Introduction
Embryonal tumor with medulloblastoma-like
histopathology (ETMH) is an embryonic-type malignancy originating
from remnants of primitive embryonic cells and is most common in
children, accounting for 20% of all childhood central nervous
system (CNS) tumors. It is rare in adults, where ETMH accounts for
<1% of adult CNS tumors (1).
Adult ETMH is prevalent in the cerebellar hemispheres and
cerebellar vermis, and adjacent to the surface of the brain or the
cerebellar tentorium (2). Adult
supratentorial ETMH is extremely rare and has atypical clinical
manifestations, which have been rarely reported in the literature.
The radiological manifestations are atypical, making diagnosis
difficult (3). The present study
reported on the diagnosis and treatment processes of an adult
patient with ETMH, which was misdiagnosed as glioblastoma
multiforme (GBM) and a combined literature review analysis was
provided to offer a reference for the diagnosis and treatment of
this disease.
Case description
A 41-year-old male patient from Xuzhou visited The
Second Affiliated Hospital of Xuzhou Medical University (Xuzhou,
China) in August 2023, due to headache, nausea and vomiting for
over a month. The symptoms worsened the week before visiting the
hospital, without an obvious cause. The patient was otherwise
healthy with no history of surgery, hypertension or family history.
Physical examination and laboratory tests revealed no
abnormalities. The patient underwent non-contrast and
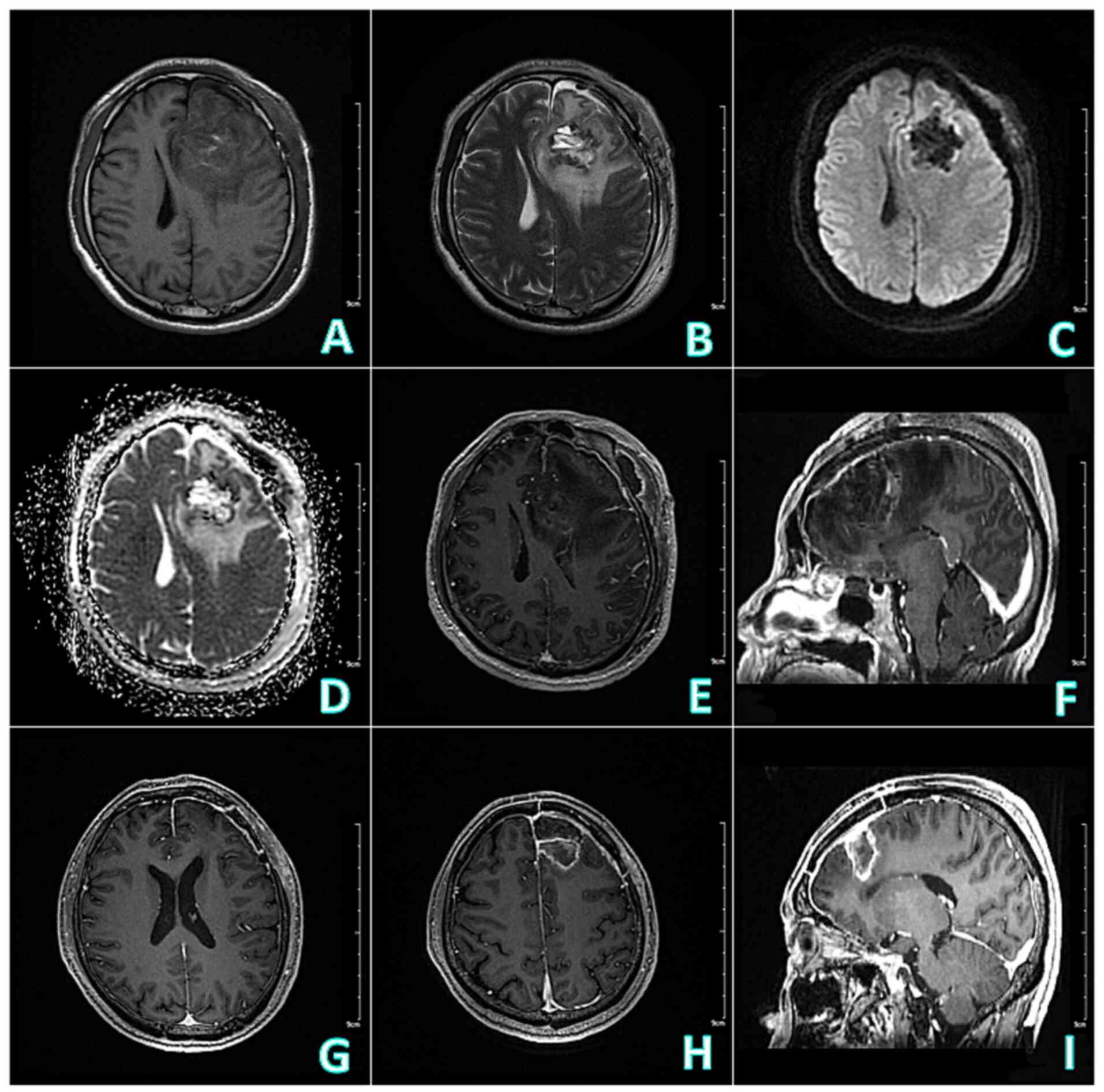
contrast-enhanced brain magnetic resonance imaging (MRI) (Fig. 1A-F), which revealed a cystic-solid
lesion in the left frontal lobe with unclear borders, measuring
~60x50x39 mm. The lesion had a large central cyst and multiple
small cysts at the margins. The solid part of the lesion exhibited
hypointensity on T1-weighted imaging (T1WI), hyperintensity on
T2-weighted imaging (T2WI), and inhomogeneous hyperintensity on
diffusion-weighted imaging (DWI). The cystic part exhibited
hypointensity on T1WI, hyperintensity on T2WI, and hypointensity on
DWI. Contrast-enhanced T1WI (CE-T1WI) revealed obvious enhancement
of the solid part of the lesion, with no enhancement of the cystic
part. The periphery of the lesion presented with large areas of
edema, marked compression and narrowing of the bilateral
ventricles, and a rightward shift of the midline structures.
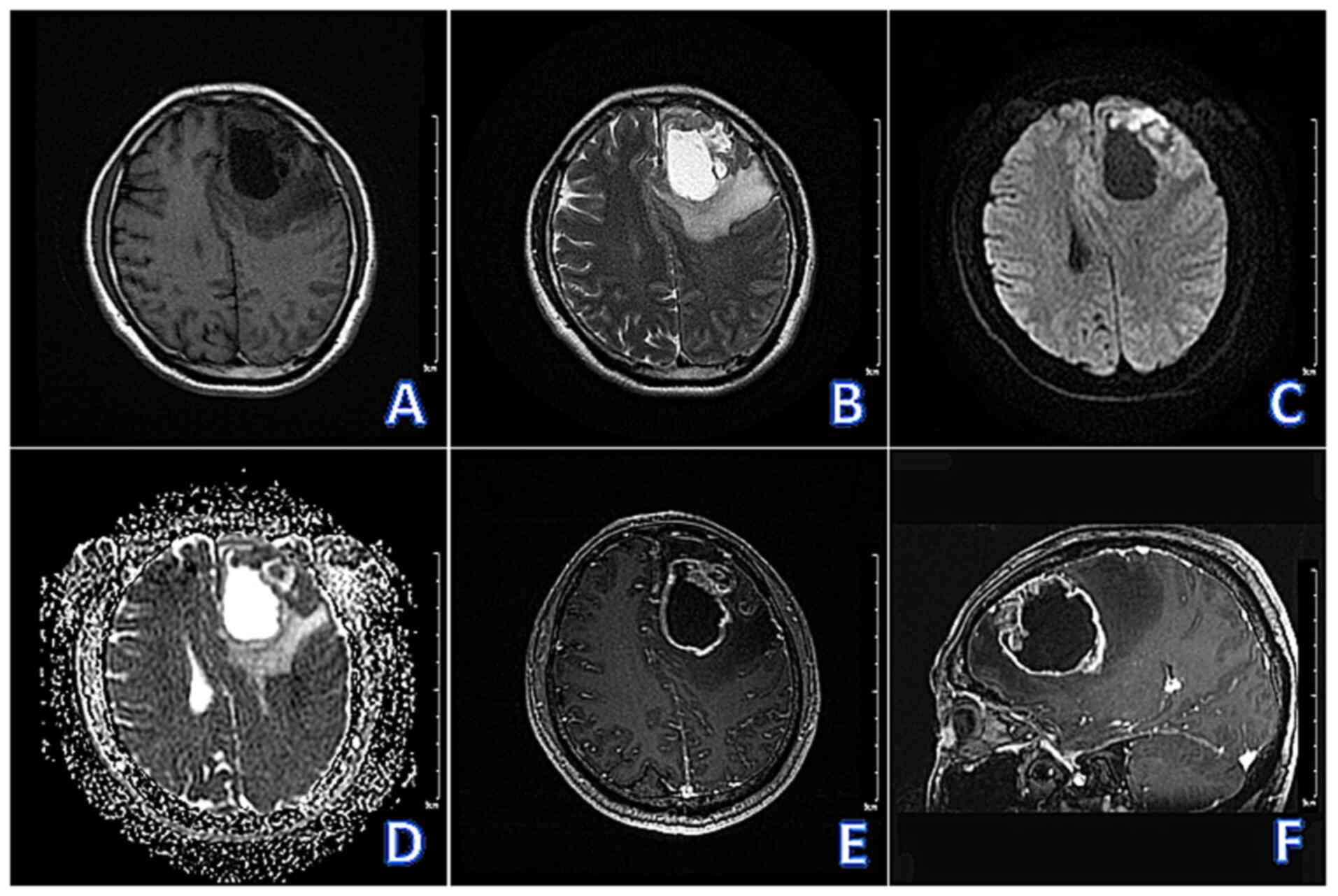 | Figure 1Pre-operative brain magnetic resonance
imaging examinations. (A-F) Sequentially, T1WI (axial), T2WI
(axial), DWI (axial), apparent diffusion coefficient (axial),
CE-T1WI (axial), CE-T1WI (sagittal) revealed: Cystic solid
occupancy in the left frontal lobe with unclear borders, with a
large capsule in the center of the lesion and multiple small
capsules at the margins. The solid part of the lesion showed
hypointensity on T1WI, hyperintensity on T2WI, and inhomogeneous
hyperintensity on DWI and the cystic part showed hypointensiy on
T1WI, hyperintensity on T2WI and hypointensity on DWI. CE-T1WI
showed obvious enhancement of the solid part of the lesion and no
enhancement of the cystic part. The periphery of the lesion showed
large areas of edema, with marked compression and narrowing of the
bilateral ventricles and a rightward shift of the midline
structures. T1WI, T1-weighted imaging; T2WI, T2-weighted imaging;
DWI, diffusion-weighted imaging; CE, contrast-enhanced. |
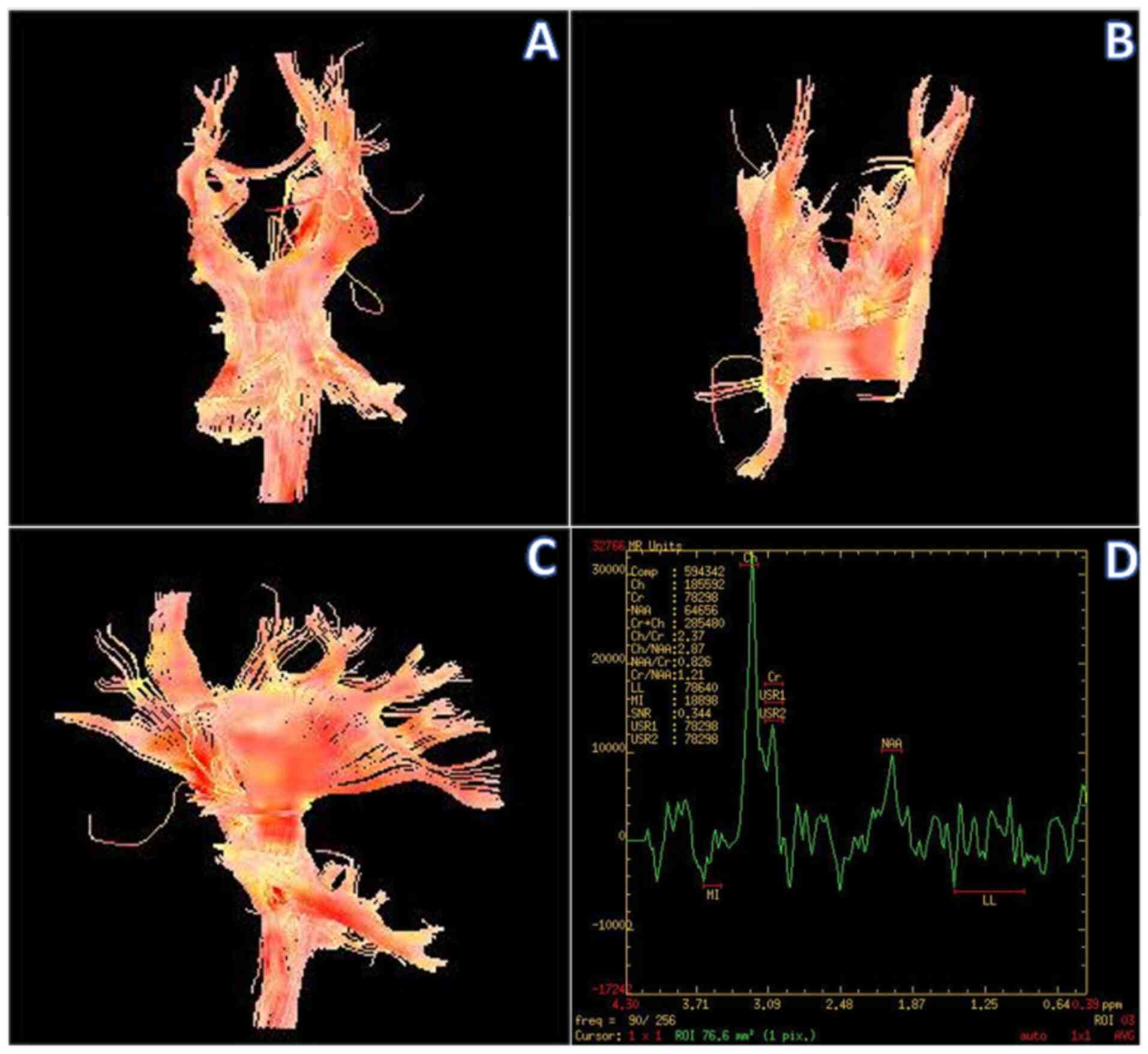
Diffusion tensor imaging (DTI) disclosed sparse and
locally interrupted conduction bundles in the white matter of the
left frontal lobe compared with the healthy side (Fig. 2A-C).
Magnetic resonance spectroscopy (MRS) of the left
frontal region of interest (solid portion) revealed markedly
elevated choline (Cho), decreased N-acetyl-aspartic acid (NAA) and
creatine (Cr), and mildly elevated lactate (Lac) (Fig. 2D).
Based on the radiological findings, including: i)
Marked enhancement of the solid part of the lesion, with absence of
enhancement of the cystic part; ii) The periphery of the lesion
presented with extensive perilesional edema; iii) DTI disclosed
sparse and focally disrupted fiber tracts in the white matter of
the left frontal lobe compared with the healthy side; iv) MRS of
the left frontal region of interest (solid portion) revealed
markedly elevated Cho, decreased NAA and Cr and therefore a
diagnosis of GBM was made.
The patient underwent a left frontal lobe tumor
resection in August 2023. Intraoperative findings revealed that the
left frontal lobe tumor was grayish-white, soft, and cystic-solid,
with a fish-like wall of the cystic portion, rich in blood supply,
measuring ~60x55x40 mm, with unclear boundaries with the
surrounding brain tissue. The tumor and part of the meninges were
resected under the microscope.
Pathological examination
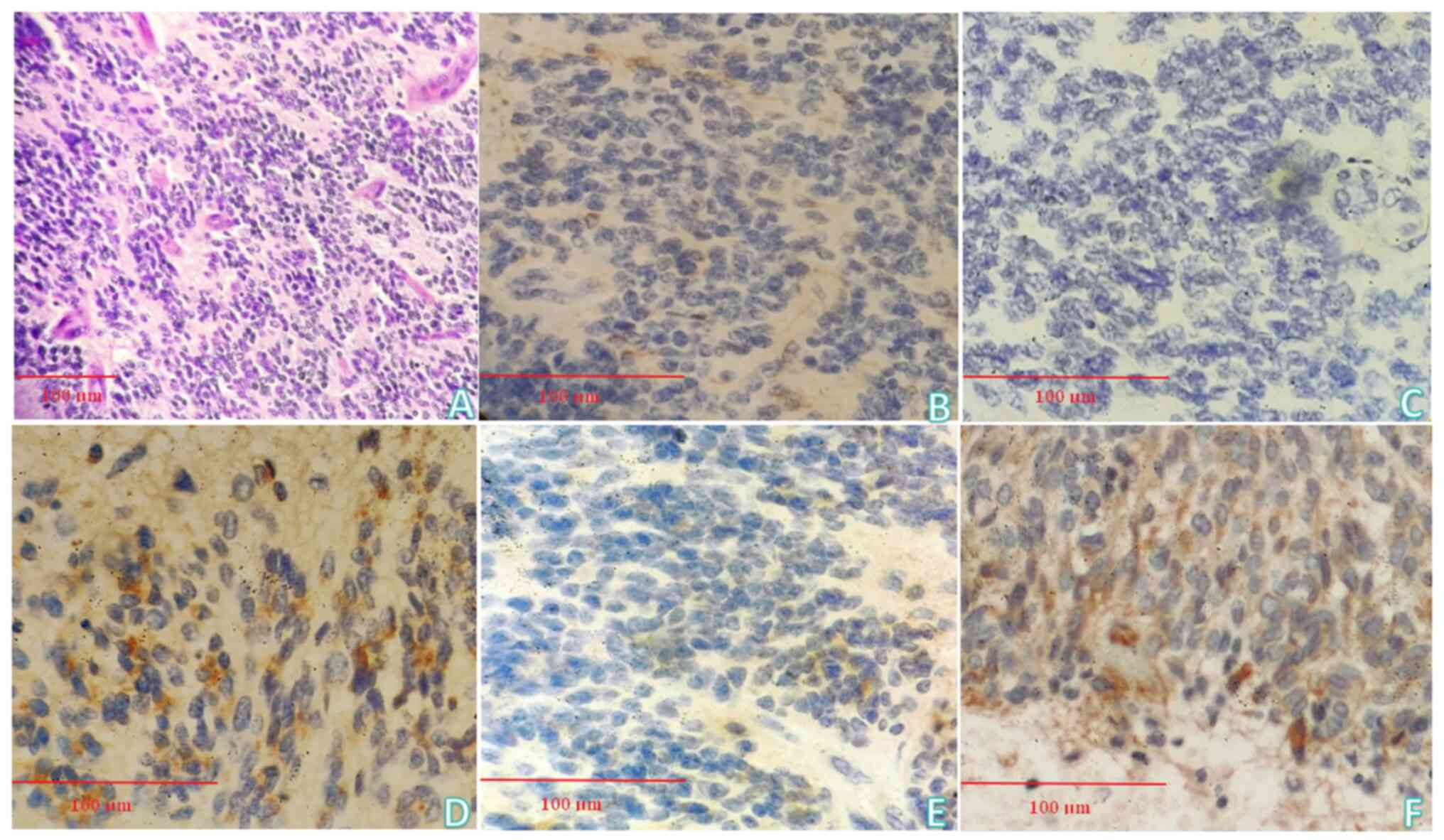
H&E staining revealed that the tumor component
of the sent tissue consisted of round and oval cells with deeply
stained nuclei and multiple chrysanthemum-shaped mass structures
were exhibited (Fig. 3A).
Immunohistochemistry (IHC)
The following findings were revealed in Fig. 3B-F: GFAP (focally +), S-100 (-),
Olig-2 (+), ATRX (-), IDH-1 (-), P53 (~50% +), EMA (focally +),
TP53 (-), NSE (-), Syn (focally +), CgA (-), CD56 (-), TIF-1 (-),
Vimentin (focally +), CK (P) (-). Combined with H&E staining
and IHC, the final pathological diagnosis was embryonal tumor, CNS
WHO grade 4.
Postoperative follow-up non-contrast and
contrast-enhanced brain MRI disclosed postoperative changes in the
left frontal lobe, measuring 46x44x35 mm, with hypointensity on
T1WI and uneven hypointensity on T2WI. The internal portion of the
lesion presented with patchy hyperintensity on T1WI and T2WI
(considering hemorrhage), and CE-T1WI revealed no enhancement
within the lesion and irregular circumferential enhancement at the
margins (Fig. 4A-F).
 | Figure 4Follow-up brain MRI of postoperative
and completion of RT. (A-F) Postoperative brain MRI: Sequentially,
T1WI (axial), T2WI (axial), DWI (axial), apparent diffusion
coefficient (axial), CE-T1WI (axial), CE-T1WI (sagittal) showed
that postoperative changes in the left frontal lobe, 46x44x35 mm in
size, with hypointensity on T1WI and uneven hypointensity on T2WI,
and the internal portion of the lesion showed patchy hyperintensity
on T1WI and T2WI (considering hemorrhage), and CE-T1WI revealed no
enhancement within the lesion and irregular circumferential
enhancement at the margins. (G-I) Follow-up contrast-enhanced brain
MRI one month after completion of RT: Sequentially, CE-T1WI
(axial), CE-T1WI (axial), CE-T1WI (sagittal) revealed that
irregular circular enhancement in the operative region, measuring
29x28x21 mm, and the edema around the lesion was less extensive
than before (postoperative), and the midline structure was roughly
centered. MRI, magnetic resonance imaging; RT, radiotherapy; T1WI,
T1-weighted imaging; T2WI, T2-weighted imaging; DWI,
diffusion-weighted imaging; CE, contrast-enhanced. |
The patient underwent a cerebrospinal fluid (CSF)
examination after surgery, and the result was negative. The patient
was treated with anti-infective, hemostatic, acid-suppressive,
antiepileptic, dehydration, brain recovery, and symptomatic
supportive therapy.
After discussion by the neuro-oncology
multidisciplinary team, the patient underwent craniospinal
radiotherapy (RT) for a total dose of 35 Gy in conventional
fractionation plus a sequential boost to residual surgical region
for a total dose of 54 Gy.
Follow-up contrast-enhanced brain MRI one month
after completion of RT revealed irregular circular enhancement in
the operative region, measuring 29x28x21 mm. The rest of the brain
exhibited no obvious abnormal enhancement and the edema around the
lesion was less extensive than before, with the midline structure
roughly centered (Fig. 4G-I).
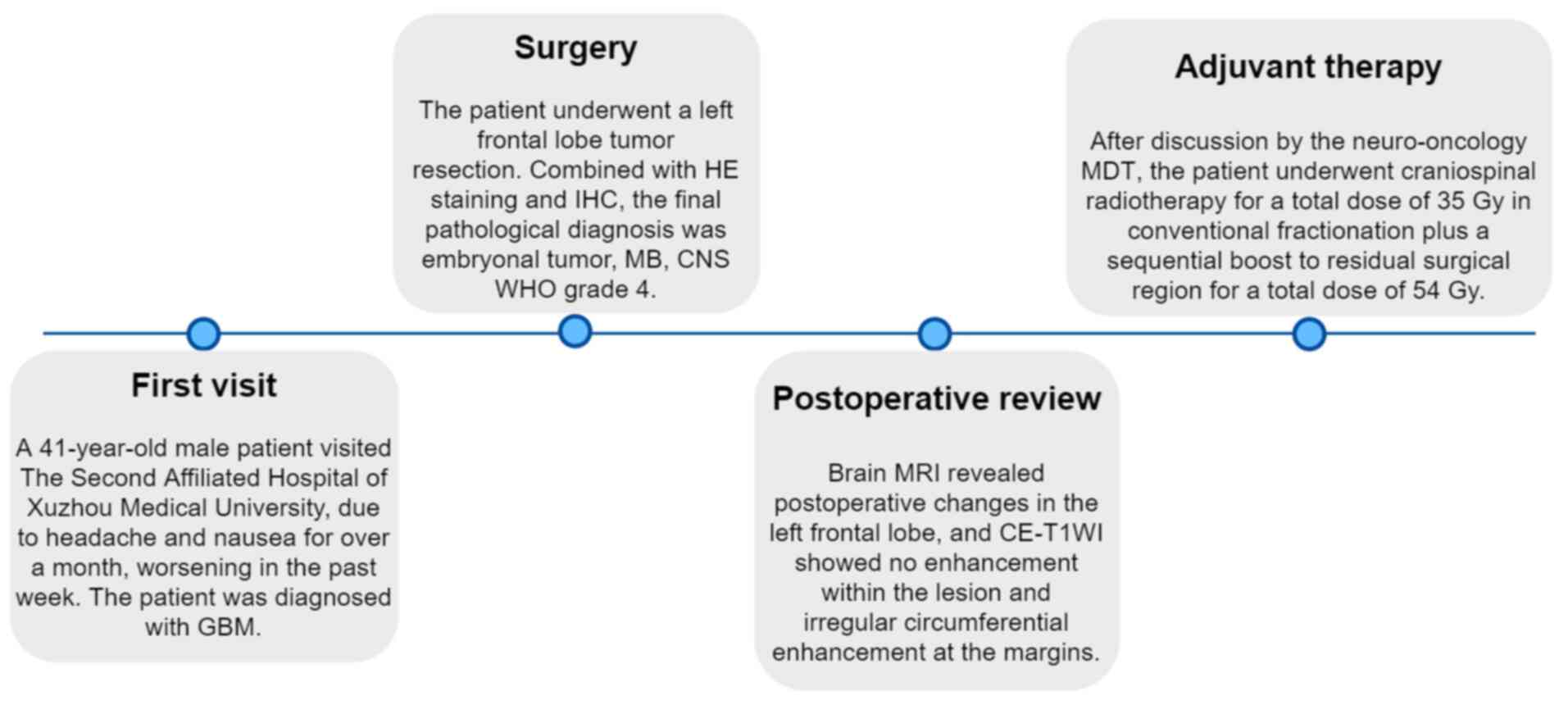
The timeline for the patient's diagnosis and
treatment is demonstrated in Fig.
5.
Discussion
ETMH is an embryonic malignant tumor originating
from residual primitive embryonic cells, commonly found in
children, accounting for 20% of all CNS tumors in children. A total
of 70% of ETMH occurs in children under the age of 10. ETMH is
relatively rare in adults, accounting for <1% of adult CNS
tumors, with the peak age of onset between 30-40 years old, and it
is more common in male patients (1-4).
ETMH often occurs in the posterior cranial fossa, with strong
invasiveness, high malignancy, rapid growth, easy detachment of
tumor cells, and the ability to produce disseminated implants along
the subarachnoid space through CSF circulation. Therefore, the
prognosis is poor and the survival period is short (5-7).
ETMH in children is most often observed in the
midline, with ~28% occurring in the cerebellar vermis, more than
half located in the dorsal cerebellar hemisphere near the pons and
a few may be located in the pontocerebellar horn region (8-10).
Adult ETMH is more common in the cerebellar hemisphere and vermis,
closely attached to the surface or tentorium of the brain, with the
most common clinical symptoms being headache, vomiting, unstable
gait, ataxia and reduced vision (11). A study summarized the MRI
manifestations of adult ETMH occurring in the cerebellum, as
follows: The tumor was circular or quasi circular and could be
multiple, prone to cystic changes, often located inside or around
the lesion; the solid part of the tumor presented with
hyperintensity on DWI, with uneven mild to moderate enhancement and
no or mild peritumoral edema (12).
Adult supratentorial ETMH is extremely rare and has
been rarely reported in the literature. The patient described in
the present case report is a 41-year-old male with a cystic-solid
lesion located in the left frontal lobe. The solid part disclosed
hypointensity on T1WI and hyperintensity on T2WI and the solid
component of the lesion was significantly enhanced on
contrast-enhanced T1WI. MRS and DTI indicated that the lesion was
an intracerebral lesion with damage to the white matter fiber
bundle and the surrounding edema range was wide. Similar to the
radiological manifestations of the case reported by Hou et
al (13), the difference is
that the surrounding edema range is larger in the present case.
The preoperative diagnosis of this case was GBM and
the possibility of ETMH was not considered. Summarizing the causes
of misdiagnosis in the present case may include the following
points. First, combining the patient's clinical symptoms and
radiological manifestations, common supratentorial brain tumors
were preferred and the imaging features of the lesion in the
present case were more consistent with GBM. Second, adult ETMH is
relatively rare and lacks a characteristic radiological
presentation and radiologists lack experience in the diagnosis of
this disease. Furthermore, adult ETMH occurs more often in the
cerebellum and less often in the supratentorial region, whereas in
this case the lesion was located in the left frontal lobe in an
atypical location, which makes it highly susceptible to
misdiagnosis (14).
Reviewing the present case and referring to the
relevant literature, it was hypothesized that the present case
still has the characteristic radiological presentation of ETMH
(15-17):
i) The solid part of the lesion presented with obvious
hyperintensity on DWI and hypointensity on apparent diffusion
coefficient (ADC). The pathological basis is that ETMH is a tumor
of small round-cell origin and the tumor cells are densely
arranged, with little cytoplasm, large and densely stained nuclei,
high nuclear-to-plasma ratio, small extracellular interstitial
space, and the tumor contains little water and the diffusion of
water molecules is significantly restricted, which mostly showed
hyperintensity on DWI and the corresponding ADC value is reduced.
ii) There are cystic and necrotic areas within the tumor. Cystic
changes are a relatively typical radiological sign of ETMH and the
rate of cystic changes can exceed 80% (18). The cause may be related to the lack
of blood supply in the fast-growing tumor or the presence of some
secretory function of the tumor. iii) The solid part of the lesion
has significantly elevated Cho, significantly decreased NAA,
significantly increased Cho/Cr and Cho/NAA ratios and mildly
elevated and partially inverted Lac, suggesting a high degree of
tumor malignancy as well as a mild hypoxic state. Although MRS does
not make the diagnosis of ETMH alone, it assists in differentiating
it from extracerebral tumors.
Key imaging features of adult supratentorial ETMH
include (19): i) CT: Ill-defined
supratentorial mass with heterogeneous density, occasionally
showing calcifications or necrosis. ii) MRI: Iso-to hypointense on
T1WI, heterogeneous hyperintensity on T2/FLAIR sequences with
surrounding vasogenic edema and restricted diffusion due to high
cellularity. iii) Contrast enhancement: Moderate to marked
heterogeneous enhancement, often with rim enhancement in cystic
areas. iv) MRS: Cho peak, reduced NAA and possible lactate/lipid
peaks, reflecting aggressive metabolism. In addition, adult
supratentorial ETMH needs to be differentiated from the following
tumors: i) GBM: GBM is prevalent in middle-aged and elderly people,
often located in the white matter of the cerebral hemisphere. It is
irregular in shape, with hypointensity on T1WI and hyperintensity
on T2WI, and is mostly inhomogeneous and prone to necrosis. The
tumor has infiltrative growth, with an unclear boundary, and the
solid part of the tumor is often mildly diffusion restricted on DWI
(degree of restriction lower than that of ETMH). Surrounding the
tumor is often accompanied by extensive edema, and CE-T1WI reveals
a wreath-like enhancement (20).
ii) Ventricular meningioma (VM): Cystic-solid parenchymal VM often
occurs in children or adolescents, and the all-parenchymal type is
more common in adults. The tumor is located in the frontoparietal
lobe, with uneven intensity, often combined with necrosis, cystic
degeneration, or calcification. The cystic portion often accounts
for >2/3 of the tumor, and the cystic wall is thin. The solid
portion is mostly located in the cortical area. The tumor shows
mild diffusion restriction on DWI with no peritumoral edema, and
the solid part of the lesion presents with obvious enhancement on
CE-T1WI (21). iii) Solitary brain
metastasis (SBM): SBM is common in middle-aged and elderly people
with a history of primary tumor. SBM is often located in the
corticomedullary junction area, and the radiological manifestations
vary depending on the primary tumor. SBM is prone to hemorrhage and
necrosis, often showing irregular ring enhancement and peritumoral
edema is more obvious (22). iv)
Primary CNS lymphoma (PCNSL). PCNSL is located in the white matter
area, with irregular morphology and can grow across the midline via
the corpus callosum. The intensity of PCNSL is mostly
uniform, presenting is equal intensity on T1WI and T2WI, obviously
diffusion-restricted on DWI, with obvious surrounding edema, and
homogeneous and obvious enhancement on CE-T1WI. Besides, the
presence of Lac and Lip, together with the elevation of the Cho/Cr
ratio, are of great value in the diagnosis of PCNSL (23). v) Meningioma: Meningiomas are
more common in female patients and often have calcification. There
are often signs of extracerebral tumors such as compression and
displacement of brain parenchyma, widening of brain cisterns, and
bone changes in the cranium. MRS can distinguish whether tumors are
intracerebral or extracerebral and has differential value (24).
Adult ETMH is relatively rare, with fewer
prospective studies in adults, and most current diagnostic
protocols refer to advances in pediatric ETMH. Surgical resection
is preferred for this disease, but complete resection is generally
difficult. The principles are to achieve maximum resection of the
tumor under the premise of minimizing damage to normal brain tissue
and re-establishing the CSF circulation (25,26).
ETMH is prone to CNS metastasis, and postoperative craniospinal RT
and/or chemotherapy (CMT) is the standard treatment for this
disease. RT/CMT should be administered as early as possible after
tumor resection, and the ideal time to start RT/CMT is within 4-6
weeks postoperatively. The patient's age, the extent of surgical
resection, the postoperative physical condition, the presence or
absence of metastasis on radiological examinations, the results of
the CSF examination, and the type of postoperative pathology should
be adequately evaluated prior to RT/CMT, and different therapeutic
strategies should then be adopted according to the clinical risk
stratification (27,28). Although comprehensive treatment with
surgery as the mainstay and RT and CMT as adjuncts has made some
progress, the prognosis of ETMH patients remains poor, and
surviving patients are still affected by serious adverse reactions.
In recent years, with the development of genomics, people have
gained a deeper understanding of the molecular typing and
development mechanisms of ETMH, and targeted therapy has become a
new research hotspot. Accurate molecular typing and personalized
targeted treatment strategies may improve the overall survival and
quality of life of patients with ETMH (29,30).
The molecular subtypes of ETMH can be divided into
four types, namely WNT type, SHH type, Group 3, and Group 4. WNT
type is defined as low-risk and can reduce the radiation dose
during RT. Chemotherapy regimens mainly include cisplatin and
cyclophosphamide to avoid overtreatment. Group 4 is of moderate
risk, with a treatment plan consisting of standard chemotherapy
(cisplatin, vincristine) and conformal RT. Group 3 is defined as
high-risk and requires intensive treatment (high-dose RT combined
with multi drug chemotherapy). SHH type is recommended for targeted
therapy, using SMO inhibitors or PI3K/mTOR inhibitors (31). The patient was recommended to
undergo more comprehensive molecular examinations, however he and
his family refused due to the high cost. The cost of tumor
molecular examination is expensive, which cannot be reimbursed by
medical insurance and needs to be borne by patients themselves.
In clinical practice, when adults present with solid
cystic lesions in the supratentorial brain that are difficult to
distinguish from GBM, clinicians also need to pay attention to the
characteristics of DWI and ADC intensities in the lesions, as well
as whether there are cystic changes and necrosis. When the lesions
show obvious hyperintensity in the solid part of DWI and obvious
hypointensity in ADC, and there are cystic changes and necrosis,
especially when multiple cystic lesions appear, the diagnosis of
ETMH should be considered (32,33).
First and foremost, enhancing the recognition
capability of ETMH in rare screenings and minimizing misdiagnosis
is crucial. This involves refining the diagnostic techniques and
algorithms to improve identification of the unique characteristics
of ETMH even in less common imaging presentations. Secondly,
standardizing the application of multimodal imaging and molecular
detection is essential. By integrating different imaging modalities
such as MRI, CT, and PET-CT, along with advanced molecular tests, a
more comprehensive understanding of the tumor can be obtained,
thereby facilitating accurate diagnosis. This standardization
should cover aspects including the sequence of tests,
interpretation criteria and data integration. Moreover, optimizing
treatment strategies, including precisely defining the RT range and
dosage, holds the key to improving patient prognosis. Tailoring the
treatment according to the individual patient's condition, tumor
size, location, and genetic profile can maximize the therapeutic
effect while minimizing side-effects (34).
The original intention of the present study was to
reveal the clinical characteristics of adult supratentorial ETMH
through typical cases, providing reference for clinical practice.
However, the authors realized that relying solely on individual
case data is difficult to support innovative conclusions. Although
lacking in innovation, the present study systematically summarized
the imaging and clinical characteristics of adult supratentorial
ETMH, providing a standardized evaluation tool for subsequent
large-scale studies. Connections with other hospitals will be
established to collect more cases of this kind, laying the
foundation for future innovative research.
WHO 2021 Classification of Tumors of the CNS has
redefined embryonal tumors based on molecular characteristics,
where traditional anatomical locations are no longer the primary
basis for classification. According to the review by Louis et
al (35), ETMH is now
classified as an ‘embryonal tumor’, and its diagnosis requires
integration of molecular subtypes (for example, WNT, SHH, Group
3/4). The IHC results in the present case suggested a neurogenic
embryonal tumor; however, the limitation of lacking molecular
subtyping data to confirm the specific subtype was acknowledged. In
developing countries, molecular testing is prohibitively expensive,
and numerous patients decline such testing due to financial
constraints, leading to limited adoption of the WHO 2021
classification criteria. In future clinical practice, the authors
will strengthen patient education to emphasize the importance of
molecular subtyping in diagnosing such tumors and recommend
comprehensive molecular testing for eligible patients.
In summary, adult ETMH is rare, and those occurring
in the supratentorial region are even rarer. The radiological
manifestations are atypical, and diagnosis is difficult. However,
conventional MRI, DWI and MRS findings still have certain
diagnostic value. Therefore, when adults are found to have
supratentorial tumors that differ from common tumor radiological
manifestations, multimodal MRI methods should be applied to improve
diagnostic accuracy, improve assistance in the formulation of
clinical treatment plans and evaluate prognosis. For the diagnosis
of supratentorial cystic-solid tumors, the possibility of ETMH
should be considered.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key R&D
Project of Xuzhou Science and Technology Bureau (grant no.
KC23208), the Development Fund Project of Xuzhou Medical University
Affiliated Hospital (grant no. XYFY202460), the Research Project of
Jiangsu Provincial Health Commission (grant no. Z2024021) and the
Clinical Technology Key Personnel Advanced Training Program of
Xuzhou.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW, AC and PD conceived and designed the study,
collected and assembled the data, and confirm the authenticity of
all the raw data. All authors read and revised the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study involving human participant was
reviewed and approved (approval no. 2024032703) by the Ethics
Committee of The Second Affiliated Hospital of Xuzhou Medical
University (Xuzhou, China) The patient provided his written
informed consent to participate in the present study.
Patient consent for publication
The patient provided informed consent for
publication of his data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cotter JA and Hawkins C: Medulloblastoma:
WHO 2021 and beyond. Pediatr Dev Pathol. 25:23–33. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bouffet E: Management of high-risk
medulloblastoma. Neurochirurgie. 67:61–68. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegfried A and Delisle MB:
Medulloblastoma. Pathology. Neurochirurgie. 67:28–38.
2021.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
4
|
Vinchon M and Leblond P: Medulloblastoma:
Clinical presentation. Neurochirurgie. 67:23–27. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Quinlan A and Rizzolo D: Understanding
medulloblastoma. JAAPA. 30:30–36. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Roussel MF and Stripay JL: Epigenetic
drivers in pediatric medulloblastoma. Cerebellum. 17:28–36.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Martirosian V, Chen TC, Lin M and Neman J:
Medulloblastoma initiation and spread: Where neurodevelopment,
microenvironment and cancer cross pathways. J Neurosci Res.
94:1511–1519. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Grassiot B, Beuriat PA, Di Rocco F,
Leblond P, Faure-Conter C, Szathmari A and Mottolese C: Surgical
management of posterior fossa medulloblastoma in children: The Lyon
experience. Neurochirurgie. 67:52–60. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Grill J, Dufour C, Guerrini-Rousseau L and
Ayrault O: New research directions in medulloblastoma.
Neurochirurgie. 67:87–89. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Archer TC and Pomeroy SL: Medulloblastoma
biology in the post-genomic era. Future Oncol. 8:1597–1604.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Franceschi E, Hofer S, Brandes AA, Frappaz
D, Kortmann RD, Bromberg J, Dangouloff-Ros V, Boddaert N, Hattingen
E, Wiestler B, et al: EANO-EURACAN clinical practice guideline for
diagnosis, treatment, and follow-up of post-pubertal and adult
patients with medulloblastoma. Lancet Oncol. 20:e715–e728.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ou YH, Bai YP, Han N, Liu G and Zhang J:
Multimodal MRI findings and misdiagnosis analysis of adult
medulloblastoma. Chin J Magn Reson Imaging. 11:360–363. 2020.
|
|
13
|
Hou HM, Xu M, Wang LM and Lv HN: Analysis
of misdiagnosis of adult supratentorial medulloblastoma: Report of
one case. J China Clin Med Imaging. 33:446–447. 2022.
|
|
14
|
Majd N and Penas-Prado M: Updates on
management of adult medulloblastoma. Curr Treat Options Oncol.
20(64)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Deng J, Xue C, Liu X, Li S and Zhou J:
Differentiating between adult intracranial medulloblastoma and
ependymoma using MRI. Clin Radiol. 78:e288–e293. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang N, Ouyang T, Kang H, Long W, Thomas
B and Zhu S: Adult medulloblastoma: Clinical characters, prognostic
factors, outcomes and patterns of relapse. J Neurooncol.
124:255–264. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Beier D, Kocakaya S, Hau P and Beier CP:
The neuroradiological spectra of adult and pediatric
medulloblastoma differ: Results from a literature-based
meta-analysis. Clin Neuroradiol. 28:99–107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Z, Ren S, Zhang H, Liao Z, Liu Z, An
X, Cheng J, Li C, Gong J, Niu H, et al: Multiparametric MRI-based
machine learning system of molecular subgroups and prognosis in
medulloblastoma. Eur Radiol: Jan 30, 2025 (Epub ahead of
print).
|
|
19
|
Aljaafary M and Alali AA: Association of
imaging biomarkers with molecular subtypes of medulloblastoma.
Neuroradiol J: 19714009241303065, 2024 (Epub ahead of print).
|
|
20
|
Sun Z, Feng D and Zhu R: A case of adult
medulloblastoma. Asian J Surg: S1015-9584(24)01979-1, 2024 (Epub
ahead of print).
|
|
21
|
Ahmed M, Nadeem M, Shahzad UB and Tariq A:
A comprehensive approach to lateral ventricular tumor resection:
Techniques, technologies, and outcomes. Neurosurg Rev.
47(489)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Lin H, Sun J, Pu R, Zhou Y and Sun
B: Texture feature differentiation of glioblastoma and solitary
brain metastases based on tumor and tumor-brain interface. Acad
Radiol. 32:400–410. 2025.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ayalew ZS, Gebregiorgis M, Azibte GT,
Hamza AK, Abdo IS and Molla BA: Primary central nervous system
lymphoma: A diagnostic challenge in a young immunocompetent patient
with limited resources. Radiol Case Rep. 19:4644–4649.
2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kalasauskas D, Kosterhon M, Kurz E,
Schmidt L, Altmann S, Grauhan NF, Sommer C, Othman A, Brockmann MA,
Ringel F and Keric N: Preoperative prediction of CNS WHO grade and
tumour aggressiveness in intracranial meningioma based on radiomics
and structured semantics. Sci Rep. 14(20586)2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Penas-Prado M, Armstrong TS and Gilbert
MR: Proposed additions to the NCCN guidelines for adult
medulloblastoma. J Natl Compr Canc Netw. 18:1579–1584.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen B, Chen C, Zhao Y, Cui W and Xu J:
The role of chemotherapy in the treatment of adult medulloblastoma.
World Neurosurg. 163:e435–e449. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Brandes AA, Bartolotti M, Marucci G,
Ghimenton C, Agati R, Fioravanti A, Mascarin M, Volpin L, Ammannati
F, Masotto B, et al: New perspectives in the treatment of adult
medulloblastoma in the era of molecular oncology. Crit Rev Oncol
Hematol. 94:348–359. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Neth BJ, Raghunathan A, Kizilbash SH, Uhm
JH, Breen WG, Johnson DR, Daniels DJ, Sener U, Carabenciov ID,
Campian JL, et al: Management and long-term outcomes of adults with
medulloblastoma: A single-center experience. Neurology.
101:e1256–e1271. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Coltin H, Sundaresan L, Smith KS, Skowron
P, Massimi L, Eberhart CG, Schreck KC, Gupta N, Weiss WA, Tirapelli
D, et al: Subgroup and subtype-specific outcomes in adult
medulloblastoma. Acta Neuropathol. 142:859–871. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhao F, Ohgaki H, Xu L, Giangaspero F, Li
C, Li P, Yang Z, Wang B, Wang X, Wang Z, et al: Molecular subgroups
of adult medulloblastoma: A long-term single-institution study.
Neuro Oncol. 18:982–990. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Harry JL, Shezi NB and Mwazha A: Molecular
classification of medulloblastoma using immunohistochemistry: A
single centre study. Ann Diagn Pathol. 76(152463)2025.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang YRJ, Wang P, Yan Z, Zhou Q, Gunturkun
F, Li P, Hu Y, Wu WE, Zhao K, Zhang M, et al: Advancing presurgical
non-invasive molecular subgroup prediction in medulloblastoma using
artificial intelligence and MRI signatures. Cancer Cell.
42:1239–1257.e7. 2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hassannejad E and Mohammadifard M,
Payandeh A, Bijari B, Shoja A, Abdollahi M and Mohammadifard M:
Correlation of ADC values of adult brain tumors with the diagnosis
and pathological grade: A cross-sectional multicenter study. Health
Sci Rep. 7(e2110)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bonm AV, Rutenberg MS, Therkelsen KE,
Herbst J, Sanaf A, Sherwood MA, Rhee JY, McGranahan TM, Cimino PJ,
Gonzalez Castro LN, et al: A multi-institutional retrospective
cohort of adult-onset medulloblastoma in the modern era. Neurooncol
Adv. 7(vdae231)2025.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251.
2021.PubMed/NCBI View Article : Google Scholar
|