1. Introduction
While Human Immunodeficiency Virus (HIV) infection
continues to be the most common cause of Acquired Immune Deficiency
Syndrome (AIDS), it is now considered a chronic condition rather
than a fatal disease. This shift is attributed to the early
initiation of prophylactic and therapeutic practices aimed at
preventing opportunistic infections and other complications related
to AIDS. However, despite these advancements, morbidity and
mortality in patients with HIV persist, primarily due to
non-AIDS-related conditions (1,2).
In this context, HIV infection has been linked to
thromboembolic events. The systemic activation of the immune system
and the inflammatory microenvironment, often intensified by
co-existing infections in patients with HIV, create a prothrombotic
state with diverse clinical manifestations (1,2).
2. HIV infection and thrombosis
HIV primarily targets CD4+ T-lymphocytes,
along with macrophages and dendritic cells, which in turn produce a
variety of cytokines and chemokines with multiple roles, including
IFN-γ, IL-6, IL-10, IL-12 and TNF-a (3). Infected and activated CD4+
T-lymphocytes exhibit elevated levels of the NF-κB factor,
promoting the genome transcription of the integrated virus. This
leads to the production of new viral particles, resulting in a
detrimental cycle of T-cell infection and activation.
Simultaneously, the virus evades regulatory immune mechanisms,
inducing apoptosis in cells mobilized to control HIV infection.
This series of events represents persistent immune system
activation and an ongoing inflammatory process, inevitably
intertwined with the coagulation pathway (3,4).
Immunothrombosis is the term used to describe this
bidirectional interaction between coagulation and inflammation,
mediated by cellular and non-cellular components of both systems
(5). Endothelial cells are the
first to be exposed to pathogens circulating in the blood via
inflammatory mediators, such as the lipopolysaccharide of the
microbial membrane. Loss of endothelial integrity results to
platelet adhesion and activation, as well as expression of tissue
factor (TF) on the endothelial surface, which acts as the initiator
of the coagulation cascade (6).
Activated platelets play multiple roles by releasing the content of
their granules, which include pro-coagulant and pro-inflammatory
substances [coagulation factors, ADP, TXA2, platelet factor 4
(PF4)] (6,7). Platelets also release microparticles
(MPs) with procoagulant properties and express adhesion molecules,
such as P-selectin, as well as negatively charged phospholipids on
their surface, such as phosphatidylserine (PS), which constitutes
the substrate to promote the coagulation process (6,7).
P-selectin binds to P-selectin glycoprotein ligand-1 of endothelial
and inflammatory cells, favoring the migration and activation of
monocytes and neutrophils (7).
P-selectin is also expressed by endothelial cells, being a site of
attachment of neutrophils to the vascular wall (5).
HIV induces endothelial injury and activation of
endothelial cells, with higher levels of endothelial MPs observed
in patients with HIV compared with controls (8). Platelets have a variety of receptors,
such as C-type lectin receptor 2, CC chemokine receptor 3 and
integrin β3, which mediate interactions with HIV and may result in
endocytosis of the virus and subsequent platelet activation
(9). Patients with HIV display a
high percentage of agranular platelets, increased plasma levels of
platelet activation markers, and platelet MPs, all indicative of
prior platelet activation (8,10).
Activated platelets express higher levels of P-selectin,
facilitating endothelial adhesion and secrete large amounts of CD40
ligand, thus recruiting monocytes and neutrophils (9). Importantly, HIV-infected individuals
exhibit increased numbers of TF-expressing monocytes, along with
higher TF plasma levels compared with controls. TF expression by
monocytes correlates not only with elevated HIV plasma levels but
also with various markers of immune system activation, such as CD38
and CD20 HLA-DR in CD8+ T-lymphocytes and excessive
sCD14 plasma levels (11).
Furthermore, platelet-neutrophil interactions lead to NETs
formation, a scaffold made of extracellular nucleic acids,
histones, enzymes, white blood cells and coagulation factors, which
further contributes to a prothrombotic state (9,12).
Co-infections, including mycobacterium tuberculosis,
cytomegalovirus and pneumocystis pneumonia, contribute to immune
system activation in patients with HIV (13). The destruction of the
gastrointestinal (GI) mucosal barrier, leading to the microbial
shift, is caused by the depletion of CD4+ memory T-cells
in the GI tract during the early stages of infection. This
depletion persists despite therapeutic interventions, resulting in
the loss of GI tract integrity, microbial translocation and
activation of the innate immune system (3,14).
Emerging evidence has indicated that gut microbiota
plays a significant role in thrombosis through its influence on
inflammation, metabolism and coagulation pathways. Gut
microbiota-derived metabolites, such as trimethylamine-N-oxide
(TMAO), choline and betaine have been associated with
cardiovascular disease (CVD) in a variety of clinical settings, by
promoting endothelial dysfunction, impairment of liver cholesterol
metabolism and platelet aggregation, mostly by increasing platelet
sensitivity to various stimuli (15). A recent study of COVID19-infected
patients has shown that 2-methylbutyrylcarnitine, a branched-chain
acylcarnitine, augments platelet hyperreactivity by binding to
platelet integrin α2β1 and potentiating phospholipase A2
activation, thus contributing to a hypercoagulable state (16). In the context of HIV infection,
HIV-related gut dysbiosis along with microbial translocation may
contribute to endothelial activation and thrombosis. However,
robust evidence still lacks. A recent study of HIV-infected women
has demonstrated that microbial metabolite imidazole-propionate has
been correlated with several inflammatory markers and carotid
artery plaque. Nonetheless, these findings warrant further
research, since development of agents targeting these pathways,
such as TMAO inhibitors, along with dietary interventions may help
reduce thrombotic complications and CVD in patients with HIV
(17).
Furthermore, HIV-associated malignancies, along with
reported acquired deficiencies in protein C, S, and antithrombin,
increased antiphospholipid antibodies, and elevated vWF levels,
contribute to the prothrombotic state observed in patients with HIV
(13).
Consequently, a variety of factors and their complex
interactions end up in the state of chronic inflammation, chronic
immune system activation, platelet and endothelial dysregulation,
thus promoting thrombosis.
3. Antiretroviral therapy (ART) and
thrombosis
Modern ART, which involves co-administering drugs
targeting distinct pathways of HIV infection, has significantly
enhanced the quality of life for patients with HIV. This is
achieved by reducing plasma viral load, preventing HIV
transmission, and curbing drug resistance, ultimately leading to a
decrease in mortality rates. Despite these benefits, ART has been
linked to thrombotic events and heightened cardiovascular risk.
Previous studies have demonstrated that markers of
platelet activation, such as sCD62P, sCD40L, CD62P and RANTES,
remain elevated, even during successful ART, and may persist for
several months (18-20).
Opposingly, a study of platelet function in patients with HIV on
ART has reported normalization of markers of platelet activation by
12 months post-ART initiation (21). A previous meta-analysis has shown
that platelet reactivity persists despite initiation of ART
(18). In particular, levels of
sCD62P and surface CD62P remained significantly elevated following
effective ART, thus suggesting that long-term HIV infection is
correlated with a prothrombotic state despite its control with ART
(18). A study has previously shown
that TF-expressing monocytes are increased in HIV-infected patients
as compared with healthy individuals and that monocyte TF levels
remain elevated, despite administration of suppressive ART, thus
indicating that the persistent coagulopathy observed in these
patients may be attributable to monocytes and TF (22).
Intriguingly, it has been shown that raltegravir, an
integrase inhibitor, is associated with reduced platelet reactivity
and reduced platelet monocyte aggregates (19,23),
whereas abacavir (ABC)-containing regimens is associated with
increased platelet activation, thus indicating that the type of
antiretroviral agent may have impact on thrombotic risk (19). Results from a large cohort of
contemporary-treated patients with HIV have indicated that recent
ABC use is associated with increased risk for CVD (24). However, a recent meta-analysis has
not found a significant association between ABC use and CVD
incidence rate (25). Although
there is ongoing debate as to whether ABC is associated with
increased thrombotic risk, recent data have suggested that ABC may
promote thrombosis by increasing prothrombin conversion and by
inducing the release of soluble low-density lipoprotein receptor-1,
which in turn leads to platelet activation (26,27).
Regarding integrase strand-transfer inhibitors (INSTIs), despite
the previous reports of raltegravir-induced reduction of platelet
reactivity, recent data have suggested that treatment with INSTIs
is associated with an elevated risk for CVD in the first 24 months
after treatment initiation, which thereafter decreases to levels
similar to those never exposed to INSTIs (28). Moreover, INSTIs regimens have been
linked to greater weight gain but improved lipid metabolism
profiles among individuals with HIV (29).
Among antiretroviral agents, protease inhibitors
(PIs) display significant thrombotic risk. Increased levels of
markers of platelet activation have remained largely unchanged in
patients with HIV following PI treatment initiation (18). PIs within ART may impact coagulation
proteins through hepatic metabolism and are associated with
abnormal coagulation parameters, especially when combined with
nucleoside reverse transcriptase inhibitors (NRTI) regimens,
resulting in elevated fibrinogen levels (10,13).
Patients on ART, particularly those on PI regimens, may experience
metabolic disorders such as lipodystrophy and various abnormalities
in lipid and glucose metabolism (30). Endothelial dysfunction has also been
observed in individuals on ART, specifically those on PI or
non-NRTI (NNRTI), attributed to reduced nitric oxide (NO) levels,
increased production of reactive oxygen species, and alterations in
lipid metabolism (8). A study
involving patients with HIV treated with PIs/NNRTIs revealed higher
serum levels of soluble endothelial dysfunction markers
(P-selectin, tissue Plasminogen Activator) compared with those not
receiving therapy (31). PIs
ritonavir and indinavir have been directly associated with
endothelial dysfunction, linked to mitochondrial DNA damage and
cell death (8).
Despite these findings, the precise pathogenetic
pathways underlying the metabolism, endothelium, and coagulation
alterations induced by ART have not been fully elucidated.
Furthermore, thrombogenic potential of novel agents incorporated in
the treatment of HIV, such as cabotegravir and lenacapavir needs to
be elucidated. LATTE-2 Study, a study of efficacy and safety of
long-acting cabotegravir and rilpivirine has reported occurrence of
thrombotic events in four patients, which however were not
drug-related (32).
Nonetheless, further research is warranted, and more
randomized clinical trials should be conducted, thus safe
conclusions, regarding the thrombogenic potential of various
antiretroviral agents can be drawn. A summary of different classes
of antiretroviral drugs and their thrombotic potential is presented
in Table I.
 | Table IA summary of different classes of
antiretroviral drugs and their thrombotic potential. |
Table I
A summary of different classes of
antiretroviral drugs and their thrombotic potential.
| Drug class | Mechanism | Thrombotic
outcomes |
Studies/comments |
|---|
| NRTIs, specifically
Abacavir | Platelet
activation, prothrombin conversion, release of soluble LOX-1 | Risk of MI and
probably other cardiovascular events | Conflicting
evidence in studies (24-27).
NRTIs combined with PIs may influence coagulation parameters
(10,13). |
| INSTIs | ↑ cardiovascular
risk during first 24 months, then ↓ | Cardiovascular
events soon after initiation of treatment | Risk reduces over
time (28). |
| Raltegravir
(INSTI) | ↓ Platelet
reactivity, ↓ platelet-monocyte aggregates | Probably reducing
thrombotic risk | Weight gain,
improved lipid profile (19,23,29) |
| PIs | Platelet
activation, ↑ fibrinogen, lipodystrophy-lipid/glucose disorders,
endothelial dysfunction | Risk of
cardiovascular events-especially MI, VTE | Ritonavir and
indinavir particularly implicated. PIs combined with NRTIs may
influence coagulation parameters. PIs combined with NNRTIs: higher
levels of endothelial dysfunction markers (8,10,13,18,30,31,39) |
| Non-NRTIs | Endothelial
dysfunction, ↓ NO, ROS production, lipid disorders | Risk of
cardiovascular events | NNRTIs combined
with PIs: higher levels of endothelial dysfunction markers
(8,31) |
| Novel agents | No mechanism
identified | No confirmed
thrombotic risk | Thrombotic events
not drug-related-LATTE 2 study (32). |
| Cabotegravir
(long-acting INSTI) | Unknown | Unknown | Further studies
required |
| Lenacapavir (capsid
inhibitor) | | | Further studies
required |
4. Thrombotic events in patients with
HIV
The multifactorial pathophysiological mechanism of
thrombosis in HIV, as depicted in Fig.
1, leads to a wide spectrum of thrombotic events, including
venous and arterial thrombosis, thrombosis in unusual sites and
thrombotic microangiopathy.
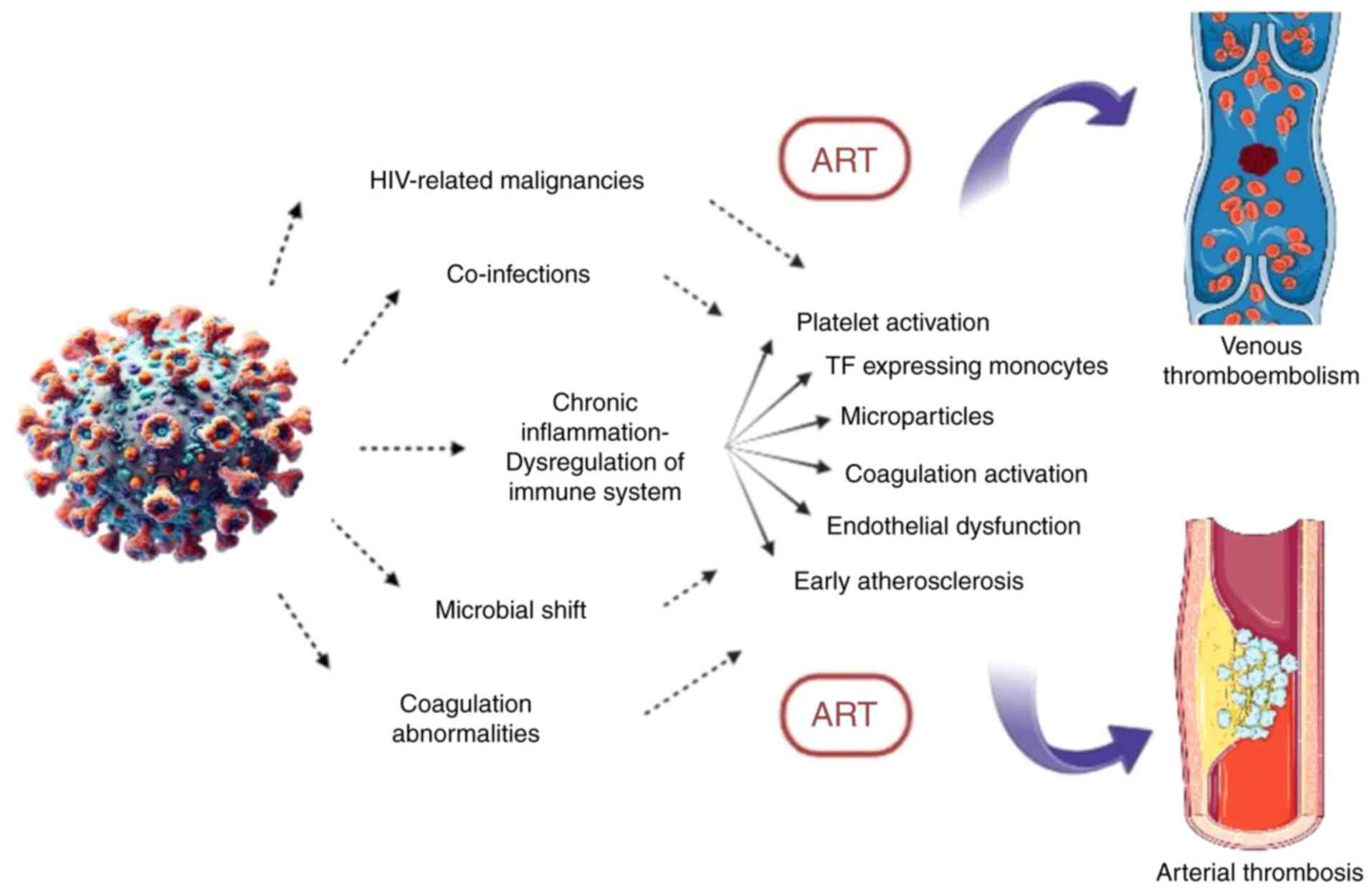 | Figure 1Pathophysiological mechanisms
involved in venous and arterial thrombosis in HIV infection
(26). The figure illustrates the
complex interplay between HIV infection, chronic inflammation and
thrombosis. Immune system dysregulation, in combination with
HIV-related malignancies, co-infections, microbial shift, and
coagulation abnormalities, trigger a cascade of prothrombotic
mechanisms: platelet activation, tissue factor-expressing
monocytes, microparticle release, coagulation activation,
endothelial dysfunction, and early atherosclerosis. ART can also
contribute and enhance these processes, ultimately leading to
increased risk of venous thromboembolism and arterial thrombosis.
ART, antiretroviral therapy. |
Venous thromboembolism (VTE)
The reported incidence of VTE in patients with HIV
ranges from 0.19-7.63% per year, compared with 0.1-0.18% per year
in general population (13,33). In a study by Sullivan et al
(34), including 42,935
HIV-infected individuals, the incidence of thrombosis was 2.6/1,000
person-years (PY), calculated over a period of average 2.4 years.
In a Danish study of 43,330 patients with HIV, the 5-year risk of
VTE was estimated at 8.0% and 1.5% for intravenous drug users (IDU)
and non-IDU individuals respectively, compared with 0.3% in the
control group (35). The observed
fluctuation in the incidence of VTE is attributed to the different
number of patients included and the different duration of the
studies. Additionally, some studies were performed before the
universal application of ART.
Patients with HIV and uninfected controls with a
history of VTE (first episode) were included in the study by Rokx
et al (36), in order to
determine the risk of recurrent VTE (36). The first episode of VTE was
unprovoked in the majority of patients with HIV (71% vs. 34% in
controls) (36,37). Recurrence rates were 5.2 and 3.1 per
100 PY of follow-up for HIV and uninfected individuals respectively
(36). The higher incidence of
recurrent VTE also applied for all HIV subgroups (men, women,
patients with provoked or unprovoked first VTE episode) (36,37).
During the five-year follow-up of study subjects, after
discontinuation of anticoagulant therapy, the risk of recurrent VTE
appeared to increase gradually, with the highest incidence at 5
years (23.4% vs. 15.3% in controls) (36,37).
VTE recurrence was markedly reduced in patients with HIV who were
immunodeficient during the first VTE episode and showed an improved
response to ART, as reflected by CD4+ T cell counts
(36,37).
Clinical manifestations and the distribution of VTE
in individuals with HIV do not differ significantly from non-HIV
individuals. This includes occurrences of low extremity deep vein
thrombosis, pulmonary embolism, and less common sites of
thrombosis, such as splanchnic vein and cerebral vein thrombosis
(CVT) (13,34). According to Sullivan et al
(34), identified risk factors for
VTE in patients with HIV include opportunistic infections, the
administration of megestrol acetate or indinavir, hospitalization
and an age of ≥45 years.
Regarding thrombosis at unusual sites, isolated
cases have been documented in the literature. In a study of 26
HIV-infected individuals with CVT in India, elevated homocysteine
levels and decreased vitamin B12 levels were noted. The clinical
course of these patients was milder compared with HIV-negative
patients with CVT, and they exhibited an improved response to
therapeutic interventions (38).
Ramanampamonjy et al (39)
reported four cases of HIV-infected patients with portal vein
thrombosis, showcasing severe immunodeficiency, PI regimens, and
co-infections (hepatitis C, tuberculosis) in some or all of these
cases.
However, although thrombotic risk is amplified in
HIV-infected individuals, the exact incidence and risk factors
still remain largely unclear.
Arterial thrombosis and CVD
CVD is one of the leading causes of morbidity and
mortality, not only worldwide, but also in patients with HIV. In
fact, in comparison individuals without HIV, patients with HIV have
twice the risk for CVD, while the global percentage of HIV-related
CVD has tripled in the last 20 years and accounts for 2.57 million
disability-adjusted life years per year (40). CVD and sudden death were also the
most common causes of death, at a rate of 19.31%, among patients
with HIV in the SMART and ESPRIT studies (41).
Interestingly, the cardiovascular profile of
patients with HIV is notably and more frequently impacted by
traditional risk factors compared with individuals without HIV.
These include factors such as obesity, hypertension, smoking, and
disorders in lipid/glucose metabolism (30).
Thus, chronic inflammation, endothelial dysfunction
and early atherosclerosis are implicated in HIV-related CVD
(42). Introduction of ART has
significantly increased survival rates of HIV-infected individuals,
making aging an additional cardiovascular risk factor in these
patients, while at the same time, ART itself has been associated
with metabolic disorders and an increased CVD risk (8,42). In
a meta-analysis of 2012, patients with HIV not receiving ART had a
1.61-fold higher risk and patients with HIV receiving ART a 2-fold
higher risk of CVD than individuals without HIV. Among HIV-infected
patients, those on ART had a 1.52-fold higher CVD risk than
treatment-naïve individuals (43).
However, although HIV and ART exacerbate traditional
cardiovascular risk factors, recent data have not indicated a
statistically significant association of increased incidence of CVD
with HIV-specific factors, such as use of specific antiretroviral
agents (ABC, efavirenz) (25).
Myocardial infraction (MI)
Focusing on MI, in a meta-analysis by Gutierrez
et al (44), patients with
HIV were at 1.6-fold higher risk of acute coronary syndrome than
HIV-negative controls (44). In
another, 2019 meta-analysis, the relative risk of MI in patients
with HIV receiving ART and in untreated patients with HIV was 1.80
and 1.25 respectively, both compared with uninfected individuals
(45). In the same study, low
CD4+ T-lymphocyte counts (<200 cells/mm3),
increased plasma viral copies (>100,000 copies/ml) and ART, more
specifically exposure to certain ART regimens and duration of
administration, were associated with an increased MI risk (44,45).
Among the different classes of antiretroviral drugs,
NRTIs and PIs were related to a greater risk of MI, with ABC (NRTI)
and indinavir (PI) having the highest relative risks (1.71 and 1.46
respectively) (45). Administration
of NNRTIs, either alone or in combination with other antiretroviral
drugs, was not associated with increased incidence of MI (45).
Stroke
In the pre-ART era, HIV-infected individuals had a
2,3-fold higher risk of stroke than uninfected individuals, with
cerebral infraction being the most common event in patients with
HIV, as shown in a retrospective study from 1990 to 1994(46). In a 2017 meta-analysis, the
estimated risk of stroke in HIV compared with non-HIV individuals
was 1.82 and 1.27, specifically for ischemic stroke (44). Regarding ART, no antiretroviral drug
class was associated with an increased risk of ischemic stroke
(44).
In addition to traditional and HIV-related
cardiovascular risk factors, as aforementioned, opportunistic
infections affecting the central nervous system, such as
tuberculosis meningitis, neurosyphilis, and varicella-zoster virus,
are also involved in the pathophysiology of ischemic attacks in
HIV-infected patients (47). This
may be one of the reasons why viral suppression in patients with
with HIV, as reflected in high CD4+ T-lymphocyte counts
and low plasma HIV-RNA levels, may reduce ischemic stroke risk
(48).
HIV-related thrombotic
thrombocytopenic purpura (TTP)
HIV can be one of the numerous causative factors of
secondary TTP, with a 15-40-fold higher incidence in this patient
group (49). It occurs more
frequently in patients with advanced HIV infection, reduced
CD4+ T-cell counts and various comorbidities, such as
Kaposi's sarcoma and cryptococcal meningitis (50).
Inflammatory cytokines participating in HIV
infection may induce the endothelial release of ultra-large von
Willebrand factor (ULVWF) multimers and downregulate the synthesis
of ADAMTS-13 protein, which is the cleaving protease of ULVWF.
Autoantibodies against ADAMTS-13 have been detected in some, but
not all, HIV-infected patients (51).
Heparin-induced thrombocytopenia (HIT)
and HIV
A higher risk of HIT was reported in patients with
HIV compared with non-HIV-infected patients, when treated with
heparin. Considering that dysregulated immune responses and various
autoimmune manifestations have been documented in individuals with
HIV, chronic activation of the immune system in these patients may
be implicated in the formation of antibodies against platelet
factor 4 (PF4)-heparin complex (52).
5. Therapeutic and prophylactic measures in
HIV-related thrombosis
Currently, there are no specific guidelines
regarding antithrombotic and thrombolytic therapy in patients with
HIV. However, in the event of an established venous or arterial
thrombotic event, therapeutic management should not deviate from
that for HIV-negative patients (13,53).
It is crucial to pay special attention to potential interactions
between ART and antithrombotic agents. Numerous antiretroviral
agents impact CYP450 metabolism, leading to fluctuations in drug
concentrations, which may affect medications like warfarin and
direct oral anticoagulants (53).
Moreover, low molecular weight heparin may be a preferable
therapeutic option, given the higher risk of developing HIT with
unfractionated heparin in patients with HIV (52). The duration of anticoagulant therapy
in a confirmed VTE case should be tailored to each patient,
generally continuing in the case of unprovoked VTE episodes,
similar to uninfected individuals (37).
Modifying traditional cardiovascular risk factors
remains an essential preventive measure in individuals with HIV.
However, there is insufficient data concerning pharmaceutical
prophylaxis with anticoagulant and antiplatelet agents in these
patients. Various antithrombotic agents, including aspirin,
clopidogrel, vorapaxar and edoxaban, have been utilized in
different studies to assess their impact on inflammation, platelet
activation and coagulation. Among these, clopidogrel has shown the
most favorable results in reducing inflammatory biomarkers
(42).
Due to the association of ART with lipid disorders,
the initiation of antilipidemic therapy, when and where
appropriate, should be integrated into the management algorithm for
these patients. This integration should always consider potential
interactions with ART agents (30).
There is a clear need for studies exploring novel
treatment options for HIV-infected patients, aiming to minimize
drug interactions and improve control of chronic inflammation and
thrombosis. With the effective HIV suppression achieved by modern
ART, future research could focus on incorporating immunomodulatory
agents that inhibit major inflammatory markers, such as IL-1β, IL-6
and TNF-α (42). IL-1β is a key
pro-inflammatory cytokine that promotes endothelial dysfunction, TF
expression and platelet activation, ultimately leading to a
prothrombotic state. Administration of canakinumab, a monoclonal
antibody against IL-1β, has been shown to significantly reduce
cardiovascular events in patients with prior MI, independent of
lipid levels (54). Similarly,
previous studies have shown reduced cardiovascular events with the
use of anti-TNF agents and IL-6 inhibitors such as sarilumab in
patients with autoimmune diseases, thus implicating a potential
role of these agents in reducing thrombotic risk (55,56).
Additionally, investigating specific biomarkers in HIV-infected
patients and their role in thrombotic events and antithrombotic
therapy, such as CD4+ T cell levels, could represent a
promising direction for future research (37) Finally, given the heterogeneity of
the underlying mechanisms driving thrombosis in patients with HIV,
individualized strategies should be adapted, which should also
include development of assessment tools for thrombotic risk
profiling, incorporating markers of platelet activation, such as
sCD40L and sCD62P as well as the type of agent used.
6. Conclusion
In summary, HIV infection represents a prothrombotic
situation, with patients being at increased risk for venous
thromboembolic events, MIs and ischemic strokes. Various
pathophysiological mechanisms are involved in the establishment of
a thrombotic event in HIV infection, with the major ones being
chronic inflammation, endothelial dysfunction and platelet and
coagulation activation, enhanced by co-existing infections,
acquired coagulation abnormalities and HIV-related malignancies.
Specific ART regimens have also emerged as significant contributors
to the HIV procoagulant state and have been incriminated for
increased MI risk. An individualized therapeutic approach, taking
into consideration the risk-benefit assessment and the potential
drug interactions with antithrombotic agents, should be made for
each patient, while further studies will explore the role of novel
anti-inflammatory agents in the HIV thrombotic process.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GD developed, planned and supervised the project,
and wrote the manuscript. MV performed data entry and evaluation
and wrote the manuscript. MP and AG developed and supervised the
current study and wrote the manuscript. TP, VZ and DS, collected
relevant literature. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Palella FJ Jr, Baker RK, Moorman AC,
Chmiel JS, Wood KC, Brooks JT and Holmberg SD: HIV Outpatient Study
Investigators. Mortality in the highly active antiretroviral
therapy era: Changing causes of death and disease in the HIV
outpatient study. J Acquir Immune Defic Syndr. 43:27–34.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Crum NF, Riffenburgh RH, Wegner S, Agan
BK, Tasker SA, Spooner KM, Armstrong AW, Fraser S and Wallace MR:
Triservice AIDS Clinical Consortium. Comparisons of causes of death
and mortality rates among HIV-infected persons: Analysis of the
pre-, early, and late HAART (highly active antiretroviral therapy)
eras. J Acquir Immune Defic Syndr. 41:194–200. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ipp H, Zemlin AE, Erasmus RT and Glashoff
RH: Role of inflammation in HIV-1 disease progression and
prognosis. Crit Rev Clin Lab Sci. 51:98–111. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Appay V and Sauce D: Immune activation and
inflammation in HIV-1 infection: Causes and consequences. J Pathol.
214:231–241. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Engelmann B and Massberg S: Thrombosis as
an intravascular effector of innate immunity. Nat Rev Immunol.
13:34–45. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Gaertner F and Massberg S: Blood
coagulation in immunothrombosis-At the frontline of intravascular
immunity. Semin Immunol. 28:561–569. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Martinod K and Deppermann C:
Immunothrombosis and thromboinflammation in host defense and
disease. Platelets. 32:314–324. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gresele P, Falcinelli E, Sebastiano M and
Baldelli F: Endothelial and platelet function alterations in
HIV-infected patients. Thromb Res. 129:301–308. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Awamura T, Nakasone ES, Gangcuangco LM,
Subia NT, Bali AJ, Chow DC, Shikuma CM and Park J: Platelet and HIV
interactions and their contribution to non-AIDS comorbidities.
Biomolecules. 13(1608)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jackson BS and Pretorius E: Pathological
clotting and deep vein thrombosis in patients with HIV. Semin
Thromb Hemost. 45:132–140. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Funderburg NT, Mayne E, Sieg SF, Asaad R,
Jiang W, Kalinowska M, Luciano AA, Stevens W, Rodriguez B,
Brenchley JM, et al: Increased tissue factor expression on
circulating monocytes in chronic HIV infection: Relationship to in
vivo coagulation and immune activation. Blood. 115:161–167.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kapoor S, Opneja A and Nayak L: The role
of neutrophils in thrombosis. Thromb Res. 170:87–96.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bibas M, Biava G and Antinori A:
HIV-associated venous thromboembolism. Mediterr J Hematol Infect
Dis. 3(e2011030)2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hunt P: HIV and inflammation: Mechanisms
and consequences. Curr HIV/AIDS Rep. 9:139–147. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Canyelles M, Borràs C, Rotllan N, Tondo M,
Escolà-Gil JC and Blanco-Vaca F: Gut microbiota-derived TMAO: A
causal factor promoting atherosclerotic cardiovascular disease? Int
J Mol Sci. 24(1940)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang K, Li Z, He X, Dai J, Huang B, Shi
Y, Fan D, Zhang Z, Liu Y, Li N, et al: Gut microbial co-metabolite
2-methylbutyrylcarnitine exacerbates thrombosis via binding to and
activating integrin α2β1. Cell Metab. 36:598–616.e9.
2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Z, Peters BA, Usyk M, Xing J, Hanna
DB, Wang T, Post WS, Landay AL, Hodis HN, Weber K, et al: Gut
microbiota, plasma metabolomic profiles, and carotid artery
atherosclerosis in HIV infection. Arterioscler Thromb Vasc Biol.
42:1081–1093. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nkambule BB, Mxinwa V, Mkandla Z, Mutize
T, Mokgalaboni K, Nyambuya TM and Dludla PV: Platelet activation in
adult HIV-infected patients on antiretroviral therapy: A systematic
review and meta-analysis. BMC Med. 18(357)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mesquita EC, Hottz ED, Amancio RT,
Carneiro AB, Palhinha L, Coelho LE, Grinsztejn B, Zimmerman GA,
Rondina MT, Weyrich AS, et al: Persistent platelet activation and
apoptosis in virologically suppressed HIV-infected individuals. Sci
Rep. 8(14999)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kiebala M, Singh MV, Piepenbrink MS, Qiu
X, Kobie JJ and Maggirwar SB: Platelet activation in human
immunodeficiency virus type-1 patients is not altered with cocaine
abuse. PLoS One. 10(e0130061)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
O'Halloran JA, Dunne E, Gurwith M, Lambert
JS, Sheehan GJ, Feeney ER, Pozniak A, Reiss P, Kenny D and Mallon
P: The effect of initiation of antiretroviral therapy on monocyte,
endothelial and platelet function in HIV-1 infection. HIV Med.
16:608–619. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schechter ME, Andrade BB, He T, Richter
GH, Tosh KW, Policicchio BB, Singh A, Raehtz KD, Sheikh V, Ma D, et
al: Inflammatory monocytes expressing tissue factor drive SIV and
HIV coagulopathy. Sci Transl Med. 9(eaam5441)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tunjungputri RN, Van Der Ven AJ,
Schonsberg A, Mathan TS, Koopmans P, Roest M, Fijnheer R, Groot PG
and de Mast Q: Reduced platelet hyperreactivity and
platelet-monocyte aggregation in HIV-infected individuals receiving
a raltegravir-based regimen. AIDS. 28:2091–2096. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jaschinski N, Greenberg L, Neesgaard B,
Miró JM, Grabmeier-Pfistershammer K, Wandeler G, Smith C, De Wit S,
Wit F, Pelchen-Matthews A, et al: Recent abacavir use and incident
cardiovascular disease in contemporary-treated people with HIV.
AIDS. 37:467–475. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Du M, Zhang S, Liu M and Liu J:
Cardiovascular disease and its risk factors among people living
with HIV: A systematic review and meta-analysis. J Infect Public
Health. 18(102654)2025.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Blanch-Ruíz MA, Sánchez-López A,
Ríos-Navarro C, Ortega-Luna R, Collado-Díaz V, Orden S,
Martínez-Cuesta MA, Esplugues JV and Álvarez Á: Abacavir causes
leukocyte/platelet crosstalk by activating neutrophil P2X7
receptors thus releasing soluble lectin-like oxidized low-density
lipoprotein receptor-1. Br J Pharmacol. 180:1516–1532.
2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yan Q, Huang S, van der Heijden W,
Ninivaggi M, van de Wijer L, de Laat-Kremers R, Van der Ven AJ, de
Laat B and de Mast Q: Abacavir use is associated with increased
prothrombin conversion. Front Immunol. 14(1182942)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Neesgaard B, Greenberg L, Miró JM,
Grabmeier-Pfistershammer K, Wandeler G, Smith C, De Wit S, Wit F,
Pelchen-Matthews A, Mussini C, et al: Associations between
integrase strand-transfer inhibitors and cardiovascular disease in
people living with HIV: A multicentre prospective study from the
RESPOND cohort consortium. Lancet HIV. 9:e474–e485. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ntsekhe M and Baker JV: Cardiovascular
disease among persons living with HIV: New insights into
pathogenesis and clinical manifestations in a global context.
Circulation. 147:83–100. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dentali F, Nicolini E and Ageno W: Venous
and arterial thrombosis associated with HIV infection. Semin Thromb
Hemost. 38:524–529. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
De Gaetano Donati K, Rabagliati R,
Tumbarello M, Tacconelli E, Amore C, Cauda R and Lacoviello L:
Increased soluble markers of endothelial dysfunction in
HIV-positive patients under highly active antiretroviral therapy.
AIDS. 17:765–768. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Smith GHR, Henry WK, Podzamczer D, Masiá
MDM, Bettacchi CJ, Arasteh K, Jaeger H, Khuong-Josses MA,
Montes-Ramírez ML, Stellbrink HJ, et al: Efficacy, safety, and
durability of long-acting cabotegravir and rilpivirine in adults
with human immunodeficiency virus type 1 infection: 5-Year results
from the LATTE-2 study. Open Forum Infect Dis.
8(ofab439)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Heit JA: Epidemiology of venous
thromboembolism. Nat Rev Cardiol. 12:464–474. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sullivan PS, Dworkin MS, Jones JL and
Hooper WC: Epidemiology of thrombosis in HIV-infected individuals.
The adult/adolescent spectrum of HIV disease project. AIDS.
14:321–324. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rasmussen LD, Dybdal M, Gerstoft J,
Kronborg G, Larsen CS, Pedersen C, Pedersen G, Jensen J, Pedersen
L, Sørensen HT and Obel N: HIV and risk of venous thromboembolism:
A Danish nationwide population-based cohort study. HIV Med.
12:202–210. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rokx C, Borjas Howard JF, Smit C, Wit FW,
Pieterman ED, Reiss P, Cannegieter SC, Lijfering WM, Meijer K,
Bierman W, et al: Risk of recurrent venous thromboembolism in
patients with HIV infection: A nationwide cohort study. PLoS Med.
17(e1003101)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shaw ML: Persons living with HIV have a
higher risk of recurrent blood clots, 2020.
|
|
38
|
Netravathi M, Jaychandran R, Bhat M,
Christopher R and Satishchandra P: Profile of 26 HIV seropositive
individuals with cerebral venous thrombosis. J Neurol Sci.
378:69–74. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ramanampamonjy RM, Ramarozatovo LS, Bonnet
F, Lacoste D, Rambeloarisoa J, Bernard N, Beylot J and Morlat P:
Portal vein thrombosis in HIV-infected patients: Report of four
cases. Rev Med Interne. 26:545–548. 2005.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
40
|
Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam
S, Clifford S, Longenecker CT, Strachan F, Bagchi S, Whiteley W, et
al: Global burden of atherosclerotic cardiovascular disease in
people living with HIV: Systematic review and meta-analysis.
Circulation. 138:1100–1112. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rodger AJ, Lodwick R, Schechter M, Deeks
S, Amin J, Gilson R, Paredes R, Bakowska E, Engsig FN, Phillips A,
et al: Mortality in well controlled HIV in the continuous
antiretroviral therapy arms of the SMART and ESPRIT trials compared
with the general population. AIDS. 27:973–979. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Perkins MV, Joseph SB, Dittmer DP and
Mackman N: Cardiovascular disease and thrombosis in HIV infection.
Arterioscler Thromb Vasc Biol. 43:175–191. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Islam FM, Wu J, Jansson J and Wilson DP:
Relative risk of cardiovascular disease among people living with
HIV: A systematic review and meta-analysis. HIV Med. 13:453–468.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gutierrez J, Albuquerque ALA and Falzon L:
HIV infection as vascular risk: A systematic review of the
literature and meta-analysis. PLoS One. 12(e0176686)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Eyawo O, Brockman G, Goldsmith CH, Hull
MW, Lear SA, Bennett M, Guillemi S, Franco-Villalobos C, Adam A,
Mills EJ, et al: Risk of myocardial infarction among people living
with HIV: An updated systematic review and meta-analysis. BMJ Open.
9(e025874)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Qureshi AI, Janssen RS, Karon JM, Weissman
JP, Akbar MS, Safdar K and Frankel MR: Human immunodeficiency virus
infection and stroke in young patients. Arch Neurol. 54:1150–1153.
1997.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bogorodskaya M, Chow FC and Triant VA:
Stroke in HIV. Can J Cardiol. 35:280–287. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chow FC, Bacchetti P, Kim AS, Price RW and
Hsue PY: Effect of CD4+ cell count and viral suppression on risk of
ischemic stroke in HIV infection. AIDS. 28:2573–2577.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Becker S, Fusco G, Fusco J, Balu R,
Gangjee S, Brennan C and Feinberg J: Collaborations in HIV Outcomes
Research/US Cohort. HIV-associated thrombotic microangiopathy in
the era of highly active antiretroviral therapy: An observational
study. Clin Infect Dis. 39 (Suppl 5):S267–S275. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Louw S, Gounden R and Mayne ES: Thrombotic
thrombocytopenic purpura (TTP)-like syndrome in the HIV era. Thromb
J. 16(35)2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Meiring M, Webb M, Goedhals D and Louw V:
HIV-associated thrombotic thrombocytopenic purpura-what we know so
far. Eur Oncol Haematol. 8:89–91. 2012.
|
|
52
|
Thompson GR III, Lawrence VA and Crawford
GE: HIV infection increases the risk of heparin-induced
thrombocytopenia. Clin Infect Dis. 45:1393–1396. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang Q, Peng F, Li M, Yi Q, Tang W and Wu
S: Elevated risk of venous thromboembolism in people living with
HIV. Viruses. 14(590)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ridker PM, Everett BM, Thuren T, MacFadyen
JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker
SD, et al: Antiinflammatory therapy with canakinumab for
atherosclerotic disease. N Engl J Med. 377:1119–1131.
2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jacobsson LTH, Turesson C, Gülfe A,
Kapetanovic MC, Petersson IF, Saxne T and Geborek P: Treatment with
tumor necrosis factor blockers is associated with a lower incidence
of first cardiovascular events in patients with rheumatoid
arthritis. J Rheumatol. 32:1213–1218. 2005.PubMed/NCBI
|
|
56
|
Gabay C, Burmester GR, Strand V, Msihid J,
Zilberstein M, Kimura T, van Hoogstraten H, Boklage SH, Sadeh J,
Graham NMH and Boyapati A: Sarilumab and adalimumab differential
effects on bone remodelling and cardiovascular risk biomarkers, and
predictions of treatment outcomes. Arthritis Res Ther.
22(70)2020.PubMed/NCBI View Article : Google Scholar
|















