Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory
rheumatic condition that can impact up to 30% of patients with
psoriasis (PsO) (1). Timely
detection and effective monitoring of PsA are essential, as prompt
therapy can limit disease progression, prevent joint erosion, joint
deformity and systemic complications, and improve quality of life.
The present study explores the potential of videocapillaroscopy as
a method for evaluating disease activity, assessing treatment
effectiveness and potentially facilitating the early diagnosis of
PsA.
Pro-inflammatory cytokines, particularly tumor
necrosis factor (TNF)-α, interleukin (IL)-23 and IL-17, are
critical in the pathogenesis of PsA, where they markedly contribute
to joint destruction. Notably, these pro-inflammatory cytokines
lead to chronic systemic inflammation and organ damage associated
with PsA. Early damage in PsA can result in irreversible joint
damage and functional impairment, highlighting the critical
importance of early diagnosis and appropriate therapeutic
management to preserve joint function and improve long-term
outcomes (2,3).
Chronic systemic inflammation in PsA can lead to a
variety of complications and negative effects on the body. It is
known that PsA is characterized by irreversible joint damage and
destruction. Chronic inflammation of the joints can cause erosion
of the cartilage and bone, leading to pain, stiffness and reduced
mobility, resulting in deformities and disability. In addition to
joint involvement, chronic inflammation in PsA can affect several
organs and systems. PsA has been associated with an increased risk
of developing cardiovascular disease, as chronic inflammation has
the potential to damage blood vessels, markedly increasing the risk
of myocardial infarction and cerebrovascular accident. Research has
indicated that individuals with PsA are at a greater risk of
cardiovascular disease because of the higher rates of risk factors,
such as hypertension, hyperlipidemia and obesity. One of the key
consequences of chronic inflammation is the development of
atherosclerosis and the impairment of endothelial cell function,
leading to endothelial dysfunction and thrombosis (4,5).
Furthermore, patients with chronic inflammation have a higher risk
of osteoporosis and fracture risk; prolonged increases in
pro-inflammatory cytokines have been shown to impact bone
metabolism. Bone mass is also often decreased in patients with PsA
due to additional variables, such as older age, disability or
immobility (caused by disease activity) and menopausal status, and,
less frequently, due to glucocorticoid therapy (6,7).
Microvascular abnormalities play a unique role in
the pathological changes associated with PsO, notably contributing
both to onset and disease progression. Although the mechanisms
underlying neoangiogenesis are not yet fully understood, it has
been considered that disruptions in the balance between
pro-angiogenic and anti-angiogenic factors may have a crucial role
(8). Angiogenesis, which refers to
the formation of new blood vessels, serves a critical role in the
pathogenesis of PsA. It is driven by various pro-inflammatory
cytokines, such as vascular endothelial growth factor (VEGF), which
are elevated in PsA (9). VEGF has a
central role in this process, stimulating the proliferation of
endothelial cells and their migration, ultimately leading to the
formation of new capillaries. This angiogenic response is further
modulated by other cytokines and growth factors, including IL-1 and
IL-23, which contribute to the dysregulation of vascular
homeostasis. Endothelial dysfunction is characterized by altered
permeability and increased leukocyte adhesion, thus contributing to
synovial inflammation and joint destruction (10). Notably, the examination of nailfold
capillaries, which serve as a non-invasive view into the state of
the microvasculature, may offer valuable insights into the vascular
changes associated with PsA. Patients with PsA often exhibit
alterations in capillary morphology, such as increased capillary
density, enlarged capillaries and the presence of neoangiogenesis.
These changes can be indicative of the underlying inflammatory
process and vascular involvement in PsA.
Nailfold videocapillaroscopy (NVC) is a non-invasive
technique that examines the microcirculation in the nailfold area;
it uses a high magnification microscope and a video camera to
visualize and analyze the nailfold capillaries through
epiluminescence, which allows light to penetrate the surface of the
skin. NVC is used to identify capillaroscopic abnormalities in
Raynaud's phenomenon and systemic sclerosis, aiding in the early
diagnosis, treatment monitoring and prognosis of disease (11-14).
Notably, in the past few years, NVC has been a subject of interest
in several other immune-mediated inflammatory diseases, such as
rheumatoid arthritis (RA), PsA and Sjogren syndrome. NVC is a safe
and quick technique, which is easily accepted by patients. A single
drop of cedar oil is usually placed on the nailfold to enhance
magnification. To minimize false-positive results, the subject
needs to follow some basic rules: They must not remove fingernail
cuticles or undergo cosmetic procedures in the month prior to NVC;
they should avoid smoking and drinking caffeinated beverages in the
previous 4 h; and they should be acclimated to room temperature
(20-24˚C) for ≥15 min before the examination. Capillaroscopy can be
performed on a number of anatomical locations; however, the
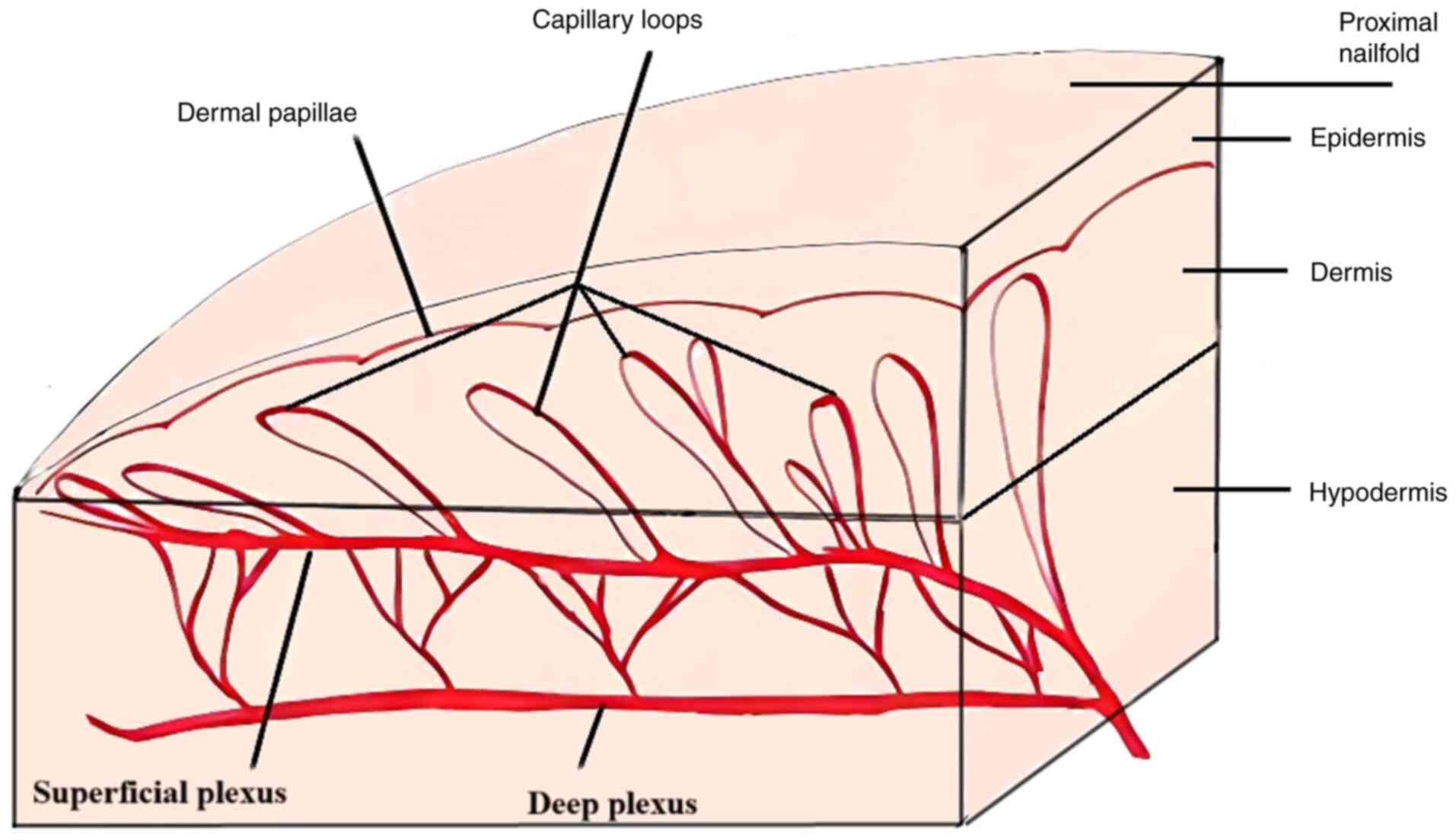
nailfold offers a distinctive longitudinal view of the capillaries
that run parallel to the surface of the skin (Fig. 1). This tool can provide information
about capillary length, width, morphology and even red blood cell
velocity (15). Magnification can
vary from 50x to 500x, but the preferable magnification is 200x.
The capillaroscope uses a cold light source (LED) to prevent
vasodilatation, and the probe should be placed gently (not pressed)
at an angle of 45-90˚. All fingers can be evaluated, but because
the skin thickness varies between fingers, the best images are
obtained from the fourth finger, whereas capillaries in the first
finger are rarely analyzed due to poor visualization (16).
Normal capillaries have a hairpin configuration, and
are uniform in shape and size, with minor morphological variations
(Table I). Normal density is
characterized as ≥7 capillaries/mm with no avascular areas. In
addition, no microhemorrhages/ hemosiderin deposits or angiogenesis
should be detected. Elongated capillaries exceed 300 µm. Tortuous
capillaries can be normal; however, crossed capillaries are
considered abnormal, as well as other shapes. An apical diameter of
<20 µm is considered to be within the normal range, whereas
dilated capillaries measure 20-50 µm and giant capillaries measure
≥50 µm.
 | Table INormal capillaroscopic pattern. |
Table I
Normal capillaroscopic pattern.
| Parameters | Normal |
|---|
| Visibility | Good capillary
visibility |
| Architecture | Uniform
distribution Parallel distribution |
| Morphology | ‘Hairpin’
appearance/reversed ‘U’ shape |
| Anomalies | None |
| Height | <300 µm |
| Capillary | Afferent loop
(arterial): 6-19 µm |
| diameter | Efferent loop
(venous): 8-20 µm |
| Venous/arterial
ratio | <2:1 |
| Capillary
density | ≥7
capillaries/mm |
| Flow | Continuous, no
stasis |
Some factors, such as lifestyle choices,
comorbidities and medication, can greatly impact capillary
morphology. Smoking can cause microvascular damage and alter
capillary morphology, which might complicate the interpretation of
videocapillaroscopy results. Notably, changes in capillary
structure due to smoking may be misattributed to underlying
diseases, leading to misdiagnosis. Furthermore, patients with
diabetes may exhibit specific microvascular changes, such as
increased capillary loop density or altered capillary structure.
The presence of diabetes can therefore make it challenging to
differentiate between capillary changes due to diabetes and those
due to other underlying conditions. Certain medications, such as
vasodilators, can also influence microcirculation and capillary
morphology (17).
Capillaroscopic abnormalities can be qualitatively
categorized as ‘scleroderma patterns’ or ‘non-scleroderma
patterns’. The scleroderma pattern is defined by the existence of
‘giant’ capillaries (apical diameter ≥50 µm), low capillary density
(<7/mm), and the presence of microhemorrhages and angiogenesis.
Non-scleroderma patterns are non-specific abnormalities that can be
visible in other rheumatic inflammatory diseases and even in some
healthy individuals (18,19). Notably, a semi-quantitative
assessment has been proposed, which analyzes >32 fields,
consisting of four 1-mm fields in eight fingers (excluding the
thumb). This assessment evaluates capillary density and diameter
(enlarged/giant), microhemorrhages and abnormal capillaries. The
rating scores are as follows: 0 (no alterations), 1 (<33% of
capillaries are altered), 2 (33-66% are altered) and 3 (>66% are
altered), and applies for each of the aforementioned parameters
(20).
Nail PsO is regarded as a predictive sign for
progression to PsA. Enthesitis, an early inflammatory alteration in
PsA, is responsible for nail abnormalities. These alterations may
result from inflammation of the distal interphalangeal (DIP)
extensor tendon enthesis, which is in close contact with the nail.
Because clinical musculoskeletal symptoms appear with an estimated
delay of 10 years, silent alterations may be detected early, using
various methods that are nail-focused, such as capillaroscopy and
ultrasound (21-25).
Frequent abnormalities have been identified in PsA, including
enlarged and tortuous capillaries, angiogenesis, microhemorrhages
and even capillary loss. Image analysis can be performed manually,
semi-manually or digitally (26,27).
Capillaroscopy has the potential to reveal subtle
changes in the microcirculation that precede clinical
manifestations of PsA. Detecting these changes could be crucial for
early intervention, potentially delaying or preventing the onset of
arthritis in susceptible individuals. Furthermore, capillaroscopic
abnormalities in patients with PsO may correlate with inflammatory
markers; this relationship could enhance the sensitivity of
capillaroscopy as a diagnostic tool for identifying patients at
risk for PsA. Barriers to early diagnosis often include a lack of
awareness among healthcare providers about the signs and symptoms
of PsA, misattribution of symptoms to other conditions and a
variability in clinical presentation. Patients may also express
apprehension regarding the necessity of medical procedures, often
attributing their symptoms to aging or overexertion (28,29).
Early diagnosis of PsA is vital for maximizing therapeutic success,
preventing joint damage, managing comorbidities and improving the
overall quality of life for patients. Patients diagnosed early tend
to have an improved prognosis, with a higher likelihood of
obtaining remission or low disease activity over time. Early
intervention can help maintain joint function and reduce the
likelihood of disability, enabling individuals to maintain their
quality of life. Moreover, the healthcare costs associated with
advanced disease management, surgeries and hospitalizations can be
significantly reduced (30).
Patients with PsA often exhibit alterations in
capillary morphology, such as decreased capillary density, enlarged
capillaries and the presence of neoangiogenesis. These changes can
be indicative of the underlying inflammatory process and vascular
involvement in PsA.
Changes observed in NVC can be correlated with
several biomarkers and tests to monitor or confirm disease activity
and progression, such as serum inflammatory markers [C-reactive
protein (CRP), erythrocyte sedimentation rate, fibrinogen and
ferritin], imaging studies [ultrasound and magnetic resonance
imaging (MRI) of the joints and entheses], patient-reported
outcomes (questionnaires that assess quality of life and pain
levels, such as Patient Global Assessment, Health-Related Quality
of Life, Dermatology Life Quality Index, American College of
Rheumatology joint count and Mander enthesis index, and composite
measures, such as Disease Activity in PsA). While
videocapillaroscopy is a useful tool for assessing microvascular
changes, its limitations must be recognized. Operator dependency,
the potential for false positives and negatives, and the influence
of patient factors can all impact the accuracy of
videocapillaroscopy findings. To mitigate these limitations, it is
essential to standardize protocols, ensure operator training and
proficiency, and consider patient factors when interpreting
results.
The present systematic review aimed to explore the
capillaroscopic findings among patients with PsO (with or without
joint involvement). Moreover, it was planned to determine whether
capillaroscopy may be useful for differential diagnosis in early
arthritis. Furthermore, the benefits, drawbacks and future of this
imaging technique in PsA management were examined.
Materials and methods
Various medical databases, including PubMed
(https://pubmed.ncbi.nlm.nih.gov/), Web
of Science (Clarivate; www.webofscience.com), Scopus (https://www.scopus.com/) and Google Scholar
(https://scholar.google.com/) were
searched with a combination of related key words. The key words
used included a combination of: ‘PsA OR PsO AND Nailfold
capillaroscopy’, ‘Videocapillaroscopy AND PsO OR PsA’, ‘Nailfold
capillary changes AND PsO OR PsA’. Only articles written in the
English language were considered. Databases were searched by the
main author (OGP) until August 2024. The articles included were
published between 2012 and 2024 with one exception, an article from
1982 (Zaric et al), which may have been the first to mention
the use of nailfold capillaroscopy in patients with PsO and PsA.
The articles were then analyzed and selected. After selection, data
were verified by two co-authors (DA and AB). Information was
extracted manually from selected papers by the main author (OGP).
Several parameters were evaluated: Total number of patients ±
controls; the presence of disorganized, tortuous, crossed,
ramified, elongated, dilated and giant capillaries; angiogenesis;
the presence of hemorrhages or hemosiderin deposits and low
capillary density ± avascular areas.
The inclusion criteria were as follows: Population
group consisting of a minimum of five patients diagnosed with PsA,
utilizing nailfold capillaroscopy (with no restrictions on
magnification) of at least one finger. The exclusion criteria were
as follows: Articles written in languages other than English,
articles that included patients with PsO with an undefined number
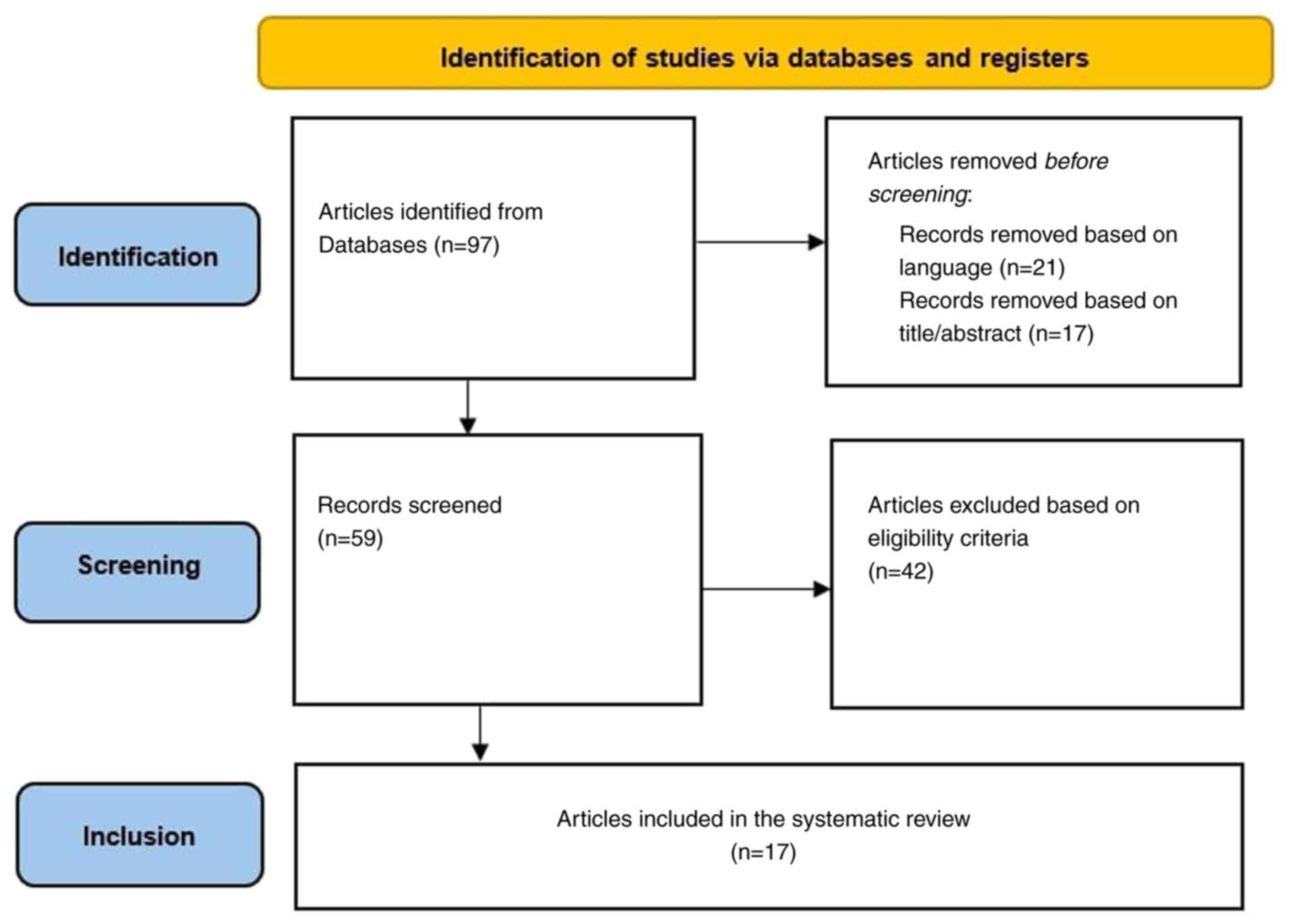
of patients with PsA (Fig. 2). To
minimize the risk of bias, the identified papers were assessed by
the main author, and were then verified by co-authors DA and AB.
Capillaroscopy mainly uses a qualitative approach for data
interpretation, meaning the presence of specific characteristics is
indicated as either ‘Yes’ or ‘No’. This method markedly reduces the
potential for bias in data interpretation. The present systematic
review was registered in the INPLASY register under accession
number INPLASY2024110112 (https://inplasy.com/inplasy-2024-11-0112/) (31).
Results
A total of 97 studies that focused on nailfold
capillaroscopy in PsA were identified. A total of 21 articles were
excluded that were written in languages other than English and 17
articles were excluded based on the title or abstract.
Additionally, 42 articles were excluded after reviewing the full
text, since they did not meet the inclusion criteria. Only 17
studies regarding nailfold capillaroscopic findings in PsA were
found eligible and were included in the present review.
A study by Ali et al (32) examined Egyptian patients with PsO,
PsA and RA. The findings revealed that patients with PsA had a
lower density of capillaries, more hemorrhages and more dilated
capillaries. Furthermore, patients with PsA had tortuous
capillaries and frequent capillary disorganization. Moreover, a
correlation between CRP titer and capillary diameter was observed.
Regarding disease activity, a strong correlation was observed
between tender joint count and capillary width, as well as with
capillary density (negative correlations) (32).
Another study by Guldberg-Møller et al
(33) identified some differences
between PsO and PsA. Giant capillaries were observed in some of the
patients with PsA and none of the patients with PsO. Low density
was also observed more frequently in patients with PsA than in
those with PsO. However, it was observed that patients with PsO had
more dilated capillaries, ramifications and microhemorrhages
compared with in patients with PsA (33).
Anghel et al (34) noted that patients with PsA had more
giant capillaries, more elongated capillaries, more hemorrhages and
more avascular areas than patients with RA. In patients with RA,
the main capillaroscopic abnormalities were crossed, tortuous and
dilated capillaries, as well as the presence of angiogenesis. No
differences between capillary density were observed between
patients with PsA and those with RA. Moreover, a correlation was
observed between CRP titer and the arterial diameter of
capillaries. Regarding reversibility with treatment, Anghel et
al (34) observed that after 12
months of anti-TNF-α therapy, there was an improvement in
capillaroscopic abnormalities, including angiogenesis, enlarged
capillaries and avascular areas. However, they did not find
significant changes tortuous, crossed or bushy capillaries
(34).
Graceffa et al (35) conducted another study that observed
the differences between patients with RA and PsA. Regarding the
diameter of blood vessels, it was revealed that patients with PsA
had larger diameters compared with those in patients with PsO, but
smaller than in those with RA. Additionally, capillary density was
smaller in PsA than RA, as was the height of the capillaries.
Regarding the tortuosity of blood vessels, this was highest in
patients with PsA and was smallest in the controls (35).
Rajaei and Dehghan (36) performed a study on patients with PsA
and identified that capillary architecture was abnormal in 22% of
patients, the venular plexus was visible in 98% of patients and
capillary density was normal in all of the patients. Scleroderma
pattern (the occurrence of the following findings: Tortuous
capillaries, abnormal architecture, angiogenesis and enlarged
loops) was observed in 27% of patients (36).
Molteni et al (37) noted in a study performed on patients
with RA and PsA that the predominant structural changes were
tortuous capillaries (with an occurrence of 90% in PsA and 100% in
RA), as well as single crossed capillaries (90% in PsA and 86% in
RA). Furthermore, multiple crossed capillaries were observed in 50%
of patients with PsA compared with in 21% of patients with RA. By
contrast, hemorrhages occurred more frequently in patients with RA
than in those with PsA. No differences were found between those
groups and healthy controls regarding capillary density, length and
distribution (37).
Lambova and Müller-Ladner (38) observed that patients with PsA had a
lower density compared with patients with other causes of
inflammatory arthritis; no other differences were observed.
In a study by Zaric et al (39) regarding capillaroscopy findings in
patients with PsA and PsO, it was observed that hemorrhages were
more prevalent in patients with PsA, as well as the presence of
subpapillary plexus in patients with PsO. Regarding capillary
length, both groups showed smaller capillaries than controls
(39).
Comparing patients with PsA to those with PsO,
Bardehle et al (40)
observed bushy capillaries (severe ramification) in patients with
PsA; however, no other differences were observed between the
groups.
Florea et al (41) observed that patients with early
arthritis, which later developed into PsA, had longer, tortuous and
dilated capillaries; however, no density abnormalities or
microhemorrhages were observed (41). Elmesiry et al (42) performed a study on patients with PsA
and PsO and highlighted that all patients with PsA had abnormal
capillary morphology and >23% had hemorrhages (42). Fukasawa et al (43) noted that patients with PsA had more
enlarged loops and hemorrhages compared with those in patients with
PsO. Moreover, it was concluded that microhemorrhages and dilated
capillaries may serve as notable indicators of the progression from
PsO to PsA (43).
A study on patients with PsO and PsA conducted by
Bhushan et al (44) observed
that capillary density was reduced in those that had nail disease
and associated DIP joint disease compared with those in the
controls. Furthermore, limb diameters (venous and arterial) were
decreased in patients with PsA that affected DIP joints (44).
Sivasankari et al (45) observed that patients with PsA had
capillary disorganization (irregular and haphazard distribution).
Ribeiro et al (46) reported
no marked differences between patients with PsO and PsA regarding
capillary density. However, avascular areas were more prevalent in
patients with PsA, as well as tortuous capillaries (46). Relhan et al (47) observed that patients with PsA had
more aberrant morphology, lower density and more avascular areas.
No correlation was observed between sex, disease duration and the
severity of PsO (47).
Kamboj et al (PsO) performed a study on 200
individuals (100 patients with PsO, of which 25 had PsA, and 100
healthy controls). It was noted that patients with PsA had a
disorganized distribution of capillaries, with loss of the usual
hairpin shape of capillaries detected; no other specific findings
were noted.
The analysis of these 17 articles revealed a
significant variability in the reporting of capillary
characteristics. While certain features, such as disorganized
capillaries (9/17), tortuous capillaries (12/17) and dilated
capillaries (8/17), were reported with relative frequency, a
considerable number of articles (ranging between 2 and 10) did not
provide data on various findings, indicating a potential gap in the
literature. Features such as elongated capillaries and
neovascularization were particularly underreported, with only 3 and
2 articles mentioning them, respectively. Moreover, the presence of
crossed and ramified capillaries also showed limited reporting.
Hemorrhages were reported in 5/17 articles, whereas in 5/17
articles they remained underexplored. The aforementioned
information is summarized in Tables
II and III.
 | Table IINailfold capillaroscopic
abnormalities found in psoriatic arthritis. |
Table II
Nailfold capillaroscopic
abnormalities found in psoriatic arthritis.
| First
author/year | Total no. of
patients | Diagnosis | Controls (no.) |
Disorganisation | Elongated | Tortuous | Crossed | Ramified | Dilated | Giant |
Neovascularisation | Hemorrhages | Low density | (Refs.) |
|---|
| Ali et al,
2019 | 40 | PsA | Yes: 20 | Yes | - | Yes | - | - | Yes | - | - | Yes | Yes | (32) |
| Guldberg-Moleler
et al, 2021 | 75 | PsA and PsO | Yes: 12 PsO and 13
osteoarthritis) | Yes | Yes | Yes | - | Yes | Yes | Yes | - | Yes | Yes | (33) |
| Anghel et
al, 2023 | 92 | PsA and RA | Yes: 34 RA and 24
healthy controls | - | Yes | Yes | - | - | Yes | Yes | - | Yes | No | (34) |
| Graceffa et
al, 2013 | 60 | PsA and RA | 30: RA | - | - | Yes | - | - | No | No | - | - | Yes | (35) |
| Rajaei et
al, 2016 | 54 | PsA | No | Yes | - | Yes | - | - | No | No | Yes | - | No | (36) |
| Molteni et
al, 2022 | 64 | PsA -20 and RA -
14 | Yes: 30 | Yes | - | Yes | Yes | Yes | No | No | - | No | - | (37) |
| Lambova et
al, 2012 | 105 | PsA - 34 RA - 62
Early arthritis - 9 | No | - | No | No | No | No | No | No | No | No | Yes | (38) |
| Zaric et al,
1982 | 135 | PsA-34 PsO-31 | Yes: 70 HC | - | No | No | No | No | No | No | No | Yes | No | (39) |
 | Table IIINailfold capillaroscopic
abnormalities found in psoriatic arthritis-continuation. |
Table III
Nailfold capillaroscopic
abnormalities found in psoriatic arthritis-continuation.
| First
author/year | Total no. of
patients | Diagnosis | Controls (no.) |
Disorganisation | Elongated | Tortuous | Crossed | Ramified | Dilated | Giant |
Neovascularisation | Hemorrhages | Low density | (Refs.) |
|---|
| Bardehle et
al, 2021 | 148 | PsA- 24 PsO-
53 | Yes: 71 | - | Yes | Yes | Yes | Yes | Yes | - | - | - | Yes | (40) |
| Florea et
al, 2015 | 21 | PsA - 1 Other
diseases -20 | Yes | Yes | - | Yes | - | - | Yes | - | - | No | No | (41) |
| Elmesiry et
al, 2021 | 225 | PsA - 175 | Yes: 25 RA + 25
SSc | - | - | Yes | - | - | No | No | No | No | No | (42) |
| Fukasawa et
al, 2023 | 449 | PsA - 213 | Yes: 236 PsO | - | - | - | - | - | Yes | - | - | Yes | - | (43) |
| Bhushan et
al, 2000 | 88 | PsO - 31 PsA -
13 | Yes: 44 HC | - | No | - | No | No | No ↓ | No ↓ | No | No | Yes (especially in
DIP joint disease and nail disease) | (44) |
| Sivasankari et
al, 2021 | 110 | PsO | No | Yes | No ↓ | Yes | Yes | No | Yes | No | No | No | No | (45) |
| Ribeiro et
al, 2012 | 96 | PsA - 7 PsO -
39 | Yes: 50 HC | Yes | - | Yes | - | - | - | - | - | - | Yes + Avascular
areas | (46) |
| Relhan et
al, 2023 | 150 | PsA - 18 | Yes: 75 HC + 57
PsO | Yes | - | Yes | Yes | No | Yes | Yes | Yes | No | Yes + Avascular
areas | (47) |
| Kamboj et
al, 2024 | 200 | 25 PsA + 75
PsO | Yes: 100 HC | Yes | - | - | - | - | - | - | - | - | - | (48) |
Discussion
PsA is a chronic rheumatic inflammatory disease,
defined by rapid joint destruction. Early diagnosis and precise
monitoring are crucial for patient outcome; notably, delayed
diagnosis of PsA can lead to permanent joint deformities and poor
physical function. In addition, due to prolonged systemic
inflammation and endothelial dysfunction, delayed diagnosis can
increase the risk of cardiovascular events (49).
Upon analyzing the gathered information (Tables II and III), the most prevalent findings in
patients with PsA were disorganized and tortuous capillaries.
Another predominant finding was the decreased capillary density in
patients with PsA compared with that in patients with PsO. In
addition, some authors reported the presence of dilated and giant
capillaries, along with crossed and ramified capillaries; however,
elongated capillaries were noted by only 2 authors.
A total of 5 authors reported a higher occurrence of
hemorrhages, whereas 7 did not observe these characteristics in
patients with PsA. The majority of the authors did not observe the
presence of neovascularization; however, studies have shown an
imbalance in angiogenic and anti-angiogenic factors in patients
with PsA, resulting in a dysregulated angiogenesis (50,51).
Microvascular abnormalities serve a distinctive role in the
pathological changes associated with PsO, serving a crucial role in
the onset and progression of disease. They supply essential
nutrients for the proliferation of keratinocytes and surrounding
tissues while facilitating the migration of inflammatory cells
(52).
In PsA, inflammation activates processes that
increase the permeability of blood vessels, allowing inflammatory
cells (macrophages, T cells and mast cells) into the affected
tissues. These cells release angiogenic factors (such as VEGF and
TNF-α). These alterations result in the formation of abnormal or
dysfunctional capillaries. The mechanisms behind neoangiogenesis
are not yet fully understood; however, it is becoming increasingly
clear that disruptions in the balance between pro-angiogenic and
anti-angiogenic factors are critical (53-55).
Nailfold capillaroscopy has received notable
interest in recent years for its potential to provide valuable
insights into PsA. Videocapillaroscopy is an inexpensive clinical
investigation, which is easily accessible to all patients with PsO.
The investigation causes no discomfort and can be completed in a
timely manner, making it easily accepted by patients (53-58).
Dermatologists have a major role in the early diagnosis of PsA. NVC
has gained marked interest, being used in a number of rheumatic
autoimmune diseases (besides systemic sclerosis). Its use reflects
the increasing recognition of its ability to offer key insights
into the vascular changes of PsA (59-62).
Notably, evidence on the use of capillaroscopy
specifically for monitoring vascular or systemic complications in
PsA is limited, and not yet part of standard clinical practice. By
utilizing capillaroscopy to analyze the capillary patterns of
patients with PsO (with or without joint involvement), physicians
may be able to identify distinctive patterns that help distinguish
between the two conditions. This imaging technique can be used
alongside ultrasonography and MRI to identify asymptomatic
individuals who may progress to PsA (63,64).
MRI provides comprehensive insights into joint pathology, but is
limited by cost and accessibility. Ultrasound is excellent in
assessing superficial structures and is less expensive and more
portable than MRI; however, none of these imaging techniques can
assess microvascular changes. A combined approach that leverages
the strengths of each imaging technique may enhance diagnostic
accuracy and improve outcomes for patients with PsA.
While there is emerging research on the use of NVC
in PsA and other conditions, the evidence base is not yet as robust
as it is for scleroderma (65).
Videocapillaroscopy requires specialized training and expertise,
which may not be available in all clinics, and thus this limits its
accessibility and widespread use. However, artificial intelligence
algorithms may be used in the future to analyze capillaroscopic
images and detect abnormalities in capillary structure. Learning
algorithms can be developed using extensive datasets to improve
diagnostic accuracy, reducing the likelihood of human error and
providing standardized assessments. The integration of these
emerging technologies can potentially optimize diagnostic
efficiency, notably decrease the duration of diagnosis, improve
patient outcomes and optimize clinical procedures. As research
progresses and these technologies become more widely adopted, they
may lead to more precise and individualized patient care (66).
The establishment of standardized protocols for
performing and interpreting nailfold capillaroscopy in PsA is
imperative for enhancing diagnostic accuracy, ensuring consistency
across practices and facilitating research. By implementing these
protocols, the microvascular changes associated with PsA can be
better understood, ultimately leading to improved patient care, and
facilitating immediate intervention and efficient therapeutic
approaches.
Overall, studies on capillaroscopic differences
between patients with PsA and PsO are important as they aid in the
early detection of PsA, which can help prevent severe joint
deformities and improve patient outcomes. Although capillaroscopy
offers valuable information about microvascular changes, it should
not be used as a standalone diagnostic tool. For a thorough
evaluation, it may work best alongside other imaging techniques,
such as ultrasonography and MRI.
Although there are few available data supporting
whether capillaroscopy can predict disease progression and joint
damage, it remains a valuable tool for assessing microvascular
changes. Long-term, large-scale (multicentric) prospective studies
with clearly defined control groups (patients with PsO and healthy
individuals) would allow for robust comparisons between patients
with PsA, healthy individuals and those with other related
autoimmune conditions. These would also help establish whether
capillaroscopy can predict disease progression, joint damage and
response to therapy (although they can sometimes lack coordination,
leading to variations in data collection and analysis methods).
Moreover, there is a lack of standardized protocols for performing
and interpreting videocapillaroscopy. Variability in techniques can
lead to inconsistencies in results and make comparisons across
studies challenging. In addition, there is often a lack of
appropriate control groups, such as individuals with other
inflammatory arthritis conditions or healthy controls, making it
difficult to distinguish specific capillaroscopic changes
associated with PsA. Addressing these gaps in future research could
enhance the understanding of the role of videocapillaroscopy in the
diagnosis and management of PsA and improve patient care.
In conclusion, upon reviewing the use of
videocapillaroscopy in patients with PsA, some key findings were
observed: Disorganized and tortuous capillaries, along with a
notable reduction in capillary density, were detected in patients
with PsA compared with in individuals with PsO. Nailfold
capillaroscopy may thus be considered a valuable tool, offering
insights into both microvascular lesions and structural
abnormalities of the nail. Based on these insights, it is
increasingly clear that integrating microvascular assessments into
the clinical management of PsA may enhance the ability to monitor
disease progression, and could also aid in evaluating treatment
efficacy, ultimately leading to improved patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
OGP conceptualized the study, developed methodology,
provided resources, performed visualization, and wrote, reviewed
and edited the manuscript. DA and AB performed formal analysis. OGP
and AB conducted investigation and project administration. OGP and
MLG curated data. OGP and VCB prepared the original draft. VCB and
MLG supervised the study. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lazar LT, Guldberg-Møller J, Lazar BT and
Mogensen M: Nailfold capillaroscopy as diagnostic test in patients
with psoriasis and psoriatic arthritis: A systematic review.
Microvasc Res. 147(104476)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lee BW and Moon SJ: Inflammatory cytokines
in psoriatic arthritis: Understanding pathogenesis and implications
for treatment. Int J Mol Sci. 24(11662)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Skougaard M, Ditlev SB, Søndergaard MF and
Kristensen LE: Cytokine signatures in psoriatic arthritis patients
indicate different phenotypic traits comparing responders and
non-responders of IL-17A and TNFα inhibitors. Int J Mol Sci.
24(6343)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pahwa R, Goyal A and Jialal I: Chronic
Inflammation. In: StatPearls. StatPearls Publishing, Treasure
Island, FL, 2024.
|
|
5
|
Zhu TY, Li EK and Tam LS: Cardiovascular
risk in patients with psoriatic arthritis. Int J Rheumatol.
2012(714321)2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Y, Song ZB, Deng XR, Zhang XH and
Zhang ZL: Risk factors associated with osteoporosis and fracture in
psoriatic arthritis. Chin Med J (Engl). 134:2564–2572.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ioniță-Radu F, Nicolau IN, Petrache OG,
Groșeanu ML, Bojincă VC, Negru MM, Bucurică S and Anghel D:
Correlation between trabecular bone score and homocysteine level in
rheumatoid arthritis patients on anti-TNF inhibitors. Life (Basel).
14(463)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen Y, Tai Z, Zhu C, Yu Q, Zhu Q and Chen
Z: Vascular endothelial growth factor A VEGFA inhibition: An
effective treatment strategy for psoriasis. Int J Mol Sci.
25(59)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Di Lorenzo B, Zoroddu S, Mangoni AA,
Paliogiannis P, Erre GL, Satta R, Carru C and Zinellu A: VEGF in
psoriatic arthritis: Systematic review and meta-analysis. Clin Chim
Acta. 567(120084)2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Butt C, Lim S, Greenwood C and Rahman P:
VEGF, FGF1, FGF2 and EGF gene polymorphisms and psoriatic
arthritis. BMC Musculoskelet Disord. 8(1)2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hofstee HM, Vonk Noordegraaf A, Voskuyl
AE, Dijkmans BA, Postmus PE, Smulders YM and Serné EH: Nailfold
capillary density is associated with the presence and severity of
pulmonary arterial hypertension in systemic sclerosis. Ann Rheum
Dis. 68:191–195. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Smith V, Ickinger C, Hysa E, Snow M, Frech
T, Sulli A and Cutolo M: Nailfold capillaroscopy. Best Pract Res
Clin Rheumatol. 37(101849)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rajaei A, Dehghan P and Amiri A: Nailfold
capillaroscopy in 430 patients with rheumatoid arthritis. Casp J
Intern Med. 8:269–274. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Anghel D, Prioteasă OG, Nicolau IN,
Bucurică S, Belinski DO, Popescu GG, Ghinescu MC, Bobircă A,
Groșeanu ML and Bojincă VC: The role of nailfold
videocapillaroscopy in the diagnosis and monitoring of interstitial
lung disease associated with rheumatic autoimmune diseases.
Diagnostics (Basel). 15(362)2025.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cutolo M, Melsens K, Herrick AL, Foeldvari
I, Deschepper E, De Keyser F, Distler O, Ingegnoli F, Mostmans Y,
Müller-Ladner U, et al: Reliability of simple capillaroscopic
definitions in describing capillary morphology in rheumatic
diseases. Rheumatology (Oxford). 57:757–759. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dinsdale G, Roberts C, Moore T, Manning J,
Berks M, Allen J, Anderson ME, Cutolo M, Hesselstrand R, Howell K,
et al: Nailfold capillaroscopy-how many fingers should be examined
to detect abnormality? Rheumatology (Oxford). 58:284–288.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Komai M, Takeno D, Fujii C, Nakano J,
Ohsaki Y and Shirakawa H: Nailfold capillaroscopy: A comprehensive
review on its usefulness in both clinical diagnosis and improving
unhealthy dietary lifestyles. Nutrients. 16(1914)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Smith V, Vanhaecke A, Herrick AL, Distler
O, Guerra MG, Denton CP, Deschepper E, Foeldvari I, Gutierrez M,
Hachulla E, et al: Fast track algorithm : how to differentiate a
‘scleroderma pattern’ from a ‘non-scleroderma pattern.’. Autoimmun
Rev. 18(102394)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kabasakal Y, Elvins DM, Ring EF and McHugh
NJ: Quantitative nailfold capillaroscopy findings in a population
with connective tissue disease and in normal healthy controls. Ann
Rheum Dis. 55:507–512. 1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Smith V, Herrick AL, Ingegnoli F, Damjanov
N, De Angelis R, Denton CP, Distler O, Espejo K, Foeldvari I, Frech
T, et al: Standardisation of nailfold capillaroscopy for the
assessment of patients with Raynaud's phenomenon and systemic
sclerosis,’ Autoimmun. Rev. 19(102458)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sandre MK and Rohekar S: Psoriatic
arthritis and nail changes: Exploring the relationship. Semin
Arthritis Rheum. 44:162–169. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Maejima H, Taniguchi T, Watarai A and
Katsuoka K: Evaluation of nail disease in psoriatic arthritis by
using a modified nail psoriasis severity score index. Int J
Dermatol. 49:901–906. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Love TJ, Gudjonsson JE, Valdimarsson H and
Gudbjornsson B: Psoriatic arthritis and onycholysis-results from
the cross-sectional Reykjavik psoriatic arthritis study. J
Rheumatol. 39:1441–1444. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
McGonagle D, Benjamin M and Tan AL: The
pathogenesis of psoriatic arthritis and associated nail disease:
Not autoimmune after all? Curr Opin Rheumatol. 21:340–347.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
McGonagle D: Enthesitis: An
autoinflammatory lesion linking nail and joint involvement in
psoriatic disease. J Eur Acad Dermatol Venereol. 23 (Suppl
1):S9–S13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Urwin S, Griffiths B and Allen J: The use
of geometric and algorithmic complexity to quantify and classify
capillaroscopy images displaying a scleroderma pattern and
controls. In: Proceedings of the British Microcirculation Society,
Newcastle-upon-Tyne, 2016.
|
|
27
|
Berks M, Dinsdale G, Murray A, Moore T,
Manning J, Taylor C and Herrick AL: Automated structure and flow
measurement-a promising tool in nailfold capillaroscopy. Microvasc
Res. 118:173–177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ohta R and Sano C: Challenges in
diagnosing psoriatic arthritis in primary care: A meta-ethnographic
study. Cureus. 15(e49443)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rida MA and Chandran V: Challenges in the
clinical diagnosis of psoriatic arthritis. Clin Immunol.
214(108390)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kavanaugh A, Helliwell P and Ritchlin CT:
Psoriatic arthritis and burden of disease: Patient perspectives
from the population-based multinational assessment of psoriasis and
psoriatic arthritis (MAPP) survey. Rheumatol Ther. 3:91–102.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Higgins JPT and Green SE: Guide to the
contents of a Cochrane protocol and review. In: Cochrane Handbook
for Systematic Reviews of Interventions. Higgins JPT and Green S
(eds). 1st edition. John Wiley & Sons, pp51-79, 2008.
|
|
32
|
Ali AM, Hamza SM, Aboud FM and El-Shahat
NM: Nailfold capillaroscopic changes in Egyptian patients with
psoriatic arthritis in comparison to rheumatoid arthritis. Egypt
Rheumatol. 41:303–307. 2019.
|
|
33
|
Guldberg-Møller J, Henriksen M, Ellegaard
K, Haedersdal M, Lazar LT, Kristensen LE and Mogensen M: Novel
application of optical coherence tomography and capillaroscopy in
psoriatic arthritis in relationship to psoriasis and hand
osteoarthritis. Rheumatol Adv Pract. 5(rkab065)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Anghel D, Sîrbu CA, Petrache OG,
Opriș-Belinski D, Negru MM, Bojincă VC, Pleșa CF and Ioniță Radu F:
Nailfold videocapillaroscopy in patients with rheumatoid arthritis
and psoriatic arthropathy on ANTI-TNF-ALPHA therapy. Diagnostics
(Basel). 13(2079)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Graceffa D, Amorosi B, Maiani E, Bonifati
C, Chimenti MS, Perricone R and Di Carlo A: Capillaroscopy in
psoriatic and rheumatoid arthritis: A useful tool for differential
diagnosis. Arthritis. 2013(957480)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rajaei A and Dehghan P: Microvascular
changes in patients with psoriatic arthritis as shown in nail fold
capillaroscopy. Int J Sci. 26:269–277. 2016.
|
|
37
|
Molteni E, Pellegrino G, Castellani C,
Reza Beigi DM, Conti F, Scrivo R and Riccieri V: Ab1355 Nailfold
capillaroscopy changes in patients with psoriatic arthritis and
rheumatoid arthritis. Ann Rheum Dis. 81 (Suppl 1):S1783–S1784.
2022.
|
|
38
|
Lambova SN and Müller-Ladner U:
Capillaroscopic pattern in inflammatory arthritis. Microvasc Res.
83:318–322. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zaric D, Clemmensen OJ, Worm AM and Stahl
D: Capillary microscopy of the nail fold in patients with psoriasis
and psoriatic arthritis. Dermatologica. 164:10–14. 1982.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bardehle F, Sies K, Enk A, Rosenberger A,
Fink C and Haenssle H: Nailfold videocapillaroscopy identifies
microvascular pathologies in psoriasis vulgaris: Results of a
prospective controlled study. J Dtsch Dermatol Ges. 19:1736–1744.
2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Florea M, Roşu A, Vreju FA, Muşetescu AE,
Criveanu C and Ciurea P: Contribution of nail fold
videocapillaroscopy in patients with early inflammatory arthritis.
Curr Health Sci J. 41:233–238. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Elmesiry AM, Mahmoud SA, Mohamed MS and
Hamoud HS: Nailfold capillaroscopy in psoriatic domains according
to Group for Research and Assessment of Psoriasis and Psoriatic
Arthritis (GRAPPA). Scientific J Al-Azhar Med Fac Girls.
5(667)2021.
|
|
43
|
Fukasawa T, Toyama S, Enomoto A,
Yoshizaki-Ogawa A, Norimatsu Y, Tateishi S, Kanda H, Miyagawa K,
Sato S and Yoshizaki A: Utility of nailfold capillary assessment
for predicting psoriatic arthritis based on a prospective
observational cohort study. Rheumatology (Oxford). 62:2418–2425.
2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bhushan M, Moore T, Herrick AL and
Griffiths CE: Nailfold video capillaroscopy in psoriasis. Br J
Dermatol. 142:1171–1176. 2000.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sivasankari M, Arora S, Vasdev V and Mary
EM: Nailfold capillaroscopy in psoriasis. Med J Armed Forces India.
77:75–81. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ribeiro CF, Siqueira EB, Holler AP,
Fabrício L and Skare TL: Periungual capillaroscopy in psoriasis. An
Bras Dermatol. 87:550–553. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Pal V, Relhan V and Sahoo B: A comparative
observational study of nailfold capillaroscopy in psoriasis
patients and healthy controls using a USB videodermatoscope.
Australas J Dermatol. 64:e400–e402. 2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kamboj P, Vij V, Prashantha GB, Sinha P,
Raj Choudhary S and Janardnan NM: A study of nailfold
capillaroscopy in different morphological variants of psoriasis in
a tertiary care hospital. J Mar Med Soc. 26:19–24. 2024.
|
|
49
|
Verhoeven F, Prati C, Demougeot C and
Wendling D: Cardiovascular risk in psoriatic arthritis, a narrative
review. Joint Bone Spine. 87:413–418. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lim MWS, Setjiadi D, Dobbin SJH, Lang NN,
Delles C and Connelly PJ: Nailfold video-capillaroscopy in the
study of cardiovascular disease: A systematic review. Blood Press
Monit. 28:24–32. 2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cantatore FP, Maruotti N, Corrado A and
Ribatti D: Angiogenesis dysregulation in psoriatic arthritis:
Molecular mechanisms. Biomed Res Int. 2017(5312813)2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Micali G, Lacarrubba F, Musumeci ML,
Massimino D and Nasca MR: Cutaneous vascular patterns in psoriasis.
Int J Dermatol. 49:249–256. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Han W and Huang F: Advancements in
psoriasis research: Skin microcirculation and its prognostic
significance. J Biosci Med (Irvine). 11:1–11. 2023.
|
|
54
|
Veale DJ and Fearon U: What makes
psoriatic and rheumatoid arthritis so different? RMD, Open.
1(e000025)2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shishkin AN and Nikolaeva AA: Features of
microcirculation in psoriatic arthritis. Regional blood circulation
and microvascularization. 20:11–17. 2021.(In Russian).
|
|
56
|
Carvalho AL and Hedrich CM: The Molecular
pathophysiology of psoriatic arthritis-the complex interplay
between genetic predisposition, epigenetics factors, and the
microbiome. Front Mol Biosci. 8(662047)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Avouac J, Vallucci M, Smith V, Senet P,
Ruiz B, Sulli A, Pizzorni C, Frances C, Chiocchia G, Cutolo M and
Allanore Y: Correlations between angiogenic factors and
capillaroscopic patterns in systemic sclerosis. Arthritis Res Ther.
15(R55)2013.PubMed/NCBI View
Article : Google Scholar
|
|
58
|
Jiaravuthisan MM, Sasseville D, Vender RB,
Murphy F and Michael CY: Psoriasis of the nail: Anatomy, pathology,
clinical presentation, and a review of the literature on therapy. J
Am Acad Dermatol. 57:1–27. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zabotti A, Tinazzi I, Aydin SZ and
McGonagle D: From psoriasis to psoriatic arthritis: Insights from
imaging on the transition to psoriatic arthritis and implications
for arthritis prevention. Curr Rheumatol Rep. 22(24)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Anghel D, Petrache OP, Groșeanu MM, Negru
MM, Pleșa CF and Radu FI: The implication of videocapillaroscopy in
rheumatoid arthritis and psoriatic patients. Int Med. 19:55–61.
2022.
|
|
61
|
Lin KM, Cheng TT and Chen CJ: Clinical
applications of nailfold capillaroscopy in different rheumatic
diseases. J Intern Med. 20:238–247. 2009.
|
|
62
|
Cutolo M, Sulli A, Secchi ME, Olivieri M
and Pizzorni C: The contribution of capillaroscopy to the
differential diagnosis of connective autoimmune diseases. Best
Pract Res Clin Rheumatol. 21:1093–1108. 2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Pennington SR and FitzGerald O: Early
origins of psoriatic arthritis: Clinical, genetic and molecular
biomarkers of progression from psoriasis to psoriatic arthritis.
Front Med (Lausanne). 8(723944)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Köhm M, Zerweck L, Ngyuen PH, Burkhardt H
and Behrens F: Innovative imaging technique for visualization of
vascularization and established methods for detection of
musculoskeletal inflammation in psoriasis patients. Front Med
(Lausanne). 7(468)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Cafaro G, Bursi R, Valentini V, Hansel K,
Perricone C, Venerito V, Bistoni O, Sebastiano M, Topini F,
Stingeni L, et al: Combined semiquantitative nail-enthesis complex
ultrasonography and capillaroscopy in psoriasis and psoriatic
arthritis. Front Immunol. 15(1505322)2025.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kassani PH, Ehwerhemuepha L, Martin-King
C, Kassab R, Gibbs E, Morgan G and Pachman LM: Artificial
intelligence for nailfold capillaroscopy analyses-a proof of
concept application in juvenile dermatomyositis. Pediatr Res.
95:981–987. 2024.PubMed/NCBI View Article : Google Scholar
|