Introduction
Helicobacter pylori (H. pylori) is a highly
prevalent infectious agent that affects ~40% of the global
population (1). H. pylori
was first discovered in 1983 by Robin Warren and Barry Marshall in
gastric mucosal biopsy samples from patients with chronic active
gastritis and peptic ulcer disease (2). Currently, this organism is recognized
as the causative agent of gastritis, peptic ulcer disease, gastric
adenocarcinoma and gastric B-cell lymphoma (MALT lymphoma)
(3).
Several in vivo and in vitro studies
have demonstrated that H. pylori can invade epithelial cells
and the lamina propria, triggering a more intense mucosal
inflammatory response than bacterial attachment to epithelial cells
alone. This process is important in the initiation and development
of gastric inflammation (4-7).
Furthermore, H. pylori that invades the gastric mucosa may
translocate to gastric lymph nodes, leading to chronic immune
system stimulation (8).
H. pylori in the lamina propria or
subepithelial regions may be underdiagnosed without an appropriate
immunohistochemical (IHC) study. Ito et al (8) found H. pylori in both the
mucous and deeper layers of gastric tissue in patients with gastric
cancer using the TMDU antibody. However, the study focused on
cancerous conditions and did not provide further descriptions or
categorizations of the distribution patterns of the bacteria.
Proton pump inhibitors (PPIs) are among the most
frequently prescribed medications to alleviate dyspeptic pain and
have demonstrated in vitro anti-H. pylori activity
(9). These drugs can reduce the
H. pylori load while inhibiting its urease activity
(10,11) and may interfere with certain H.
pylori detection tests, potentially leading to false-negative
results (12).
Following PPI therapy, rod-shaped H. pylori
can convert into its coccoid form (13,14).
Research has indicated that coccoid H. pylori may contribute
to bacterial transmission and the recurrence of infection, even
following antimicrobial treatment (13-16).
However, the exact mechanisms underlying the pathogenesis of
coccoid H. pylori remain poorly understood.
The detection of coccoid H. pylori requires
IHC staining because simple histochemical stains cannot reliably
detect low numbers of coccoid or intracellular bacteria, nor can
they differentiate coccoid H. pylori from artefacts or other
bacteria. IHC stains offer high specificity, enabling accurate
identification of H. pylori and exclusion of other
similar-shaped organisms (17).
It was hypothesized that PPI treatment for acid
suppression may modify the gastric mucosal microenvironment,
influencing the distribution of H. pylori in both the
surface and subepithelial compartments. Using IHC staining, it was
aimed to identify distinct infection patterns and their potential
associations with clinical outcomes.
Materials and methods
The study protocol complied with the principles of
the Declaration of Helsinki and was approved by the Institutional
Ethics Committee of Navamindradhiraj University (approval no.
120/2566; Bangkok, Thailand). Gastric tissue biopsies from patients
diagnosed with gastritis, who underwent endoscopic examination at
Vajira Hospital (Faculty of Medicine, Navamindradhiraj University,
Bangkok, Thailand) between October and December 2022, were reviewed
retrospectively.
Inclusion criteria were as follows: i) Availability
of at least four biopsies obtained from both the antrum and corpus
as part of routine clinical practice; ii) diagnosis of gastritis
confirmed by histopathological evaluation; and iii) sufficient
formalin-fixed paraffin-embedded (FFPE) tissue for both hematoxylin
and eosin (H&E) and IHC analysis. Additional exclusion criteria
included: vii) inadequate biopsy samples, and viii) poor tissue
preservation precluding histological interpretation.
As part of standard clinical practice, at least four
biopsies were collected from both the gastric antrum and corpus.
Patients were not specifically recruited for the present study, and
no additional tissue samples were collected. FFPE blocks were
processed for histopathological and IHC analysis. Clinical data
were also extracted and recorded between April and May 2024. All
identifying information was removed.
The patients were divided into two groups according
to PPI therapy: Those receiving PPIs for at least a 14-day period
prior to the procedure (PPI-treated group) and those not receiving
PPIs or receiving them for a shorter period (PPI-untreated
group).
Exclusion criteria
Patients were excluded from the analysis on the
basis of the following criteria: i) Previous use of antibiotics,
histamine (H2) receptor blockers, statins, aspirin, or
non-steroidal anti-inflammatory drugs; ii) a prior history of H.
pylori infection or triple therapy eradication; iii) the
presence of underlying conditions, including autoimmune diseases,
diabetes mellitus, cerebrovascular disease, peptic ulcers, HIV
infection, or gastrointestinal cancers; iv) use of
immunosuppressive drugs prior to endoscopy; v) diagnosis of
Helicobacter heilmannii infection based on morphological
criteria (for example, straight appearance, corkscrew-shaped
spirals, or exceeding 3 µm in length) (18); and vi) cases of gastritis with
specific etiologies other than H. pylori infection, such as
reactive gastropathy or autoimmune gastritis.
H. pylori eradication therapy
H. pylori eradication treatment was defined
as the prescription of either a PPI with at least two types of
antibiotics initiated simultaneously or a fixed-dose triple
therapy. Eradication was considered successful if no second
treatment was prescribed after the initial treatment. Treatment
failure, defined as the prescription of a second eradication
treatment within 12 months, was used as a proxy for antimicrobial
resistance.
Histopathology
All the paraffin blocks were sectioned at 3-µm
thickness and stained with H&E in August 2023. Staining was
performed at room temperature (~22-25˚C), with hematoxylin applied
for 5-10 min and eosin for 1-2 min, following standard histological
protocols.
Two pathologists (CS and KL) independently reviewed
the H&E-stained slides. Cases with discordant results were
reviewed together and discussed until consensus was reached.
Neutrophilic and mononuclear inflammation, glandular atrophy, and
intestinal metaplasia were assessed and graded (0=none, 1=mild,
2=moderate, 3=severe) according to the updated Sydney System
(19).
Gastritis was classified on the basis of
inflammatory process status (chronic and/or active) and the
presence or absence of structural changes (intestinal metaplasia,
atrophy, ulcers). This resulted in four distinct groups: i) Chronic
nonactive gastritis without structural change, ii) chronic active
gastritis without structural change, iii) chronic non-active
gastritis with structural change, and iv) chronic active gastritis
with structural change.
To analyze the associations between pathologic
features and H. pylori presence, the cases were further
regrouped as ‘non-active gastritis’ (groups i and iii) and ‘active
gastritis’ (groups ii and iv). Additionally, they were regrouped as
‘without structural change’ (groups i and ii) and ‘with structural
change’ (groups iii and iv).
IHC
IHC tests were performed on all cases in August 2023
using the Leica Bond-Max automated IHC staining platform (Leica
Microsystems, Inc.) with 2-µm-thick consecutive sections prepared
from FFPE tissue, in accordance with the manufacturer's
instructions. Tissue sections were deparaffinized using Leica Bond
Dewax Solution (cat. no. AR9222), and rehydration was performed
automatically by the Bond-Max system using a graded ethanol series
integrated into the protocol. Antigen retrieval was conducted using
Epitope Retrieval Solution 2 (ER2; pH 9.0; cat. no. AR9640) for 20
min at 100˚C under standard automated conditions.
Endogenous peroxidase activity was blocked using the
Peroxidase Block reagent included in the Leica Bond Polymer Refine
Detection kit (cat. no. DS9800), which contains ~3% hydrogen
peroxide. This blocking step was carried out for 5 min at room
temperature (~22-25˚), according to the system's default
protocol.
The following primary antibodies targeting H.
pylori were used: mouse monoclonal anti-H. pylori
antibody (1:800; clone TMDU-D8; cat. no. D369-3; MBL International
Co.), monoclonal anti-H. pylori antibody (1:300; clone
ULC3R; cat. no. MU880-5UCE; BioGenex Laboratories), polyclonal
anti-H. pylori antibody (1:100; cat. no. 215A-76; Cell
Marque), and polyclonal anti-H. pylori antibody (1:50; cat.
no. B0471; Dako; Agilent Technologies, Inc.). Primary antibody
incubation was performed at room temperature for 15 min, consistent
with Leica's standard program for the Bond-Max system. BioGenex
demonstrated superior diagnostic performance for H. pylori
detection and was therefore used as the reference standard (gold
standard) in the present study (Table
SI).
Immunoreactivity was visualized using the Leica Bond
Polymer Refine Detection kit (cat. no. DS9800), with incubation
performed at room temperature for 15-30 min, as per the
manufacturer's protocol. Positive (H. pylori-infected
gastric biopsy) and negative (gastric tissue from sleeve
gastrectomy patients without gastritis) controls were included.
Stained slides were examined using a Nikon Eclipse E200 light
microscope.
Detection of H. pylori
Two pathologists (CS and KL) independently evaluated
the H. pylori IHC slides. Disagreements were resolved
through joint discussion until a consensus was reached. Bacterial
density was assessed separately for surface epithelial and
subepithelial locations. The surface epithelial H. pylori
density was graded using the updated Sydney System's standardized
visual analogue scale, which classifies bacteria into four
categories (19): S0: normal (no
bacteria); S1: individual bacteria or small groups of <1/3 of
the mucosal surface; S2: moderate (bacteria count greater than mild
but less than severe); S3: severe (large groups of bacteria
covering >2/3 of the mucosal surface).
The subepithelial H. pylori location (either
intracellular or interstitial) was examined under high
magnification (0.196 mm2 area, high-power field) using a
Nikon Eclipse E200 light microscope. Subepithelial signals were
counted below the lower border of the basement membrane, which
served as the histological landmark.
For signal selection, only those exhibiting an
intensity comparable to surface-attached bacilli morphology in the
external or internal positive controls and showing a negative
signal in the negative control were included. Homogeneous,
indiscrete clumps were counted as a single signal, whereas
heterogeneous, discrete signals within clumps were counted
individually.
When the signal density varied across the slide, the
region with the highest density (hot spot) was designated for
counting. The subepithelial bacterial density was then graded on
the basis of the number of immunopositive signals: I0: no signal;
I1: 1-20 signals/HPF; I2: 20-50 signals/HPF; and I3: >50
signals/HPF.
Patterns of H. pylori
The antibodies demonstrating the highest diagnostic
accuracy served as the gold standard for classifying H.
pylori patterns. The classification, derived from combining
bacterial presence in both surface epithelial and subepithelial
locations, resulted in the following five groups: Group 1: Isolated
surface epithelial pattern (S1-S3 and I0); Group 2: Isolated
subepithelial pattern (I1-I3 and S0); Group 3: Predominant surface
epithelial pattern (S3 and only I1); Group 4: Predominant
subepithelial pattern (I3 and only S1); Group 5: Non-specific
pattern (S1 and I1-I2, S2 and I1-I3, or S3 and I2-I3).
Statistical analyses
All the statistical analyses were conducted using
Stata (version 13; StataCorp LP). The diagnostic performance of
each IHC study was assessed by calculating the sensitivity,
specificity, positive predictive value and negative predictive
value.
Categorical variables were compared using the
chi-square test or Fisher's exact test, as appropriate. Univariate
and multivariate analyses utilized the Cox proportional hazards
model. The time to clinical improvement (cumulative clinical
improvement rate) was calculated as the duration between the onset
of upper abdominal symptoms at diagnosis and either clinical
improvement or the last follow-up. These data were compared between
groups via Kaplan-Meier plots and the log-rank test.
To assess the associations between H. pylori
infection patterns and treatment success, modified Poisson
regression was used to estimate the relative risk (RR), with robust
standard errors used to calculate 95% confidence intervals (CIs).
Statistically significant difference was set at P<0.05. Only
patients with consistent follow-up visits every 1-2 months for at
least 6 months were included in the analysis.
Results
Demographic and histopathological
characteristics of the study population
The present study included 255 patients with chronic
gastritis diagnosed from tissue biopsies. The mean age of the
patients was 47 years, and 130 (51.0%) were female. Nearly half
(115 patients, 45.1%) had received PPI therapy, with a median
treatment duration of 2.7 years. There were no significant
differences in age or sex between the PPI-treated and untreated
groups.
The demographic and histopathological
characteristics of the study population are summarized in Table I. Among all participants, the most
common type of gastritis, considering both inflammatory activity
and structural changes, was chronic non-active gastritis without
structural changes (68.2%). This was followed by chronic nonactive
gastritis with structural changes (15.7%), chronic active gastritis
without structural changes (12.2%), and chronic active gastritis
with structural changes (3.9%). These trends were similar in
patients receiving PPI treatment. However, among patients not using
PPIs, chronic nonactive gastritis without structural changes was
most common (66.4%). Chronic non-active gastritis with structural
changes and chronic active gastritis without structural changes
were equally prevalent (14.3%), whereas chronic active gastritis
with structural changes was the least common (5.0%).
 | Table IClassification of 255 patients
according to age, sex, histopathological findings, and H.
pylori status by BioGenex (n=255). |
Table I
Classification of 255 patients
according to age, sex, histopathological findings, and H.
pylori status by BioGenex (n=255).
| Variables | Total, (n=255) | PPI positive
(n=115) | PPI negative
(n=140) | P-value |
|---|
| Age, years | | | | | | | |
|
<60 | 159 | (62.4) | 68 | (59.1) | 91 | (65.0) | |
|
≥60 | 96 | (37.6) | 47 | (40.9) | 49 | (35.0) | 0.336a |
| Sex, n (%) | | | | | | | |
|
Female | 130 | (51.0) | 66 | (57.4) | 64 | (45.7) | 0.063a |
|
Male | 125 | (49.0) | 49 | (42.6) | 76 | (54.3) | |
| Histology | | | | | | | |
|
Chronic
active gastritis with structural change | 10 | (3.9) | 3 | (2.6) | 7 | (5.0) | 0.519b |
|
Chronic
non-active gastritis with structural change | 40 | (15.7) | 20 | (17.4) | 20 | (14.3) | 0.497a |
|
Chronic
active gastritis without structural change | 31 | (12.2) | 11 | (9.6) | 20 | (14.3) | 0.251a |
|
Chronic
non-active gastritis without structural change | 174 | (68.2) | 81 | (70.4) | 93 | (66.4) | 0.494a |
| H. pylori
status | | | | | | | |
|
H.
pylori positive | 83 | (32.5) | 31 | (27.0) | 52 | (37.1) | 0.107a |
|
Any
surface | 60 | (23.5) | 26 | (22.6) | 34 | (24.3) | 0.753a |
|
Any
subepithelium | 75 | (29.4) | 25 | (21.7) | 50 | (35.7) | 0.015a |
H. pylori detection rate
Among the 255 gastric biopsies, the H. pylori
detection rate by BioGenex was 32.5%. Bacteria were detected in the
surface epithelium in 23.5% of the cases (72.3% of positive cases)
and in the subepithelial location in 29.4% of the cases (90.4% of
positive cases). The performance of all four antibodies is
illustrated in Fig. 1.
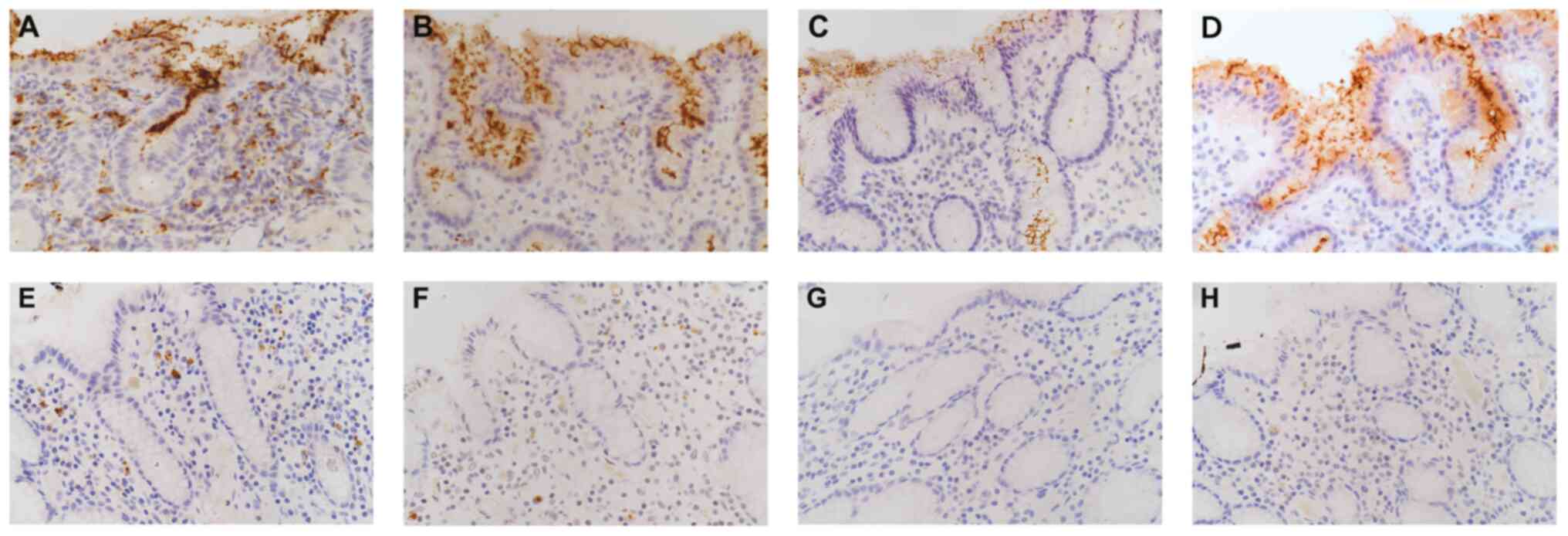 | Figure 1Topographic distribution of
immunoreactive H. pylori according to surface epithelial and
subepithelial locations, using BioGenex, TMDU, Cell Marque and
DAKO. Although the detection frequency and staining intensity
varied, the immunoreactive H. pylori did not differ
morphologically among these antibodies when detected in the surface
epithelium. Remarkable differences were observed in the IHC results
for subepithelial H. pylori in the lamina propria. (A-D)
Scattered dot-like particles were found with (A) BioGenex and, to
lesser extent, with (B) TMDU, a few particles with (C) Cell Marque,
and no reactivity with (D) DAKO. (E-H) In the isolated
subepithelial pattern, (E) BioGenex and (F) TMDU identified
subepithelial bacteria while (G) Cell Marque and (H) DAKO did not
identify any bacteria. This also clarifies that all four markers
were negative for surface bacteria (original magnification, x60).
H. pylori, Helicobacter pylori. |
PPI use and H. pylori detection
A significantly lower H. pylori detection
rate was observed among patients not using PPIs than among those in
the PPI-treated groups: 21.7% vs. 35.7% for overall subepithelial
staining (P=0.015) and 3.8% vs. 19.4% for the isolated epithelial
pattern (P=0.047), respectively. However, no statistically
significant associations were found between PPI treatment and other
mixed histopathology patterns of gastritis. The results are
summarized in Table II.
 | Table IIComparison of histopathological
characteristics and group classification between PPI and non-PPI
users in the H. pylori-positive cases, using BioGenex as the
gold standard. |
Table II
Comparison of histopathological
characteristics and group classification between PPI and non-PPI
users in the H. pylori-positive cases, using BioGenex as the
gold standard.
| Variables | Total (n=83) | PPI positive
(n=31) | PPI negative
(n=52) | P-value |
|---|
| Histology | | | | | | | |
|
Chronic
active gastritis with morphological change | 10 | (12.0) | 3 | (9.7) | 7 | (13.5) | 0.737b |
|
Chronic
nonactive gastritis with morphological change | 17 | (20.5) | 8 | (25.8) | 9 | (17.3) | 0.353a |
|
Chronic
active gastritis without morphological change | 26 | (31.3) | 10 | (32.3) | 16 | (30.8) | 0.888a |
|
Chronic
nonactive gastritis without morphological change | 30 | (36.1) | 10 | (32.3) | 20 | (38.5) | 0.569a |
|
HP
positive | 83 | (100) | 31 | (100) | 52 | (100) | NA |
|
Any
surface staining | 60 | (72.3) | 26 | (83.9) | 34 | (65.4) | 0.069a |
|
Any
subepithelial staining | 75 | (90.4) | 25 | (80.6) | 50 | (96.2) | 0.047b |
| Isolated surface
epithelial pattern | 8 | (9.6) | 6 | (19.4) | 2 | (3.8) | 0.047b |
| Isolated
subepithelial pattern | 23 | (27.7) | 5 | (16.1) | 18 | (34.6) | 0.069a |
| Predominant surface
epithelial pattern | 17 | (20.5) | 7 | (22.6) | 10 | (19.2) | 0.879a |
| Predominant
subepithelial pattern | 8 | (9.6) | 2 | (6.5) | 6 | (11.5) | 0.704b |
| Non-specific
pattern | 27 | (32.5) | 11 | (35.5) | 16 | (30.8) | 0.657a |
Mucosal density and location of H.
pylori and grades based on the Sydney system visual analogue
scale
Using BioGenex as a reference, a weak correlation
(r=0.36) was observed between the surface epithelial density of
H. pylori (evaluated via IHC) and active inflammation in the
epithelial compartment (gastric mucosa) (Table III). However, H. pylori
density was moderately correlated (r=0.45) with chronic
inflammation and not correlated with active inflammation in the
subepithelial compartment.
 | Table IIICorrelation between H.
pylori-positive cases and histologic grades according to the
updated Sydney System, using BioGenex as the gold standard. |
Table III
Correlation between H.
pylori-positive cases and histologic grades according to the
updated Sydney System, using BioGenex as the gold standard.
| | Surface | Subepithelium |
|---|
| Sydney system | + | - | + | - |
|---|
| Chronic
inflammation | | | | |
|
0 | 0 | 0 | 0 | 0 |
|
1 | 0 | 0 | 0 | 0 |
|
2 | 4 | 1 | 2 | 3 |
|
3 | 22 | 4 | 23 | 3 |
|
ra | 0.046 | 0.451 |
|
P-value | 0.805 | 0.011 |
| PMN
infiltration | | | | |
|
0 | 13 | 5 | 13 | 5 |
|
1 | 0 | 0 | 0 | 0 |
|
2 | 3 | 0 | 2 | 1 |
|
3 | 10 | 0 | 10 | 0 |
|
ra | 0.363 | 0.296 |
|
P-value | 0.045 | 0.105 |
| Glandular
atrophy | | | | |
|
0 | 18 | 3 | 18 | 3 |
|
1 | 4 | 1 | 3 | 2 |
|
2 | 3 | 1 | 3 | 1 |
|
3 | 1 | 0 | 1 | 0 |
|
ra | -0.065 | -0.149 |
|
P-value | 0.727 | 0.424 |
| Intestinal
metaplasia | | | | |
|
0 | 22 | 3 | 20 | 5 |
|
1 | 0 | 1 | 1 | 0 |
|
2 | 4 | 1 | 4 | 1 |
|
3 | 0 | 0 | 0 | 0 |
|
ra | -0.200 | 0.027 |
|
P | 0.281 | 0.887 |
| H. pylori
density | | | | |
| Surface | | | | |
|
0 | 0 | 5 | 5 | 0 |
|
1 | 11 | 0 | 5 | 6 |
|
2 | 7 | 0 | 7 | 0 |
|
3 | 8 | 0 | 8 | 0 |
|
ra | 0.663 | 0.285 |
|
P-value | <0.001 | 0.120 |
| Subepithelium | | | | |
|
0 | 6 | 0 | 0 | 6 |
|
1 | 10 | 4 | 14 | 0 |
|
2 | 6 | 1 | 7 | 0 |
|
3 | 4 | 0 | 4 | 0 |
|
ra | 0.021 | 0.727 |
|
P-value | 0.912 | <0.001 |
Factors predicting H. pylori
distribution patterns
Multivariate analysis (Table IV) revealed that PPI use and a
histologically active pattern were independently associated with
the isolated surface epithelial pattern [hazard ratio (HR), 8.02
and 3.55, respectively] and inversely associated with the isolated
subepithelial pattern (HR, 0.24 and 0.05, respectively).
Additionally, a histologically active pattern was independently
associated with the predominant surface epithelial pattern (HR,
5.18) and the non-specific pattern (HR, 3.12). No statistically
significant associations were observed between PPI treatment
(treated vs. untreated groups) and age, sex, or the histopathology
of gastritis.
 | Table IVMultivariable analyses of factors
associated with Helicobacter pylori distribution
patterns. |
Table IV
Multivariable analyses of factors
associated with Helicobacter pylori distribution
patterns.
| | Multivariable
analysis (G1) | Multivariable
analysis (G2) | Multivariable
analysis (G3) | Multivariable
analysis (G4) | Multivariable
analysis (G5) |
|---|
| Variables |
ORadj2 | 95% CI | P-value |
ORadj2 | 95% CI | P-value |
ORadj2 | 95% CI | P-value |
ORadj2 | 95% CI | P-value |
ORadj2 | 95% CI | P-value |
|---|
| Age, years | | | | | | | | | | | | | | | |
|
<60 | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | |
|
≥60 | 1.01 | (0.34-3.02) | 0.989 | 1.04 | (0.32-3.33) | 0.953 | 1.30 | (0.41-4.06) | 0.657 | 2.09 | (0.38-11.56) | 0.398 | 0.80 | (0.30-2.12) | 0.655 |
| Sex | | | | | | | | | | | | | | | |
|
Male | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | |
|
Female | 1.08 | (0.38-3.12) | 0.881 | 0.68 | (0.21-2.16) | 0.509 | 3.82 | (1.25-11.68) | 0.019 | 0.77 | (0.16-3.65) | 0.738 | 0.73 | (0.28-1.89) | 0.518 |
| Treatment | | | | | | | | | | | | | | | |
|
PPI
negative | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | |
|
PPI
positive | 8.02 | (2.62-24.50) | <0.001 | 0.24 | (0.07-0.84) | 0.026 | 0.59 | (0.19-1.86) | 0.366 | 0.60 | (0.11-3.40) | 0.565 | 1.09 | (0.41-2.89) | 0.869 |
| Histology | | | | | | | | | | | | | | | |
| Active
inflammation | | | | | | | | | | | | | | | |
| Non-active
patterna | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | | 1.00 | Reference | |
| Active
patterna | 3.55 | (1.18-10.72) | 0.024 | 0.05 | (0.01-0.25) | <0.001 | 5.18 | (1.73-15.51) | 0.003 | 0.36 | (0.07-1.96) | 0.237 | 3.12 | (1.22-8.00) | 0.018 |
| Structural
alteration | | | | | | | | | | | | | | | |
| No structural
changeb | | | | | | | | | | | | | | | |
| With structural
changeb | | | | | | | | | | | | | | | |
Clinical data and follow-up
Of the 255 patients included in the
histopathological and IHC studies, 83 patients with H.
pylori infection had complete clinical follow-up for a minimum
of 3 months and were included in the outcome analyses.
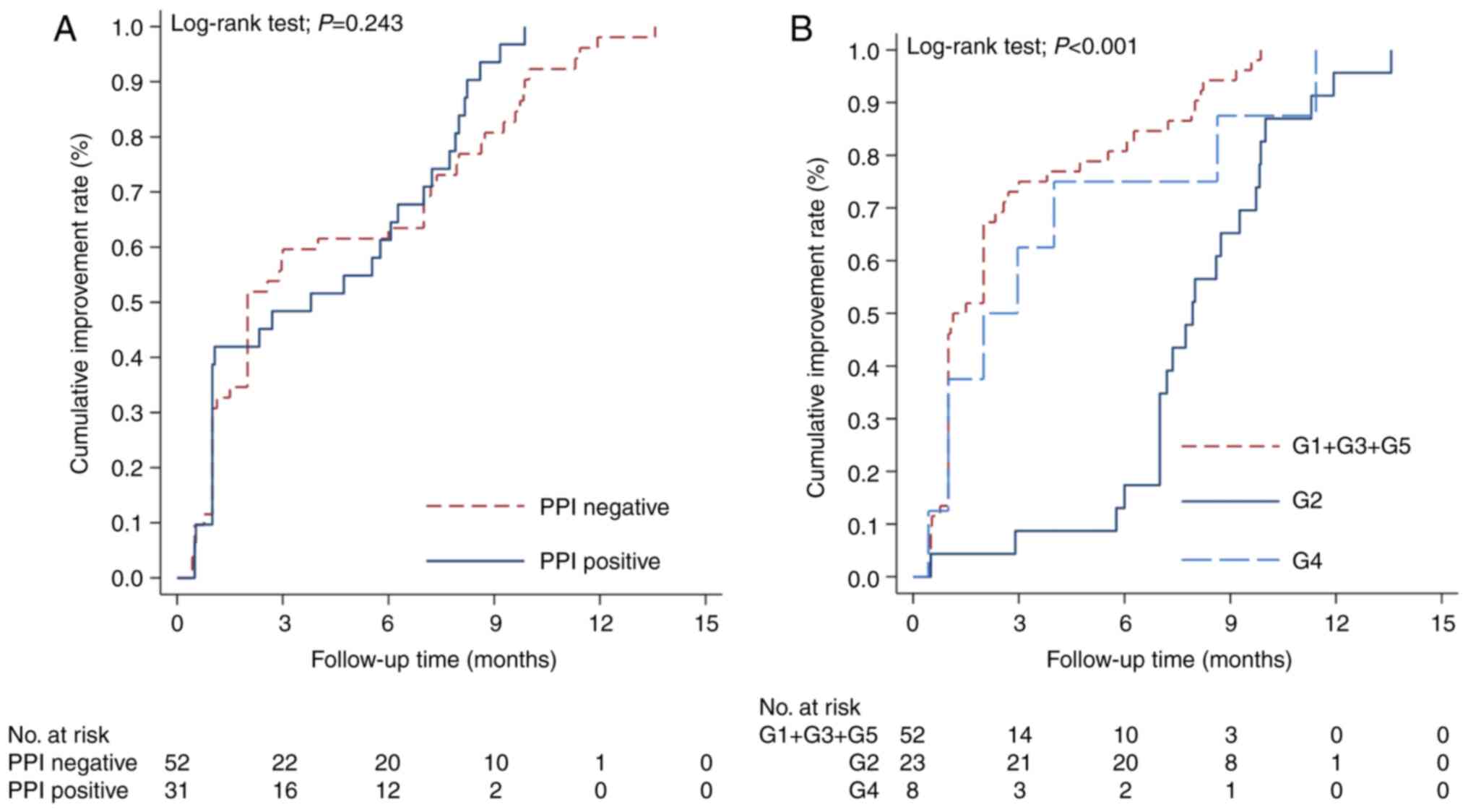
Overall, 55.4% (46 patients) achieved clinical
improvement (Table V). The clinical
outcomes of patients on the basis of the clinical and pathological
features of gastritis, as well as the H. pylori pattern, are
presented in Table VI. Univariable
analysis revealed that patients with chronic gastritis with active
inflammation experienced greater clinical improvement than those
with non-active infections did (improvement rate: 80.6; 95% CI:
66.42-91.44; P=0.005), whereas patients with the isolated
subepithelial pattern had the lowest improvement rate compared with
the other groups (improvement rate: 8.7; 95% CI: 2.25-30.51;
P<0.001). However, only the isolated subepithelial pattern was
confirmed as an independent factor for clinical outcomes through
multivariate analysis (HR, 0.25; 95% CI: 0.12-0.50; P<0.001).
Notably, PPI treatment was not found to be associated with clinical
outcomes. The Kaplan-Meier survival curves of patients according to
their H. pylori distribution pattern is included in Fig. 2.
 | Table VUnivariable analyses of the clinical
improvement rate at 3 months in the Helicobacter
pylori-positive cases according to various clinicopathologic
factors (n=83). |
Table V
Univariable analyses of the clinical
improvement rate at 3 months in the Helicobacter
pylori-positive cases according to various clinicopathologic
factors (n=83).
| | | Clinical
improvement rate at 3 months |
|---|
| Variables | N | Number of
improvements | Rate | (95% CI) | P-value |
|---|
| Total | 83 | 46 | 55.42 | (45.14-66.28) | |
| Age, years | | | | | |
|
<60 | 31 | 14 | 45.16 | (29.74-64.03) | 0.474 |
|
≥60 | 52 | 32 | 61.54 | (48.66-74.57) | |
| Sex | | | | | |
|
Female | 39 | 23 | 58.97 | (44.24-74.31) | 0.521 |
|
Male | 44 | 23 | 52.27 | (38.55-67.49) | |
| Treatment | | | | | |
|
PPI
negative | 52 | 31 | 59.62 | (46.74-72.89) | 0.243 |
|
PPI
positive | 31 | 15 | 48.39 | (32.64-66.96) | |
| Histopathology | | | | | |
| Active
inflammation | | | | | |
|
Non-active
patterna | 47 | 17 | 36.17 | (24.27-51.57) | |
|
Active
patterna | 36 | 29 | 80.56 | (66.42-91.44) | 0.005 |
| Structural
alteration | | | | | |
|
No
structural changeb | 56 | 31 | 55.36 | (42.98-68.58) | |
|
With
structural changeb | 27 | 15 | 55.56 | (38.25-74.44) | 0.841 |
| Topographic
distribution | | | | | |
|
Isolated
surface or predominant surface epithelial or non-specific
patterns | 52 | 39 | 75.00 | (62.74-85.73) | <0.001 |
|
Isolated
subepithelial pattern | 23 | 2 | 8.70 | (2.25-30.51) | |
|
Predominant
subepithelial pattern | 8 | 5 | 62.50 | (32.56-91.3) | |
 | Table VIUnivariable and multivariable Cox
proportional hazards regression survival analyses of factors
associated with clinical improvement in Helicobacter
pylori-positive cases. |
Table VI
Univariable and multivariable Cox
proportional hazards regression survival analyses of factors
associated with clinical improvement in Helicobacter
pylori-positive cases.
| | Univariable
analysis | Multivariable
analysis |
|---|
| Variables | HR | 95%CI | P-value |
HRadj | 95%CI | P-value |
|---|
| Age (years) | | | | | | |
|
<60 | 1.00 | Reference | | 1.00 | Reference | |
|
≥60 | 0.85 | (0.54-1.35) | 0.497 | 0.97 | (0.58-1.63) | 0.921 |
| Sex | | | | | | |
|
Male | 1.00 | Reference | | 1.00 | Reference | |
|
Female | 1.15 | (0.74-1.78) | 0.541 | 1.00 | (0.64-1.57) | 0.984 |
| Treatment | | | | | | |
|
PPI
negative | 1.00 | Reference | | 1.00 | Reference | |
|
PPI
positive | 1.30 | (0.82-2.06) | 0.268 | 0.78 | (0.44-1.37) | 0.388 |
| Histopathology | | | | | | |
| Active
inflammation | | | | | | |
|
Non-active
patterna | 0.55 | (0.35-0.86) | 0.009 | 1.13 | (0.66-1.93) | 0.664 |
|
Active
patterna | 1.00 | Reference | | 1.00 | Reference | |
| Structural
alteration | | | | | | |
|
No
structural changeb | 1.00 | Reference | | | | |
|
With
structural changeb | 1.05 | (0.66-1.66) | 0.848 | | | |
| Topographic
distribution | | | | | | |
|
Isolated
surface or predominant surface epithelial or non-specific
patterns | 1.00 | Reference | | 1.00 | Reference | |
|
Isolated
subepithelial pattern | 0.30 | (0.17-0.51) | <0.001 | 0.25 | (0.12-0.50) | <0.001 |
|
Predominant
subepithelial pattern | 0.56 | (0.26-1.22) | 0.147 | 0.49 | (0.22-1.12) | 0.093 |
H. pylori eradication therapy
Among the H. pylori-positive patients, 73.5%
(61 patients) received eradication therapy, while 26.5% (22
patients) were left untreated. Among the untreated patients, the
majority (81.8%, 18 patients) had an isolated subepithelial
pattern. Compared with the isolated surface epithelial pattern or
mixed patterns, the isolated subepithelial pattern was associated
with a lower eradication success rate. Modified Poisson regression
analysis revealed that compared with the isolated subepithelial
pattern, the surface epithelial pattern or mixed patterns had a
greater risk ratio (RR) of successful H. pylori eradication,
with RR values ranging from 1.46-1.67 (RR=1) (Table VII).
 | Table VIIFactors associated with the success
rates of H. pylori eradication. |
Table VII
Factors associated with the success
rates of H. pylori eradication.
| Patterns of H.
pylori | Number of patients
treated | Eradication rate
(%) | Treatment failure
(%) | RR | 95% CI | P-value |
|---|
| Isolated surface
epithelial pattern | 8 | 100 | 0 | 1.67 | (0.81-3.43) | 0.165 |
| Isolated
subepithelial pattern | 5 | 60 | 40 | 1.00 | Reference | |
| Predominant surface
epithelial pattern | 15 | 93.3 | 6.7 | 1.56 | (0.75-3.24) | 0.238 |
| Predominant
subepithelial pattern | 8 | 87.5 | 12.5 | 1.46 | (0.68-3.14) | 0.336 |
| Non-specific
pattern | 25 | 92 | 8 | 1.53 | (0.74-3.18) | 0.252 |
Discussion
In the present study, the prevalence of H.
pylori infection, as determined by IHC in 255 patients, was
32.5%, which is lower than the 40-45% reported in previous studies
(1,20,21).
This discrepancy may be attributed to differences in study periods,
with more recent research indicating a decline in prevalence
compared with earlier studies (1,20,21).
This reduction in infection rates could be due to demographic
variations, improved personal hygiene, improved living conditions,
increased public awareness, and active H. pylori eradication
efforts (1,20,21).
The ability to detect H. pylori and its
specific localization may vary depending on the antibody used for
IHC staining. Certain antibodies typically detect H. pylori
only in superficial areas and not beneath the epithelium. Ito et
al (8) examined the presence
and invasive behavior of H. pylori via various methods,
including IHC, PCR, bacterial culture and immunoelectron
microscopy. They found H. pylori in both the mucous layer
and the lamina propria in gastric cancer patients using the
TMDU-mAb. In the present study, the diagnostic accuracy of BioGenex
was comparable with that of TMDU, with BioGenex showing greater
sensitivity in detecting H. pylori in deeper tissue, where
TMDU was detected in only 68.9% of cases.
H. pylori was initially considered a
non-invasive pathogen that resides solely in the gastric lumen and
affects only gastric epithelial cells. However, most previous
studies focused primarily on the surface epithelium. Subsequent
in vivo and in vitro studies have shown that H.
pylori is invasive and can also be found in the lamina propria
(4-8).
Expanding the focus to include the subepithelial location may
provide a more accurate representation of H. pylori
prevalence.
The presence of H. pylori in the
subepithelial region correlated more strongly with the severity of
chronic inflammation than surface bacteria in active gastritis,
which aligns with the findings of Ito et al (8). This suggests a location-dependent
immunological response, potentially involving macrophage
interaction and contributing to the induction and maintenance of
chronic inflammation in H. pylori-infected gastric mucosa.
While surface epithelial H. pylori typically induces IL-12
production and Th1 cell accumulation (22), subepithelial bacteria, interacting
with lamina propria macrophages and T cells, may elicit a distinct
chronic inflammation. The present study observed a significantly
higher prevalence of isolated subepithelial H. pylori
(27.7%) compared with Ito et al's (2%). Notably, Ito et
al (8) reported 46 PCR-positive
H. pylori cases in the stomach; however, 4 of these cases
exhibited negative IHC results for both surface and subepithelial
bacteria, with only 1 showing isolated subepithelial positivity.
The differences in findings may be explained by several factors.
Firstly, Ito et al (8)
employed more thorough tissue sampling, which may have led to an
increased detection of surface bacteria.
In contrast to surface-attached bacilli,
subepithelial H. pylori presented as dot-like signals, which
may represent bacterial casts or coccoid forms. Additionally, the
enhanced sensitivity of BioGenex used in the present study may
explain the discrepancies. BioGenex utilizes the ULC3R clone to
identify specific epitopes of H. pylori that are resistant
to intracellular digestion and capable of detecting antigenic forms
of H. pylori resembling the bacillary type, even in coccoid
forms. BioGenex detected all TMDU-positive cases in the
subepithelium, indicating greater sensitivity. Furthermore,
differing positivity cutoffs (1 in the present study vs. ≥10
signals in Ito et al's) could contribute to the observed
variation. Consequently, the precise prevalence of isolated
subepithelial H. pylori remains inconclusive, necessitating
further validation.
A significantly lower detection rate of H.
pylori in the subepithelial compartment was observed in
patients treated with PPIs than in those not treated with PPIs.
These findings suggest that PPI use may inhibit H. pylori
infiltration into the lamina propria.
The underlying mechanism may involve inhibiting
MMP-2/TIMP-3 interactions. These effects could protect against
gastric mucosal injury, thereby reducing the degree of epithelial
damage that facilitates bacterial translocation (23).
In addition to the diagnostic challenges of
detecting subepithelial bacteria, their presence may have
implications for clinical outcomes. First, subepithelial particles
could signify residual bacteria from the surface that remain viable
in unbiopsied areas of the stomach, potentially causing persistent
symptoms. Second, immunoreactive signals in subepithelial regions
may represent debris from dead bacteria, which can continue to
trigger inflammation and contribute to ongoing inflammatory
responses.
Third, subepithelial immunoreactive particles may
reflect a transformation from the usual bacillary form of H.
pylori to an atypical coccoid form under stressful conditions.
These coccoid forms could revive and revert to culturable bacilli.
This transformation may contribute to treatment failure and
relapse, as coccoid forms may evade immune detection while
remaining susceptible to antibiotics (13-16).
These findings may explain our results, which revealed that the
eradication rate of H. pylori treatment was greater in
patients with isolated surface epithelial or mixed infection
patterns than in those with an isolated subepithelial pattern. The
presence of the latter pattern may provide indirect evidence
supporting the consideration of a step-up eradication therapy
regimen. However, establishing a definitive link between the
isolated subepithelial pattern and treatment success or failure
remains challenging due to the small sample size.
Unlike treated patients, most untreated H.
pylori-positive patients exhibited an isolated subepithelial
pattern, likely due to underdiagnosis. Most treated patients are
diagnosed using the rapid urease test (RUT), H&E staining,
Giemsa, or polyclonal DAKO IHC, which are routinely employed in
Vajira Hospital. By contrast, untreated patients predominantly
presented with an isolated subepithelial pattern, which typically
yields negative results for H&E, Giemsa, or polyclonal DAKO
IHC. A total of 5 patients were treated on the basis of positive
RUT results. However, RUT is rarely performed at our institution;
treatment decisions are typically based on histopathology
(H&E), special stains, or IHC results. This underscores the
potential for significant underdiagnosis in real-world clinical
practice.
Whether this staining represents live subepithelial
bacteria or merely debris from dead bacteria remains unclear.
Nevertheless, our results suggest that this immunoreactive signal
should not be overlooked and warrants further investigation.
The current reporting system for H. pylori
may benefit from the incorporation of immuno-stained subepithelial
bacteria as a prognostic factor. The authors propose reporting
H. pylori-IHC as an ancillary study alongside the Updated
Sydney System to provide a clearer summary of H. pylori
density and improve clinical communication. Specifically, infection
patterns should be reported under the heading ‘H.
pylori-positive cases’, including specific location details.
This would enable clinicians to accurately identify infection types
and guide treatment decisions. The isolated subepithelial pattern
may suggest the necessity for step-up therapy, closer monitoring,
or additional testing, such as urea breath tests or stool H.
pylori antigen tests, in accordance with current guidelines
(24). Using IHC, H. pylori
density grading can be easily summarized on the basis of the groups
described in the methodology for clinical implications.
The overall pattern should be reported under the
heading ‘H. pylori-positive cases’ followed by a description
of the specific location. This approach would improve communication
with clinicians, for example, ‘isolated surface epithelial pattern
(surface bacteria 3, negative subepithelial signal)’ or
‘non-specific pattern (surface bacteria 3, subepithelial signal
2)’. This pattern group system aims to improve description of H.
pylori behavior and could guide further management,
particularly for the isolated subepithelial pattern group. In such
cases, patients should undergo a urea breath test or a stool H.
pylori antigen test, according to guidelines. The presence of
this pattern suggests that step-up therapy and close follow-up or
further investigation should be considered. By contrast, the
surface-attached groups had a greater likelihood of successful
treatment than did the isolated subepithelial pattern group.
Although the present study provides valuable
insights into H. pylori, it has several limitations. The
primary limitations are its retrospective design and the small
number of patients with complete clinical follow-up data, which may
affect the generalizability of the findings. Additionally, since
only omeprazole is included in the national reimbursement program,
other PPIs could have varying effects on the outcomes. Further
prospective studies with larger cohorts and broader medication
coverage are needed to validate and expand upon these findings.
In conclusion, H. pylori, particularly in
subepithelial locations, can be underdiagnosed even with IHC tests.
The detection rate is influenced by the sensitivity of the primary
antibody used and the location of the bacteria. The effect of PPIs
might prevent surface bacterial translocation. This feature should
be included in pathological assessments and reports as an ancillary
study alongside the updated Sydney System. Subepithelial H.
pylori infection could serve as an independent prognostic
factor for clinical outcomes and could guide further patient
management.
Supplementary Material
Sensitivity, specificity, positive
predicted value, negative predicted value, accuracy, and kappa
measure of agreement for BioGenex, TMDU, Cell Marque and DAKO.
Acknowledgements
The authors would like to thank Mrs Unaporn
Sitthivilai (Department of Anatomical Pathology, Faculty of
Medicine, Vajira Hospital, Navamindradhiraj University, Bangkok,
Thailand) for her technical assistance and Professor Siriwan
Tangjitgamol (Department of Obstetrics and Gynecology, Faculty of
Medicine, Vajira Hospital, Navamindradhiraj University, Bangkok,
Thailand; Obstetrics and Gynecology Section, MedPark Hospital,
Bangkok, Thailand) for her support in manuscript preparation.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KL and CS conceived and designed the study,
conducted the literature review, analyzed and interpreted the data.
CS wrote the manuscript. KL revised the manuscript and performed
the statistical analysis. CS and KL confirm the authenticity of all
the raw data. Both authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the ethics
committee of the Navamindrajhiraj University (approval no.
120/2566; Bangkok, Thailand) before study initiation. The Ethics
Committee/Institutional Review Board waived the requirement of
written informed consent for participation from the participants or
the participants' legal guardians/next of kin because due to the
retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Choi H, Leung K, Jiang F, Graham DY
and Leung WK: Global prevalence of Helicobacter pylori
infection between 1980 and 2022: A systematic review and
meta-analysis. Lancet Gastroenterol Hepatol. 8:553–564.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marshall B: Helicobacter pylori-a
nobel pursuit? Can J Gastroenterol. 22:895–896. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Malfertheiner P, Camargo MC, El-Omar E,
Liou JM, Peek R, Schulz C, Smith SI and Suerbaum S: Helicobacter
pylori infection. Nat Rev Dis Primers. 9(19)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gasbarrini G and Bonvicini F: Interaction
between Helicobacter pylori and human gastric mucosa
revisited by electron microscopy: Still something new to debate?
Eur Rev Med Pharmacol Sci. 22:5312–5316. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chauhan M, Nahar J, Zafar Y and
El-Halawany H: Acute gastroenteritis revealing Helicobacter
bacteremia: How common is this? Am J Gastroenterol. 113
(Suppl)(S1392)2018.
|
|
6
|
Dudley J, Wieczorek T, Selig M, Cheung H,
Shen J, Odze R, Deshpande V and Zukerberg L: Clinicopathological
characteristics of invasive gastric Helicobacter pylori. Hum
Pathol. 61:19–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang Y, Wang QL, Cheng DD, Xu WT and Lu
NH: Adhesion and invasion of gastric mucosa epithelial cells by
Helicobacter pylori. Front Cell Infect Microbiol.
6(159)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ito T, Kobayashi D, Uchida K, Takemura T,
Nagaoka S, Kobayashi I, Yokoyama T, Ishige I, Ishige Y, Ishida N,
et al: Helicobacter pylori invades the gastric mucosa and
translocates to the gastric lymph nodes. Lab Invest. 88:664–681.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Scott DR, Sachs G and Marcus EA: The role
of acid inhibition in Helicobacter pylori eradication.
F1000Res 5: F1000 Faculty Rev-1747, 2016.
|
|
10
|
Siavoshi F, Saniee P, Khalili-Samani S,
Hosseini F, Malakutikhah F, Mamivand M, Shahreza S and Sharifi AH:
Evaluation of methods for H. pylori detection in PPI
consumption using culture, rapid urease test and smear examination.
Ann Transl Med. 3(11)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Saniee P, Shahreza S and Siavoshi F:
Negative effect of proton-pump inhibitors (PPIs) on Helicobacter
pylori growth, morphology, and urease test and recovery after
PPI removal-an in vitro study. Helicobacter. 21:143–152.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Malfertheiner P, Megraud F, O'Morain CA,
Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton
J, Graham DY, et al: Management of Helicobacter pylori
infection-the maastricht V/florence consensus report. Gut. 66:6–30.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sarem M and Corti R: Role of
Helicobacter pylori coccoid forms in infection and
recrudescence. Gastroenterol Hepatol. 39:28–35. 2016.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
14
|
Ierardi E, Losurdo G, Mileti A, Paolillo
R, Giorgio F, Principi M and Di Leo A: The puzzle of coccoid forms
of Helicobacter pylori: Beyond basic science. Antibiotics
(Basel). 9(293)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gladyshev N, Taame M and Kravtsov V:
Clinical and laboratory importance of detecting Helicobacter
pylori coccoid forms for the selection of treatment. Prz
Gastroenterol. 15:294–300. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Krzyżek P and Grande R: Transformation of
Helicobacter pylori into coccoid forms as a challenge for
research determining activity of antimicrobial substances.
Pathogens. 9(184)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rupp S, Papaefthymiou A, Chatzimichael E,
Polyzos SA, Spreitzer S, Doulberis M, Kuntzen T and Kountouras J:
Diagnostic approach to Helicobacter pylori-related gastric
oncogenesis. Ann Gastroenterol. 35:333–344. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Heilmann KL and Borchard F: Gastritis due
to spiral shaped bacteria other than Helicobacter pylori:
Clinical, histological, and ultrastructural findings. Gut.
32:137–140. 1991.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis. The updated sydney
system. International workshop on the histopathology of gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Uchida T, Miftahussurur M, Pittayanon R,
Vilaichone RK, Wisedopas N, Ratanachu-Ek T, Kishida T, Moriyama M,
Yamaoka Y and Mahachai V: Helicobacter pylori infection in
Thailand: A nationwide study of the CagA phenotype. PLoS One.
10(e0136775)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen YC, Malfertheiner P, Yu HT, Kuo CL,
Chang YY, Meng FT, Wu YX, Hsiao JL, Chen MJ, Lin KP, et al: Global
prevalence of Helicobacter pylori infection and incidence of
gastric cancer between 1980 and 2022. Gastroenterology.
166:605–619. 2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bagheri N, Salimzadeh L and Shirzad H: The
role of T helper 1-cell response in Helicobacter
pylori-infection. Microb Pathog. 123:1–8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rudra DS, Pal U, Chowdhury N, Maiti NC,
Bagchi A and Swarnakar S: Omeprazole prevents stress induced
gastric ulcer by direct inhibition of MMP-2/TIMP-3 interactions.
Free Radic Biol Med. 181:221–234. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chey WD, Howden CW, Moss SF, Morgan DR,
Greer KB, Grover S and Shah SC: ACG clinical guideline: Treatment
of Helicobacter pylori infection. Am J Gastroenterol.
119:1730–1753. 2024.PubMed/NCBI View Article : Google Scholar
|