Introduction
Rituximab (RTX), a monoclonal antibody targeting the
B-cell-specific CD20 antigen, is an effective treatment agent for
B-cell non-Hodgkin's lymphoma (NHL) (1,2).
However, its application for the treatment of central nervous
system (CNS) lymphoma is still controversial since the blood-brain
barrier (BBB) is relatively impermeable to antibodies with high
molecular weights (3,4). Conversely, RTX could exhibit
therapeutic effects on CNS lymphoma when the permeability of the
blood-tumor barrier increases.
The primary therapeutic option for CNS lymphoma is
intravenous high-dose methotrexate (MTX)-based chemotherapy
(5–7). Previously, RTX and temozolomide
showed significant therapeutic effects against relapsed primary CNS
lymphoma (8,9). However, there are no indisputable
data showing successful combination of MTX with RTX. We
hypothesized that MTX normalizes the increased permeability of the
blood-tumor barrier and thus reduces the accessibility of RTX to
CNS lymphoma (10). In the present
study, we administered RTX to a CNS lymphoma animal model prior to
MTX treatment to avoid the normalizing effects of MTX on the
blood-tumor barrier, and our novel treatment modality conferred
synergistic therapeutic effects against CNS lymphoma.
Materials and methods
Cell culture and reagents
Raji human Burkitt lymphoma cells (American Type
Culture Collection, Manassas, VA) were grown in RPMI-1640 (Gibco
BRL, Grand Island, NY) supplemented with 10% FBS, 2 mM L-glutamine,
penicillin (100 U/ml), and streptomycin (100 μg/ml). MTX and
RTX (Mabthera) were purchased from Mayne Pharmaceuticals and Roche
Pharmaceuticals, respectively.
Primary CNS lymphoma animal model
Anesthetized 6-week-old male Balb/c-nu mice were
secured in a rodent stereotactic frame, a hollow guide screw was
implanted into a small drill hole made 2 mm right and 1 mm anterior
to the bregma, and 5×104, 5×105 and
5×106 Raji cells in 5 μl HBSS were injected
through this guide screw into the white matter at a depth of 3 mm
[anterior/posterior (AP) +1.0 mm, medial/lateral (ML) −1.7 mm,
dorsal/ventral (DV) −3.2 mm]. All of the experiments where mice
received antitumor therapy were performed using an inoculum of
5×105 Raji cells. Animal experiments were approved by
the appropriate Institutional Review Boards of the Samsung Medical
Center, Seoul, Korea and conducted in accordance with the ‘National
Institute of Health Guide for the Care and Use of Laboratory
Animals’ (NIH publication no. 80-23, revised in 1996).
Evaluation of antitumor activity
Twelve days after the intracerebral injections of
Raji cells, mice were randomly assigned to receive sham treatment
(Group A), MTX administration followed by RTX treatment (Group B),
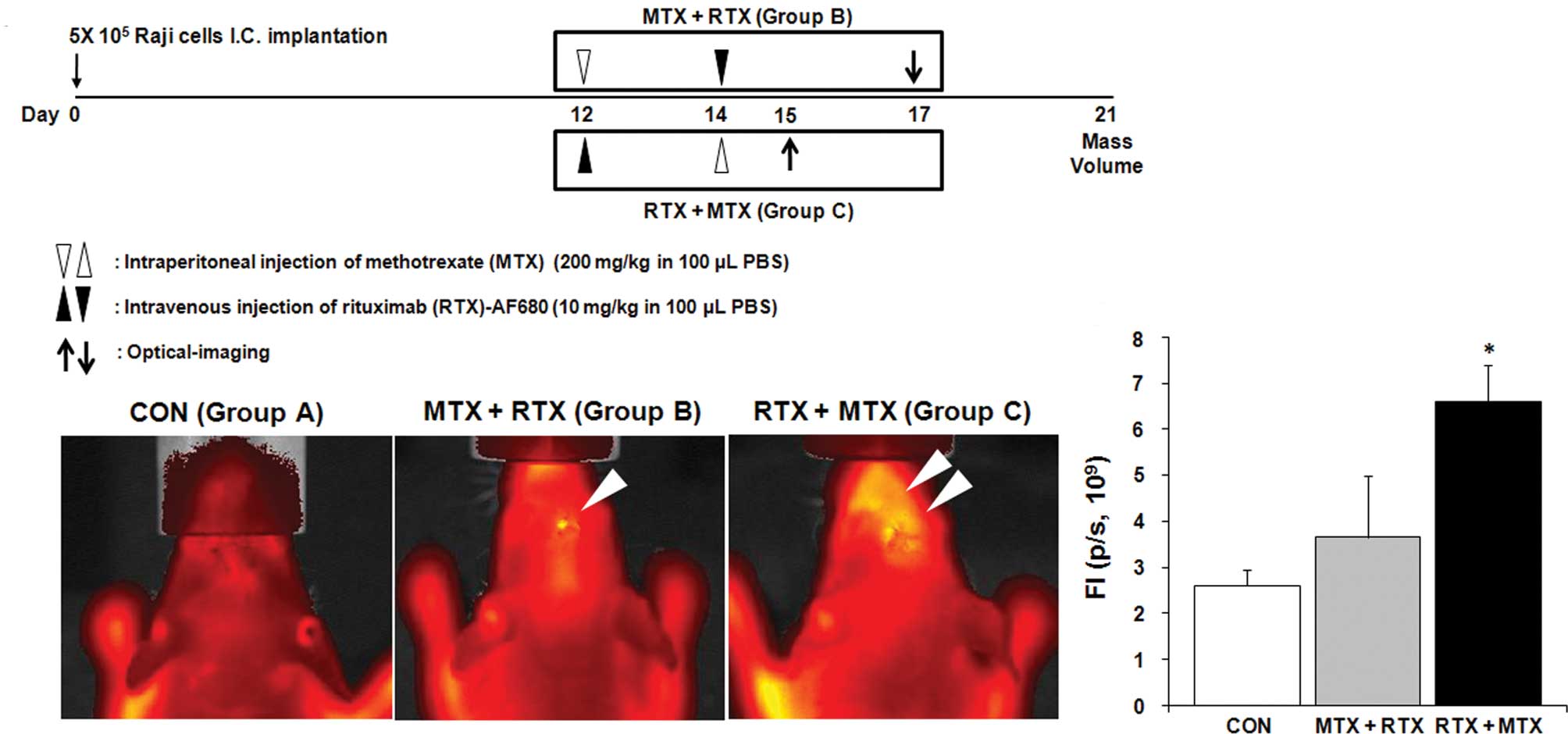
or RTX administration followed by MTX treatment (Group C) (Fig. 3A). Twenty-one days after the
injection of Raji cells, the mice were sacrificed. Brains were
harvested and processed for paraffin embedding. The tumor volume
was calculated by measuring the section with the largest tumor
portion applying the formula: (width)2 × length ×
0.5.
In vivo quantification of the penetration
of RTX into the CNS lymphoma
To evaluate the penetration of RTX into the mouse
brains bearing CNS lymphoma, RTX conjugated with Alexa Fluor 680
(RTX-AF680) was utilized. RTX was conjugated with Alexa Fluor 680
according to the manufacturer's protocol (SAIVI Alexa Fluor 680
antibody/protein 1 mg labeling kit; Invitrogen, CA, USA). Three
days after i.v. injection of RTX-AF680 (Fig. 3A), mice were anesthetized with 2–3%
isoflurane. The signal from RTX-AF680 was detected in the region of
the brain using a prototype Xenogen IVIS® Spectrum in
vivo imaging system (Caliper Life Science). Fluorescence
intensity was analyzed as photons per second (p/s) by Living Image
3.1 software (Caliper Life Science).
Statistical analyses
Statistical comparisons of the RTX penetration and
tumor regression between groups were analyzed by one-way analysis
of variance (ANOVA) followed by the least significant difference
(LSD) test. All data were presented as means ± SEM. A values of
P<0.05 was considered statistically significant.
Results
CNS lymphoma animal model
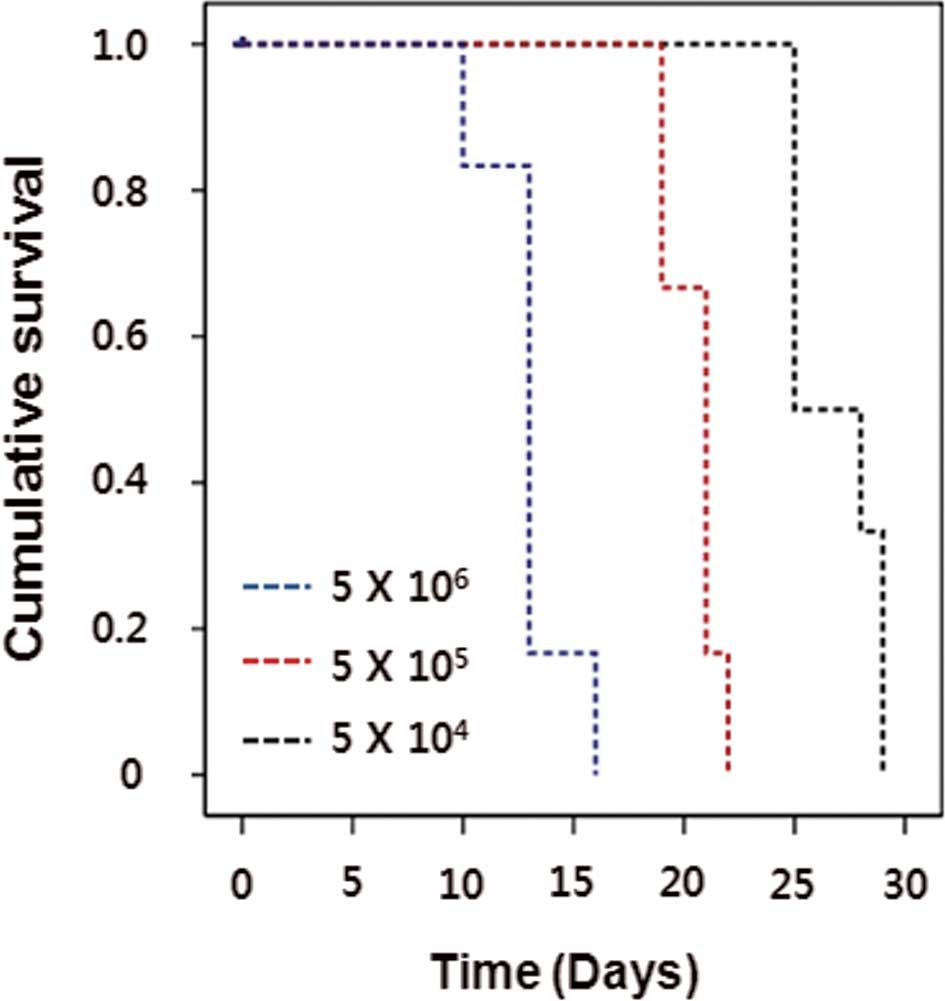
To establish the CNS lymphoma animal model, Raji
human Burkitt lymphoma cells were stereotactically injected into
the brains of immune compromised Balb/c-nu mice with a range of
cell doses (0.5, 5 and 50×105 cells/mouse), and mice
were observed for the development of irreversible neurological
symptoms before euthanization. Nude mouse survival times were
dependent on the cell dose (Fig.
1). The optimal cell dose of Raji cells was determined by the
length of survival. A cell dose of 5×105 resulted in
100% mortality within 21 days (19–23 days) after implantation and
was used in all of the subsequent in vivo experiments. Huge
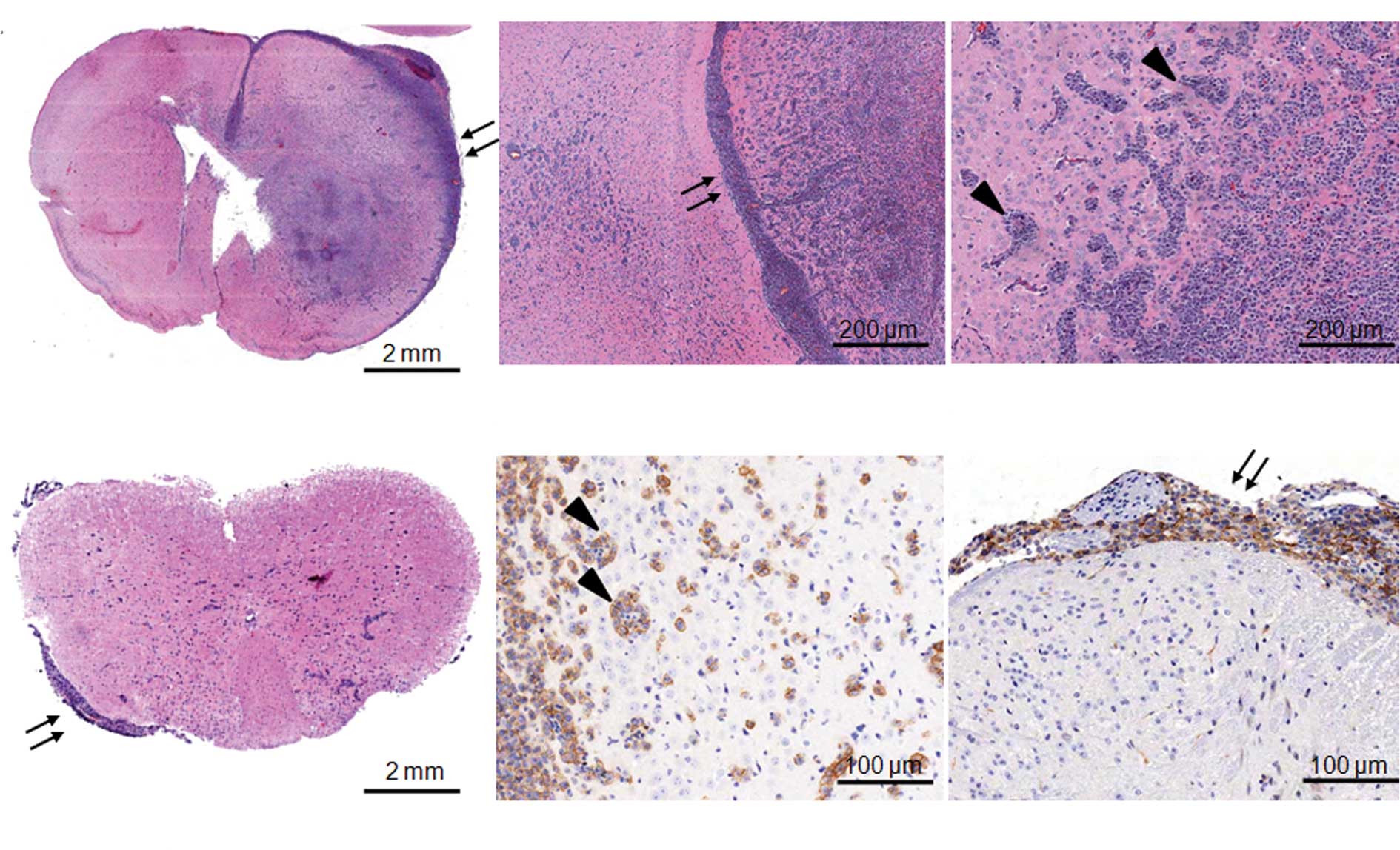
infiltrative intracerebral tumors were observed in the brain three
weeks after the tumor cell injection (Fig. 2A). Pathologic characteristics of
CNS lymphoma such as extensive leptomeningeal seeding (arrows in
Fig. 2A) and perivascular cuffing
at the periphery of tumors (arrow heads in Fig. 2A) were obvious. In addition to
cerebral involvement, lymphoma cells were clearly observed in the
leptomeninges of the spinal cord but not in the parenchyma (arrows
in Fig. 2B and D, thoracic spinal
cord). Most lymphoma cells highly expressed CD20, a B-cell marker
(Fig. 2C for perivascular cuffing
in the brain, Fig. 2D for
leptomeningeal seeding in the spinal cord).
Effects of MTX treatment on the RTX
penetration across the blood-tumor barrier
To evaluate our hypothesis, we administrated MTX and
RTX to the mice bearing lymphoma cells in their brains according to
different schedules (Fig. 3A).
Administered RTX was labeled with AF680 to visualize the
distribution in the brain in vivo. Each mouse was examined 3
days after RTX treatment (Group B; Day 17, Group C; Day 15), while
mice from Group A were examined twice at Day 15 and 17 (Fig. 3A). We did not observe fluorescent
signals from the brains of the mice not administered RTX (Group A,
Fig. 3B). When mice received MTX
treatment followed by RTX (Group B), RTX was not accumulated in the
brain (Group B, Fig. 3B). In
contrast, penetration of RTX across the BBB or the blood-tumor
barrier was significantly increased by changing the treatment order
from MTX + RTX to RTX + MTX (Group C, Fig. 3B and C).
Therapeutic effects of RTX and MTX
combination treatments on CNS lymphoma
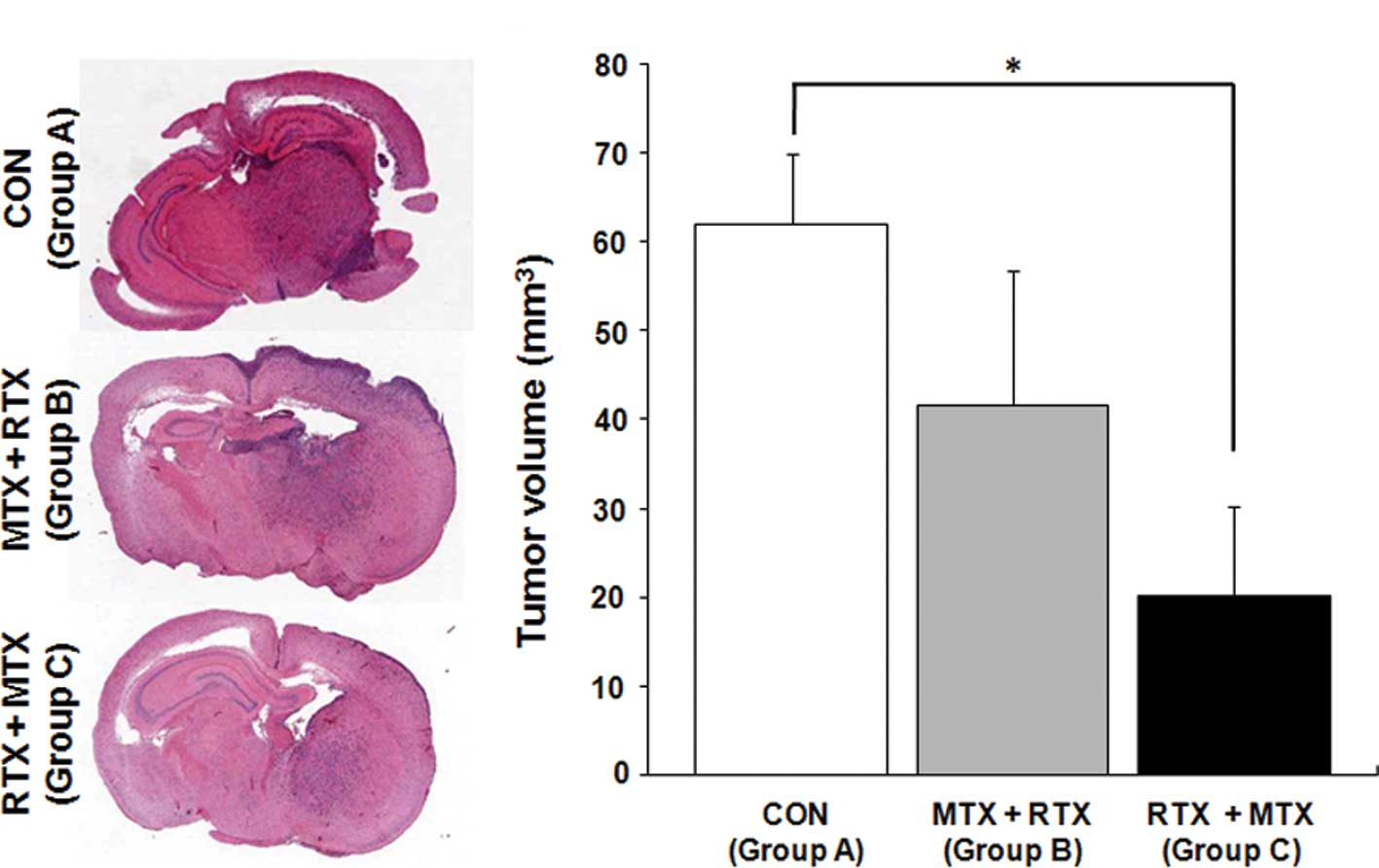
Next, we analyzed whether RTX penetration is
associated with therapeutic effects by measuring tumor volumes at
Day 21 (Fig. 3A). The RTX and MTX
combination treatment, in which MTX administration was followed by
RTX injection (Group B), reduced tumor volume by 32%, but this was
not statistically significant (Fig. 4A
and B; Group A, 62±8.0 mm3; Group B, 41.7±15.1
mm3). In contrast, mice that received RTX treatment
followed by MTX administration showed a significantly reduced tumor
volume (Fig. 4A and B; Group C,
20.1±10.2 mm3, 68% tumor volume reduction;
P<0.05).
Discussion
The investigation of CNS lymphoma treatment,
biology, pathophysiology, and pathology in humans is troublesome,
mainly due to the rarity of the disorder. Our data demonstrated
that the intracerebral implantation of Raji cells into
immune-compromised mouse brain provides an animal model that
closely mimics the morphological and molecular characteristics of
human CNS lymphoma (11). Here, we
demonstrated that RTX can be successfully combined with MTX using
an alternative treatment schedule that allows increased
distribution of RTX in CNS lymphoma.
The BBB is relatively impermeable to high molecular
weight antibodies (3,4). However, the blood-tumor barrier of
brain tumors is more permissive to the penetration of antibodies
into tumors, which would enable the application of therapeutic
antibodies to brain tumors (12,13).
We hypothesized that prior MTX treatment reverses the permissive
characteristics of the tumor-blood barrier as there are massive
tumor cell apoptosis and normalization of brain tissue structure
caused by MTX. By in vivo imaging of RTX distribution, we
provided data showing that the treatment schedule significantly
alters the accessibility of therapeutic antibodies to brain
tumors.
Due to the different mechanisms of action of MTX and
RTX, a synergistic effect with improved response rate could be
postulated by the combination. However, previous studies have
failed to demonstrate the superiority of MTX and RTX combination
treatment over MTX or RTX monotherapy (10). In the present study, we
demonstrated that MTX influences the penetration of RTX across the
blood-tumor barrier, which consequently abolishes the expected
synergistic effects. In addition, we developed a novel combination
method in which RTX treatment was followed by MTX administration.
By applying the altered combination therapy, both accumulation of
RTX in the brains bearing CNS lymphoma and strengthening of
therapeutic effects of MTX chemotherapy on CNS lymphoma were
achieved.
We report the successful combination of MTX and RTX
for the treatment of CNS lymphoma in the translational setting.
Clinical relevance of this strategy needs to be further elucidated
through clinical trials.
Acknowledgements
This study was supported by a grant of
the Korea Healthcare Technology R&D Project, Ministry for
Health and Welfare Affairs, Republic of Korea (A092255) (D.-H. Nam)
and the Ministry of Health and Welfare of Korea
(0405-MNO1-0604-0007) (S.W. Seo).
References
|
1.
|
McLaughlin P, Grillo-Lopez AJ, Link BK, et
al: Rituximab chimeric anti-CD20 monoclonal antibody therapy for
relapsed indolent lymphoma: half of patients respond to a four-dose
treatment program. J Clin Oncol. 16:2825–2833. 1998.
|
|
2.
|
Maloney DG, Grillo-Lopez AJ, Bodkin DJ, et
al: IDEC-C2B8: results of a phase I multiple-dose trial in patients
with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 15:3266–3274.
1997.PubMed/NCBI
|
|
3.
|
Neuwelt EA, Barnett PA, McCormick CI,
Remsen LG, Kroll RA and Sexton G: Differential permeability of a
human brain tumor xenograft in the nude rat: impact of tumor size
and method of administration on optimizing delivery of biologically
diverse agents. Clin Cancer Res. 4:1549–1555. 1998.
|
|
4.
|
Neuwelt EA: Mechanisms of disease: the
blood-brain barrier. Neurosurgery. 54:131–142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Herrlinger U, Kuker W, Uhl M, et al:
NOA-03 trial of high-dose methotrexate in primary central nervous
system lymphoma: final report. Ann Neurol. 57:843–847. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Abrey LE, Yahalom J and DeAngelis LM:
Treatment for primary CNS lymphoma: the next step. J Clin Oncol.
18:3144–3150. 2000.PubMed/NCBI
|
|
7.
|
DeAngelis LM, Seiferheld W, Schold SC,
Fisher B and Schultz CJ: Combination chemotherapy and radiotherapy
for primary central nervous system lymphoma: Radiation Therapy
Oncology Group Study 93-10. J Clin Oncol. 20:4643–4648. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Enting RH, Demopoulos A, DeAngelis LM and
Abrey LE: Salvage therapy for primary CNS lymphoma with a
combination of rituximab and temozolomide. Neurology. 63:901–903.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wong ET, Tishler R, Barron L and Wu JK:
Immunochemotherapy with rituximab and temozolomide for central
nervous system lymphomas. Cancer. 101:139–145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Jahnke K, Muldoon LL, Varallyay CG, et al:
Efficacy and MRI of rituximab and methotrexate treatment in a nude
rat model of CNS lymphoma. Neuro Oncol. 11:503–513. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wang W, Kardosh A, Su YS, Schonthal AH and
Chen TC: Efficacy of celecoxib in the treatment of CNS lymphomas:
an in vivo model. Neurosurg Focus. 21:E142006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Barnett PA, Roman-Goldstein S, Ramsey F,
et al: Differential permeability and quantitative MR imaging of a
human lung carcinoma brain xenograft in the nude rat. Am J Pathol.
146:436–449. 1995.PubMed/NCBI
|
|
13.
|
Neuwelt EA, Barnett PA, Hellstrom KE,
Hellstrom I, McCormick CI and Ramsey FL: Effect of blood-brain
barrier disruption on intact and fragmented monoclonal antibody
localization in intracerebral lung carcinoma xenografts. J Nucl
Med. 35:1831–1841. 1994.
|