Introduction
Gastric cancer (GC) is one of the most common and
lethal malignancies in Japanese and East Asian populations, and the
second most common cause of cancer-related death in the world
(1,2). Although the incidence and mortality
rate of GC located outside the cardia have been decreasing over the
last few decades, a considerable percentage of patients still have
advanced disease at diagnosis, and some of these patients are not
candidates for curative surgery. Each GC tumor often has a
different biological behavior, which leads them to have differing
clinical phenotypes, prognosis and response to treatment. These
differences may be partly explained by certain host genetic
differences.
MicroRNAs (miRNAs) are 21- to 24-nucleotide-long
small noncoding RNA gene products that regulate gene expression by
base pairing with target mRNAs at the 3′-untranslated region,
leading to mRNA cleavage or translational repression (3–5). It
has been suggested that miRNAs are involved in various biological
processes, including cell proliferation, cell death, stress
resistance and fat metabolism (6).
Several reports have shown that miRNAs participate in human
tumorigenesis as tumor suppressors or oncogenes (7–9). For
example, miR-143 and miR-145, targeting ERK4, are down-regulated in
GC (10), whereas miRNA-106b,
miR-93 and miR-25, clustering at MCM7 intron, were reported to be
overexpressed in GC (11).
Single-nucleotide polymorphisms (SNPs), or mutations
in the miRNA sequence, may alter miRNA expression and/ or
maturation. Recently, Hu et al performed a screening for
common SNPs in miRNA sequences and identified four SNPs (rs2910164,
rs2292832, rs11614913 and rs3746444) located at the pre-miRNA
regions of miR-146a, miR-149, miR-196a2 and miR-499, respectively
(12). Among the above four SNPs,
the rs11614913 SNP in miR-196a2 was found to be associated with
shortened survival time of non-small cell lung cancer patients
through the altered expression of mature miR-196a and the binding
activity of target mRNA (12).
Consequently, the rs11614913 SNP in miR-196a2 contributed to lung
cancer susceptibility (13). In
carcinomas in other organs, the rs2910164 SNP within miR-146a was
associated with papillary thyroid (14) and hepatocellular carcinomas
(15). Moreover, the rs11614913
SNP in miR-196a2 and the rs3746444 SNP in miR-499 were both
associated with the risk of breast cancer (16). In the case of stomach cancer, a
preliminary association was initially reported for rs11614913 in
miRNA-146a in a Chinese population (17).
Since SNPs in miRNAs are closely associated with GC
susceptibility, it is necessary to clarify whether these SNPs are
also associated with the clinical characteristics of GC.
Accordingly, this study was designed to evaluate the possible
association between three common SNPs (rs11614913, rs2910164 and
rs3746444) in pre-miRNAs (miR-196a2, miR-146a and miR-499) that had
previously been shown to contribute to human cancer susceptibility
(13–17), and various clinicopathological
characteristics of GC.
We also investigated the association between these
SNPs and the CpG island hypermethylation (CIHM) status in tumor
suppressor genes, which constitutes a distinct tumor subtype in GC
(18,19). Furthermore, we investigated the
association between these SNPs and overall survival in GC
patients.
Materials and methods
Patients, tissue samples, DNA extraction
and Helicobactor pylori infection status
The studied population comprised 127 patients with
GC being treated at the Endoscopy Center of Fujita Health
University Hospital. The GCs were histologically diagnosed and
classified according to Lauren's classification (20). Detailed information was obtained
concerning lymph node, peritoneal, liver and distant metastases.
Data on venous and lymphatic invasion were also obtained in 98
resected cases. Based on this information, early-stage GC was
defined as GC localized within the mucosa or submucosa,
irrespective of lymph node metastasis (21), and all other cases were defined as
advanced-stage GC. According to its morphological appearance in an
endoscopic image or surgically resected specimen, early GC was
divided into two groups: polypoid or elevated type, and depressed
type. Advanced GC was also classified according to Borrmann's
classification. H. pylori infection status was assessed by
serologic or histological analysis, or by the urea breath test.
Patients were diagnosed as infected when at least one of the
diagnostic tests was positive. All patients underwent an upper
endoscopy with a biopsy from both the cancer lesion and the
non-pathological mucosa, and the biopsy specimens were immediately
frozen and stored at −80°C. Genomic DNA was isolated from the
frozen specimens using proteinase K. Patients with severe systemic
diseases were not included in the study. The experimental protocol
was approved by the Ethics Committee of the Fujita Health
University School of Medicine, and written informed consent was
obtained from all participating subjects.
Genotyping
Using genomic DNA extracted from non-pathological
mucosa, the rs11614913 (C>T), rs2910164 (G>C) and rs3746444
(A>G) SNPs were determined by polymerase chain reaction
(PCR)-based restriction fragment length polymorphism (RFLP) assays,
as described by Hu et al (12). Genotypes were determined by
evaluation of the gel images by two independent investigators, who
were blinded to the names and phenotype of the patients.
Bisulfite modification and
methylation-specific PCR (MSP)
To detect CIHM, four candidate promoter CpG islands
were selected, whose CIHM has been reported in GC (22–27):
p14, p16, DAP-kinase and CDH1. For the examination of DNA
methylation, genomic DNA from the cancer lesion was treated with
sodium bisulfite using the BislFast DNA Modification kit for
methylated DNA detection (Toyobo Co., Ltd., Osaka, Japan). CIHM
status of four candidate promoter CpG islands was examined by MSP,
as described previously (28). The
primer pairs and experimental conditions for MSP were the same as
those in our recent studies (18,19).
CIHM was defined as the presence of a positive methylation band,
separated by electrophoresis in 2.5% agarose gels under UV
illumination using ethidium bromide staining, showing signals
approximately equivalent to or greater than that of the size marker
(10 ng/μl: 100 bp DNA Ladder; Takara Bio Inc., Shiga, Japan),
irrespective of the presence of unmethylated bands. Samples giving
faint positive signals were analyzed a further two times, and only
those samples with a consistent positive methylation band were
considered to be CIHM. In addition, we measured the fluorescence
intensities of methylated bands in 50 randomly selected CHIM
samples using a digital densitometer (Lane Analyzer; ATTO, Tokyo,
Japan) and confirmed that the fluorescence intensities of all 50
methylated bands were approximately equivalent to or greater than
the size marker (data not shown).
Statistical analysis
Genotype frequencies were calculated by direct
counting. Differences in genotype frequencies among different
clinicopathological subtypes were determined by the χ2
test. The odds ratios (ORs) and 95% confidence intervals (CI) were
calculated by logistic regression analysis, with adjustment for age
and gender. Survival among the different genotypes was assessed
using the Kaplan-Meier method and compared using the log-rank test.
A probability value of <0.05 was considered statistically
significant in all analyses.
Results
Characteristics of the subjects and
association between SNPs in miRNAs and GC clinicopathological
characteristics
The characteristics of 127 GC patients are shown in
Table I, and the association
between SNPs in miRNAs and clinicopathological characteristics of
GC are shown in Table II. The
rs11614913 (T>C), rs2910164 (C>G) and rs3746444 (A>G) SNPs
were successfully genotyped in all subjects.
 | Table I.Clinicopathologic characteristics of
the GC patients. |
Table I.
Clinicopathologic characteristics of
the GC patients.
| Variable (n) | |
|---|
| Mean age ± SD
(years) | 65.2±12.1 |
| Gender
(Male:Female) | 90:37 |
| Lauren's histologic
subtype | |
| Intestinal
type | 73 |
| Diffuse type | 54 |
| H. pylori
infection status | |
| H. pylori
(+) | 102 |
| H. pylori
(−) | 25 |
| Stage | |
| Early cancer | 57 |
| Advanced
cancer | 70 |
| Morphology (early
cancer) | |
| Polypoid or
elevated type | 23 |
| Depressed
type | 34 |
| Morphology
(advanced cancer) | |
| Borrmann type
I | 4 |
| Borrmann type
II | 24 |
| Borrmann type
III | 34 |
| Borrmann type
IV | 8 |
 | Table II.Associations between rs11614913,
rs2910164 and rs3746444 SNPs and clinicopathological subtypes of
GC. |
Table II.
Associations between rs11614913,
rs2910164 and rs3746444 SNPs and clinicopathological subtypes of
GC.
| Variables (n) | rs11614913 genotype
| rs2910164 genotype
| rs3746444 genotype
|
|---|
| TT | TC | CC | CC | CG | GG | AA | AG | GG |
|---|
| Overall GC
(127) | 36 | 63 | 28 | 64 | 49 | 14 | 82 | 33 | 12 |
| Lauren's
classification (127) | | | | | | | | | |
| Diffuse type
(54) | 16 | 27 | 11 | 25 | 25 | 4 | 37 | 12 | 5 |
| Intestinal type
(73) | 20 | 36 | 17 | 39 | 24 | 10 | 45 | 21 | 7 |
| Staging (127) | | | | | | | | | |
| Early (57) | 19 | 27 | 11 | 28 | 20 | 9 | 36 | 16 | 5 |
| Advanced
(70) | 17 | 36 | 17 | 36 | 29 | 5 | 46 | 17 | 7 |
| Lymphatic invasion
(98) | | | | | | | | | |
| Negative
(37) | 9 | 18 | 10 | 17 | 13 | 7 | 22 | 11 | 4 |
| Positive
(61) | 19 | 32 | 10 | 31 | 25 | 5 | 39 | 17 | 5 |
| Venous invasion
(98) | | | | | | | | | |
| Negative
(65) | 20 | 32 | 13 | 31 | 24 | 10 | 42 | 17 | 6 |
| Positive
(33) | 8 | 18 | 7 | 17 | 14 | 2 | 19 | 11 | 3 |
| Lymph node
metastasis (127) | | | | | | | | | |
| Negative
(65) | 18 | 34 | 13 | 32 | 24 | 9 | 42 | 17 | 6 |
| Positive
(62) | 18 | 29 | 15 | 32 | 25 | 5 | 40 | 16 | 6 |
| Peritoneal
dissemination (127) | | | | | | | | | |
| Negative
(101) | 31 | 49 | 21 | 48 | 41 | 12 | 64 | 28 | 9 |
| Positive
(26) | 5 | 14 | 7 | 16 | 8 | 2 | 18 | 5 | 3 |
| Liver or distant
metastasis (127) | | | | | | | | | |
| Negative
(120) | 35 | 60 | 25 | 59 | 48 | 13 | 76 | 32 | 12 |
| Positive (7) | 1 | 3 | 3 | 5 | 1 | 1 | 6 | 1 | 0 |
| Morphology (early
GC) (57) | | | | | | | | | |
| Polypoid or
elevated type (23) | 7 | 9 | 7 | 12 | 8 | 3 | 14 | 6 | 3 |
| Depressed type
(34) | 12 | 18 | 4 | 16 | 12 | 6 | 22 | 10 | 2 |
| Morphology
(advanced GC) (70) | | | | | | | | | |
| Borrmann type I
(4) | 1 | 2 | 1 | 2 | 2 | 0 | 3 | 0 | 1 |
| Borrmann type II
(24) | 6 | 13 | 5 | 12 | 10 | 2 | 14 | 8 | 2 |
| Borrmann type III
(34) | 9 | 17 | 8 | 18 | 14 | 2 | 22 | 8 | 4 |
| Borrmann type IV
(8) | 1 | 4 | 3 | 4 | 3 | 1 | 7 | 1 | 0 |
In the comparison of genotype frequencies among
different clinicopathological subtypes, we found only a significant
marginal association between the rs11614913 CC genotype and
polypoid or elevated type morphology in the early-stage GC
(depressed type vs. polypoid or elevated type, rs11614913 CC vs.
TT+TC; age- and gender-adjusted OR=6.29, 95% CI 1.18–33.47,
p=0.03), while other subtypes, such as Lauren's classification,
staging, lymphatic and venous invasion, lymph node, peritoneal,
liver and distant metastasis, were not associated with any of the
three SNPs in the miRNAs.
Association between SNPs in miRNAs and GC
CIHM status
We assessed the association between the three SNPs
in the miRNAs and the CIHM status of GC, which has been reported to
be involved in the biological characteristics of GC (18,19).
The results are shown in Table
III. All 127 GC samples were available for MSP analysis. Among
the subjects, CIHM for p14 was found in 59 (46.4%) patients, CIHM
for p16 in 26 (20.5%), DIHM for CDH1 in 87 (68.5%) and CIHM for
DAP-kinase in 114 (89.8%) patients. The rs2910164 CC and CG
genotypes were associated with increased susceptibility to CIHM of
DAP-kinase (DAP-kinase unmethylated vs. hypermethylated; rs2910164
CC+CG vs. GG, age- and gender-adjusted OR=5.48, 95% CI 1.30–23.10,
p=0.02; rs2910164 CC vs. CG+GG, age- and gender-adjusted OR=6.93,
95% CI 1.37–35.02, p=0.02; rs2910164 CG vs. CC+GG, age- and
gender-adjusted OR=4.24, 95% CI 0.87–20.78, p=0.07). The 11614913
TT and TC genotypes were also found to be associated with a higher
number of CIHM (no. of CIHM 0–1 vs. 2–4; rs11614913 TT+TC vs. CC,
age- and gender-adjusted OR=3.67, 95% CI 0.98–13.72, p=0.05;
rs11614913 TC vs. TT+CC, age- and gender-adjusted OR=4.08, 95% CI
1.04–15.97, p=0.04).
 | Table III.Associations between rs11614913,
rs2910164 and rs3746444 SNPs and GC CIHM status. |
Table III.
Associations between rs11614913,
rs2910164 and rs3746444 SNPs and GC CIHM status.
| Variables (n) | rs11614913 genotype
| rs2910164 genotype
| rs3746444 genotype
|
|---|
| TT | TC | CC | CC | CG | GG | AA | AG | GG |
|---|
| CIHM status
(127) | | | | | | | | | |
| p14 | | | | | | | | | |
| Unmethylated
(68) | 19 | 35 | 14 | 37 | 22 | 9 | 47 | 17 | 4 |
| Hypermethylated
(59) | 17 | 28 | 14 | 27 | 27 | 5 | 35 | 16 | 8 |
| p16 | | | | | | | | | |
| Unmethylated
(101) | 30 | 48 | 23 | 50 | 39 | 12 | 64 | 27 | 10 |
| Hypermethylated
(26) | 6 | 15 | 5 | 14 | 10 | 2 | 18 | 6 | 2 |
| CDH1 | | | | | | | | | |
| Unmethylated
(40) | 11 | 21 | 8 | 22 | 16 | 2 | 27 | 9 | 4 |
| Hypermethylated
(87) | 25 | 42 | 20 | 42 | 33 | 12 | 55 | 24 | 8 |
| DAP-kinase | | | | | | | | | |
| Unmethylated
(13) | 3 | 7 | 1 | 4 | 5 | 4 | 8 | 4 | 1 |
| Hypermethylated
(114) | 33 | 54 | 27 | 60 | 44 | 10 | 74 | 29 | 11 |
| No. of CIHM | | | | | | | | | |
| 0–1 (30) | 9 | 18 | 3 | 16 | 12 | 2 | 20 | 7 | 3 |
| 2–4 (97) | 27 | 45 | 25 | 48 | 37 | 12 | 62 | 26 | 9 |
Association between survival curves
estimated by the Kaplan-Meier method and SNPs in the miRNAs
Of the 125 patients, including 21 unresectable and
104 resectable cases, overall survival, defined as the time from
the date of surgery for resectable cases and the date of initial
chemotherapy for unresectable cases, was characterized using the
Kaplan-Meier method and was compared using the log-rank test for
different genotypes of the three SNPs in miRNAs. The median
follow-up period of the 125 patients was 30 months. Of the 21
unresectable patients, TS-1-based chemotherapy was performed in 18
cases and chemotherapy of other regimens was performed in the
remaining three patients. No association was found between three
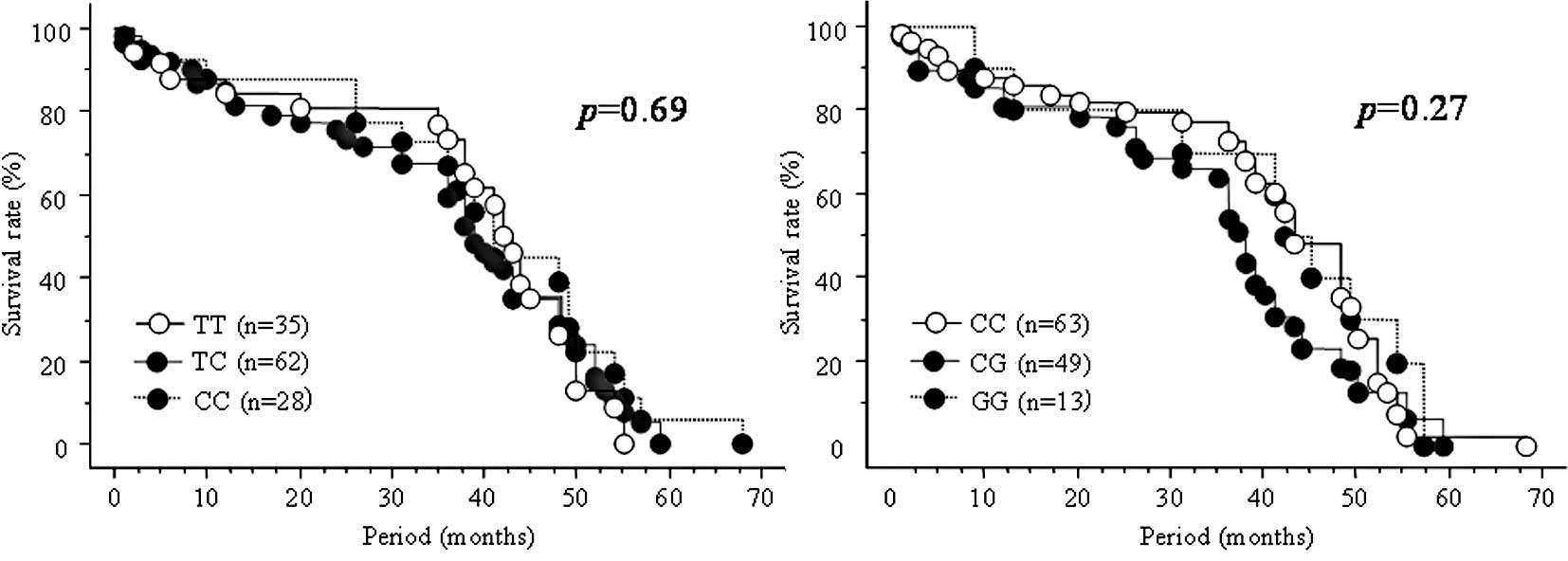
SNPs and overall survival in any of the 125 subjects (Fig. 1A–C).
When subjects were divided according to age group,
the combined rs11614913 TT+TC genotype tended to be associated with
worse overall survival than the CC genotype among patients younger
than 65 years of age (p=0.05, Fig.
1D).
Also, the combined rs2910164 CG+GG genotype tended
to be associated with worse overall survival than the CC genotype
in the same age group (p=0.09, Fig.
1E). On the other hand, no such association was found for the
rs3746444 genotype (data not shown).
Discussion
Although a number of studies have evaluated the
association between SNPs in protein-coding genes and susceptibility
to GC and its prognosis or response to treatment, association
studies regarding SNPs in miRNA genes are relatively rare. However,
it is increasingly recognized that SNPs in miRNAs may have
important phenotypic consequences in human diseases.
Here, we evaluated the effect of three selected SNPs
(rs11614913, rs2910164 and rs3746444) in pre-miRNAs (miR-196a2,
miR-146a and miR-499), which were shown to contribute to human
cancer susceptibility in recent association studies (12–17)
on various subtypes of GC, including CIHM status and overall
survival. In various clinicopathological subtypes, a significant
association between the rs11614913 CC genotype and polypoid or
elevated type morphology in early-stage GC was found. The
morphological appearance of both early and advanced GC has been
reported to be closely related to its histological subtypes,
prognosis and survival, and these differences have also been
characterized by several genetic and epigenetic alterations
(19). Although no association was
found between the rs11614913 CC genotype and other
clinicopathological subtypes, which provide more distinct
biological characteristics of GC such as lymphatic and venous
invasion or metastasis, our data provide initial evidence that the
rs11614913 CC genotype in miR-196a2 may contribute to the
characterization of certain phenotypes of GC. Polypoid or elevated
type morphology in early-stage GC usually shows well-differentiated
histopathology (29) and is
unlikely to present lymph node metastasis in small size, compared
to depressed type (30). In this
context, the rs11614913 CC genotype may be associated with a rather
mild phenotype of GC.
We also investigated whether the three SNPs
(rs11614913, rs2910164 and rs3746444) are associated with the CIHM
status of GC. We found that the rs2910164 CC and CG genotypes were
associated with increased susceptibility to CIHM of DAP-kinase. In
addition, the 11614913 TT and TC genotypes were associated with a
higher number of CIHM.
CIHM is now accepted as an important mechanism in
gene silencing, and CIHM of tumor suppressor genes is also highly
involved in gastric carcinogenesis; CIHM of p14, p16, CDH1 and
DAP-kinase genes assessed in this study frequently occur in GC
tissue, as well as in pre-malignant lesions (3–8).
Therefore, they are thought to be susceptible candidate genes for
CIHM in GC. Furthermore, it has been suggested that this epigenetic
change may also constitute a certain distinct biological behavior
of GC.
CIHM of DAP-kinase and a higher number of CIHM have
been associated with worse survival of GC patients (19). These epigenetic alterations have
also been correlated with the morphological appearance of GC
(18).
Moreover, a higher number of CIHM in neoplastic
gastric mucosa has been closely associated with the severity of
H. pylori-related gastritis (31) and GC occurrence (32), suggesting that rs2910164 and
11614913 SNPs may be an important influencing factor for
CIHM-related gastric carcinogenesis.
We also demonstrated that combined rs11614913 TT+TC
genotypes were weakly associated with worse overall survival than
the CC genotype in patients younger than 65 years of age. A similar
trend was also found for the combined rs2910164 CG+GG genotype for
patients in the same age group, suggesting that these genotypes may
be predictors of worse prognosis of GC, particularly for younger
patients. Due to its variable biological behavior, GC often
presents various clinical phenotypes, prognosis and response to
treatment. Therefore, in order to focus on disease heterogeneity,
it is necessary for the physician to more appropriately conduct a
clinical evaluation for each patient. Our preliminary results
showed that more longitudinal studies are required to investigate
the clinical usefulness of SNPs in miRNAs as a molecular biomarker
for the prediction of prognosis in GC patients.
In conclusion, we demonstrated that the rs11614913
and rs2910164 SNPs in pre-miRNAs (miR-196a2 and miR-146a) may have
an effect on the clinicopathological characteristics of GC,
including its morphological appearance, CIHM status and overall
survival. However, it should be noted that our associations were
found in a subgroup stratification analysis of a small sample;
thus, the statistical power was not sufficient. In addition, the
association of CIHM with subtypes of GC was found to vary in other
studies, according to the different CpG islands assessed, even in
the same genes (33,34). Moreover, our study is best viewed
as hypothesis-generating rather than hypothesis-testing, as both
the underlying in vitro and in vivo mechanisms of the
three miRNAs in carcinogenesis of the stomach are largely unknown.
Further characterization of miRNA SNPs, miRNAs, target mRNAs and
their compensational or redundancy role in GC are required to
confirm our results.
Abbreviations:
|
miRNA
|
microRNA
|
|
GC
|
gastric cancer
|
|
H. pylori
|
Helicobacter pylori
|
|
PCR
|
polymerase chain reaction
|
|
RFLP
|
restricted fragment length
polymorphism
|
|
CIHM
|
CpG island hypermethylation
|
|
MSP
|
methylation-specifc PCR
|
References
|
1.
|
Parkin DM, Whelan SL, Ferlay J, Raymond L
and Young J: Cancer Incidence in Five Continents. Volume VII. IARC
Press; Lyon: 1997
|
|
2.
|
Parkin DM, Pisani P and Ferlay J:
Estimates of the worldwide incidence of 25 major cancers in 1990.
Int J Cancer. 80:827–841. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lee RC and Ambros V: An extensive class of
small RNAs in Caenorhabditis elegans. Science. 294:862–864.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ambros V: MicroRNA pathways in flies and
worms: growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lu J, Getz G, Miska EA, et al: MicroRNA
expression profiles classify human cancers. Nature. 435:834–838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Esquela-Kerscher A and Slack FJ: Oncomirs
– microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269.
2006.
|
|
9.
|
Kumar MS, Lu J, Mercer KL, Golub TR and
Jacks T: Impaired microRNA processing enhances cellular
transformation and tumorigenesis. Nat Genet. 39:673–677. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Petrocca F, Visone R, Onelli MR, et al:
E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest
and apoptosis in gastric cancer. Cancer Cell. 13:272–286. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Takagi T, Iio A, Nakagawa Y, Naoe T,
Tanigawa N and Akao Y: Decreased expression of microRNA-143 and
-145 in human gastric cancers. Oncology. 77:12–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Hu Z, Chen J, Tian T, et al: Genetic
variants of miRNA sequences and non small cell lung cancer
survival. J Clin Invest. 118:2600–2608. 2008.PubMed/NCBI
|
|
13.
|
Tian T, Shu Y, Chen J, et al: A functional
genetic variant in microRNA-196a2 is associated with increased
susceptibility of lung cancer in Chinese. Cancer Epidemiol
Biomarkers Prev. 18:1183–1187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:7269–7274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Xu T, Zhu Y, Wei QK, et al: A functional
polymorphism in the miR-146a gene is associated with the risk for
hepatocellular carcinoma. Carcinogenesis. 29:2126–2131. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hu Z, Liang J, Wang Z, et al: Common
genetic variants in premicroRNAs were associated with increased
risk of breast cancer in Chinese women. Hum Mutat. 30:79–84. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Peng S, Kuang Z, Sheng C, et al:
Association of microRNA-196a-2 gene polymorphism with gastric
cancer risk in a Chinese population. Dig Dis Sci. 55:2288–2293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tahara T, Shibata T, Arisawa T, et al: CpG
island promoter methylation (CIHM) status of tumor suppressor genes
correlates with morphological appearances of gastric cancer.
Anticancer Res. 30:239–244. 2010.
|
|
19.
|
Tahara T, Shibata T, Nakamura M, et al:
Association between cyclin D1 polymorphism with CpG island promoter
methylation status of tumor suppressor genes in gastric cancer. Dig
Dis Sci. April 16–2010.(E-pub ahead of print).
|
|
20.
|
Lauren P: The two histological main types
of gastric carcinoma: diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
21.
|
Gotoda T, Yanagisawa A, Sasako M, et al:
Incidence of lymph node metastasis from early gastric cancer:
estimation with a large number of cases at two large centers.
Gastric Cancer. 3:219–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Toyota M, Ahuja N, Suzuki H, et al:
Aberrant methylation in gastric cancer associated with the CpG
island methylator phenotype. Cancer Res. 59:5438–5442.
1999.PubMed/NCBI
|
|
23.
|
Kang GH, Shim YH, Jung HY, Kim WH, Ro JY
and Rhyu MG: CpG island methylation in premalignant stages of
gastric carcinoma. Cancer Res. 61:2847–2851. 2001.PubMed/NCBI
|
|
24.
|
Chan AO, Lam SK, Wong BC, et al: Promoter
methylation of E-cadherin gene in gastric mucosa associated with
Helicobacter pylori infection and in gastric cancer. Gut.
52:502–506. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Raveh T and Kimchi A: DAP kinase – a
proapoptotic gene that functions as a tumor suppressor. Exp Cell
Res. 264:185–192. 2001.
|
|
26.
|
Schildhaus HU, Krockel I, Lippert H,
Malfertheiner P, Roessner A and Schneider-Stock R: Promoter
hypermethylation of p16INK4a, E-cadherin, O6-MGMT, DAPK and FHIT in
adenocarcinomas of the esophagus, esophagogastric junction and
proximal stomach. Int J Oncol. 26:1493–1500. 2005.PubMed/NCBI
|
|
27.
|
Waki T, Tamura G, Sato M, Terashima M,
Nishizuka S and Motoyama T: Promoter methylation status of
DAP-kinase and RUNX3 genes in neoplastic and non-neoplastic gastric
epithelia. Cancer Sci. 94:360–364. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kang GH, Lee HJ, Hwang KS, Lee S, Kim JH
and Kim JS: Aberrant CpG island hypermethylation of chronic
gastritis, in relation to aging, gender, intestinal metaplasia, and
chronic inflammation. Am J Pathol. 163:1551–1556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Song SY, Kim S, Kim DS, Son HJ, Rhee JC
and Kim YI: Abnormal expression of E-cadherin in early gastric
carcinoma: its relationship with macroscopic growth patterns and
catenin alpha and beta. J Clin Gastroenterol. 38:252–259. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Namieno T, Koito K, Higashi T, Takahashi
M, Yamashita K and Kondo Y: Assessing the suitability of gastric
carcinoma for limited resection: endoscopic prediction of lymph
node metastases. World J Surg. 22:859–864. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Tahara T, Arisawa T, Shibata T, et al:
Increased number of methylated CpG islands correlates with
Helicobacter pylori infection, histological and serological
severity of chronic gastritis. Eur J Gastroenterol Hepatol.
21:613–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Tahara T, Shibata T, Nakamura M, et al:
Increased number of CpG island hypermethylation in tumor suppressor
genes of nonneoplastic gastric mucosa correlates with higher risk
of gastric cancer. Digestion. 82:27–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Zhang KL, Sun Y, Li Y, et al: Increased
frequency of CpG island methylator phenotype and CDH1 methylation
in a gastric cancer high-risk region of China. Transl Oncol.
1:28–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Luo D, Zhang B, Lv L, et al: Methylation
of CpG islands of p16 associated with progression of primary
gastric carcinomas. Lab Invest. 86:591–598. 2006.PubMed/NCBI
|