Introduction
Diabetes mellitus (DM) is a chronic metabolic
disorder characterized by persistent hyperglycemia, which arises
due to either insufficient insulin production (type 1 DM) or
insulin resistance [type 2 DM (T2DM)]. T2DM is the most prevalent
form of diabetes, accounting for ~90% to 96% of all diabetes cases
worldwide. In 2021, an estimated 529 million individuals globally
were living with diabetes, with 96% of these cases attributed to
T2DM (1). T2DM is identified by the
resistance to insulin, leading to persistently increased blood
glucose levels. The impact of elevated glucose concentrations on
cellular membranes, signaling and function has been extensively
studied using in vitro models, particularly in the context
of DM (2-4).
Several previous studies have reported that
prolonged exposure to hyperglycemic conditions affects the
structural and functional integrity of cells. These effects
influence the shedding of small, membrane-bound particles called
extracellular vesicles (EVs) (5-7).
EVs [formerly called microparticles (MPs)] are particles that are
released from cells and delimited by a lipid bilayer that cannot
replicate on their own. The size of EVs range from 100-1,000 nm in
diameter. EVs can be classified by distinct properties such as
molecular markers, size and their functions (8). Moreover, they have gained increasing
attention as key mediators of intercellular communication and as
critical players in several physiological and pathological
processes, including DM (9,10). These vesicles are involved in
regulating immune responses, inflammation and cellular metabolism,
all of which are highly relevant to the pathogenesis of DM
(11).
Studies have reported that platelet-derived EVs
(PEVs) notably contribute to the inflammatory processes associated
with DM. For example, studies have reported that PEVs carry
pro-inflammatory molecules, such as cytokines and chemokines, which
can modulate endothelial cell function and promote vascular
inflammation (11,12). In patients with T2DM, PEVs have been
reported to be associated with an increased level of
pro-inflammatory markers, such as interleukin-1β and tumor necrosis
factor-α (TNF-α), which contribute to insulin resistance and the
development of atherosclerosis (13). This inflammatory environment is
critical for the development of diabetic complications, such as
cardiovascular disease, which is more prevalent in individuals with
DM (12).
However, whilst several studies have assessed the
role of PEVs in DM research, few studies have evaluated the role of
red blood cell (RBC)-derived EVs (REVs) in this field. In diabetes
milieu, RBCs undergo several alterations, including changes in
membrane integrity, membrane glycation, size and overall
morphology. It has been proposed that these disruptions lead to the
generation of REVs, which have marked implications for cellular
communication and disease progression (13).
On RBCs, there are several surface receptors that
control cell physiology. CD47 is one of these crucial membrane
proteins which is involved in preventing the phagocytosis of RBCs
by macrophages (14). CD47
expression confers prevention RBC from phagocytosis, whereas the
decreased CD47 levels on aged RBCs mark them for clearance by
hepatic and splenic macrophage (15). The reduction in CD47 expression and
increase in phosphatidylserine (PS) expression are associated with
RBC clearance in mechanical stress condition. However, previous
research reported no significant decreases in CD47 expression among
aged RBCs (16). Moreover, in
inflammatory-induced conditions, an increase in PS expression on
RBC membrane has been reported to be altered (17). However, there is no evidence of an
association between CD47 expression and membrane vesiculation under
inflammatory-induced conditions such as DM.
Recent studies have highlighted the role of EVs in
the progression of DM and its associated complications (5-7,11).
However, the specific populations of EVs and their precise impacts
on DM remain underexplored. Therefore, the aim of the present study
was to assess the effects of hyperglycemia on the integrity and
characteristics of RBC membranes under hyperglycemic conditions.
Additionally, the study aimed to evaluate the potential mechanisms
driving membrane vesiculation and the shedding of REVs using an
in vitro model.
Materials and methods
Participant recruitment and ethical
considerations
The participants were recruited at hematology
laboratory, School of Allied Health Sciences, Walailak University,
between 6th June 2023 to 5th June 2024. The
protocol was approved (approval no. WUEC-23-143-01) by the Walailak
University Institutional Review Board (Tha Sala, Thailand). Written
informed consents were obtained from all participants prior to the
commencement of the study. Participants with no history of
hyperglycemia or underlying medical conditions were recruited for
the study. Individuals who were pregnant, had recently recovered
from a febrile illness, or were taking therapeutic medications were
excluded. A total of 6 ml whole blood was collected from 10 healthy
participants in 3.2% sodium citrate at ratio of 9:1 following a
12-h fasting period. Clinicopathological characteristics including
age and sex distribution are mentioned in Table I.
 | Table ICharacteristics and hematological
parameters of the enrolled subjects (n=9). |
Table I
Characteristics and hematological
parameters of the enrolled subjects (n=9).
| Parameter | Value | Reference
values |
|---|
| Sex | | |
|
Male | 3 (0.3) | - |
|
Female | 6 (0.7) | - |
| Age, years | 20±0.24 | - |
| Fasting blood
glucose, mg/dl | 82.4±5.7 | 70-99 mg/dl |
| RBC,
106/µl | 4.6±0.5 | Male: 4.7-6.1,
Female: 4.2-5.4 |
| Hb, g/dl | 13.6±0.8 | Male: 13.8-17.2;
Female: 12.1-15.1 |
| Hematocrit, % | 39.4±1.4 | Male: 40-54;
Female: 36-48 |
| Mean corpuscular
volume, fl | 86.7±6.4 | 80-100 |
| Mean corpuscular
Hb, pg | 28.8±2.5 | 27-33 pg |
| Mean corpuscular Hb
concentration, % | 33.9±2.0 | 32-36% |
| RBC distribution
width-CV (%) | 13.8±1.3 | 11.5-14.5% |
| White blood cells,
103/µl | 5.4±0.7 |
4-11x103/µl |
| Platelet count,
103/µl | 211±86.1 |
150-450x103/µl |
RBC preparation and culture
RBCs were prepared for in vitro culture as
previously described by Viskupicova et al (17) and Loyola-Leyva et al
(18), with certain modifications.
Briefly, whole bloods were centrifuged at 1,500 x g for 10 min at
4˚C. The plasma was collected for preparing the complete media
culture. Packed RBC pellets were washed three times with sterilized
0.9% NaCl. Washed RBC pellets (1 ml) were then resuspended in 1 ml
Dulbecco's Modified Eagle Medium (DMEM; Sigma-Aldrich; Merck KGaA)
with 10% v/v autologous plasma (4,500 µl DMEM with 500 µl of
filtered plasma). Subsequently, washed RBCs were seeded in T25
tissue culture flasks and the glucose-DMEM was replenished at
several concentrations (5, 45 and 100 mM) to generate a 30% v/v
culture (900 µl washed RBC with 3 ml medium). This in vitro
hyperglycemic condition ensured consistency with previous studies
by Batista da Silva et al (19) which demonstrated that in
vitro glucose treatment induces phenotypic and biochemical
changes resembling those in patients with diabetes. The conditioned
medium and treated RBCs were collected at 24 and 48 h after
treatment for further experiments. Each culture was performed in
duplicate.
Quantification of REVs
Glucose-treated RBCs were collected at specific time
points and stained, followed by the quantification of REVs using
flow cytometry. The staining protocol for REVs was performed as
previously described (19,20), with certain modifications. Briefly,
the treated RBCs were centrifuged at 500 x g for 10 min at 4˚C and
RBC pellets were collected for PS expression analysis. The
supernatant was collected, centrifuged further at 14,000 x g for 2
min at 4˚C to obtain platelet-free plasma, then centrifuged further
at 14,000 x g for 45 min at 4˚C to obtain non-purified MP pellets
(including EVs, apoptotic bodies, or other extracellular
structures) for REV quantification. All monoclonal antibodies and
staining buffers were purchased from BioLegend, Inc. A total of
10-µl supernatant was stained with 2 µl anti-CD235-PE (cat. no.
349106; BioLegend, Inc.), 2 µl Annexin V FITC and 41 µl 1X annexin
V binding buffer (cat. no. 42220; BioLegend, Inc.), which was then
mixed and incubated for 15 min at room temperature. The stained
REVs were resuspended with 350 µl annexin V binding buffer to make
up a final volume of 400 µl. The absolute numbers of REV were
measured using CountBright beads (Molecular Probes; Thermo Fisher
Scientific, Inc.). Following the manufacturer's instructions, 50-µl
well-mixed CountBright beads were added to the stained mixture,
mixed and then subjected to flow cytometry (BD
FACSVerse™ Flow Cytometer; BD Biosciences). The EV gate
was created using standard beads of 0.88 µm (Spherotech, Inc.),
positive for annexin V+ (Fig. 1A). Subsequently, the events (number
of acquisition signals) CD235+/AnnexinV+ were
counted and acquired for 1,000 events of counting beads in
CountBright gate (Fig. 1B and
C). The absolute number of REVs was
calculated as follows: REV (particle/µl) which was obtained using
the number of REV counts per acquired count beads, was multiplied
by the pre-calculated bead number per resuspension volume (Fig. 1D).
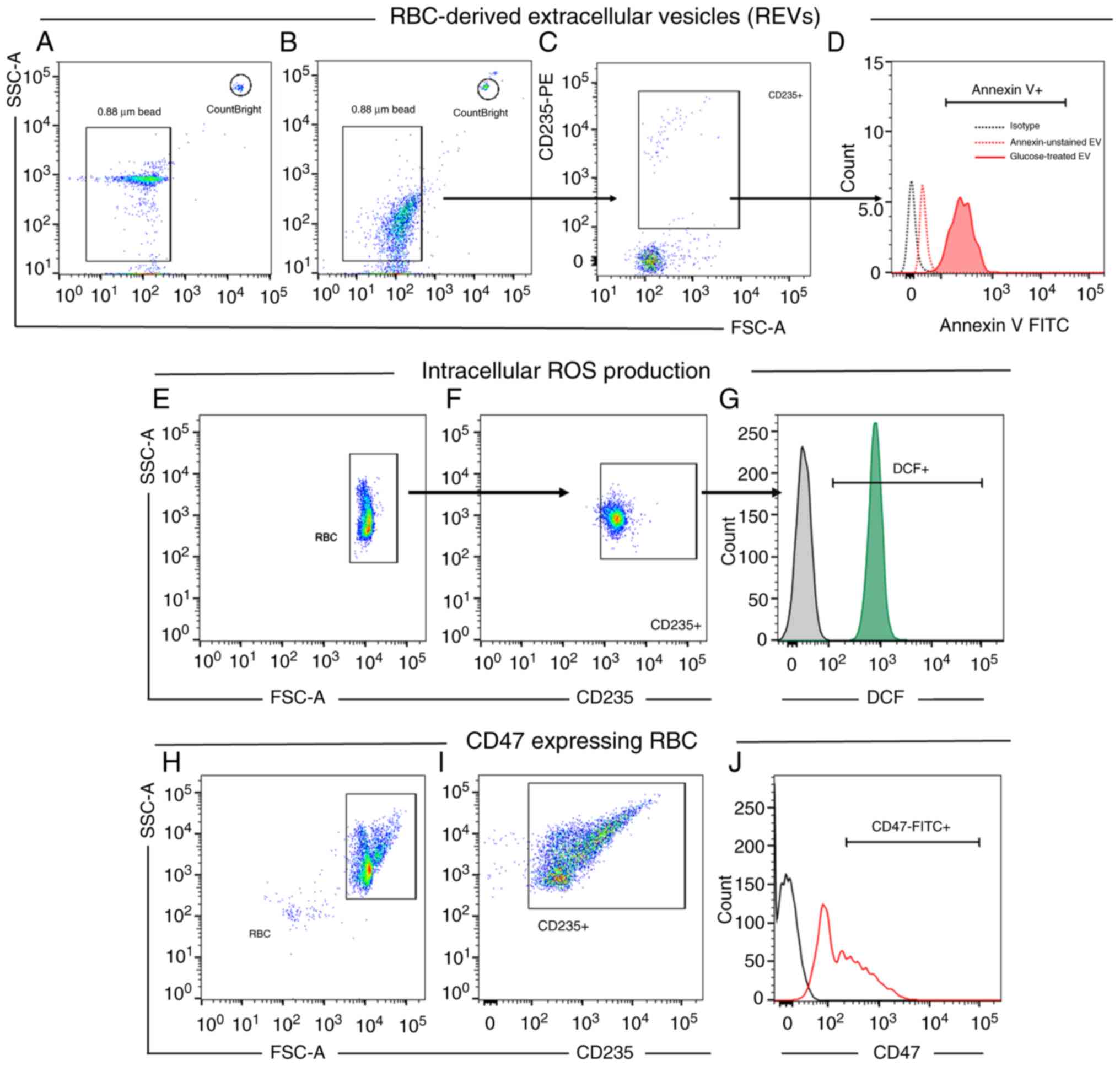 | Figure 1Gating strategies used for flow
cytometric analysis. Gating strategies for REV quantification. (A)
Dot plot representing size-calibrated beads (0.88 µm) and
CountBright bead used in the study. (B) The EV gate was defined
using size-calibrated standard beads (0.88 µm). (C) EV events
within the EV gate were analyzed to identify REVs, characterized as
CD235-PE+. (D) CD235+ RBCs were gated for
Annexin-V expression. The number of Annexin V+ events
was assessed for REV calculation as follows: (Number of REV
events/number of bead events) x number of beads counted per volume.
Gating strategies for intracellular ROS production in cultured
RBCs. (E) Dot plot demonstrated gating of RBCs from FSC/SSC. (F)
RBCs were selected based on CD235+ expression. (G) A histogram was
generated to calculate the MFI of DCF fluorescence, which indicates
ROS production. The isotype control, shown as a black histogram,
served as a negative control. (H and I) A dot plot represents the
RBC population gated based on (H) FSC/SSC and (I) CD235+
expression. (J) A histogram was generated to determine the MFI of
CD47-FITC. The isotype control, shown as a black histogram, served
as a negative control. EV, extracellular vesicle; RBC, red blood
cell; REV, RBC-derived EV; MFI, mean fluorescence intensity; DCF,
dichlorofluorescein; ROS, reactive oxygen species; FSC, forward
side scatter; SSC, side scatter. |
Measurement of intracellular reactive
oxygen species (ROS) in cultured-RBCs
The levels of ROS production were measured
intracellularly using a 2',7'-dichlorofluorescin diacetate
(DCFH-DA) assay as previously described, with certain modifications
(21). Briefly, the cultured RBCs
under several hyperglycemic conditions were washed twice with 1X
PBS, then cultured with 2 µM working DCFH-DA. RBCs were washed
twice with 1X PBS to remove the excess DCFH-DA. A minimum of 10,000
CD235-positive events were recorded per sample then DCF
fluorescence expression was assessed using flow cytometry (Fig. 1E-G). Untreated RBCs and RBCs treated
with H2O2 (100 µM) were used as negative and
positive controls, respectively.
Expression of CD47 in RBCs
The expression of CD47 in cultured-RBCs were
determined using flow cytometry. A total of 50 µl treated RBCs was
mixed with 5 µl anti-CD47 FITC (cat. no. 323106) and 2 µl CD235 PE
(cat. no. 349106; both from BioLegend, Inc.), and incubated for 15
min at 25˚C. The cells were washed twice with 1X PBS and
resuspended in 1% paraformaldehyde prior to flow cytometry (BD FACS
Verse™ Flow Cytometer; BD Biosciences). The proportions
of CD47+ RBCs were gated from CD235+
(Fig. 1H and I). Mean fluorescence intensities (MFI) of
CD47 expressing RBCs were compared among the treatment conditions
(Fig. 1J).
N-acetylcysteine (NAC) treatment
NAC is a precursor for glutathione synthesis, a
potent cellular antioxidant (22).
In the present study, RBCs pretreated with NAC were used to assess
the effect of ROS production on REV shedding during hyperglycemia.
NAC was purchased from Sigma-Aldrich; Merck KGaA and a stock
solution of 100 mM was prepared by dissolving NAC in sterile PBS,
which was then aliquoted and stored at -20˚C until use. RBCs were
then resuspended in DMEM supplemented with 10% fetal bovine serum
(Hyclone; Cytiva) at a hematocrit of 2%. RBCs were treated with NAC
at a final concentration of 1 mM, then incubated at 37˚C in a 5%
CO2 incubator. Subsequently, the cells were incubated
with NAC for 24 h. RBCs were then washed three times with PBS to
remove residual NAC and resuspended in conditioned medium that was
formulated with two concentrations of glucose, 5 and 100 mM, as
euglycemia and severe hyperglycemia, respectively. Oxidative stress
levels were evaluated using flow cytometry. Control cells were
treated with an equal volume of PBS without NAC.
Statistical analysis
In the present study, the statistics were calculated
using GraphPad Prism version 7 (Dotmatics). REV numbers, ROS
production and CD47 expression on the RBCs under hyperglycemic
conditions were compared with the euglycemia control (5 mM). REV
numbers and ROS generation in RBCs under NAC-treated conditions
were compared with untreated groups. The differences in glucose
concentrations between 24- and 48-h cultures were assessed using
the Wilcoxon signed-rank test. The dose-dependent effects of
glucose on ROS production, CD47 expression, and REV numbers were
evaluated by Kruskal-Wallis test, followed by Dunn's multiple
comparisons test for post hoc analysis. The correlation between REV
number and ROS production were analyzed using the Spearman rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Participants demographics
A total of 10 healthy volunteers were recruited in
the present study. The screening of blood glucose after fasting was
measured using the Accu-check® Performa II (Roche
Diagnostics GmbH). A total of 1 volunteer, who showed an abnormal
lipid profile and blood glucose levels, was excluded from the
study. Finally, 9 recruited participants (6 women and 3 men) were
included in the study. The mean age of participants was 20±0.24
years, and the median fasting blood glucose level was 85.21±2.52
mg/dl. Complete blood count parameters among the recruited
participants were within the normal reference ranges (Table I).
RBCs demonstrate elevated ROS levels
under hyperglycemic conditions
Prolonged hyperglycemia is considered as a factor
driving ROS production (23,24).
Therefore, RBCs were treated with several concentrations of
glucose. Treated RBCs, which were gated from CD235+,
were measured for ROS production by the expression of DCF using
flow cytometry (Fig. 1E-G). The
results showed a significant increase in ROS production under
hyperglycemic conditions. Elevated ROS production was observed at
100 mM glucose (P=0.0004) after 24 h of culture, as well as at 45
mM (P=0.0426) and 100 mM (P=0.0195) after 48 h of culture (Fig. 2A). Mean fluorescence intensities
(MFIs) of DCF fluorescence were compared among glucose
concentrations at 24 h and 48 h of culture (Fig. 2B and C). The current findings are consistent
with previous study, demonstrating dosage-dependent effects on ROS
production under hyperglycemic conditions (23) and our supplementary data (Fig. S1G-I). However, the comparison of
the time of glucose exposure demonstrated a significant elevation
of ROS production specifically in the euglycemic stage (P=0.0273)
(Fig. 2D-G).
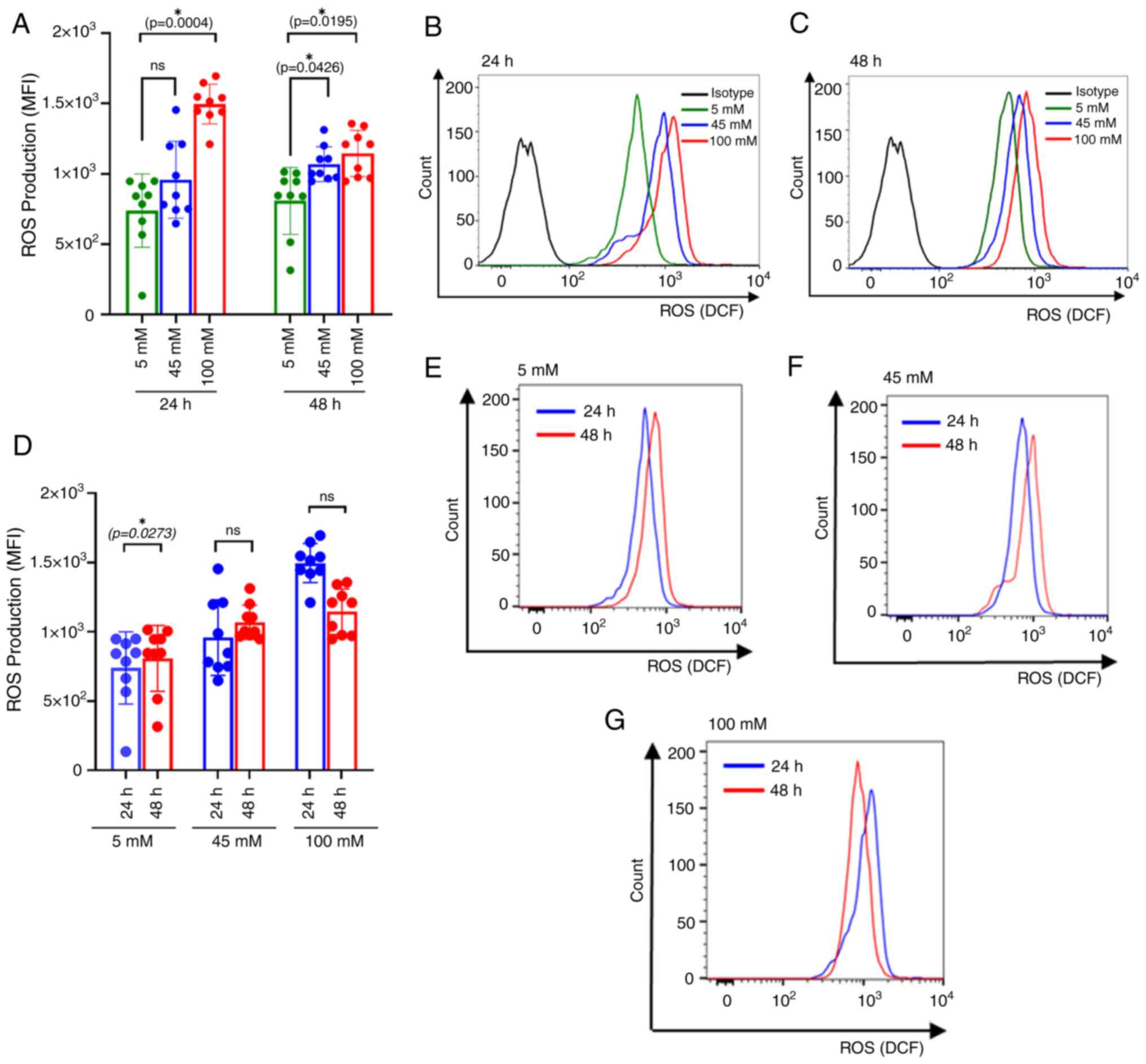 | Figure 2Intracellular ROS production in
glucose-treated RBCs. (A) Bar plot comparing intracellular ROS
production among different glucose concentrations at 24 and 48 h of
incubation. The colors, green, blue and red, correspond with the
different treatment groups: Euglycemic control (5 mM), intermediate
level (45 mM) and severe hyperglycemia (100 mM), respectively. The
significantly increased ROS production was observed at 100 mM
glucose concentrations compared with 5 mM after 24 h of treatment
(P=0.0004). Similarly, ROS production was significantly higher at
45 mM and 100 mM compared with 5 mM after 48 h of treatment
(P=0.0426 and P=0.0034, respectively). (B) Representative histogram
comparing the ROS production of glucose-treated RBC. MFIs of DCF in
5, 45 and 100 mM were compared at 24 h of incubation. (C)
Representative histogram comparing the ROS production of
glucose-treated RBC. MFIs of DCF in 5, 45 and 100 mM were compared
at 48 h of incubation. (D) Bar plots represent the levels of ROS
production among different treatment groups: Euglycemic control (5
mM), intermediate level (45 mM) and severe hyperglycemia (100 mM)
The colors, blue and red, correspond with 24 and 48 h of
incubation, respectively. The significance increased ROS production
was observed at 5 mM glucose concentration between 24 h and 48 h of
culture (P=0.0273), whereas no significance differences of ROS
production between 24 and 48 h of glucose culture at 45 and 100 mM.
(E-G) Representative histogram comparing ROS production at (E) 5
mM, (F) 45 mM and (G) 100 mM between glucose culture at 24 and 48 h
of incubation. Effect of incubation time and glucose concentrations
on ROS production at distinct glucose concentrations, compared
using the Wilcoxon sign-rank test. Data are presented as the mean ±
standard error of the mean, with individual dots indicating
measurements from separate samples. *Indicates
statistical significance. ROS, reactive oxygen species; RBC, red
blood cell; MFI, mean fluorescence intensity; M, molar. |
Hyperglycemic conditions alter CD47
expression on RBCs
CD47 is an integral membrane protein that is a
member of the Rh complex. The major role of CD47 on RBCs is the
molecular switch that controls the phagocytosis of RBCs. The
binding of CD47 with signal-regulatory protein (SIRP)-α that is
expressed on macrophages prevents RBCs from phagocytosis by
macrophages. A previous study reported a decreased CD47 expression
in long-term storage RBCs (15).
However, the impact of varying glucose concentrations has not been
fully elucidated.
In the present study, glucose-treated RBCs were
collected and stained for CD47 expression, represented as the MFI
(Fig. 1H-J). The results
demonstrated that there was a significant decrease in the CD47
expression under different glycemic conditions. The significant
were observed at 48 h of incubation at 100 mM glucose culture
compared with 5 mM, (P=0.0003) (Fig.
3A). Mean fluorescence intensities (MFIs) of CD47expression on
RBCs were compared among glucose concentrations at 24 h and 48 h of
culture (Fig. 3B and C) Moreover, the significant decrease in
CD47 expression was observed for both intermediate (P=0.0078) and
severe hyperglycemic treatment (P=0.0078) conditions at 24 and 48 h
after glucose treatment (Fig.
3D-G). This suggests that in vitro hyperglycemic conditions
altered CD47 expression on the RBC membrane. Interestingly, the
decreased CD47 expression was coincided with the downregulation of
CD235 which is RBC membrane biomarkers (Fig. S2).
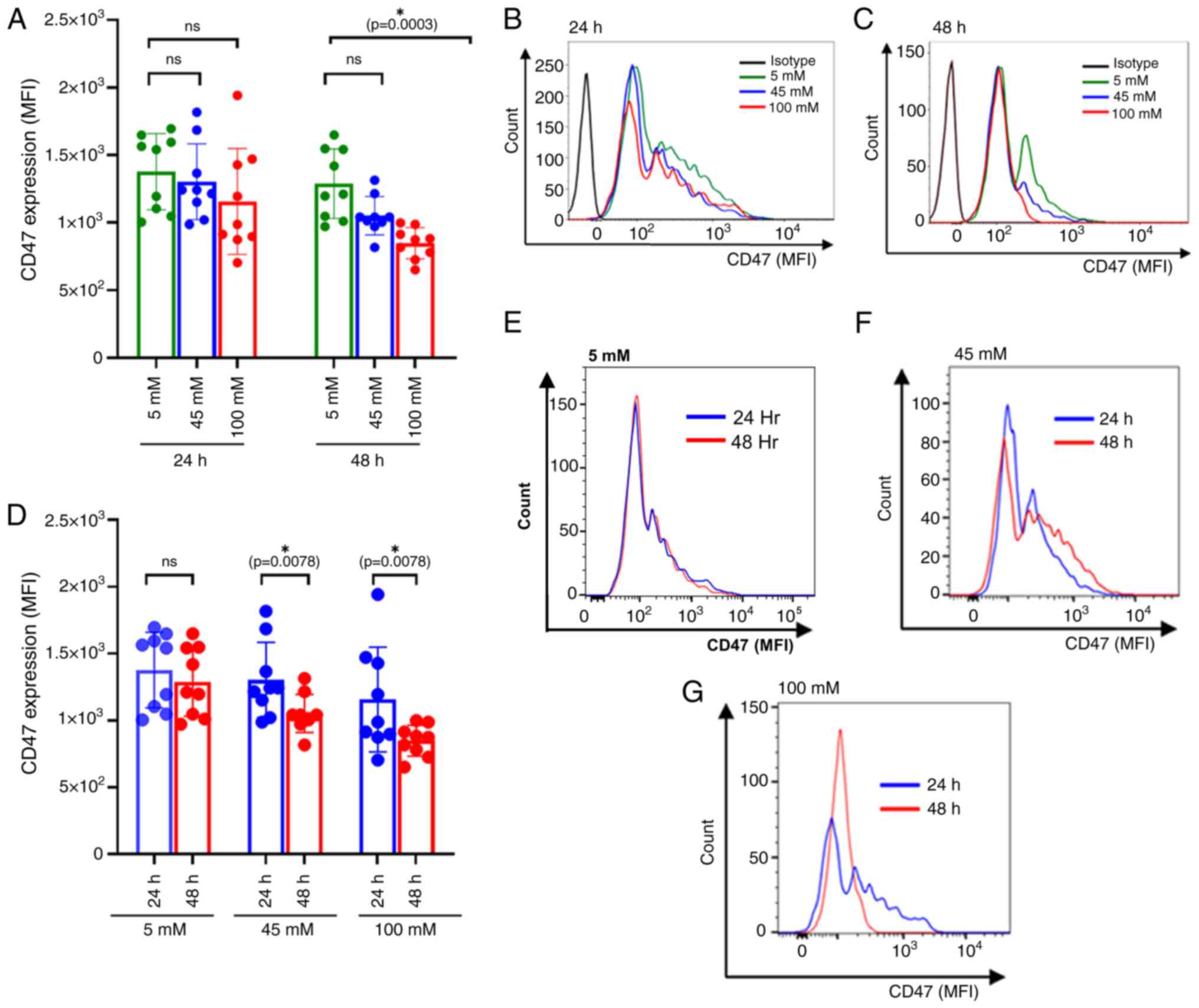 | Figure 3CD47 expression of glucose-treated
RBCs. (A) Bar plot comparing the dosage effect of glucose on CD47
expression at 24 and 48 h of incubation. The colors, green, blue
and red, correspond with the different treatment groups: Euglycemic
control (5 mM), intermediate level (45 mM) and severe hyperglycemia
(100 mM), respectively. The significantly decreased CD47 expression
was observed at 100 mM glucose concentrations compared with 5 mM
after 48 h of treatment (P=0.0003). However, no statistically
significant differences of CD47 expressions were observed at 45 and
100 mM compared with 5 mM at 24 h. (B) Representative histogram
comparing CD47 expression of glucose-treated RBC. MFIs of CD47 in
5-, 45- and 100-mM glucose treatment were compared at 24 h of
incubation. (C) Representative histogram comparing CD47 expression
of glucose-treated RBC. MFIs of CD47 in 5-, 45- and 100-mM glucose
treatment were compared at 48 h of incubation. (D) Bar plots
represent CD47 expression among different treatment groups:
Euglycemic control (5 mM), intermediate level (45 mM) and severe
hyperglycemia (100 mM). The colors, blue and red, correspond with
24 and 48 h of incubation, respectively. CD47 expression
significantly downregulated at 45 and 100 mM of glucose treatment,
(P=0.0078 and P=0.0078, respectively). However, no significant
differences in CD47 expression were observed at 5 mM glucose
concentration between 24 and 48 h of culture. (E-G) Representative
histograms comparing CD47 expression at (E) 5 mM, (F) 45 mM and (G)
100 mM glucose concentrations after 24 and 48 h of incubation.
Effect of incubation time on and glucose concentrations on CD47
expression at distinct glucose concentrations, compared using the
Wilcoxon sign-rank test. Data are presented as the mean ± standard
error of the mean, with individual dots indicating measurements
from separate samples. *Indicates statistical
significance. ROS, reactive oxygen species; RBC, red blood cell;
MFI, mean fluorescence intensity; M, molar. |
Increased REV production in
hyperglycemic treated conditions
RBCs obtained from healthy participants were treated
with different concentrations of glucose. At 5 mM, the in
vitro model mimicked euglycemia, whereas treatment with 100 mM
represented severe hyperglycemic milieu. To assess the effect of
glucose concentrations on the duration of exposure, RBCs were
treated for 24 and 48 h. The cultured RBCs were collected at
specific time points and quantified for REV production. Compared
with the euglycemic state, the results revealed a significant
increase in REV numbers under 100 mM (P<0.0001) glucose
treatment conditions after 24 h of culture, with similar findings
at 48 h (P<0.0001) (Fig. 4A-D).
This finding indicates a possible dose-dependent effect that
becomes apparent at higher glucose levels (Fig. S1A-C).
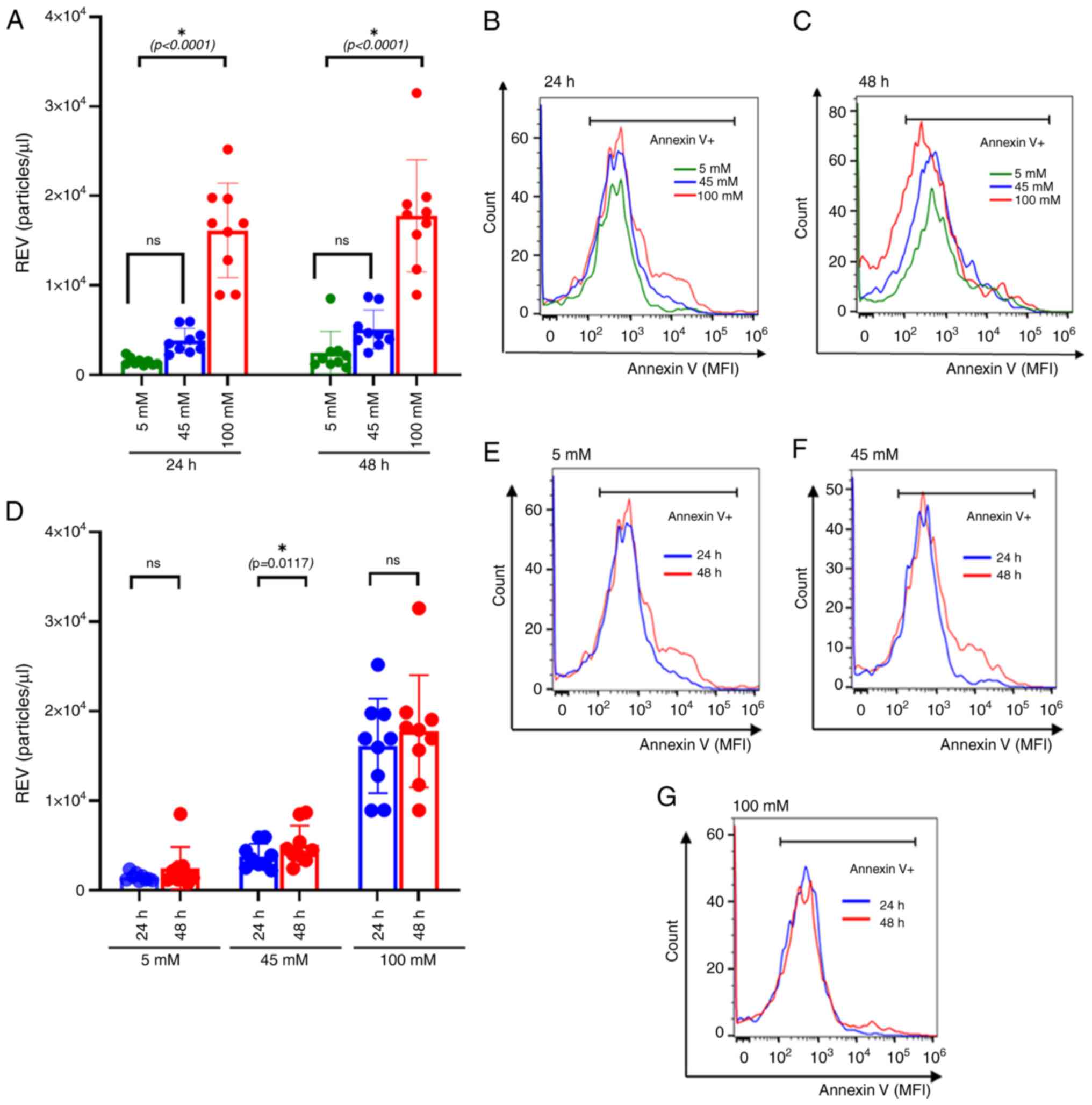 | Figure 4REV formation under different glucose
treatments. (A) Bar plot compares REV production at different
concentrations. The colors, green, blue and red, correspond with
the different treatment groups: Euglycemic control (5 mM),
intermediate level (45 mM) and severe hyperglycemia (100 mM),
respectively. The significantly elevated REV production was
observed at 100 mM glucose concentrations compared with 5 mM either
24 h of treatment (P<0.0001) or 48 h of treatment (P<0.0001).
(B and C) Representative histogram compares annexin V expressing
EVs that are used for REV calculations. MFIs of annexin V in 5, 45
and 100 mM of glucose were compared at (B) 24 h and (C) 48 h of
incubation. (D) Bar plots compared REV production among different
treatment groups: Euglycemic control (5 mM), intermediate level (45
mM) and severe hyperglycemia (100 mM). The colors, blue and red,
correspond with 24 and 48 h of incubation, respectively. REV
significantly increased at 45 mM of glucose treatment, (P=0.0117).
However, no significant differences in REV production were observed
between 24 and 48 h of culture at 5 and 100 mM glucose
concentration. (E-G) Representative histograms comparing annexin V
expressing EVs at (E) 5 mM, (F) 45 mM and (G) 100 mM glucose
concentrations after 24 and 48 h of incubation. Effect of the
incubation time and glucose concentrations on REV production were
compared using the Wilcoxon sign-rank test. Data are presented as
mean ± standard error of the mean, with individual dots indicating
measurements from separate samples. *Indicates
statistical significance. REV, red blood cell-derived extracellular
vesicle; MFI, mean fluorescence intensity; M, molar. |
NAC reduces intracellular ROS
production and REV formation in RBCs under hyperglycemic
conditions
To assess the impact of the inhibition of
intracellular ROS and REV production, cultured RBCs were pretreated
with 1 mM NAC for 24 h. Subsequently, NAC-pretreated RBCs were
cultured in glucose concentrations of 5, 45 and 100 mM, and
incubated for 24 h.
The significant differences of ROS production were
observed among the NAC-treated group in the intermediate (P=0.0039)
and severe hyperglycemic conditions (P=0.0039) (45- and 100-mM
glucose; Fig. 5A-D). This suggests
that the intracellular oxidative stress augmented by hyperglycemic
conditions may have been involved in REV production.
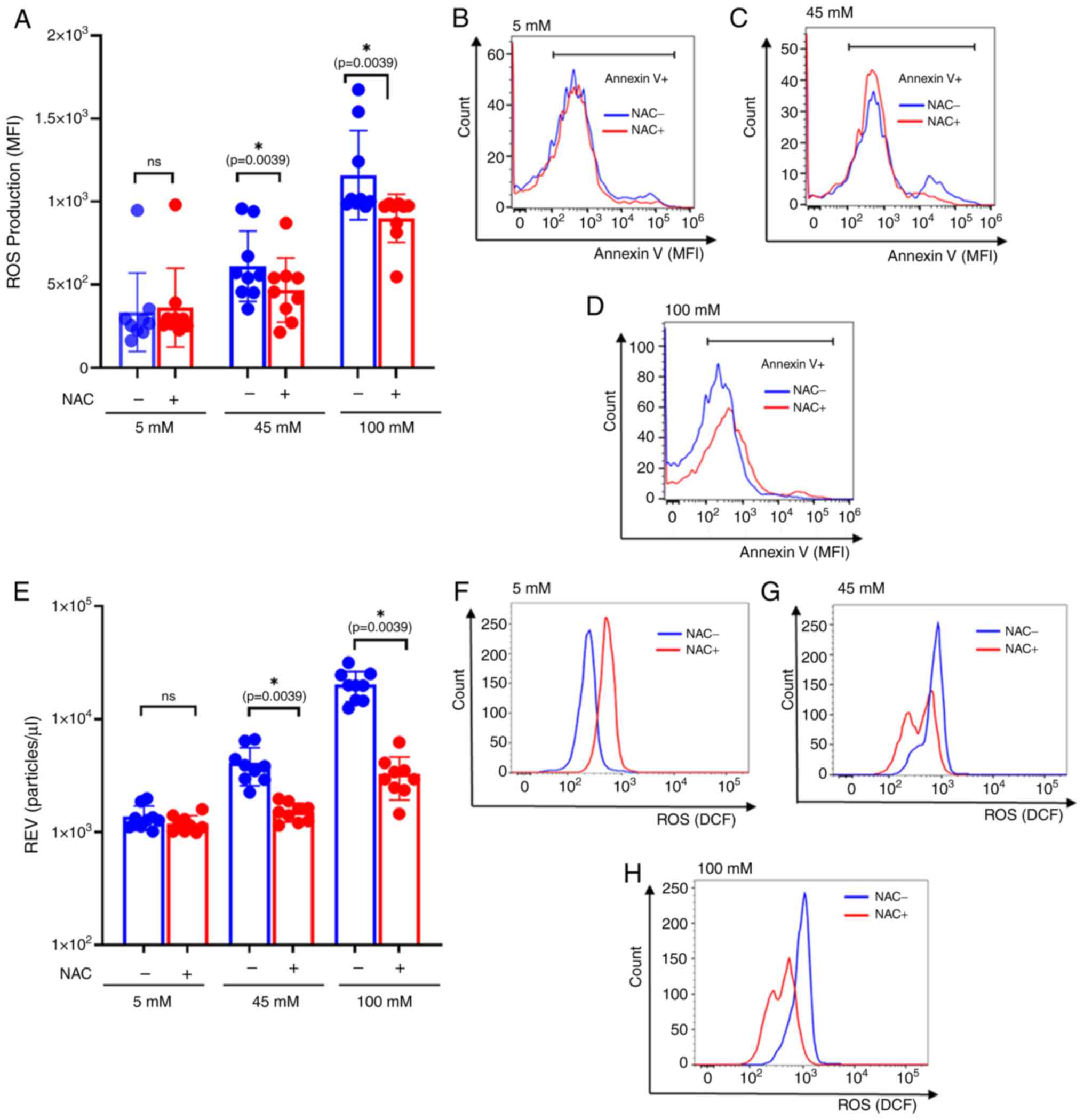 | Figure 5Comparison of REV and ROS production
between NAC-pretreated RBCs and the untreated group. (A) Bar plots
represent REV production comparing between untreated and
NAC-pretreated RBCs after 24 h of incubation. The colors, blue and
red and correspond with the different treatment groups: untreated
and NAC-pretreated RBC, respectively. NAC-pretreated significantly
decreased REV production at 45 mM and 100 mM of glucose
concentrations, (P=0.0039 and P=0.0039, respectively). However, no
significant differences of REV production was observed between
untreated and pretreating NAC condition at 5 mM of glucose culture
condition. (B-D) Representative histogram compares annexin V
expressing EVs that are used for REV calculations. MFIs of annexin
V were compared between untreated and NAC-pretreated conditions at
(B) 5 mM, (C) 45 mM and (D) 100 mM of glucose culture. (E) Bar
plots represent ROS production comparing between untreated and
NAC-pretreated RBCs after 24 h of incubation. The colors, blue and
red and correspond with the different treatment groups: untreated
and NAC-pretreated RBC, respectively. NAC-pretreated significantly
decreased ROS production at 45 mM and 100 mM of glucose
concentrations, (P=0.0039 and P=0.0039, respectively). However, no
significant differences of REV production were observed between
untreated and pretreating NAC condition at 5 mM of glucose culture
condition. (F-H) Representative histograms comparing ROS production
between untreated and NAC-pretreated under (F) 5 mM, (G) 45 mM and
(H) 100 mM glucose concentrations. The effect of the incubation
time and glucose concentration on REV and ROS production, were
compared using the Wilcoxon sign-rank test. Data are presented as
the mean ± standard error of the mean, with individual dots
indicating measurements from separate samples.
*Indicates statistical significance. ROS, reactive
oxygen species; RBC, red blood cell; REV, RBC-derived extracellular
vesicle; NAC, N-acetylcysteine, MFI, mean fluorescence intensity;
M, molar. |
REV productions were measured using flow cytometry,
comparing between the NAC-pretreated RBCs and untreated conditions.
The results revealed a significant decrease in REV production
observed in NAC-pretreated RBC at 45- and 100-mM glucose levels
(P=0.0039 and P=0.0039, respectively, whereas no significant
difference of REV production following NAC treatment at 5 mM
glucose culture (Fig. 5E-H).
Increased REV production is associated
with elevated ROS production and decreased CD47 expression
Elevated ROS production is a common feature of
hyperglycemic conditions (23).
However, there is no evidence of the factors associated with ROS
production on REV biogenesis, to the best of our knowledge.
Therefore, the association between ROS production and REV formation
was assessed using correlation analysis. The data represented in
Fig. 2 (ROS production) and
Fig. 4 (REV production) were used
for calculation. The results revealed that there was no
statistically significant correlation between ROS production and
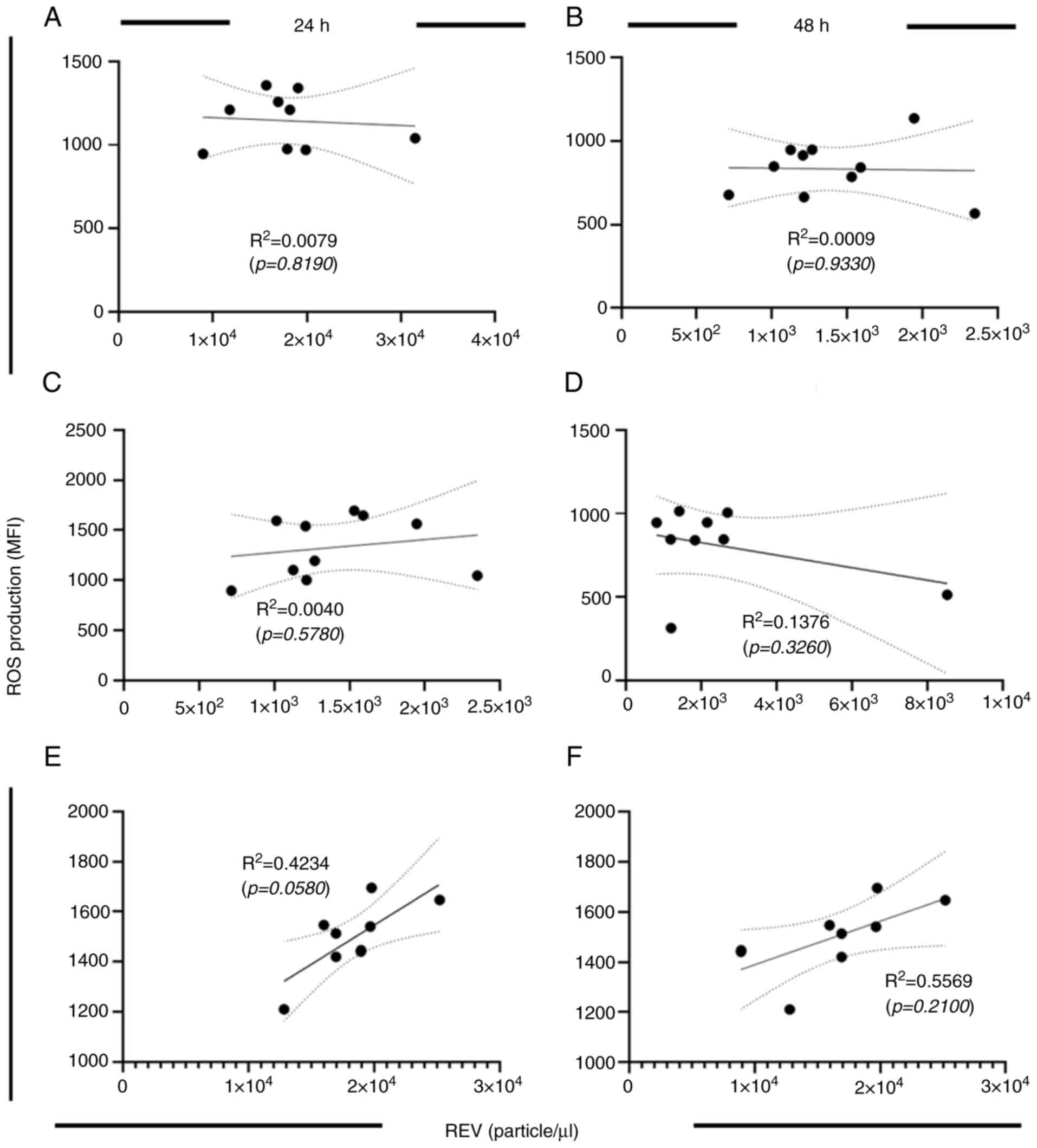
REV production in the study (Fig.
6A-D). These findings suggested that elevated ROS levels may
contribute to increased REV formation under hyperglycemic
conditions at either 24 or 48 h of incubation (Fig. 6E and F), highlighting the potential impact of
oxidative stress on RBC integrity.
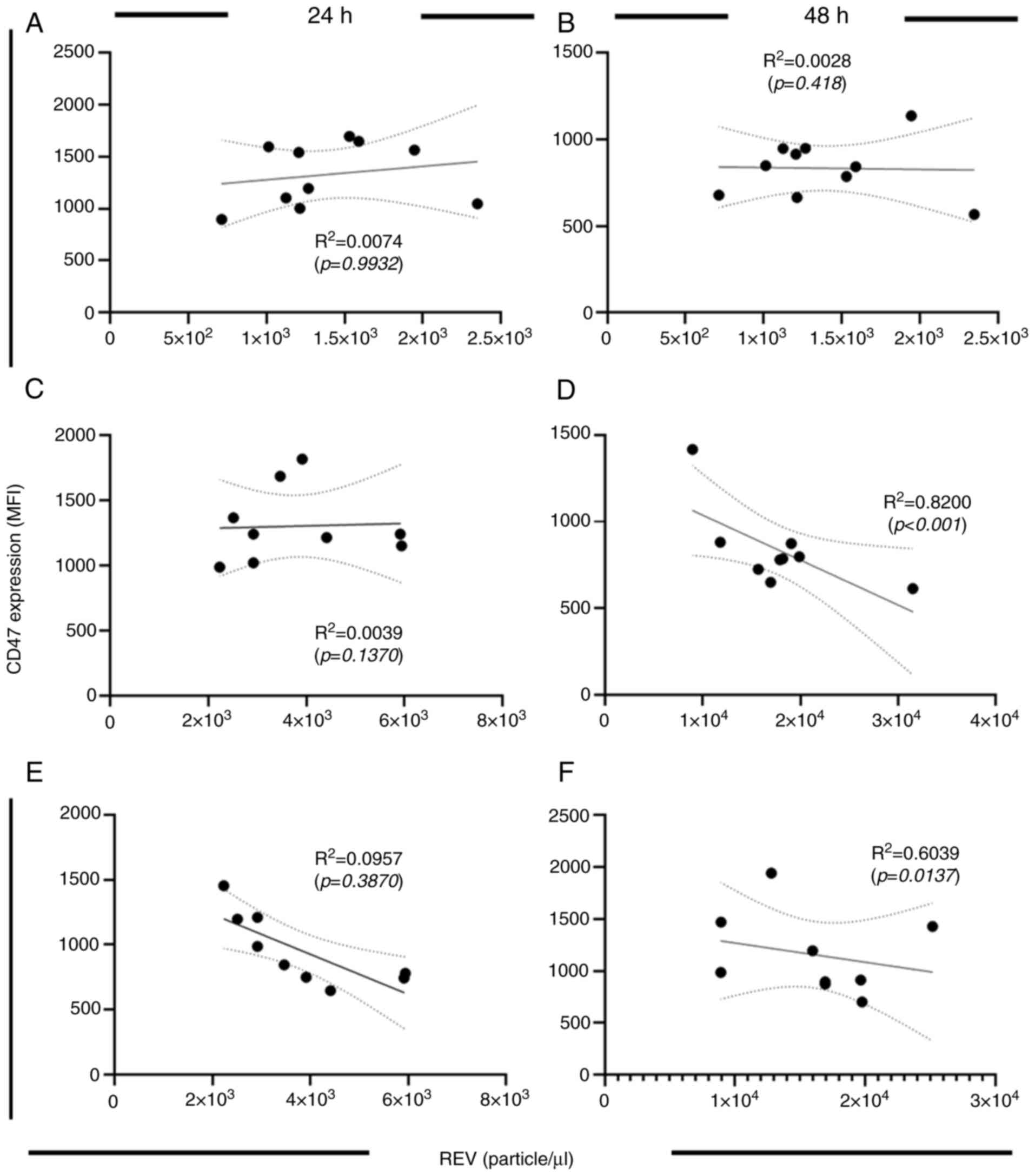
Furthermore, the findings of the present study
demonstrated a downregulation of CD47 expression in glucose-treated
RBCs. This emphasizes the role of CD47 in REV physiology.
Therefore, to further evaluate this relationship, correlation
analysis was performed to assess the association between CD47
expression and REV production under distinct culture conditions.
The data represented in Fig. 3
(CD47 expression) and Fig. 4 (REV
production) were used for calculation. The results revealed that
there was no significant correlation between CD47 expression and
REV production after 24 h of glucose culture (Fig. 7A, C
and E). At 48 h of glucose culture,
there was no significant correlation between CD47 expression and
REV production at euglycemic condition (Fig. 7B). By contrast, a significant
decrease in CD47 expression was observed in the intermediate
hyperglycemic condition at 48 h of culture (Fig. 7D). Moreover, an inverse correlation
between CD47 expression and REV production was demonstrated in the
severe glucose concentration condition at 48 h of culture (Fig. 7F). These results indicate the
physiologic alterations of REV and RBCs in different glucose
treatments. ROS increased in a dose-related pattern, which was
observed at the early phase of glucose exposure. By contrast to
CD47 expression, which was altered later at 48 h of glucose
treatment. These results suggest that increased ROS production is
associated with REV production in a dose-related manner, whereas
CD47 expression is associated with a time-dependent effect on
REV.
Discussion
Hyperglycemia is a common feature of DM which is a
major concern and non-communicable disease globally. Chronic
inflammation is one of the proposed mechanisms that drive the
progression of the disease (3,4).
Therefore, prolonged and uncontrolled hyperglycemia are considered
factors that drive multi-organ dysfunctions which are the major
complications of DM. Previous research has reported that increased
pro-inflammatory responses are observed in patients with both
prediabetic conditions and DM (15,25).
Chronic inflammation is closely associated with cellular and tissue
damage, particularly affecting the endothelial structures, which
are commonly involved in microvascular complications in patients
with DM (1-3).
The shedding of cell membrane fragments, known as EVs, is
implicated in several pathological processes. This has attracted
significant attention regarding the characteristics and biogenesis
of EVs in several disease conditions (6,7).
EVs are small particles shed from cells, and the
mechanisms underlying EV biogenesis and characteristics are of
increasing interest in understanding disease pathogenesis,
particularly in malignancies, autoimmune diseases and hematologic
disorders (10). The present study
assessed the effects of hyperglycemia on RBC membrane
microvesiculation and the shedding of EVs. EVs are involved in
modulating inflammatory responses by carrying bioactive molecules
such as proteins, lipids and nucleic acids to distant sites
(25). EVs, previously known as
MPs, are small membrane-bound particles characterized by PS
expression, commonly used as a marker for EV identification
(8). Whereas MPs were traditionally
associated with functions especially thrombosis. Recently, it was
found that EVs exhibit broader biological roles. Therefore, the
Minimal Information for Studies of EVs 2023 guidelines recommended
using the term ‘EVs’ instead of ‘MPs’ (8,26).
The present study demonstrated an increase in
intracellular ROS and REVs under hyperglycemic conditions. The
glucose concentrations used in the present study were selected
based on prior research that demonstrated significant alterations
in RBC membrane properties under hyperglycemic conditions (17-19).
Our results showed that the increased PS-expressing REV and
downregulation of CD235 align with the increased percentage of
eryptosis (17,19) and decreased cell viability (18). These results reinforcing the notion
that prolonged glucose exposure contributes to RBC membrane
alterations observed by the elevated REV production. These
comparisons support the validity of our in vitro model and
highlight the relevance of our observations in the context of
diabetes-related RBC dysfunction.
The present findings showed elevated REV production
following oxidative stress induction (Fig. S1). Oxidative stress is a
well-established driver of EV release, and prior studies have
reported that ROS overproduction in RBCs can trigger this process
(26,27). In particular, ROS overproduction has
been shown to promote oxidative stress that leads to the
modification and internalization of membrane proteins such as
CD33(28). Moreover, oxidative
stress can activate inflammatory pathways, leading to the release
of cytokines such as TNF-α, which further alter protein expression
and compromise membrane stability (28). Compared with the current findings, a
significant increase ROS production was observed at the 100-mM
glucose concentration compared with control, indicating a
dose-related effect at higher concentrations. Whereas no
significant difference was found between medium dose (45 mM) and
control at 24 h of culture. However, at 48 h of culture, a
time-dependent trend was observed with increased ROS production at
45- and 100-mM glucose (P=0.0426 and P0.0034, respectively)
(Fig. 2A). These results suggested
that prolonged hyperglycemia elevates the risk of EV release. These
findings further confirm that prolonged glucose exposure enhances
REV production.
The results of the present study are important from
the perspective of their clinical implications, particularly for
patients with poorly controlled DM, who often experience prolonged
hyperglycemic episodes (6). This is
associated with serious complications, including diabetic
nephropathy, diabetic retinopathy and microvascular damage
(9,11). Vascular injuries have been proposed
as characteristics of chronic inflammation in DM. The expression of
PS on endothelial cells activates platelets, thereby facilitating
the activation of primary hemostasis. Previous studies have
reported that PS-expressing REVs contribute to thrombogenicity and
procoagulant activity (29-31).
Moreover, PS provides binding sites for certain coagulation
factors, such as factor Xa and factor Va, which are essential for
prothrombin formation (29).
Aberrant expression of PS in these conditions is commonly observed
in patients with DM (30). However,
evidence of PS expression on RBCs under these conditions remains
limited.
Alongside with the alteration of RBC membrane
integrity, which can be explained by the increased REV production
and aberrant RBC membrane phenotypes. In the present study, CD235
was selected as surrogate markers for RBCs. CD235a (CD235)
(Glycophorin A) is a glycosylated sialoglycoprotein expressed
abundantly on the surface of mature red blood cells (RBCs). It
plays a crucial role in maintaining the structural integrity of the
RBC membrane, enabling flexibility and stability. Functionally,
CD235 is also involved in the regulation of RBC shape and the
modification of membrane protein complexes during RBC aging
(32). This makes it a valuable
marker for identifying erythroid cells in experimental studies,
including flow cytometry assays, due to its high and stable
expression throughout the life cycle of the RBC (33). CD47, another key protein present on
RBCs membrane complex, plays a significant role in regulating
immune interactions. The role of CD47 and its interactions in
diabetes and inflammation have been proposed in several ways. For
example, CD47-SIRP signaling promotes the release of
proinflammatory cytokines, such as TNF-α and IL-6, contributing to
systemic inflammation (15).
The present study explored the potential
relationship between CD235a and CD47 in the context of RBC membrane
dynamics. Changes in the expression of either CD47 or CD235a could
affect RBC survival and contribute to pathological conditions. The
present study demonstrated that CD47 dysregulation and
downregulation of CD235 were associated with aberrant ROS
production. The downregulation of CD47 expression at higher glucose
concentrations suggests a glucose-induced disruption in RBC
membrane integrity, which could have critical implications for RBC
survival, particularly in hyperglycemic conditions (13). Increased glucose levels enhance the
production of ROS, leading to oxidative damage. Oxidative stress
can affect membrane proteins such as CD47, resulting in their
degradation or reduced surface expression. One potential mechanism
underlying this decrease is glucose-induced oxidative stress, which
is known to be elevated in high-glucose environments. Elevated
glucose levels promote the generation of ROS, which contribute to
oxidative damage of membrane lipids and proteins in several cell
types, including RBCs (27,28). This oxidative damage alters membrane
integrity and increases membrane rigidity, both of which are known
to facilitate vesiculation (34,35).
This is in line with the findings of the present study, where RBCs
exposed to 100 mM glucose exhibited significantly lower CD47
levels, likely due to oxidative damage.
Although CD235 and CD47 expressed distinct
localization on RBC membrane. Both CD235a and CD47 are expressed on
the outer surface of RBCs, they do not directly interact in terms
of membrane linkage. However, indirect interactions may occur
through their involvement in membrane remodeling and vesiculation.
A previous study suggested that in response to oxidative stress or
membrane damage, both proteins may be internalized or redistributed
within the membrane. For instance, oxidative stress might lead to
vesiculation, where both CD235a and CD47 are incorporated into EVs,
potentially influencing cellular communication and immune
modulation (36).
Reduced CD47 levels typically exhibited in senescent
RBCs, making them more susceptible to clearance by macrophages
(37,38). A decrease in CD47 expression may
indicate early-stage membrane remodeling associated with vesicle
shedding and immune recognition, rather than terminal RBC
senescence.
The present results are thus more indicative of
acute membrane injury caused by oxidative stress rather than the
gradual deterioration seen in physiological RBC aging. However,
further investigations are needed to directly confirm the
functional consequences of CD47 downregulation, including the
potential mechanisms on immune activation and RBC clearance.
Additionally, glucose-induced modifications in key membrane
proteins, such as CD47, may disrupt the balance of intracellular
signaling pathways regulating vesicle shedding (37). Previous studies have shown that
oxidative stress can lead to cytoskeletal destabilization and PS
externalization, both of which promote vesicle formation and
release (39,40). In the present study, the observed
reduction in CD47 expression following glucose exposure may reflect
membrane remodeling events that precede vesiculation. Therefore,
the significant downregulation of CD47 in the 100 µM glucose
treatment group could reflect an accelerated aging process, which
may lead to increased RBC turnover in hyperglycemic conditions.
Hyperglycemia can disrupt protein glycosylation
processes. Glycosylation is critical for maintaining protein
stability and function, and hyperglycemia has been reported to
cause abnormal glycosylation patterns in several proteins, leading
to compromised cellular function (37). In the case of CD47, altered
glycosylation in a high-glucose environment may impair its proper
localization to the RBC membrane, thereby reducing its expression
(38). This mechanism has been
supported by previous research, which emphasizes the role of
glycosylation in regulating membrane protein stability (35).
Considering the findings related to REV formation,
ROS production and CD47 expression, the results of the present
study demonstrated a significant association between REV production
and ROS levels during the early phase of glucose treatment.
However, further investigation is required to fully elucidate the
interplay between these markers in EV genesis, as well as to assess
their potential complications, sources of EV generation and
functions in DM. The alteration in CD47 expression suggests a
connection to senescent phenotypes associated with prolonged
hyperglycemic conditions, which could potentially serve as novel
biomarkers for monitoring DM.
A key finding of the present study is the
significant change in membrane integrity observed under
hyperglycemic conditions, demonstrated by the downregulation of
CD47 and CD235. The reduction of these markers was associated with
increased REV counts. However, the patterns of decreased CD235 are
different to the CD47 downregulations. This aligns with a previous
study reporting that chronic high-glucose exposure increased RBC
membrane rigidity and decreased deformability-both of which are
critical factors contributing to microvascular dysfunction
(41). To our observation, CD47
significantly decreased at 100 mM only observed at 48 h of
incubation (P=0.0003) whereas CD235 significantly decreased
expression were observed both 24 h and 48 h of culture at different
glucose concentrations. Suggesting that the underlying mechanisms
of membrane destabilization under hyperglycemic stress between
these membrane proteins possibly different, requires further
investigations.
Additionally, the findings of the present study
highlight the importance of maintaining glucose homeostasis in
preserving RBC integrity. Chronic hyperglycemia, as observed in
diabetes, could exacerbate the downregulation of CD47, contributing
to increased RBC clearance and potentially leading to anemia
(42). The differential response to
glucose concentrations suggests that even moderate increases in
glucose levels can disrupt RBC function, emphasizing the need for
tight glycemic control to prevent such effects. However, the
precise molecular pathways through which glucose mediates CD47
downregulation should be investigated further.
A complex relationship was also demonstrated in the
present study, involving elevated intracellular ROS production,
decreased CD47 expression and REV production. However, there are
certain limitations of the present study that need to be
considered. The first limitation is the specific markers of EVs
characterization using flow cytometry. In the present study, PS was
used as a marker for EVs due to its well-established role in
identifying membrane vesicles shed from cells under stress
conditions. While PS exposure is commonly utilized for detecting
and isolating EVs, it is not exclusive to specific EV subtypes, as
apoptotic bodies can also externalize PS (8). However, in the context of the present
study, the use of PS alone as a marker was considered sufficient
for identifying EV populations of interest, as the primary
objective was to assess changes in vesiculation under hyperglycemic
conditions rather than to distinguish between different EV
subtypes. The observed alterations in PS-expressing EVs provide
meaningful insights into RBC membrane dynamics in response to
glucose exposure. Nevertheless, for enhanced specificity, future
studies should incorporate additional markers, such as tetraspanins
(CD9, CD63 and CD81) for exosomes or other membrane proteins
characteristic of different EV subpopulations (43). Another limitation is the in
vitro cell culture models were used, which offer controlled
conditions for evaluating specific biological mechanisms. However,
there several limitations to this approach, especially when
compared with cross-sectional studies performed in real-world
populations. The in vitro cell culture systems lack the
complexity of whole organisms: Whilst they provide valuable
insights into cellular processes in a controlled environment, they
do not fully replicate the physiological interactions found in
living organisms. Factors such as immune responses, cell signaling
between different tissues and systemic metabolic influences are
absent in vitro. The applicability of these findings to
physiological conditions may be limited. Therefore, further
investigation and characterization of these findings in diabetic
patients are recommended to enhance clinical relevance and
understanding.
The role of ROS production in this context points to
the potential benefits of incorporating antioxidants into treatment
regimens. Previous studies have reported the potential benefits of
several antioxidant-rich foods in managing DM and its
complications, such as vitamin C in combination with vitamin E and
CoQ10 (44,45). Additionally, exploring affordable
nutritional supplements in combination with current treatments
could be a promising approach. Preventing cellular damage through
antioxidants and identifying effective types of antioxidants could
enhance the quality of life for patients with DM and reduce the
risk of serious complications in patients with uncontrolled
hyperglycemia.
In summary, understanding the role of EVs in DM
provides benefits in several ways, such as identifying biomarkers
for monitoring the complications of DM and developing novel
therapeutic strategies aimed at modulating EV production, release
and uptake. Future research is expected to further elucidate the
molecular mechanisms underlying EV-mediated effects in DM, paving
the way for innovative treatments that can mitigate the burden of
this chronic disease.
Supplementary Material
Dosage effect of glucose concentration
on REV production. The colors, blue and red, correspond with the
different concentrations of H2O2 stimulation: 0.2 and 2 μM,
respectively. Bar plot represents the REV production under (A) 5
mM, (B) 45 mM and (C) 100 mM glucose concentration. The significant
increase in REV production were observed in 45 mM and 100 mM
glucose concentrations (P=0.0102 and P=0.0248, respectively).
However, no significant differences of REV productions were
observed at 5 mM glucose cultures using different H2O2
concentrations. (D-F) Representative histogram compares annexin V
expressing EVs that are used for REV calculations. MFIs of annexin
V expressing EVs after stimulation with 0.2 and 2 μM H2O2
for 24 h were compared in (D) 5, (E) 45 and (F) 100 mM of glucose
culture. (G-I) Representative histogram of ROS production using
different concentrations of H2O2 stimulation under different
glucose concentrations including (G) 5 mM, (H) 45 mM and (I) 100
mM. Data are presented as the mean ± standard error of the mean,
with individual dots indicating measurements from separate samples.
*Indicates statistical significance. REV, red blood
cell-derived extracellular vesicle; DM, diabetes mellitus; MFI,
mean fluorescence intensity; M, molar; ROS, reactive oxygen
species.
CD235 expression of glucose?treated
RBCs. (A) Bar plot comparing the dosage effect of glucose on CD235
expression at 24 and 48 h of incubation. The colors, green, blue
and red, correspond with the different treatment groups: Euglycemic
control (5 mM), intermediate level (45 mM) and severe hyperglycemia
(100 mM), respectively. The significantly decreased CD235
expression was observed at 45-mM glucose concentrations compared
with 5 mM after 24 h of treatment (P=0.0078). Whereas at 48 h of
treatment, the significant decrease of CD235 was observed at 45-
and 100-mM glucose concentration. (B) Representative histogram
comparing CD235 expression of glucose-treated RBC. MFIs of CD235 in
5-, 45- and 100- mM glucose treatment were compared at 24 h of
incubation. (C) Representative histogram comparing CD235 expression
of glucose-treated RBC. MFIs of CD235 in 5-, 45- and 100 mM glucose
treatment were compared at 48 h of incubation. (D) Bar plots
represent CD235 expression among different treatment groups:
Euglycemic control (5 mM), intermediate level (45 mM) and severe
hyperglycemia (100 mM). The colors, blue and red, correspond with
24 and 48 h of incubation, respectively. CD235 expression
significantly downregulated at 100 mM (P=0.0039). However, no
significant differences in CD47 expression were observed at 5 mM
and 45 mM glucose concentration between 24 and 48 h of culture.
(E-G) Representative histograms comparing CD235 expression at (E)
5-mM, (F) 45-mM and (G) 100-mM glucose concentrations after 24 and
48 h of incubation. Effect of incubation time on and glucose
concentrations on CD235 expression at distinct glucose
concentrations, compared using the Wilcoxon sign-rank test. Data
are presented as the mean ± standard error of the mean, with
individual dots indicating measurements from separate samples.
*Indicates statistical significance. ROS, reactive
oxygen species; RBC, red blood cell; MFI, mean fluorescence
intensity; M, molar.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Thailand Science
Research and Innovation Fund (grant no. FF-WU67-19) and partially
supported by Walailak University under the international research
collaboration (grant no. WU-CIA-05007/2024).
Availability of data and materials
The data generated in the present study are not
publicly available due to the ethical consideration but may be
requested from the corresponding author.
Authors' contributions
SS conceived, designed and supervised the study,
conducted experiments, acquired funding, interpreted data, drafted
and critically revised the manuscript for important intellectual
content. WC contributed to research design, laboratory work and
data analysis. TN contributed to research design, data acquisition,
laboratory experiments and data analysis. TB performed statistical
analysis and data interpretation. SJ was involved in laboratory
work, data collection and analysis. SYC assisted with data
acquisition and statistical analysis. NWo contributed to research
design and data interpretation. NWa was involved in literature
review, data analysis and manuscript preparation. DP contributed to
laboratory work, and data analysis. IP supervised the study,
conducted project administration, acquired funding and provided
final approval of the manuscript. All authors contributed to the
revision, read and approved the final version of the manuscript and
agree to be accountable for all aspects of the work. SS and IP
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The protocol was approved (approval no.
WUEC-23-143-01) by the Walailak University Institutional Review
Board (Tha Sala, Thailand). Written informed consents were obtained
from all participants prior to the commencement of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xie D, Shen Z, Yang L, Zhou D, Li C and
Liu F: Global, regional, and national burden of type 2 diabetes
mellitus attributable to particulate matter pollution from 1990 to
2021: An analysis of the Global Burden of Disease Study 2021.
Diabetes Res Clin Pract. 218(111934)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lu X, Xie Q, Pan X, Zhang R, Zhang X, Peng
G, Zhang Y, Shen S and Tong N: . Type 2 diabetes mellitus in
adults: pathogenesis, prevention and therapy. Signal Transduct
Target Ther. 9(262)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lima JEBF, Moreira NCS and Sakamoto-Hojo
ET: Mechanisms underlying the pathophysiology of type 2 diabetes:
From risk factors to oxidative stress, metabolic dysfunction, and
hyperglycemia. Mutat Res Genet Toxicol Environ Mutagen.
874-875(503437)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jia G, Bai H, Mather B, Hill MA, Jia G and
Sowers JR: Diabetic vasculopathy: Molecular mechanisms and clinical
insights. Int J Mol Sci. 25(804)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang M, Wang L and Chen Z: Research
progress of extracellular vesicles in type 2 diabetes and its
complications. Diabet Med. 39(e14865)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gauthier BR, Cobo-Vuilleumier N and
López-Noriega L: Roles of extracellular vesicles associated
non-coding RNAs in diabetes mellitus. Front Endocrinol (Lausanne).
13(1057407)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gustafson D, DiStefano PV, Wang XF, Wu R,
Ghaffari S, Ching C, Rathnakumar K, Alibhai F, Syonov M,
Fitzpatrick J, et al: Circulating small extracellular vesicles
mediate vascular hyperpermeability in diabetes. Diabetologia.
67:1138–1154. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Welsh JA, Goberdhan DCI, O'Driscoll L,
Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks
TAP, Erdbrügger U, et al: Minimal information for studies of
extracellular vesicles (MISEV2023): From basic to advanced
approaches. J Extracell Vesicles. 13(e12404)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wei J, Wang Z, Han T, Chen J, Ou Y, Wei L,
Zhu X, Wang K, Yan Z, Han YP and Zheng X: Extracellular
vesicle-mediated intercellular and interorgan crosstalk of
pancreatic islet in health and diabetes. Front Endocrinol
(Lausanne). 14(1170237)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sun Y, Tao Q, Wu X, Zhang L, Liu Q and
Wang L: The utility of exosomes in diagnosis and therapy of
diabetes mellitus and associated complications. Front Endocrinol
(Lausanne). 12(756581)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pretorius L, Thomson GJA, Adams RCM, Nell
TA, Laubscher WA and Pretorius E: Platelet activity and
hypercoagulation in type 2 diabetes. Cardiovasc Diabetol.
17(141)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gustafson D, Veitch S and Fish JE:
Extracellular vesicles as protagonists of diabetic cardiovascular
pathology. Front Cardiovasc Med. 4(71)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Collado A, Humoud R, Kontidou E, Eldh M,
Swaich J, Zhao A, Yang J, Jiao T, Domingo E, Carlestål E, et al:
Erythrocyte-derived extracellular vesicles induce endothelial
dysfunction through arginase-1 and oxidative stress in type 2
diabetes. J Clin Invest. 135(e180900)2025.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Garcia-Herreros A, Yeh YT, Peng Z and Del
Álamo JC: Cyclic mechanical stresses alter erythrocyte membrane
composition and microstructure and trigger macrophage phagocytosis.
Adv Sci (Weinh). 9(e2201481)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Catan A, Turpin C, Diotel N, Patche J,
Guerin-Dubourg A, Debussche X, Bourdon E, Ah-You N, Le Moullec N,
Besnard M, et al: Aging and glycation promote erythrocyte
phagocytosis by human endothelial cells: Potential impact in
atherothrombosis under diabetic conditions. Atherosclerosis.
291(8798)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sudnitsyna J, Skverchinskaya E, Dobrylko
I, Nikitina E, Gambaryan S and Mindukshev I: Microvesicle formation
induced by oxidative stress in human erythrocytes. Antioxidants
(Basel). 9(929)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Viskupicova J, Blaskovic D, Galiniak S,
Soszyński M, Bartosz G, Horakova L and Sadowska-Bartosz I: Effect
of high glucose concentrations on human erythrocytes in vitro.
Redox Biol. 5:381–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Loyola-Leyva A, Alcántara-Quintana LE,
Terán-Figueroa Y and González FJ: The in vitro effect of high
glucose concentrations on erythrocyte morphology assessed by
scanning electron microscopy. Micron. 154(103179)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Batista da Silva MV, Alet AI, Castellini
HV and Riquelme BD: A new protocol for in vitro red blood cell
glycation. Comp Biochem Physiol A Mol Integr Physiol.
264(111109)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Piwkham D, Pattanapanyasat K, Noulsri E,
Klaihmon P, Bhoophong P and Prachongsai I: The in vitro red blood
cell microvesiculation exerts procoagulant activity of blood cell
storage in Southeast Asian ovalocytosis. Heliyon.
9(e12714)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bass DA, Parce JW, Dechatelet LR, Szejda
P, Seeds MC and Thomas M: Flow cytometric studies of oxidative
product formation by neutrophils: a graded response to membrane
stimulation. J Immunol. 130:1910–1917. 1983.PubMed/NCBI
|
|
22
|
Tenório MCDS, Graciliano NG, Moura FA,
Oliveira ACM and Goulart MOF: N-acetylcysteine (NAC): Impacts on
human health. Antioxidants (Basel). 10(967)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fiorentino TV, Prioletta A, Zuo P and
Folli F: Hyperglycemia-induced oxidative stress and its role in
diabetes mellitus related cardiovascular diseases. Curr Pharm Des.
19:5695–5703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Amer J, Goldfarb A and Fibach E: Flow
cytometric measurement of reactive oxygen species production by
normal and thalassaemic red blood cells. Eur J Haematol. 70:84–90.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bazzoni R, Takam Kamga P, Tanasi I and
Krampera M: Extracellular vesicle-dependent communication between
mesenchymal stromal cells and immune effector cells. Front Cell Dev
Biol. 8(596079)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Doyle LM and Wang MZ: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells.
8(727)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhuge Z, McCann Haworth S, Nihlén C,
Carvalho LRRA, Heuser SK, Kleschyov AL, Nasiell J, Cortese-Krott
MM, Weitzberg E, Lundberg JO and Carlström M: Red blood cells from
endothelial nitric oxide synthase-deficient mice induce vascular
dysfunction involving oxidative stress and endothelial arginase I.
Redox Biol. 60(102612)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gonzalez Y, Herrera MT, Soldevila G,
Garcia-Garcia L, Fabián G, Pérez-Armendariz EM, Bobadilla K,
Guzmán-Beltrán S, Sada E and Torres M: High glucose concentrations
induce TNF-α production through the down-regulation of CD33 in
primary human monocytes. BMC Immunol. 13(19)2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Reddy EC and Rand ML: Procoagulant
phosphatidylserine-exposing platelets in vitro and in
vivo. Front Cardiovasc Med. 7(15)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Su Y, Chen J, Dong Z, Zhang Y, Ma R, Kou
J, Wang F and Shi J: Procoagulant activity of blood and endothelial
cells via phosphatidylserine exposure and microparticle delivery in
patients with diabetic retinopathy. Cell Physiol Biochem.
45:2411–2420. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nagata S, Sakuragi T and Segawa K:
Flippase and scramblase for phosphatidylserine exposure. Curr Opin
Immunol. 62:31–38. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Brugnara C, Williams A and Van Hove J:
Hematologic disorders and the biology of glycophorin A. Blood
Reviews. 35:1–15. 2019.
|
|
33
|
Liu J, Guo X, Mohandas N, Chasis JA and An
X: Membrane remodeling during reticulocyte maturation. Blood.
115:2021–2027. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bartosz G: Reactive oxygen species:
Destroyers or messengers? Biochem Pharmacol. 77:1303–1315.
2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Asaro RJ and Cabrales P: Red blood cells:
Tethering, vesiculation, and disease in micro-vascular flow.
Diagnostics (Basel). 11(971)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tian X, Yu H and Zhao Y: Oxidative stress
and red blood cell vesiculation: A link to immune modulation. Free
Radical Biol Med. 167:122–131. 2021.
|
|
37
|
Willekens FL, Werre JM, Groenen-Döpp YA,
Were JM, Groenen-Döpp YA, Roerdinkholder-Stoelwinder B, de Pauw B
and Bosman GJ: Erythrocyte vesiculation: A self-protective
mechanism? Br J Haematol. 141:549–556. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Burger P, Hilarius-Stokman P, de Korte D,
van den Berg TK and van Bruggen R: CD47 functions as a molecular
switch for erythrocyte phagocytosis. Blood. 119:5512–5521.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bosman GJ, Were JM, Willekens FL and
Novotný VM: Erythrocyte aging in vivo and in vitro: Structural
aspects and implications for transfusion. Transfus Med. 18:335–347.
2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Oldenborg PA, Zheleznyak A, Fang YF,
Lagenaur CF, Gresham HD and Lindberg FP: Role of CD47 as a marker
of self on red blood cells. Science. 288:2051–2054. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhu H, Liao SD, Shi JJ, Chang LL, Tong YG,
Cao J, Fu YY, Chen XP, Ying MD, Yang B, et al: DJ-1 mediates the
resistance of cancer cells to dihydroartemisinin through reactive
oxygen species removal. Free Radical Biology and Medicine.
71:121–132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Arkew M, Asmerom H, Gemechu K and Tesfa T:
Global prevalence of anemia among type 2 diabetic adult patients: A
systematic review and meta-analysis. Diabetes Metab Syndr Obes.
16:2243–2254. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kobayashi H, Shiba T, Yoshida T, Bolidong
D, Kato K, Sato Y, Mochizuki M, Seto T, Kawashiri S and Hanayama R:
Precise analysis of single small extracellular vesicles using flow
cytometry. Sci Rep. 14(7465)2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Balbi ME, Tonin FS, Mendes AM, Borba HH,
Wiens A and Fernandez-Llimos F: Antioxidant effects of vitamins in
type 2 diabetes: A meta-analysis of randomized controlled trials.
Diabetol Metab Syndr. 10(18)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kolahdouz Mohammadi R, Hosseinzadeh-Attar
MJ, Eshraghian MR, Nakhjavani M, Khorami E and Esteghamati A: The
effects of coenzyme Q10 supplementation on glycemic control and
lipid profile. J Diabetes Metab Disord. 19:99–109. 2019.
|