1. Introduction
Precision medicine embodies a paradigm shift in
healthcare delivery, centered on the development of personalized
treatment strategies that integrate individual genomic profiles,
environmental exposures and lifestyle factors. This paradigm shift
signifies an evolution from traditional ‘one-size-fits-all’
protocols to more patient-specific and precise medical
interventions. The exponential growth of ‘omics’ technologies,
particularly genomics and metabolomics, highlights the substantial
promise of precision medicine across disease prevention, diagnostic
frameworks and therapeutic intervention strategies (1).
Machine learning (ML) and algorithms facilitate
high-throughput analysis of biological and clinical datasets.
Within this framework, the rapid advancement of ML technologies
demonstrates strong capabilities for data processing in precision
medicine contexts. ML systems can efficiently handle complex
biological and medical data, thereby enabling clinicians to gain
comprehensive insights into individual patient characteristics and
develop more targeted treatment protocols. For instance, in
oncology, ML algorithms predict tumor progression risk and
therapeutic response by analyzing genomic profiles, supporting
personalized treatment planning (2). Furthermore, ML contributes to
accelerating drug discovery processes and optimizing clinical trial
design, thereby enhancing both operational efficiency and
therapeutic effectiveness in medical practice (3).
However, despite the significant potential of ML in
precision medicine, multiple challenges remain. Data security
remains a paramount concern, requiring robust safeguards to ensure
the confidentiality and integrity of medical data. Additionally,
ethical dilemmas are emerging as prominent concerns, particularly
regarding patient trust in ML-driven decision-making processes and
transparency in data usage (4).
Furthermore, clinical integration of ML remains impeded by diverse
technical barriers, underscoring the necessity to validate ML
efficacy and reliability (5). To
offer a clearer overview of real-world applications and constraints
of ML in precision medicine, the present review introduces a
comparative table. The table systematically compares three core
domains-genomics, medical imaging and personalized treatment-along
dimensions of data types, key ML methodologies, representative
applications and major challenges (Table I).
 | Table IML applications in addressing
precision medicine challenges. |
Table I
ML applications in addressing
precision medicine challenges.
| Field | Data type | Main ML methods | Main challenges |
|---|
| Genomics | Genomic sequences,
gene expression profiles and other multi-omics numbers | ML, deep
learning | The problem of
imbalance between high-dimensional data and samples makes it
difficult to integrate cross-omicsdata. Genetic data is highly
sensitive, and there are challenges in data sharing and privacy
protection |
| Medical imaging | Medical imaging and
pathological tissue sections, ultrasound images | Deep learning,
traditional image processing and radiomics analysis methods | The demand for
large-scale labeled data is high, and different devices and imaging
protocols bring challenges to model generalization. Challenges in
integrating regulatory approval and clinical processes |
| Precision
medicine | Electronic health
records (medical records), clinical phenotypes, lifestyle data,
multi-omics and drug response data | ML, deep learning,
reinforcement learning, network models | The fusion and
standardization of heterogeneous data are highly challenging. The
explainability of ML results is insufficient. Ethical compliance
(such as obtaining consent) and clinical adoption barriers |
In summary, while the integration of ML within
precision medicine offers transformative potential, it is
accompanied by multifaceted challenges. The current narrative
review was executed through a systematic literature review
framework. PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science
(https://clarivate.com/academia-government/scientific-and-academic-research/research-discovery-and-referencing/web-of-science/)
and IEEE Xplore (https://ieeexplore.ieee.org/Xplore/home.jsp) were
systematically queried for English-language articles published
between 2015 and 2025. Search terms encompassed ‘artificial
intelligence (AI)’, ‘ML’, ‘precision medicine’, ‘genomics’, ‘deep
learning’, ‘clinical integration’ and ‘data privacy.’ Article
selection prioritized relevance to ML applications in precision
medicine, with emphasis on studies focusing on algorithm
development, medical ethics and data interoperability. Editorials,
opinion pieces and redundant publications were excluded. The
present study aims to deliver a comprehensive review and critical
analysis of the current landscape, opportunities and barriers
associated with ML in precision medicine, thereby providing a
foundational resource for future research and clinical
implementation.
2. ML and precision medicine
Utilization of AI in genomic data
analysis. Genomic data mining
Genomic data mining constitutes a critical component
of contemporary medical research, particularly within the domain of
large-scale data analysis leveraging ML. Advancements in
high-throughput sequencing technology have led to an exponential
increase in genomic data volume, surpassing the analytical capacity
of traditional methods. ML technologies, notably ML, excel in
processing and interpreting these complex genomic datasets,
yielding critical insights. For instance, deep learning algorithms
identify genomic patterns and variations potentially associated
with specific diseases (6).
Additionally, ML integrates heterogeneous literature-derived data
through text mining approaches, enabling researchers to identify
candidate biomarkers and therapeutic targets (7). This capacity to process and analyze
data not only enhances research efficiency but also accelerates
precision medicine progress, expanding genomic applications.
Association of genetic variations with
diseases. Elucidating the relationship between genetic
diversity and disease susceptibility represents a cornerstone of
genomic research. The integration of ML has significantly enhanced
both the depth and efficiency of analytical capabilities in this
field. Leveraging ML algorithms, researchers can now analyze
large-scale genomic datasets to identify genetic variations
associated with specific diseases. For instance, ML enables the
detection of distinct gene mutations linked to tumor mutation
burden, which play pivotal roles in complex disease progression
(8). Furthermore, ML demonstrates
the capacity to integrate multi-omics data, including genomic,
transcriptomic and metabolomic information, thereby facilitating a
more comprehensive understanding of disease mechanisms (9). This multi-layered analytical framework
not only deepens insights into the genetic basis of diseases but
also provides actionable insights for early diagnosis and
personalized treatment strategies within clinical settings.
ML and precision diagnosis. ML exhibits
remarkable capabilities within precision diagnostics, particularly
for complex diseases such as cancer and genetic disorders. ML
enables researchers to rapidly and accurately assess disease risks
through comprehensive analysis of patients' genomic data. For
example, ML reliably identifies ovarian cancer by analyzing
epigenomic modifications in circulating free DNA, thereby
supporting the development of personalized treatment strategies for
individuals (10). Furthermore, ML
integration into image analysis enables innovative methodologies in
pathology, exemplified by automated analysis of histopathological
images, which significantly enhances diagnostic precision for
conditions such as gastric cancer (11). Collectively, ML not only augments
diagnostic efficiency but also facilitates improved prognostic
outcomes and therapeutic responses for patients, marking a
transformative era in medical diagnostics.
ML in personalized treatment. Models
for predicting drug response
The integration of AI in predicting patient
responses to pharmacological interventions is garnering increasing
attention. Leveraging ML methodologies, researchers can develop
sophisticated predictive models capable of analyzing patients'
genomic data, clinical features and historical medication
responses. These models play a pivotal role in identifying key
factors influencing drug efficacy, thereby enabling healthcare
providers to develop personalized treatment plans. For instance,
multiple studies have shown that multilayer cell line drug response
network models can reliably predict therapeutic responses in breast
cancer patients (12).
Additionally, predictive models for acute myeloid leukemia drug
responses demonstrate significant promise, offering critical
insights to inform clinical decision-making (13). By implementing these models,
clinicians can gain deeper insights into interpatient variability,
ultimately enhancing pharmacological treatment effectiveness.
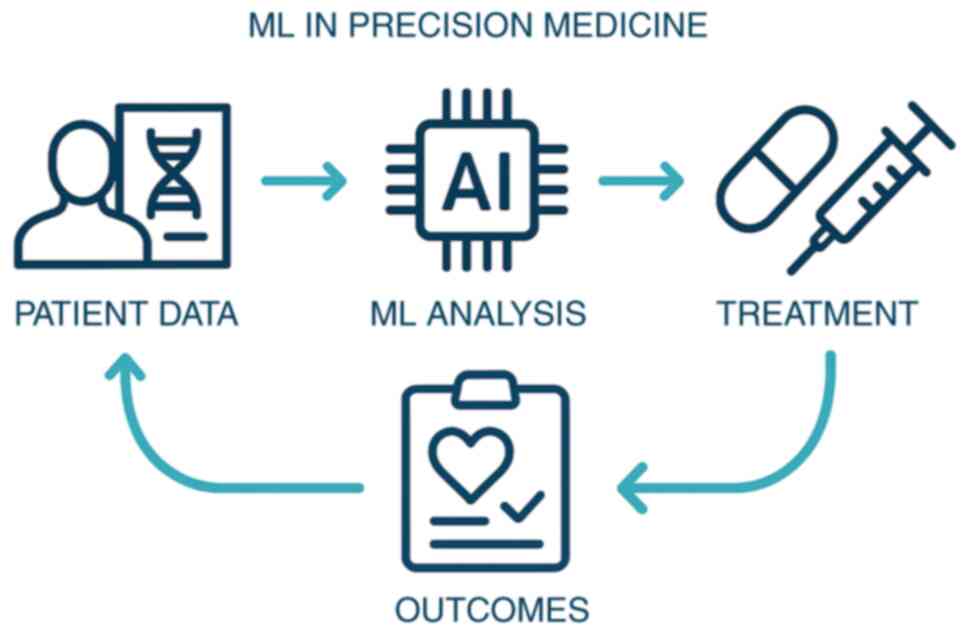
Within precision medicine, ML functions through four sequential
processes: Patient data acquisition, ML-driven analysis,
personalized treatment implementation and outcome feedback,
collectively forming a closed-loop system (Fig. 1).
Precision medicine for oncology. Personalized
cancer treatment, synonymous with precision medicine, represents a
paradigm-shifting strategy that tailors therapeutic interventions
to the unique characteristics of each patient. This approach
integrates multidimensional factors-including genetic
predisposition, environmental exposures and lifestyle
determinants-that collectively influence cancer development and
progression. By focusing on these individualized parameters,
healthcare providers can identify optimal treatment modalities for
specific patients (14). Through
analysis of tumor-specific biomarkers and genetic mutations,
clinicians can develop tailored therapeutic regimens that not only
enhance therapeutic efficacy but also mitigate the risk of adverse
effects (15). This patient-centric
paradigm not only improves clinical outcomes but also fosters
greater patient engagement and adherence to therapeutic regimens.
Recent advancements in genomic sequencing and bioinformatics have
significantly enhanced the feasibility of implementing personalized
treatment protocols. Consequently, oncologists are now better
equipped to make evidence-based decisions grounded in a
comprehensive understanding of each patient's tumor molecular
profile. Ultimately, precision medicine holds substantial promise
for improving survival rates and quality of life, marking a
transformative shift toward individualized and molecularly targeted
therapeutic options in oncology.
Optimizing treatment outcomes and dynamic
monitoring. ML plays a critical role in optimizing treatment
outcomes and enabling dynamic monitoring. By integrating real-time
data analytics with patient-reported feedback, ML demonstrates the
capacity to iteratively adjust therapeutic protocols in response to
changes in patient conditions. For instance, in diabetes
management, the integration of continuous glucose monitoring with
ML-driven algorithms allows clinicians to dynamically optimize
medication dosing, thereby improving patients' quality of life
(16-18).
Furthermore, in psychiatry, ML enhances treatment adherence and
efficacy through continuous oversight of pharmacotherapy regimens
(19). As ML technologies continue
to advance, an expanding array of ML-based tools is expected to
emerge, enabling healthcare providers to refine treatment
strategies and monitor patient health more effectively, ultimately
delivering more precise and personalized medical care.
Promise of ML in disease prediction
and early diagnosis. Application of advanced image recognition
technology in disease screening
The integration of advanced image recognition
technology within disease screening is advancing rapidly,
particularly in medical imaging analysis. Leveraging deep learning
and ML algorithms, ML demonstrates proficiency in extracting
complex features from medical images, thereby improving diagnostic
accuracy. For example, in ophthalmology, ML has been applied to
screen for diabetic retinopathy, with studies indicating that ML
systems achieve diagnostic precision comparable to experienced
ophthalmologists and, in certain cases, exceed the performance of
specialists (20). Furthermore, ML
exhibits significant potential in detecting hepatic pathologies,
accurately identifying early-stage conditions such as non-alcoholic
fatty liver disease through analysis of ultrasonography and
computed tomography scans (21).
This advanced image recognition technology not only enhances
screening efficiency but also diminishes reliance on manual
interpretation, thereby facilitating broader access to healthcare
services.
Collaboration of biomarkers and ML models.
The integration of biomarker identification with ML models has
paved the way for novel approaches in early disease diagnosis. By
leveraging multi-omics data-including genomics and proteomics-ML
demonstrates proficiency in detecting biomarkers linked to specific
disease states (22). For instance,
in early-stage cancer screening, ML can evaluate individual cancer
risk and develop tailored surveillance protocols through analysis
of multiple serum biomarkers. Additionally, ML identifies potential
biomarkers by analyzing large-scale datasets, providing critical
insights for novel drug development and disease prediction
(23). This synergy not only
improves the accuracy of biomarker discovery but also establishes a
robust foundation for advancing precision medicine.
Enhancing early intervention and prognostic
assessment capabilities. ML demonstrates significant potential
in enhancing early intervention and prognostic assessment
capabilities. Through comprehensive analysis of patient data, ML
can identify individuals at elevated risk and develop tailored
intervention strategies. For instance, in the clinical management
of cardiovascular diseases, ML predicts the probability of cardiac
events by analyzing patients' historical data and physiological
parameters, enabling timely interventions (24). Furthermore, ML supports prognostic
evaluations; by integrating clinical data with imaging results, it
provides personalized prognostic assessments, enabling healthcare
professionals to develop more effective treatment plans (25). This progress not only enhances
patient outcomes but also reduces medical resource waste, thereby
enhancing the overall efficiency of healthcare services.
Challenges of ML in the security and
privacy of medical data. Data encryption and access control
In the domain of medical data security, the
implementation of data encryption and access control is recognized
as a critical technical strategy. The rapid advancement of ML has
driven transformative changes in the storage and transmission of
medical data, thereby posing new challenges to traditional security
practices. Data encryption serves as a formidable barrier to
prevent unauthorized access and ensure patient confidentiality. For
instance, the application of homomorphic encryption enables
computations to be performed without necessitating data decryption,
thereby effectively safeguarding data privacy (26). Homomorphic encryption is a
cryptographic technique that enables data to be analyzed in its
encrypted form, facilitating secure analysis without exposing raw
patient information. Moreover, the Attribute-Based Access Control
model has gained significant traction in the administration of
medical big data sharing, enabling flexible governance of access
permissions based on user-specific attributes, thereby
strengthening data security and adaptability (27). However, despite the potential these
technologies offer for protecting medical information, challenges
such as implementation complexities must be mitigated. Thus,
healthcare organizations are encouraged to perform thorough
evaluations when choosing the most suitable technologies.
Regulations of data privacy and ethical
compliance. Adherence to data privacy regulations and ethical
compliance frameworks is paramount in the deployment of ML
technologies. With the enactment of legislation such as the General
Data Protection Regulation (GDPR), medical institutions are
mandated to ensure that their data processing practices align with
legal standards to protect patient privacy rights (28). Furthermore, ethical compliance
transcends mere legal obligations, encompassing the imperative to
respect and safeguard patient autonomy through informed consent.
When leveraging ML for patient data processing, healthcare
providers must guarantee transparent communication regarding data
utilization purposes and obtain explicit patient consent (29). However, practical implementation
reveals significant challenges for healthcare organizations,
largely attributable to the complexity and ambiguity inherent in
legal frameworks. A critical tension also persists between
advancing technological innovation and preserving patient
confidentiality (30).
Significance of patient informed consent.
Patient informed consent constitutes a cornerstone of ethical
practice in healthcare, particularly regarding ML applications.
Patients must be granted the autonomy to understand how their
personal health information will be utilized, including associated
risks and benefits (31). In the
context of ML implementation, informed consent transcends mere
legal compliance-it serves as a critical mechanism for fostering
patient trust and safeguarding rights (32). As ML systems increase in complexity,
patients may struggle to comprehend underlying algorithms and data
processing methodologies. Healthcare organizations are therefore
advised to implement transparent communication strategies that
effectively convey technical details in accessible language
(33). Ensuring informed patient
consent and adhering to data privacy regulations such as GDPR
remain essential. Dynamic consent platforms enable patients to
control data-sharing preferences; while addressing ML biases
requires vigilance-imbalanced training datasets may perpetuate
healthcare disparities. Homomorphic encryption and federated
learning represent promising solutions for enabling secure,
decentralized data analysis.
Limitations of ML applications. Algorithmic
bias has emerged as a critical ethical challenge in the application
of ML within healthcare. When ML models are trained on datasets
lacking demographic diversity or featuring imbalanced
representations, they may produce biased outputs, resulting in
disparate healthcare outcomes across diverse patient populations.
For instance, empirical studies indicate that predictive models
frequently exhibit suboptimal performance for racial minority
groups or patients with rare diseases, attributable to inadequate
representation in training datasets. Such disparities raise
significant ethical concerns, as they risk perpetuating existing
inequities in care access and diagnostic precision. To address this
challenge, developers are advised to implement fairness audits,
integrate diverse datasets, and employ bias-detection frameworks
throughout the model development and validation lifecycle.
Challenges in technology integration
and implementation. Data compatibility among diverse systems
In the modern healthcare ecosystem, ensuring data
compatibility across heterogeneous systems is critical for the
provision of high-quality medical care. Currently, the healthcare
sector faces a multiplicity of data formats and standards, creating
significant barriers to information exchange between institutions.
A persistent challenge in healthcare has been the standardization
of electronic health record systems, with the lack of interoperable
data standards impeding the exchange and utilization of critical
clinical information (34). To
address this challenge, researchers have proposed various
strategies, including the adoption of structural and semantic
interoperability frameworks to improve communication among
healthcare information systems (33). Furthermore, given the increasing
demand for cross-border medical data compatibility, the development
of health data warehouse systems has emerged as a pivotal focus of
research. This initiative not only enhances the efficiency of data
sharing but also facilitates cross-border collaboration in
healthcare initiatives (36).
Nevertheless, despite continuous technological advancements,
concerns regarding data privacy and security remain critical
barriers to implementation. The lack of adherence to
standardization protocols can give rise to data breaches and
potential misuse (37).
Consequently, advancing data compatibility across healthcare
systems requires collaborative efforts from policymakers,
technology developers and healthcare providers. Adopting
international standards such as Fast Healthcare Interoperability
Resources (FHIR) facilitates data interoperability by defining
standardized resource definitions and interfaces. FHIR structures
patient, diagnosis and medication information into standardized
resources, thereby enabling data exchange through JSON/XML formats
and RESTful APIs. This eliminates system barriers, allowing
applications across devices or platforms to access unified clinical
data.
Recognition of ML by the healthcare insurance
system. The integration of ML within the healthcare sector is
progressing at a rapid pace; however, the current healthcare
insurance system's recognition of these innovations poses a
significant barrier to their widespread adoption. Presently,
healthcare insurance frameworks often exhibit delays in adapting to
the evolving demands of emerging technologies, thereby hindering
the implementation of ML applications (38). For instance, many insurance
providers maintain skepticism regarding the clinical efficacy and
reliability of ML-driven diagnostic tools, creating significant
financial burdens for healthcare organizations seeking to deploy ML
solutions (39). To enable the
scalable adoption of ML technologies, it is imperative to redesign
current healthcare insurance frameworks and establish rigorous
evaluation systems capable of comprehensively assessing the
clinical value and cost-effectiveness of ML interventions (40). Furthermore, regulatory bodies should
facilitate collaboration between healthcare providers and
technology developers by launching pilot programs to generate
empirical evidence of ML's effectiveness, thereby providing a
foundation for necessary insurance system reforms (41). Only through enhancing the
adaptability of healthcare insurance frameworks can the full
potential of ML in medical practice be realized.
Training of medical personnel and acceptance of
ML. The professional development of healthcare workers and
their readiness to adopt technological innovations are critical
factors influencing the success of technological integration. While
the adoption of emerging technologies holds potential to enhance
healthcare efficiency and quality, limited comprehension and trust
among clinical staff regarding these tools frequently lead to
implementation resistance (42).
Consequently, training programs for healthcare professionals must
extend beyond technical proficiency, emphasizing foundational
principles to build confidence and foster acceptance of novel
technologies (43). For instance,
some institutions have implemented simulation-based training and
virtual reality platforms to facilitate comprehensive understanding
of technological applications, demonstrating significant
improvements in staff technological readiness (44). Additionally, organizational
leadership should provide enhanced support mechanisms to encourage
healthcare professionals to contribute insights on technological
implementations, thereby promoting engagement and ownership
(45). Only through structured
training and open communication can the effective adoption of
innovative technologies in clinical practice be guaranteed.
3. Discussion
The integration of ML into precision medicine
presents transformative opportunities while simultaneously posing
substantial challenges. Across genomics, oncology and clinical
integration, ML-driven approaches have demonstrated the capacity to
accelerate biomarker discovery, enhance diagnostic accuracy, and
enable dynamic, individualized treatment strategies. For instance,
in genomics, deep learning models have improved the identification
of pathogenic variants and disease-associated patterns beyond the
capacity of traditional bioinformatics pipelines. Similarly, in
oncology, predictive modeling has facilitated patient-specific drug
response forecasting, thereby optimizing therapeutic regimens.
However, despite these advances, several systemic limitations
remain evident. The reliance on heterogeneous and often imbalanced
training datasets heightens the risk of algorithmic bias,
potentially exacerbating disparities in diagnostic precision and
therapeutic outcomes among underrepresented populations.
Furthermore, the limited interoperability of healthcare data
infrastructure, coupled with the reluctance of insurance frameworks
to reimburse ML-driven interventions, constrains large-scale
clinical adoption. Ethical concerns, including patient informed
consent and the transparency of decision-support algorithms, also
demand continuous oversight. Addressing these limitations requires
a multi-stakeholder framework involving clinicians, policymakers
and developers to standardize data protocols, mandate bias audits,
and ensure rigorous clinical validation. Such coordinated efforts
are essential for translating ML-enabled precision medicine from a
conceptual paradigm into a scalable, equitable component of
clinical care.
4. Conclusion
The integration of ML into precision medicine offers
transformative potential, optimizing genomic analysis workflows,
enabling personalized treatment planning, and enhancing disease
prediction accuracy. These technological advancements not only
improve therapeutic outcomes but also provide patients with more
tailored healthcare solutions. However, the rapid advancement of ML
necessitates addressing several critical challenges.
Primarily, challenges related to data privacy and
security have become increasingly prominent. The effectiveness of
ML systems relies heavily on large-scale patient datasets; thus,
protecting sensitive information from misuse or unauthorized access
has emerged as an urgent priority. Additionally, the complexities
of technology integration demand careful consideration, as
interoperability between heterogeneous ML systems and legacy
healthcare infrastructure remains a significant barrier to seamless
implementation. Achieving interoperability between diverse ML
platforms and their effective integration into legacy healthcare
systems is essential for realizing the full potential of precision
medicine. Furthermore, ethical dimensions require rigorous
scrutiny, particularly concerning patient informed consent and
transparency in algorithmic decision-making processes.
Looking ahead, conducting comprehensive assessments
of both the capabilities and risks associated with ML applications
in precision medicine is imperative. Balancing technological
innovation with ethical practices necessitates multistakeholder
collaboration among research institutions, healthcare providers and
regulatory authorities. Standardizing data formats, ensuring
algorithmic fairness, and engaging clinicians and patients in
co-design processes will maximize ML's clinical impact. Through
rigorous governance frameworks, ML can realize its potential to
deliver personalized, equitable and efficient care. To accelerate
clinical translation and equitable adoption, clinicians should
strengthen ML literacy through training programs, policymakers
should develop unified data standards and insurance reforms to
facilitate ML integration, and developers must ensure transparency,
fairness and multi-center validation of their models. Coordinated
implementation of these measures will be essential for transforming
ML from an emerging innovation into a sustainable, equitable and
indispensable component of precision medicine.
In summary, while the integration of ML within
precision medicine holds significant promise, addressing the
introduced multifaceted challenges remains imperative. A
multidimensional framework encompassing technological, ethical and
security dimensions is essential to ensure the sustainable
evolution of precision medicine, ultimately delivering equitable
benefits to diverse patient populations.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Talent Cultivation Project (grant no. RC20189) from
Tianjin Municipal Health Commission.
Availability of data and materials
Not applicable.
Authors' contributions
QZ, GL and KD conceptualized the study and wrote the
manuscript. All authors read and approved the final version of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hamamoto R, Suvarna K, Yamada M, Kobayashi
K, Shinkai N, Miyake M, Takahashi M, Jinnai S, Shimoyama R, Sakai
A, et al: Application of artificial intelligence technology in
oncology: Towards the establishment of precision medicine. Cancers
(Basel). 12(3532)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lin PC, Tsai YS, Yeh YM and Shen MR:
Cutting-edge AI technologies meet precision medicine to improve
cancer care. Biomolecules. 12(1133)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Naik K, Goyal RK, Foschini L, Choi WC,
Thielscher C, Zhu H, Lu J, Lehár J, Pacanoswki MA, Terranova N, et
al: Current status and future directions: The application of
artificial intelligence/machine learning for precision medicine.
Clin Pharmacol Ther. 115:673–686. 2024.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Tsopra R, Fernandez X, Luchinat C,
Alberghina L, Lehrach H, Vanoni M, Dreher F, Sezerman OU, Cuggia M,
de Tayrac M, et al: A framework for validating AI in precision
medicine: Considerations from the European ITFoC consortium. BMC
Med Inform Decis Mak. 21(274)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Singh AV, Chandrasekar V, Paudel N, Laux
P, Luch A, Gemmati D, Tisato V, Prabhu KS, Uddin S and Dakua SP:
Integrative toxicogenomics: Advancing precision medicine and
toxicology through artificial intelligence and OMICs technology.
Biomed Pharmacother. 163(114784)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu J and Zhao Y: Machine learning
technology in the application of genome analysis: A systematic
review. Gene. 705:149–156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bose S, Banerjee S, Kumar S, Saha A, Nandy
D and Hazra S: Review of applications of artificial intelligence
(AI) methods in crop research. J Appl Genet. 65:225–240.
2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun X, Xue A, Qi T, Chen D, Shi D, Wu Y,
Zheng Z, Zeng J and Yang J: Tumor mutational burden is polygenic
and genetically associated with complex traits and diseases. Cancer
Res. 81:1230–1239. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Y, Pang D, Wang Z, Ma L, Chen Y,
Yang L, Xiao W, Yuan H, Chang F and Ouyang H: An integrative
analysis of genotype-phenotype correlation in Charcot Marie Tooth
type 2A disease with MFN2 variants: A case and systematic review.
Gene. 883(147684)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bahado-Singh RO, Ibrahim A, Al-Wahab Z,
Aydas B, Radhakrishna U, Yilmaz A and Vishweswaraiah S: Precision
gynecologic oncology: Circulating cell free DNA epigenomic
analysis, artificial intelligence and the accurate detection of
ovarian cancer. Sci Rep. 12(18625)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Choi S and Kim S: Artificial intelligence
in the pathology of gastric cancer. J Gastric Cancer. 23:410–427.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huang S, Hu P and Lakowski TM: Predicting
breast cancer drug response using a multiple-layer cell line drug
response network model. BMC Cancer. 21(648)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Trac QT, Pawitan Y, Mou T, Erkers T,
Östling P, Bohlin A, Österroos A, Vesterlund M, Jafari R, Siavelis
I, et al: Prediction model for drug response of acute myeloid
leukemia patients. NPJ Precis Oncol. 7(32)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Avci CB, Bagca BG, Shademan B, Takanlou
LS, Takanlou MS and Nourazarian A: Machine learning in oncological
pharmacogenomics: Advancing personalized chemotherapy. Funct Integr
Genomics. 24(182)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chiffelle J and Harari A: Personalized
cancer T-cell therapy takes the stage, mirroring vaccine success. J
Exp Med. 221(e20240854)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Horgan R, Hage Diab Y, Fishel Bartal M,
Sibai BM and Saade G: Continuous glucose monitoring in pregnancy.
Obstet Gynecol. 143:195–203. 2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shi Y, Wang Y, Niu K, Zhang W, Lv Q and
Zhang Y: How CLSPN could demystify its prognostic value and
potential molecular mechanism for hepatocellular carcinoma: A
crosstalk study. Comput Biol Med. 172(108260)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu J, Shi Y, Lan S, Wang J, Jiang F, Tang
C, Cai Y, Pan Z, Jian H, Fang H, et al: Dissection of
pyroptosis-related prognostic signature and CASP6-mediated
regulation in pancreatic adenocarcinoma: New sights to clinical
decision-making. Apoptosis. 28:769–782. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Biso L, Aringhieri S, Carli M, Scarselli M
and Longoni B: Therapeutic drug monitoring in psychiatry: Enhancing
treatment precision and patient outcomes. Pharmaceuticals (Basel).
17(642)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cheung CY, Tang F, Ting DSW, Tan GSW and
Wong TY: Artificial intelligence in diabetic eye disease screening.
Asia Pac J Ophthalmol (Phila). 8:158–164. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li XH, Liu F and Rao HY: New advances of
artificial intelligence in the diagnosis of non-alcoholic fatty
liver disease. Zhonghua Gan Zang Bing Za Zhi. 30:443–446.
2022.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
22
|
Wang HY, Lin WY, Zhou C, Yang ZA, Kalpana
S and Lebowitz MS: Integrating artificial intelligence for
advancing multiple-cancer early detection via serum biomarkers: A
narrative review. Cancers (Basel). 16(862)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reitsam NG, Enke JS, Vu Trung K, Märkl B
and Kather JN: Artificial intelligence in colorectal cancer: From
patient screening over tailoring treatment decisions to
identification of novel biomarkers. Digestion. 105:331–344.
2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huang JD, Wang J, Ramsey E, Leavey G,
Chico TJA and Condell J: Applying artificial intelligence to
wearable sensor data to diagnose and predict cardiovascular
disease: A review. Sensors (Basel). 22(8002)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tan YY, Kang HG, Lee CJ, Kim SS, Park S,
Thakur S, Da Soh Z, Cho Y, Peng Q, Lee K, et al: Prognostic
potentials of AI in ophthalmology: Systemic disease forecasting via
retinal imaging. Eye Vis (Lond). 11(17)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo L, Gao W, Cao Y and Lai X: Research on
medical data security sharing scheme based on homomorphic
encryption. Math Biosci Eng. 20:2261–2279. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gupta R, Kanungo P, Dagdee N, Madhu G,
Sahoo KS, Jhanjhi NZ, Masud M, Almalki NS and AlZain MA: Secured
and privacy-preserving multi-authority access control system for
cloud-based healthcare data sharing. Sensors (Basel).
23(2617)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bertolaccini L, Falcoz PE, Brunelli A,
Batirel H, Furak J, Passani S and Szanto Z: The significance of
general data protection regulation in the compliant data
contribution to the European society of thoracic surgeons database.
Eur J Cardiothorac Surg. 64(ezad289)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Miller DC and Smith CC: Spine Intervention
Society's Patient Safety Committee. Informed consent. Pain Med:
pnaa112, 2020 (Epub ahead of print).
|
|
30
|
Zhang J and Zhang ZM: Ethics and
governance of trustworthy medical artificial intelligence. BMC Med
Inform Decis Mak. 23(7)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Khatiwada P, Yang B, Lin JC and Blobel B:
Patient-generated health data (PGHD): Understanding, requirements,
challenges, and existing techniques for data security and privacy.
J Pers Med. 14(282)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Antwi WK, Akudjedu TN and Botwe BO:
Artificial intelligence in medical imaging practice in Africa: A
qualitative content analysis study of radiographers' perspectives.
Insights Imaging. 12(80)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sheth S, Baker HP, Prescher H and Strelzow
JA: Ethical considerations of artificial intelligence in health
care: Examining the role of generative pretrained transformer-4. J
Am Acad Orthop Surg. 32:205–210. 2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Walker DM, Tarver WL, Jonnalagadda P,
Ranbom L, Ford EW and Rahurkar S: Perspectives on challenges and
opportunities for interoperability: Findings from key informant
interviews with stakeholders in ohio. JMIR Med Inform.
11(e43848)2023.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Pournik O, Ahmad B, Lim Choi Keung SN,
Peake A, Rafid S, Tong C, Laleci Erturkmen GB, Gencturk M, Akpinar
AE and Arvanitis TN: Interoperable E-health system using structural
and semantic interoperability approaches in CAREPATH. Stud Health
Technol Inform. 305:608–611. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gavrilov G, Vlahu-Gjorgievska E and
Trajkovik V: Healthcare data warehouse system supporting
cross-border interoperability. Health Informatics J. 26:1321–1332.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ishimoto K, Arafune T, Washio T, Haishima
Y, Matsumoto K, Uematsu M, Nomura Y, Yokoi H, Sato H, Murakami M,
et al: Japanese regulatory considerations for interoperability of
medical devices. Ther Innov Regul Sci. 57:104–108. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nitiéma P: Artificial intelligence in
medicine: Text mining of health care workers' opinions. J Med
Internet Res. 25(e41138)2023.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Baric-Parker J and Anderson EE: Patient
data-sharing for AI: Ethical challenges, catholic solutions.
Linacre Q. 87:471–481. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Müller L, Kloeckner R, Mildenberger P and
Pinto Dos Santos D: Validation and implementation of artificial
intelligence in radiology: Quo vadis in 2022? Radiologie (Heidelb).
63:381–386. 2023.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
41
|
Shen TT, Liu CF and Wu MP: Implementation
of a machine learning model in acute coronary syndrome and stroke
risk assessment for patients with lower urinary tract symptoms.
Taiwan J Obstet Gynecol. 63:518–526. 2024.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Barchielli C, Marullo C, Bonciani M and
Vainieri M: Nurses and the acceptance of innovations in
technology-intensive contexts: The need for tailored management
strategies. BMC Health Serv Res. 21(639)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Le Duff M, Michinov E, Bracq MS, Mukae N,
Eto M, Descamps J, Hashizume M and Jannin P: Virtual reality
environments to train soft skills in medical and nursing education:
A technical feasibility study between France and Japan. Int J
Comput Assist Radiol Surg. 18:1355–1362. 2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Abril-Jiménez P, Merino-Barbancho B,
Lombroni I, Villanueva-Mascato S, Mallo I, Vera-Muñoz C, Arredondo
MT and Fico G: Design of a training model for remote management of
patients hospitalized at home. J Med Biol Eng. 40:610–617.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lafferty L, Smith K, Causer L, Andrewartha
K, Whiley D, Badman SG, Donovan B, Anderson L, Tangey A, Mak D, et
al: Scaling up sexually transmissible infections point-of-care
testing in remote aboriginal and torres strait islander
communities: Healthcare workers' perceptions of the barriers and
facilitators. Implement Sci Commun. 2(127)2021.PubMed/NCBI View Article : Google Scholar
|