Introduction
Breast cancer remains a serious health issue in the
US. It is estimated that more than one-fourth of all cancer
patients in 2009 were breast cancer patients, and breast cancer is
the second leading cause of cancer-related mortality. According to
clinical cancer statistics, approximately 182,500 new cases are
diagnosed each year, with 40,500 patients succumbing to breast
cancer in 2009 (1).
Epidemiological studies suggest that there are many risk factors
associated with breast cancer including age, relative body weight,
the number and timing of reproductive events and lactation,
exogenous and endogenous hormone concentrations, exposure to
radiation, alcohol consumption and family history of breast cancer
(2,3). More risk factors are still being
discovered.
Zeranol (Z), which is marketed as Ralgro®
(Merck-Schering-Plough Corp., Kenilworth, NJ, USA), is synthesized
from the mycotoxin zearalenone and is a nonsteroidal agent with
potent estrogenic activities. It has been widely used as a growth
promoter in the US beef feedlot industry to accelerate weight gain,
improve feed conversion efficiency and increase the lean
meat-to-fat ratio (4,5). Previous investigations revealed that,
although Z has adverse effects on the human breast, the use of Z as
a growth promoter was safe for humans (6). However, the safety of using Z as a
growth promoter has been debated ever since it was approved by the
US Food and Drug Administration (FDA). Due to these health
concerns, the European Union has banned imports of beef products
from animals that have been administered any of the six
growth-promoting hormones from the US. Subsequently, the US
government challenged the ban with the World Trade Organization in
early 1996. Recently, it has received increasing attention due to
speculations concerning the possible etiological role of Z in
breast cancer development. Zearalenone and Z are able to bind to
the active site of human estrogen receptor (ER) α and ER β in a
similar manner as 17 β-estradiol (E2) (7). Epidemiological investigation found
that the sperm quality in sons of 'high beef consumers' was lower
than that in males whose mothers ate less beef during their
pregnancy (8).
It has been reported that a higher red meat intake
in adolescence increases the risk of premenopausal breast cancer
(9). Our laboratory previously
reported that Z was able to transform human normal breast
epithelial cells and increase human breast cell growth in a
dose-dependent manner (10). Z has
the ability to down-regulate estrogen-regulated human breast cancer
candidate suppressor gene, protein tyrosine phosphatase γ
expression (11). More recently,
we found that the growth of pre-adipocytes derived from heifer ears
implanted for two months with 72 mg Z was approximately 12-fold
faster than that from the control heifers, and the response of the
former cells to Z treatment was more sensitive compared to the
latter. Following the investigation, the expression of cyclin D was
found to be up-regulated and p53 down-regulated in the former cells
(12). Our preliminary data showed
that 2.5% of Z-containing serum (ZS) harvested from heifers 60 days
post Z implantation (72 mg) was capable of transforming human
normal breast epithelial cell line MCF-10A to neoplastic breast
cancer cells after a 21-day culture. Additionally, we showed that
leptin, which plays a role in breast cancer development in obesity,
induces human breast cancer epithelial cell sensitivity to Z
(13). Therefore, it is crucial to
clarify whether the consumption of beef products with residues of
biologically active Z or its metabolites has any relationship with
breast cancer.
The present study investigated the effects of
biological samples directly harvested from heifers implanted with Z
on the proliferation of the human breast cancer cell line MCF-7 as
well as the underlying mechanisms. Our experimental results, for
the first time, revealed that sera harvested from the heifers after
one month of Z implantation significantly stimulated MCF-7 cell
growth compared to sera harvested from the same heifers before Z
implantation and the control heifers. The stimulatory effect on
MCF-7 cells appears to be through the up-regulation of cyclin D1
and down-regulation of p53 and p21 expression at the mRNA and
protein levels in MCF-7 cells. Further investigation in primary
cultured human normal and cancerous breast epithelial cells is
currently in progress in our laboratory. Our results suggest a
potential risk of consuming beef products with biologically active
Z or its metabolites in breast cancer initiation, promotion and
progression.
Materials and methods
Animal treatment and blood sampling
The experimental design and sample collection method
have been previously described (12). Briefly, 100 ml of blood was
harvested from the Z-implanted heifers at day 0 (ZS-D0, prior to Z
implantation), and day 30 post-Z implantation (ZS-D30) and from the
control heifers at day 0 (NZS-D0) and day 30 (NZS-D30). The sera
were immediately transferred to our laboratory and stored at 4°C
overnight. After the clot from the sides of the tube was carefully
loosened using a glass Pasteur, the serum was separated by
centrifugation of the blood in a 50-ml centrifuge tube at 4000 rpm
for 20 min at 4°C. The separated serum was sterilized through a
50-ml conical filter tube (Nalge Nunc International, New York, NY,
USA) and stored at −20°C. Part of the sterilized ZS-D30 and NZS-D30
was treated with dextran-coated charcoal (DCC, dextran T-70, Sigma;
charcoal, Sigma, USA) and stored at −20°C.
Cell culture
The MCF-7 cells were purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in
phenol red-free Dulbecco's modified Eagle's medium and Ham's F12
medium (1:1) (DMEM/F12) (1.05 mM CaCl2) containing 10%
fetal bovine serum (FBS) and an antibiotic-antimycotic (100 U/ml
penicillin G sodium, 100 μg/ml streptomycin sulfate and 0.25
μg/ml amphotericin B) (Invitrogen, Carlsbad, CA, USA) in a
75 cm2 culture flask in a humidified incubator (5%
CO2, 95% air, 37°C). When the cells reached 85–90%
confluence, they were subcultured in 75 cm2 culture
flasks at a ratio of 1:3 flasks as described above. The cells were
dissociated using 3 ml of 0.25% trypsin-5.3 mM EDTA (Invitrogen) in
PBS for 1 min at 37°C. The trypsinization was neutralized by the
addition of 10 ml of culture medium with 10% FBS. The dissociated
cells were collected and transferred to a 15-ml centrifuge tube and
centrifuged at 1200 rpm for 5 min. The supernatant was discarded,
and the cell pellets were resuspended in the culture medium with
10% FBS and subcultured in 75 cm2 culture flasks.
Cell proliferation assay
The MCF-7 cells were seeded into 96-well plates at a
density of 3,000 viable cells per well in 100 μl DMEM/F12
medium supplemented with 10% FBS and incubated at 37°C overnight.
The medium was then replaced with 100 μl DMEM/F12
supplemented with 0.2% bovine serum albumin (BSA, Sigma, USA), and
the plate was incubated at 37°C for a further 24 h. The cells were
then treated with 0.5, 2.5 and 12.5% ZS-D0 and ZS-D30 or NZS-D0 and
NZS-D30 in DMEM/F12 medium supplemented with 0.2% BSA for 24 h. The
cells treated with DMEM/F12 medium supplemented with 0.2% BSA were
the control groups. Treatment of ZS-D30 and NZS-D30 with or without
DCC pre-treatment at 0, 0.02, 0.1, 0.5 and 2.5% in cultured medium
was also carried out. Following 24 h of treatment, a cell
proliferation (MTS) assay was performed as described in the
manufacturer's protocol (Promega, Madison, WI, USA).
Cell treatment for RNA isolation and cDNA
synthesis
A total of 105 viable MCF-7 cells/well
were seeded in 6-well plates in 5 ml DMEM/F12 medium with 10% FBS
and cultured overnight. The medium was replaced with DMEM/F12
supplemented with 10% DCC-treated FBS, and the cells were cultured
overnight again. Then, MCF-7 cells were treated with 0, 0.2, 1 and
5% of ZS-D0, ZS-D30, NZS-D0 and NZS-D30 in culture medium for 24 h.
After treatment, total RNA was isolated from each well using 1 ml
TRIzol® reagent (Invitrogen) according to the
manufacturer's instructions. RNA concentration was measured using
an ND-1000 spectrophotometer (Thermo Fisher Scientific Inc., USA).
RNA (1 μg) from each treatment group was reverse transcribed
with 200 units M-MLV Reverse Transcriptase (Invitrogen) at 37°C for
50 min and then at 70°C for 15 min in the presence of 1 μl
10 mM dNTP (10 mM each dATP, dGTP, dCTP and dTTP at a neutral pH)
(Invitrogen), 1 μl 50 μM random hexamer (Amersham
Bioscience, Buckinghamshire, UK), 10 μl 5X First Strand
buffer, 5 μl 0.1 M DTT and 1 μl RNase inhibitor
(Invitrogen) in a total volume of 50 μl in a gradient
master-cycle (Eppendorf®, USA).
Quantitative real-time PCR
Real-time PCR was conducted to measure cyclin D1,
p53 and p21 expression. The PCR conditions were optimized for each
primer pair and performed in Stratagene Mx3005p (Agilent
Technologies, TX, USA). Newly synthesized cDNA (2 μl) was
used as a template for the reaction in a total volume of 20
μl reactants, which included 10 μl of 2X real-time
PCR master mix (Applied Biosystems, Warrington, UK), 3 μl
ultra-pure water and 5 μl of primer mixer. The reactants
were first incubated at 95°C for 10 min, and then 40 cycles of
amplification were carried out with each cycle consisting of
denaturing at 95°C for 30 sec, annealing at 55°C for 1 min and
elongation at 72°C for 1 min. A dissociation curve was created at
the completion of the PCR in order to ensure that the reaction
produced the correct products as anticipated. The primer sequences
for cyclin D1 were 5′-TTG GTTACAGTAGCGTAG-3′ (sense) and
5′-TTATAGTAGC GTATCGTAGG-3′ (antisense). The primer sequences for
p53 were 5′-GACAATGGCAGCATCTAC-3′ (sense) and 5′-GAA
GGTGTAATCAGTCTCC-3′ (antisense). The primer sequences for p21 were
5′-GGAAGGAAGCAGGAAGAC-3′ (antisense) and 5′-AGCAGAGATACAAGGAAGG-3′
(antisense). The primer sequences for 36B4 were 5′-ACATGCTCA
ACATCTCCC-3′ (sense) and 5′-GCGGCACTTCTCCTGCT CC-3′ (antisense).
The results of the relative mRNA expression (cyclin D1, p53 and p21
to 36B4) in the MCF-7 cells were analyzed using the ΔΔCt method
(14).
Western blot assay
The MCF-7 cells were separately seeded in a 100-mm
dish (1x106 cells/dish) in DMEM/F12 medium supplemented
with 10% FBS and cultured overnight. The medium was then replaced
with DMEM/F12 supplemented with DCC-stripped 10% FBS. After being
cultured for 24 h, the cells were treated with 0, 0.2, 1 and 5% of
ZS-D30 and NZS-D30 in culture medium for 24 h. Protein extraction,
measurement of concentration, separation and Western blot analysis
were carried out as previously described (15). For immunoblotting, the following
primary antibodies were used: rabbit polyclonal antibodies against
cyclin D1, p53 and p21 (Cell Signaling Technology, Inc., Beverly,
MA, USA) and goat polyclonal antibody against β-actin (Santa Cruz
Biotechnology, Santa Cruz, CA, USA). The secondary antibody for
cyclin D1, p53 and p21 detection was ECL™ anti-rabbit IgG linked to
horseradish peroxidase (Amersham Biosciences, Buckinghamshire, UK)
and a donkey anti-goat IgG HRP for β-actin detection (Santa Cruz
Biotechnology). Images were captured using FujiFilm LAS-3000 image
system (FujiFilm Medical Systems USA, Inc., TX, USA). The densities
of specific bands were quantified by ImageQuant software (Molecular
Dynamics, Sunnyvale, CA, USA).
Results
ZS exhibits stronger stimulation of MCF-7
cell proliferation than NZS
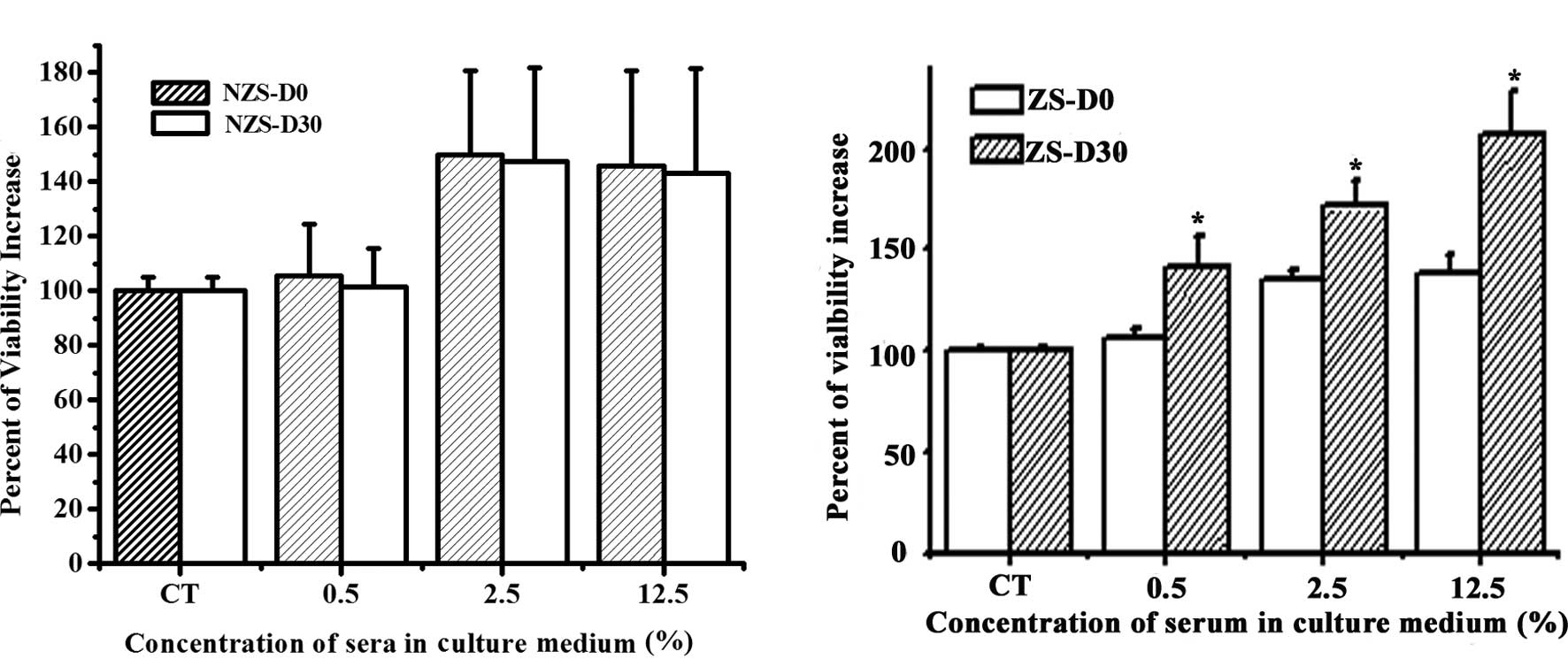
In order to investigate whether ZS exhibits
biological activity on MCF-7 cell growth, MCF-7 cells were treated
with sera harvested from control and experimental heifers before
and after Z implantation. Following 24 h of treatment, it was
discovered that all the sera stimulated MCF-7 cell growth in a
dose-dependent manner as compared to the control group (Fig. 1). No statistical difference was
noted between NZS-D0 and NZS-D30 regarding their effects on MCF-7
cell proliferation at the same concentrations (Fig. 1A). However, a significant
difference was found between ZS-D0 and ZS-D30 at the same
concentration (Fig. 1B). ZS-D30
significantly stimulated the proliferation of MCF-7 cells compared
to ZS-D0 at all doses. These data suggest that after one month of Z
implantation, the serum contains certain biologically active
components resulting in a more significant effect on the
stimulation of MCF-7 cell proliferation.
DCC treatment suppressed the stimulatory
effects of ZS-D30 on MCF-7 cell proliferation
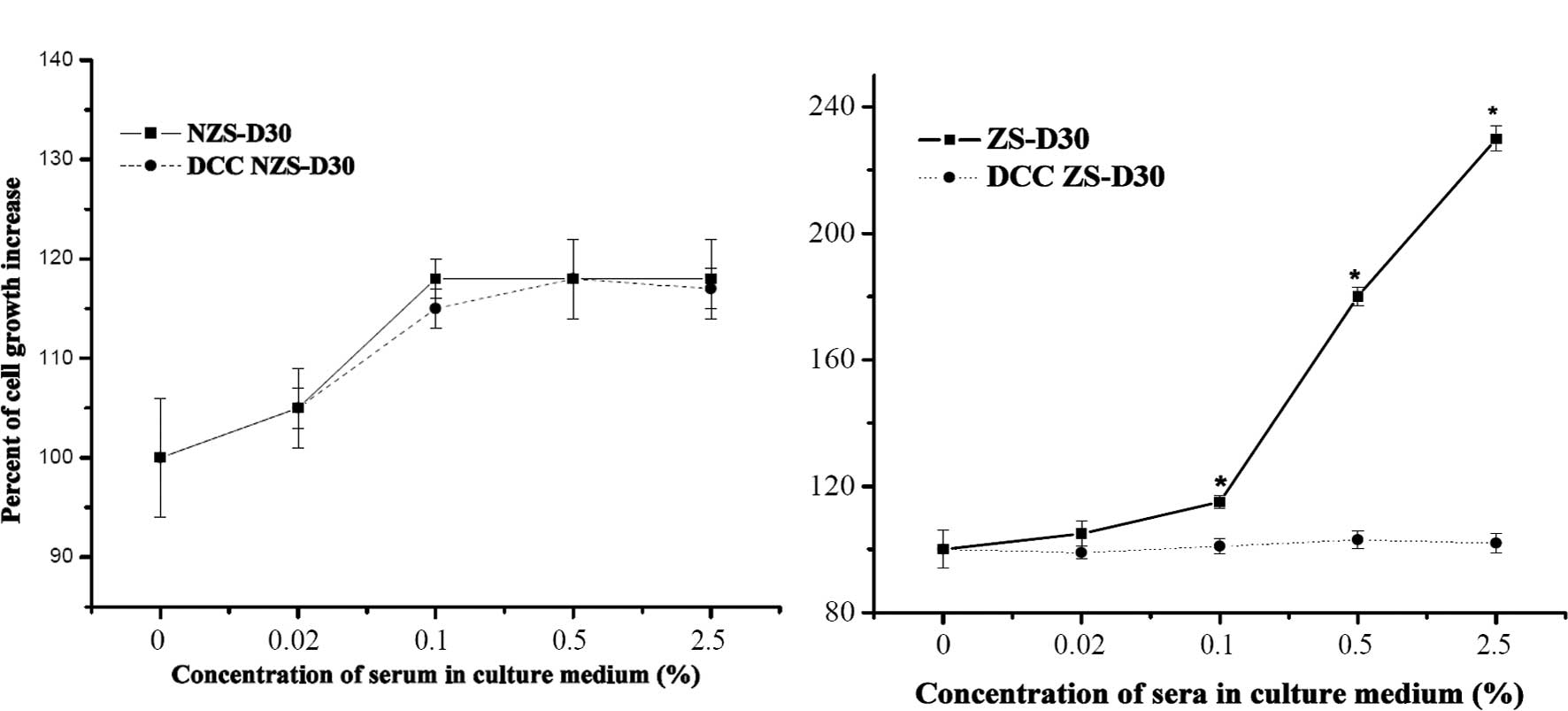
In order to confirm whether ZS-D30 contains
biological active components, ZS-D30 and NZS-D30 were treated with
DCC, which has been reported to effectively strip hormones and
growth factors from serum (16),
and the effect on MCF-7 cell growth was evaluated. As shown in
Fig. 2A, there was no significant
difference in the proliferation of MCF-7 cells after treatment with
NZS-D30 stripped or not using DCC. However, the ZS-D30 not stripped
by DCC significantly increased MCF-7 cell growth, compared to the
MCF-7 cells treated with ZS-D30 stripped by DCC. These results
revealed that ZS-D30 stripped by DCC removed the biologically
active components thus suppressing the stimulatory effects of
ZS-D30 on MCF-7 cell growth.
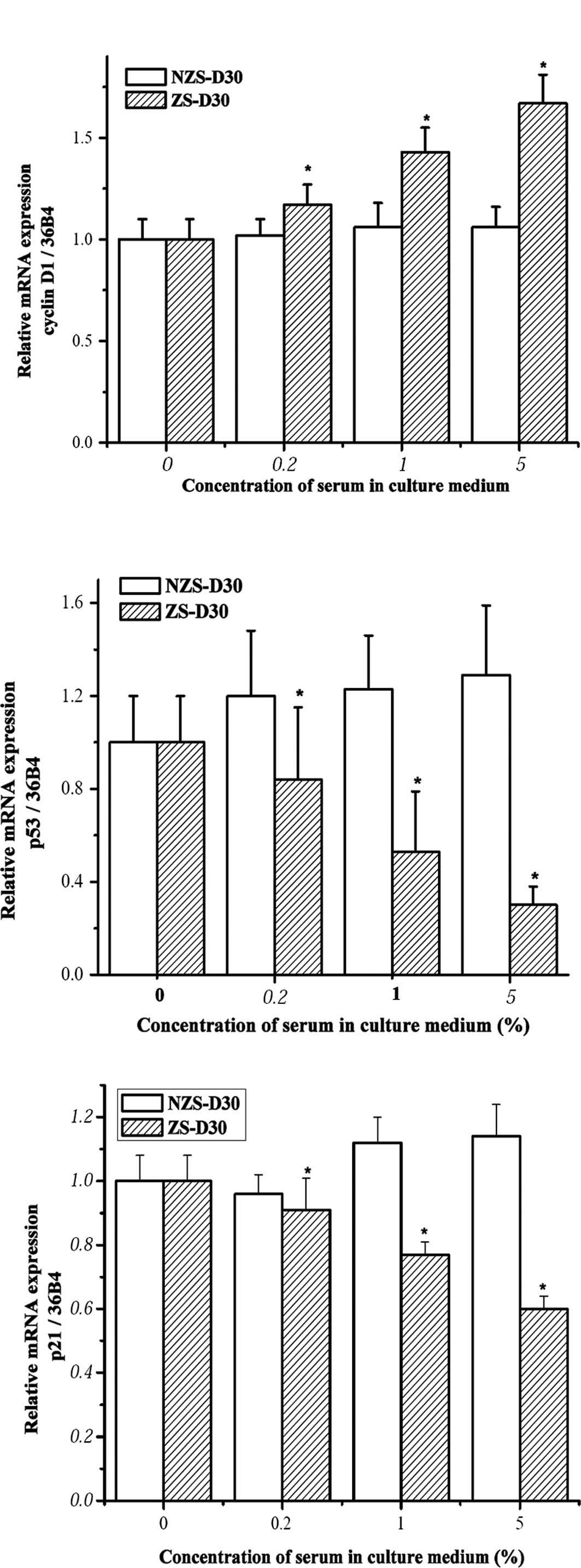
ZS-D30 up-regulates cyclin D1 and
down-regulates p53 and p21 mRNA expression in MCF-7 cells
Previous results showed that zearnol treatment
regulated the mRNA expression of cyclin D1 and p53 in
pre-adipocytes derived from heifers two months after implantation
of Z (12). Yuri et al
found that treatment with 50 nM Z accelerated MCF-7 cell growth
through down-regulated p21 expression in the cells (17). To explore the mechanisms involved
in MCF-7 cell growth stimulated by ZS-D30, the mRNA expression of
cyclin D1, p53 and p21 in MCF-7 cells after treatment with
different concentrations of ZS-D30 as well as NZS-D30 was
investigated using real-time PCR. Treatment with NZS-D30 for 24 h
did not significantly alter the mRNA expression of cyclin D1, p53
or p21 in MCF-7 cells (Fig. 3).
However, ZS-D30 treatment significantly up-regulated cyclin D1 and
down-regulated p53 and p21 mRNA in a dose-dependent manner.
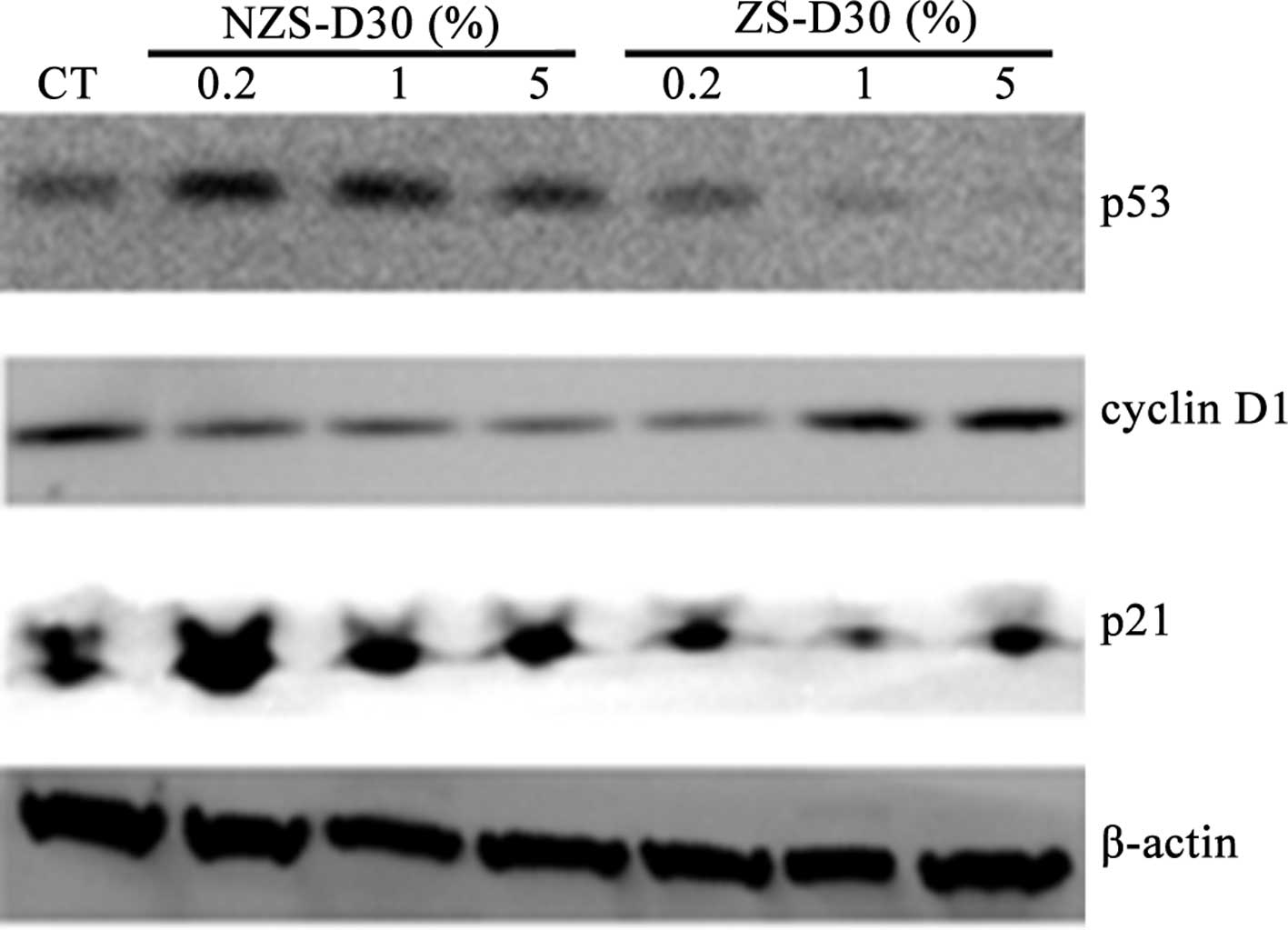
ZS-D30 up-regulates cyclin D1 and
down-regulates p53 and p21 protein expression in MCF-7 cells
The effects of NZS-D30 and ZS-D30 on the regulation
of protein expression of cyclin D1, p53 and p21 in MCF-7 cells was
investigated. Following the treatment of MCF-7 cells with 0.2, 1
and 5% NZS-D30 and ZS-D30 for 24 h, the protein was extracted and
Western blot analysis was performed (Fig. 4). Similar to the mRNA expression,
the NZS-D30 treatment did not significantly alter the protein
expression of cyclin D1, p53 or p21 at the three concentrations.
However, ZS-D30 at the three concentrations increased cyclin D1 and
down-regulated p53 and p21 protein expression in the MCF-7 cells
after 24 h of treatment (Fig. 4).
Taken together, the data suggest that the stimulatory effects of
ZS-D30 on MCF-7 cell growth may mediate the regulation of cell
cycle-related gene expression in the cells. Further mechanisms are
yet to be elucidated.
Discussion
Zeranol has been widely used as a growth promoter in
certain countries, including the US and Canada, since it
accelerates weight gain, improves feed conversion efficiency and
increases the lean meat-to-fat ratio particularly in cattle. A
previous investigation found that the use of Z in cattle was safe
for human consumption (18).
However, these results were generated using and based on outdated
techniques. With the development of molecular biology and medicine,
it was demonstrated that these previous findings may be
inconclusive. This scenario has previously occurred with
diaethylstilbestrol (DES). DES is an estrogen that was approved by
the US FDA in 1947 for the prevention of miscarriage and was widely
prescribed by doctors in the USA until 1971 and in European
countries until the late 1970s (19). Researchers also reported that DES
generally increased the weight gain of cattle by 15% and feed
conversion improvement was normally approximately 10% (20). Additionally, DES increases leanness
(20). Due to its growth
stimulation and improved feed utilization, orally administered DES
for cattle was approved by the US FDA in 1954. Later, an
investigation found that DES was not only associated with cancer in
women to whom DES was prescribed during their pregnancy, but also
in their daughters (21–23). These findings led the FDA to remove
oral DES for cattle from the market in 1972 and implants the
following year. A previous investigation among workers
occupationally exposed to Z was conducted due to a variety of
reported breast symptoms, including sharp pain, tingling, burning,
aching and irritation. In addition, two former workers (one male,
one female) had boys aged under 5 years who developed gynaecomastia
and presented unusual growth spurts. These two boys were exposed to
Z through their parents workclothes. The symptoms in the two boys
abated after their parents changed work to control the Z exposure
(6). The investigation clearly
illustrated the relationship between breast symptoms and exposure
to Z, particularly in children since they may be more sensitive to
Z than adults. Human exposure to Z occurs through ingestion of beef
products that contain pharmacological active residuals or its
metabolites. Current research is raising concerns regarding the
relationship between the consumption of beef products and adverse
health risks associated with breast cancer development. Our
previous data revealed that pre-adipocytes derived from heifers two
months post-Z-implantation were sensitive to Z treatment. This
prompted us to investigate whether biological samples such as serum
and meat extracts harvested from the heifers after Z implantation
have an effect on human breast cancer cell growth.
The present study revealed that the sera harvested
from the heifers one month post-Z implantation significantly
stimulated MCF-7 cell proliferation compared to the sera harvested
from the same heifers prior to Z implantation as well as that from
the control heifers. The results imply that the residues of
biologically active Z or its metabolites contained in the sera
exhibit a stimulatory effect on MCF-7 cells, and their activities
can be suppressed by DCC treatment. The preliminary data also
showed that the muscle extracts derived from the heifers one month
post-Z implantation stimulated MCF-7 cell proliferation. We
detected Z concentrations in the muscles, and the concentrations in
sera will be detected using ELISA method once its antibody has
developed. It is difficult to claim that the consumption of beef
products with residues of biologically active Z or its metabolites
is a risk factor in breast cancer. More evidence is required in
order to clarify this crucial health issue. Further investigation
of the impacts of biologically active Z and its metabolites on
primary cultured human normal breast epithelial cells, stromal
cells, pre-adipocytes and stem/progenitor cells is in progress in
our laboratory.
Cell cycle regulation plays a very important role in
mammary gland development and carcinogenesis. Numerous researchers
have found that cyclin D1, p21 and p53 are associated with cell
cycle regulation in breast cancer initiation, promotion and
progression. Cyclin D1 is one of the most frequently overexpressed
proteins and one of the most commonly amplified genes in breast
cancer (24,25). It is able to regulate the growth of
estrogen-responsive tissues by activating the estrogen receptor in
a ligand-independent manner (26).
The tumor suppressor p53 is able to regulate cell proliferation and
apoptosis. An imbalance between cell proliferation and apoptosis
results in a rapid increase in cell numbers, the most prominent
characteristic of tumors. Normal breast epithelial cells induce
p53-dependent apoptosis and p53-independent cell cycle arrest of
breast cancer cells (27). Tumor
angiogenesis is considered a multi-pathway process, while p21
(WAF1/CiP1) is a cyclin-dependent kinase inhibitor involved in cell
division and survival. It is activated by p53 and is a downstream
effector for p53 function by inducing G1 arrest when normal breast
cells are exposed to DNA-damaging agents (28). The expression of the p21 gene has
been found to be regulated by estrogen in estrogen
receptor-positive human breast cancer cells (29).
We demonstrated that ZS-D30 increased cyclin D1 and
down-regulated p53 and p21 expression in MCF-7 cells at the mRNA
and protein levels, when compared to NZS-D30. This may partially
explain why ZS-D30 significantly stimulated MCF-7 cell
proliferation. Other mechanisms involved in cell cycle regulation
may also exist, but further investigation is required to elucidate
them. The data show that certain as yet undefined growth factors
that are responsible for the stimulatory action of MCF-7 cell
proliferation may be secreted into the blood circulation of heifers
upon Z implantation. We attribute the stimulatory effect of ZS on
MCF-7 cell growth to the implantation of Z.
In conclusion, sera directly harvested from heifers
one month post-Z implantation exhibited a potent stimulatory effect
on MCF-7 cell growth that was mediated through an increase in
cyclin D1 and a decrease in p53 and p21 expression at the mRNA and
protein levels. Our results, to a certain degree, suggest the
association between the biological samples derived from heifers
implanted with Z and the potential adverse health risk of breast
cancer. We require further evidence to clarify this critical health
issue.
Acknowledgements
We thank Dr Walter R. Threlfall and
his team members for collecting the biological samples from the
control and experimental heifers. We also thank manager Martin
Mussard and his team members for taking care of the heifers in the
beef cattle barn located in the Ohio State University Livestock
Facilities. This research was supported by NIH Grant R01 ES
015212.
References
|
1.
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2009. CA Cancer J Clin. 59:225–249. 2009. View Article : Google Scholar
|
|
2.
|
Brekelmans CT: Risk factors and risk
reduction of breast and ovarian cancer. Curr Opin Obstet Gynecol.
15:63–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Delort L, Satih S, Kwiatkowski F, et al:
Evaluation of breast cancer risk in a multigenic model including
low penetrance genes involved in xenobiotic and estrogen
metabolisms. Nutr Cancer. 62:243–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Thonney ML: Growth, feed efficiency and
variation of individually fed Angus, polled Hereford and Holstein
steers. J Anim Sci. 65:1–8. 1987.PubMed/NCBI
|
|
5.
|
Prichard DL, Hargrove DD, Olson TA, et al:
Effects of creep feeding, zeranol implants and breed type on beef
production: I. Calf and cow performance. J Anim Sci. 67:609–616.
1989.PubMed/NCBI
|
|
6.
|
Aw TC, Smith AB, Stephenson RL, et al:
Occupational exposure to zeranol, an animal growth promoter. Br J
Ind Med. 46:341–346. 1989.PubMed/NCBI
|
|
7.
|
Takemura H, Shim JY, Sayama K, Tsubura A,
Zhu BT and Shimoi K: Characterization of the estrogenic activities
of zearalenone and zeranol in vivo and in vitro. J Steroid Biochem
Mol Biol. 103:17–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Swan SH, Liu F, Overstreet JW, et al:
Semen quality of fertile US males in relation to their mothers'
beef consumption during pregnancy. Hum Reprod. 22:1497–1502.
2007.
|
|
9.
|
Linos E, Willett WC, Cho E, et al: Red
meat consumption during adolescence among premenopausal women and
risk of breast cancer. Cancer Epidemiol Biomarkers Prev.
17:2146–2151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Liu S and Lin YC: Transformation of
MCF-10A human breast epithelial cells by zeranol and
estradiol-17beta. Breast J. 10:514–521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Liu S, Sugimoto Y, Kulp SK, et al:
Estrogenic down-regulation of protein tyrosine phosphatase gamma
(PTP gamma) in human breast is associated with estrogen receptor
alpha. Anticancer Res. 22:3917–3923. 2002.PubMed/NCBI
|
|
12.
|
Ye W, Xu P, Threlfall WR, et al: Zeranol
enhances the proliferation of pre-adipocytes in beef heifers.
Anticancer Res. 29:5045–5052. 2009.PubMed/NCBI
|
|
13.
|
Xu P, Ye W, Jen R, et al: Mitogenic
activity of zeranol in human breast cancer cells is enhanced by
leptin and suppressed by gossypol. Anticancer Res. 29:4621–4628.
2009.PubMed/NCBI
|
|
14.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ye W, Chang HL, Wang LS, et al: Modulation
of multidrug resistance gene expression in human breast cancer
cells by (-)-gossypol-enriched cottonseed oil. Anticancer Res.
27:107–116. 2007.PubMed/NCBI
|
|
16.
|
Darbre P, Yates J, Curtis S, et al: Effect
of estradiol on human breast cancer cells in culture. Cancer Res.
43:349–354. 1983.PubMed/NCBI
|
|
17.
|
Yuri T, Tsukamoto R, Miki K, et al:
Biphasic effects of zeranol on the growth of estrogen
receptor-positive human breast carcinoma cells. Oncol Rep.
16:1307–1312. 2006.PubMed/NCBI
|
|
18.
|
Kuiper-Goodman T, Scott PM and Watanabe H:
Risk assessment of the mycotoxin zearalenone. Regul Toxicol
Pharmacol. 7:253–306. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Giusti RM, Iwamoto K and Hatch EE:
Diethylstilbestrol revisited: a review of the long-term health
effects. Ann Intern Med. 122:778–788. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Dinusson WE, Andrews FN and Beeson WM: The
effects of stilbestrol, testosterone, thyroid alteration and
spaying on the growth and fattening of beef heifers. J Anim Sci.
9:321–330. 1950.PubMed/NCBI
|
|
21.
|
Andrews FN, Beeson WM and Harper C: The
effect of stilbestrol and testosterone on the growth and fattening
of lambs. J Anim Sci. 8:578–582. 1949.
|
|
22.
|
Beral V and Colwell L: Randomised trial of
high doses of stilboestrol and ethisterone in pregnancy: long-term
follow-up of mothers. Br Med J. 281:1098–1101. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Herbst AL, Ulfelder H and Poskanzer DC:
Adenocarcinoma of the vagina. Association of maternal stilbestrol
therapy with tumor appearance in young women. New Eng J Med.
284:878–881. 1971. View Article : Google Scholar
|
|
24.
|
Ellis PE, Maclean AB, Crow JC, et al:
Expression of cyclin D1 and retinoblastoma protein in Paget's
disease of the vulva and breast: an immunohistochemical study of
108 cases. Histopathology. 55:709–715. 2009.PubMed/NCBI
|
|
25.
|
Millar EK, Dean JL, McNeil CM, et al:
Cyclin D1b protein expression in breast cancer is independent of
cyclin D1a and associated with poor disease outcome. Oncogene.
28:1812–1820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zwijsen RM, Buckle RS, Hijmans EM, et al:
Ligand-independent recruitment of steroid receptor coactivators to
estrogen receptor by cyclin D1. Genes Dev. 12:3488–3498. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Toillon RA, Chopin V, Jouy N, et al:
Normal breast epithelial cells induce p53-dependent apoptosis and
p53-independent cell cycle arrest of breast cancer cells. Breast
Cancer Res Treat. 71:269–280. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Brugarolas J and Jacks T: Double
indemnity: p53, BRCA and cancer. p53 mutation partially rescues
developmental arrest in Brca1 and Brca2 null mice, suggesting a
role for familial breast cancer genes in DNA damage repair. Nat
Med. 3:721–722. 1997.
|
|
29.
|
Mandal S and Davie JR: Estrogen regulated
expression of the p21 Waf1/Cip1 gene in estrogen receptor positive
human breast cancer cells. J Cell Physiol. 224:28–32.
2010.PubMed/NCBI
|