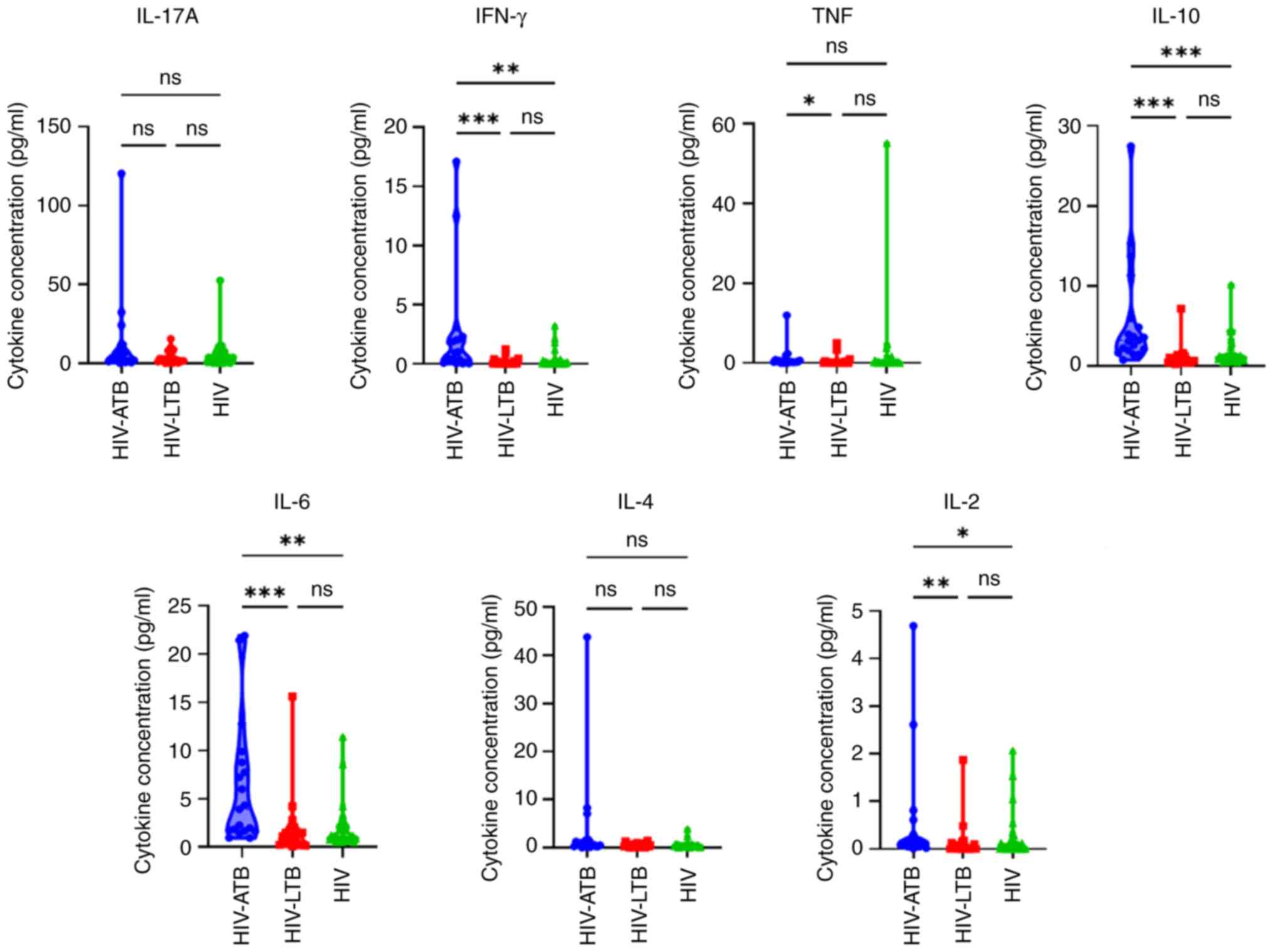 | Figure 1IL-17A, IFN-γ, TNF, IL-10, IL-6, IL-4
and IL-2 levels in HIV-ATB, HIV-LTB, and HIV groups.
*P<0.05, **P<0.01 and
***P<0.001. HIV, human immunodeficiency virus; LTB,
latent tuberculosis; ATB, active tuberculosis; ns, not
significant. |
Cytokine levels in patients with CD4 count <350
cells/µl was next assessed (Table
III). Median concentration of IFN-γ, IL-10, IL-6 and IL-2 was
significantly different between HIV-ATB, HIV-LTB and HIV groups.
IFN-γ, IL-10, IL-6, and IL-2 levels were also significantly higher
in HIV-ATB compared to HIV-LTB and HIV groups. By contrast, only
TNF and IL-10 levels differed significantly between the HIV-ATB,
HIV-LTB, and HIV groups in patients with with CD4 count ≥350
cells/µl (Table IV). However, post
hoc analysis revealed no statistical difference (data not shown).
For CD4 count <350 cells/µl, IFN-γ, IL-10 and IL-6 levels were
significantly higher in HIV-ATB compared with HIV-LTB and HIV
groups (Fig. 2).
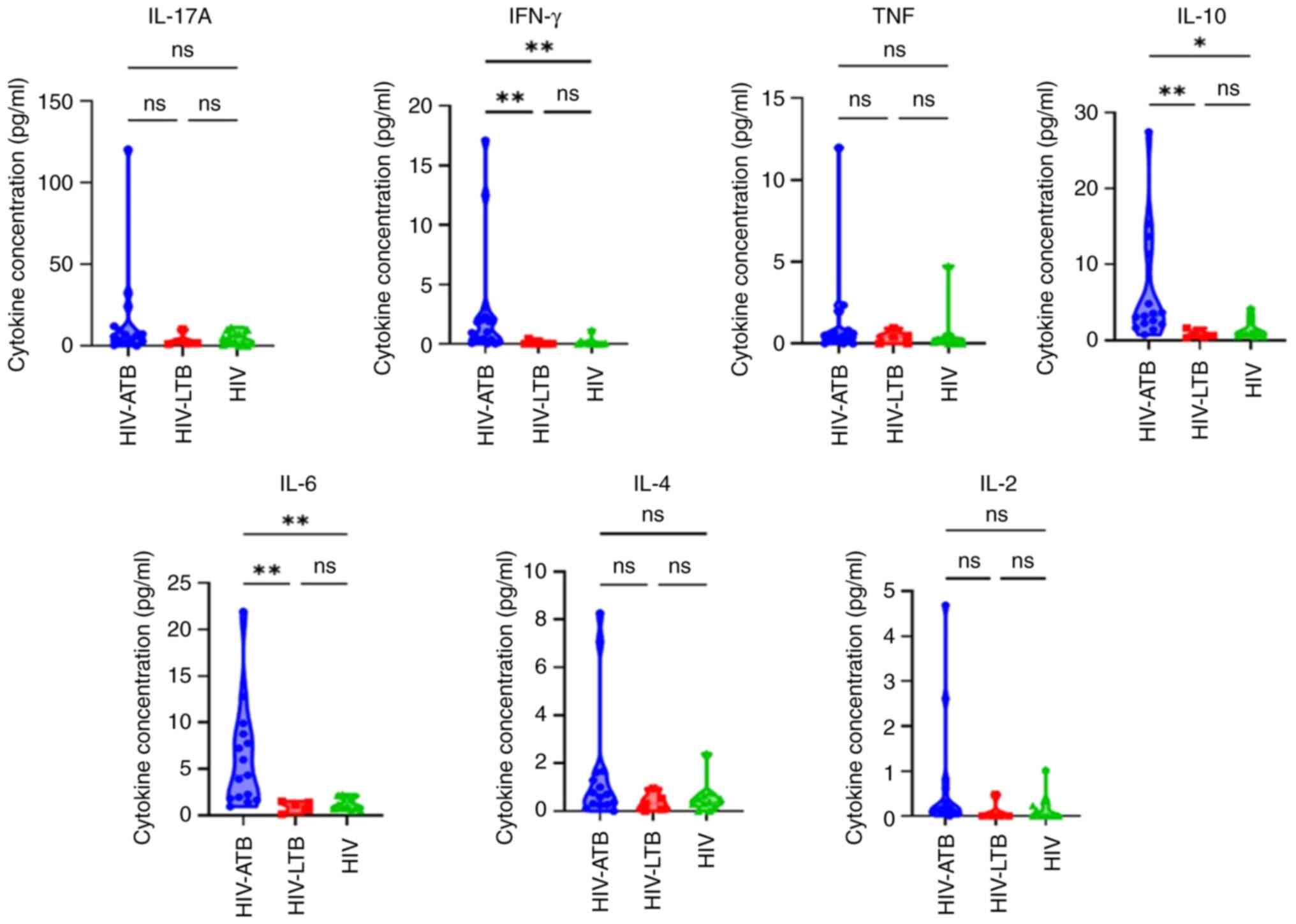 | Figure 2IL-17A, IFN-γ, TNF, IL-10, IL-6, IL-4
and IL-2 levels in HIV-ATB, HIV-LTB and HIV in patients with
CD4+ count <350 cells/µl. *P<0.05 and
**P<0.01. HIV, human immunodeficiency virus; LTB,
latent tuberculosis; ATB, active tuberculosis; ns, not
significant. |
 | Table IIICytokine production stratified by CD4
count <350 cells/µl. |
Table III
Cytokine production stratified by CD4
count <350 cells/µl.
| Cytokine,
pg/ml | HIV-ATB (n=15) | HIV-LTB (n=5) | HIV (n=11) | P-value |
|---|
| IL-17A | 6.23
(0.00-120.5) | 1.27
(1.21-10.16) | 3.37
(0.00-17.12) | 0.270 |
| IFN-γ | 0.97
(0.00-17.12) | 0.00
(0.00-0.44) | 0.06
(0.00-1.17) | <0.001 |
| TNF | 0.48
(0.00-11.99) | 0.43
(0.00-0.96) | 0.16
(0.00-4.75) | 0.240 |
| IL-10 | 3.24
(0.77-27.46) | 0.66
(0.36-1.65) | 0.99
(0.48-4.30) | 0.001 |
| IL-6 | 4.35
(0.96-21.92) | 1.13
(0.15-1.54) | 1.06
(0.54-2.26) | <0.001 |
| IL-4 | 0.70
(0.00-8.27) | 0.31
(0.00-0.95) | 0.39
(0.00-2.40) | 0.260 |
| IL-2 | 0.16
(0.00-4.69) | 0.00
(0.00-0.48) | 0.00
(0.00-0.48) | 0.030 |
 | Table IVCytokine production stratified by CD4
count >350 cells/µl. |
Table IV
Cytokine production stratified by CD4
count >350 cells/µl.
| Cytokine,
pg/ml | HIV-ATB (n=15) | HIV-LTB (n=5) | HIV (n=11) | P-value |
|---|
| IL-17A | 1.48
(0.57-3.06) | 2.11
(0.00-15.5) | 3.26
(0.00-52.52) | 0.660 |
| IFN-γ | 0.36
(0.01-1.89) | 0.00
(0.00-1.25) | 0.09
(0.00-3.23) | 0.060 |
| TNF | 0.34
(0.23-0.35) | 0.05
(0.00-5.08) | 0.26
(0.00-55.14) | 0.030 |
| IL-10 | 2.97
(1.44-5.96) | 0.74
(0.24-7.17) | 1.10
(0.48-10.18) | 0.008 |
| IL-6 | 1.71
(0.96-21.45) | 1.04
(0.10-15.63) | 1.15
(0.54-11.44) | 0.240 |
| IL-4 | 0.37
(0.19-43.81) | 0.37
(0.00-1.49) | 0.39
(0.00-3.91) | 0.880 |
| IL-2 | 0.13
(0.20-0.21) | 0.03
(0.00-1.87) | 0.06
(0.00-2.07) | 0.430 |
Discussion
In the present study, IFN-γ, TNF, IL-10, IL-6 and
IL-2 expression was significantly different in individuals infected
with HIV-ATB compared with HIV-LTB.
Previous study showed that T cell cytokine profiles
can be used to indicate certain disease states, including ATB and
LTB. The secretory balance of pro- and anti-inflammatory cytokines
generated by Th1 (IFN-γ, TNF and IL-2) and Th2 cells (IL-10, IL-6
and IL-4) during LTB is regulated to prevent reactivation into ATB
(30). Its imbalance in patients
testing positive for HIV with MTB co-infection affects the function
of the immune system (19,31).
IFN-γ, TNF and IL-2 serve important roles in
controlling the growth of MTB by activating innate and adaptive
immune cells and modulating formation of granuloma (32,33).
Compared with previous studies, a higher concentration of IFN-γ,
TNF and IL-2 was here observed in HIV-ATB compared with HIV-LTB and
HIV groups (15,31). This finding suggests that host
protective responses against MTB infection by increasing the
expression of pro-inflammatory cytokines is insufficient to prevent
reactivation into ATB (34). In
accordance with other reports, the present results also indicated
that the expression of anti-inflammatory cytokines, particularly
IL-10 and IL-6, in individuals with HIV-ATB was higher compared
with that in HIV-LTB and HIV-alone (15,19,31).
This could indicate that a shift towards the promotion of
anti-inflammatory Th2 cytokines is associated with failure to
curtail the growth of MTB in HIV-LTB, thereby facilitating
progression into ATB.
Here, only IFN-γ, IL-10 and IL-6 were significiantly
different in patients with HIV-ATB and HIV-LTB when the CD4 count
was <350 cells/µl. Furthermore, expression of IFN-γ, IL-10 and
IL-6 was not influenced by low CD4 count. The median concentration
of IL-10 and IL-6 was higher than other cytokines in patients with
HIV-ATB, suggesting a dominance of anti-inflammatory over
pro-inflammatory cytokines.
To the best of our knowledge, the present study is
the first to investigate expression of IL-17A, IFN-γ, TNF, IL-10,
IL-6, IL-4 and IL-2 in individuals with HIV-ATB, HIV-LTB and
HIV-alone.
There are certain limitations in the present study.
The cross-sectional study design limited investigation of cytokine
expression dynamics in the progression from LTB into ATB. In
addition, there was a small sample size due to difficulty acquiring
subjects that met the inclusion criteria within the study period.
Therefore, long-term cohort studies with larger subject population
are required to observe changes in over time, especially when other
associated opportunistic infections, such as aspergillosis and
cryptococcosis are present (35).
In conclusion, the present study revealed
differences in the cytokine profiles, particularly IFN-γ, IL-10 and
IL-6, in patients with HIV-ATB and HIV-LTB, especially when the CD4
count was <350 cells/µl. However, further investigations are
required to elucidate the mechanism of pro- and anti-inflammatory T
cell cytokines in pathogenesis of immune activation during HIV-MTB
co-infection.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a grant-in-aid for
Riset Kompetensi Dosen Unpad from Universitas Padjadjaran (grant
no. 1959/UN6.3.1/PT.00/2021).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ARI, DKT and RW designed the study. ARI, AS, DKT
confirm the auntheticity of all the raw data. AS, JH, NMDR and SM
recruited study participants. AS, JH, NMDR and SM conducted
experiments and collect study data. ARI, AS, JH, NMDR and SM
analyzed the study data. ARI, AS, JH, NMDR, SM and DKT wrote the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Health
Research Ethics Committee, Faculty of Medicine, Universitas
Padjadjaran (approval no. 834/UN6.KEP/EC/2018; Bandung, Indonesia)
and conducted in accordance with the Declaration of Helsinki.
Written informed consent was obtained from all participants
included in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao L, Zhou F, Li X and Jin Q: HIV/TB
co-infection in mainland China: A meta-analysis. PLoS One.
5(e10736)2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pendse R, Gupta S, Yu D and Sarkar S:
HIV/AIDS in the South-East Asia region: Progress and challenges. J
Virus Erad. 2 (Suppl 4):S1–S6. 2016.PubMed/NCBI
|
|
3
|
Mandal S, Bhatia V, Sharma M, Mandal PP
and Arinaminpathy N: The potential impact of preventive therapy
against tuberculosis in the WHO South-East Asian Region: A
modelling approach. BMC Med. 18(163)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bagcchi S: WHO's Global Tuberculosis
Report 2022. Lancet Microbe. 4(e20)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pawlowski A, Jansson M, Sköld M,
Rottenberg ME and Källenius G: Tuberculosis and HIV co-infection.
PLoS Pathog. 8(e1002464)2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kusnanto K, Arifin H and Kurniawati Y:
Determinant of BCG vaccine coverage among Indonesian children aged
0-2 months. Child Youth Serv Rev. 116(105238)2020.
|
|
7
|
Whittaker E, Nicol MP, Zar HJ, Tena-Coki
NG and Kampmann B: Age-related waning of immune responses to BCG in
healthy children supports the need for a booster dose of BCG in TB
endemic countries. Sci Rep. 8(15309)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Reuter MA, Pombo C and Betts MR: Cytokine
production and dysregulation in HIV pathogenesis: Lessons for
development of therapeutics and vaccines. Cytokine Growth Factor
Rev. 23:181–191. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kaur R, Dhakad MS, Goyal R, Bhalla P and
Dewan R: Study of TH1/TH2 Cytokine Profiles in HIV/AIDS Patients in
a Tertiary Care Hospital in India. J Med Microbiol Diagn.
5(214)2016.
|
|
10
|
French MA, Cozzi-Lepri A, Arduino RC,
Johnson M, Achhra AC and Landay A: INSIGHT SMART Study Group.
Plasma levels of cytokines and chemokines and the risk of mortality
in HIV-infected individuals: A case-control analysis nested in a
large clinical trial. AIDS. 29:847–851. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Indrati AR, Sumarpo A, Atmadja P, Wisesa
RR, Ghozali M, Judistiani RTD and Setiabudiawan B: Exploring
alternative cytokines as potential biomarkers for latent
tuberculosis infection in pregnant women. PLoS One.
17(e0270552)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zeng G, Zhang G and Chen X: Th1 cytokines,
true functional signatures for protective immunity against TB? Cell
Mol Immunol. 15:206–215. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lyadova IV and Panteleev AV: Th1 and Th17
cells in tuberculosis: Protection, pathology, and biomarkers.
Mediators Inflamm. 2015(854507)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chandra P, Grigsby SJ and Philips JA:
Immune evasion and provocation by Mycobacterium tuberculosis. Nat
Rev Microbiol. 20:750–766. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nosik M, Ryzhov K, Rymanova I, Sobkin A,
Kravtchenko A, Kuimova U, Pokrovsky V, Zverev V and Svitich O:
Dynamics of plasmatic levels of pro- and anti-inflammatory
cytokines in HIV-infected individuals with M. Tuberculosis
Co-infection. Microorganisms. 9(2291)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Murray LW, Satti I, Meyerowitz J, Jones M,
Willberg CB, Ussher JE, Goedhals D, Hurst J, Phillips RE, McShane
H, et al: Human immunodeficiency virus infection impairs Th1 and
Th17 mycobacterium tuberculosis-specific T-Cell responses. J Infect
Dis. 217:1782–1792. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dawany N, Showe LC, Kossenkov AV, Chang C,
Ive P, Conradie F, Stevens W, Sanne I, Azzoni L and Montaner LJ:
Identification of a 251 gene expression signature that can
accurately detect M. tuberculosis in patients with and without HIV
co-infection. PLoS One. 9(e89925)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sharan R, Bucşan AN, Ganatra S, Paiardini
M, Mohan M, Mehra S, Khader SA and Kaushal D: Chronic immune
activation in TB/HIV Co-infection. Trends Microbiol. 28:619–632.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sullivan ZA, Wong EB, Ndung'u T,
Kasprowicz VO and Bishai WR: Latent and active tuberculosis
infection increase immune activation in individuals Co-Infected
with HIV. EBioMedicine. 2:334–340. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Del Amo J, Malin AS, Pozniak A and De Cock
KM: Does tuberculosis accelerate the progression of HIV disease?
Evidence from basic science and epidemiology. AIDS. 13:1151–1158.
1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Appay V and Sauce D: Immune activation and
inflammation in HIV-1 infection: Causes and consequences. J Pathol.
214:231–241. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Queiroz ATL, Araújo-Pereira M,
Barreto-Duarte B, Gomes-Silva A, Costa AG, Andrade AMS,
Miguez-Pinto JP, Spener-Gomes R, Souza AB, Benjamin A, et al:
Immunologic biomarkers in peripheral blood of persons with
tuberculosis and advanced HIV. Front Immunol.
13(890003)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Benjamin R, Banerjee A, Sunder SR, Gaddam
S, Valluri VL and Banerjee S: Discordance in CD4+T-Cell Levels and
Viral Loads with Co-Occurrence of Elevated Peripheral TNF-α and
IL-4 in Newly Diagnosed HIV-TB Co-Infected Cases. PLoS One.
8(e70250)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lungu P, Njelesani E, Sukwa T, Ngalamika
O, Munsaka S, Kilembe W, Lakhi S and Mwaba P: Immune correlates of
Mycobacterium Tuberculosis patients in Zambia stratified by HIV
serostatus and level of immunity-a cross-sectional analytical
laboratory based study. PLoS One. 17(e0262454)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pollock KM, Montamat-Sicotte DJ, Grass L,
Cooke GS, Kapembwa MS, Kon OM, Sampson RD, Taylor GP and Lalvani A:
PD-1 expression and cytokine secretion profiles of Mycobacterium
tuberculosis-specific Cd4+ T-cell subsets; potential correlates of
containment in HIV-TB co-infection. PLoS One.
11(e0146905)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chiacchio T, Delogu G, Vanini V, Cuzzi G,
De Maio F, Pinnetti C, Sampaolesi A, Antinori A and Goletti D:
Immune characterization of the HBHA-specific response in
mycobacterium tuberculosis-infected patients with or without HIV
infection. PLoS One. 12(e0183846)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Duffy FJ, Thompson EG, Scriba TJ and Zak
DE: Multinomial modelling of TB/HIV co-infection yields a robust
predictive signature and generates hypotheses about the HIV+TB+
disease state. PLoS One. 14(e0219322)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nursalam N, Sukartini T, Kuswanto H,
Setyowati S, Mediarti D, Rosnani R, Pradipta RO, Ubudiyah M, Mafula
D, Klankhajhon S and Arifin H: Investigation of discriminatory
attitude toward people living with HIV in the family context using
socio-economic factors and information sources: A nationwide study
in Indonesia. PeerJ. 10(e13841)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rudeeaneksin J, Srisungngam S, Klayut W,
Bunchoo S, Bhakdeenuan P and Phetsuksiri B: QuantiFERON-TB Gold
Plus and QuantiFERON-TB Gold In-tube assays for detecting latent
tuberculosis infection in Thai healthcare workers. Rev Inst Med
Trop Sao Paulo. 65(e13)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
O'Garra A, Redford PS, McNab FW, Bloom CI,
Wilkinson RJ and Berry MP: The Immune Response in Tuberculosis.
Annu Rev Immunol. 31:475–527. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nosik M, Belikova MG, Ryzhov K, Avdoshina
D, Sobkin A, Zverev V and Svitich O: Unique profile of
proinflammatory cytokines in plasma of drug-naïve individuals with
advanced HIV/TB Co-Infection. Viruses. 15(1330)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bruchfeld J, Correia-Neves M and Källenius
G: Tuberculosis and HIV Coinfection. Cold Spring Harb Perspect Med.
5(a017871)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lahey T, Sheth S, Matee M, Arbeit R,
Horsburgh CR, Mtei L, Mackenzie T, Bakari M, Vuola JM, Pallangyo K
and von Reyn CF: Interferon γ responses to mycobacterial antigens
protect against subsequent HIV-associated tuberculosis. J Infect
Dis. 202:1265–1272. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Tadokera R, Meintjes G, Skolimowska KH,
Wilkinson KA, Matthews K, Seldon R, Chegou NN, Maartens G, Rangaka
MX, Rebe K, et al: Hypercytokinaemia accompanies HIV-tuberculosis
immune reconstitution inflammatory syndrome. Eur Respir J.
37:1248–1259. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vrinceanu D, Dumitru M, Patrascu OM,
Costache A, Papacocea T and Cergan R: Current diagnosis and
treatment of rhinosinusal aspergilloma (Review). Exp Ther Med.
22(1264)2021.PubMed/NCBI View Article : Google Scholar
|
















