Introduction
Matrix metalloproteases (MMPs), also termed
matrixins, are a family of zinc-dependent endopeptidases that
degrade proteins of the extracellular matrix (ECM). The timely
breakdown of ECM is essential for a variety of processes, including
embryonic development, morphogenesis, angiogenesis, reproduction,
osteogenesis, tissue resorption and vascular remodeling.
MMP-1 (collagenase 1) hydrolyzes collagen types I,
II, III, VII, VIII, X and XI, as well as gelatin, fibronectin,
vitronectin, laminin, tenascin and aggrecan, and links protein,
myelin basic protein and versican. MMP-2 (gellatinase) degrades
collagen types I, II, III, IV, V, VII, X and XI, gelatin, elastin,
fibronectin, vitronectin, laminin, entactin, tenascin, SPARC and
aggrecan, and links protein, galectin-3, versican, decanin and
myelin basic protein (1,2). One of the most important differences
between theses two metalloproteases is the possibility of the
hydrolysis of elastin and collagen type IV by MMP-2, but not by
MMP-1.
The endothelium, a single layer of cells
constituting the inner surface of blood vessels, was long
considered to be merely a barrier between blood and smooth muscle
cells of the vessel wall. Since then, the ability of the
endothelium to synthesize and release various substances with
multidirectorial biological functions has been elucidated, and it
is now considered the biggest endocrine gland of the human body.
The endothelium plays a crucial role in the regulation of
vasomotorics and haemostasis. Substances produced by endothelial
cells are also involved in angiogenesis and inflammation
processes.
Atherosclerosis is a systemic multifocal disease
leading to various clinical events, depending on the vascular site
where it is most pronounced: coronary arteries, cerebral arteries
or iliac and lower limb arteries. Endothelial dysfunction is
considered a key factor preceding atherosclerotic lesions. The
theory of unified response to injury, formulated by Ross (3), postulates a stereotypic vascular wall
reaction generated by various factors, leading, not only to
endothelial damage, but also to endothelial dysfunction.
Metalloproteases are produced by endothelial cells
and are involved in various vascular pathologies, including
atherosclerosis and aortal aneurysm. The latter is presently
considered the equivalent of coronary artery disease (CAD), placing
such patients in a group of secondary CAD prevention, independently
of the presence or absence of coronary heart disease itself
(4–9).
Elastin-derived peptides (EDPs) are generated as a
result of a degradation of elastin fibres. Elastin has a slow
metabolism, which is accelerated in atherosclerosis, lung
emphysema, neoplasms or arthritis (10). Oligopeptide sequences VCVAPG are
detected in both insoluble elastin and EDPs. These sequences
activate the elastin receptor and exert a multitude of biological
effects. EDPs stimulate the synthesis and release of
metalloproteases. In experimental studies, rabbits receiving
injections of EDPs developed atherosclerosis (11). Studies indicate that disturbances
of elastin metabolism leading to increased serum levels of EDPs are
one of the risk factors of atherosclerosis. Since κ-elastin is an
acknowledged EDP, numerous experiments have evaluated its influence
on aorta and endothelial cells (12–14).
The aim of our study was to compare the production
of MMP-1 and MMP-2 in cultured human arterial endothelial cells
derived from vascular pathologies localized at three different
sites, the coronary artery, iliac artery and aorta, measured as
their concentration in cell culture medium. The second aim was to
evaluate the influence of κ-elastin on the production of the
evaluated metalloproteases in the three studied endothelial cell
lines.
Materials and methods
Cell culture
Human endothelial cells isolated from the coronary
artery, iliac artery or aorta were purchased from Lonza. The cells
were subcultured according to the manufacturer's recommendations.
Briefly, the cells were maintained in EBM-2 medium with 5% FBS and
endothelial cell-specific supplements (IGF, VEGF and heparin) in a
95% CO2 atmosphere at 37°C. Cells were used in
experiments on 3-4 split. After trypsinization, the cells were
grown to confluence on 24-well plates. Subsequently, they were
incubated with κ-elastin at concentrations of 0.1, 0.4, 1.0, 2.5 or
5.0 μg/ml, respectively, for 24 h. Next, the cell culture
medium was removed, centrifuged for 15 min at 3,000 rpm and stored
at −70°C for subsequent analyses.
ELISA
MMP-1 and MMP-2 concentrations were determined using
commercially available kits (GE Healthcare). The antibodies used
for detection were specific for active MMP forms only. Due to the
high concentration of MMP-2 in the cell lysates, analytes were
diluted twenty times just before determination. Resulting optical
densities were plotted against standards, and the absolute
concentration in ng/ml was obtained and used for the statistical
analyses.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). Statistical significance was calculated using the parametric
one-way ANOVA test for normal distributions, assuming the
homogeneity and heterogeneity of variances, followed by the Tukey
HSD test. The Kruskal-Wallis rank test was applied in the case of
non-normality of distributions, followed by the Steel-Dwass test.
Dunnett's test or Steel's test were used to compare each group to
the control. Prior to the parametrical analyses, the normal
distribution was verified with the Shapiro-Wilk test. The
homogeneity of variance was analyzed using the Levene test. The
accepted level of statistical significance was p<0.05.
Results
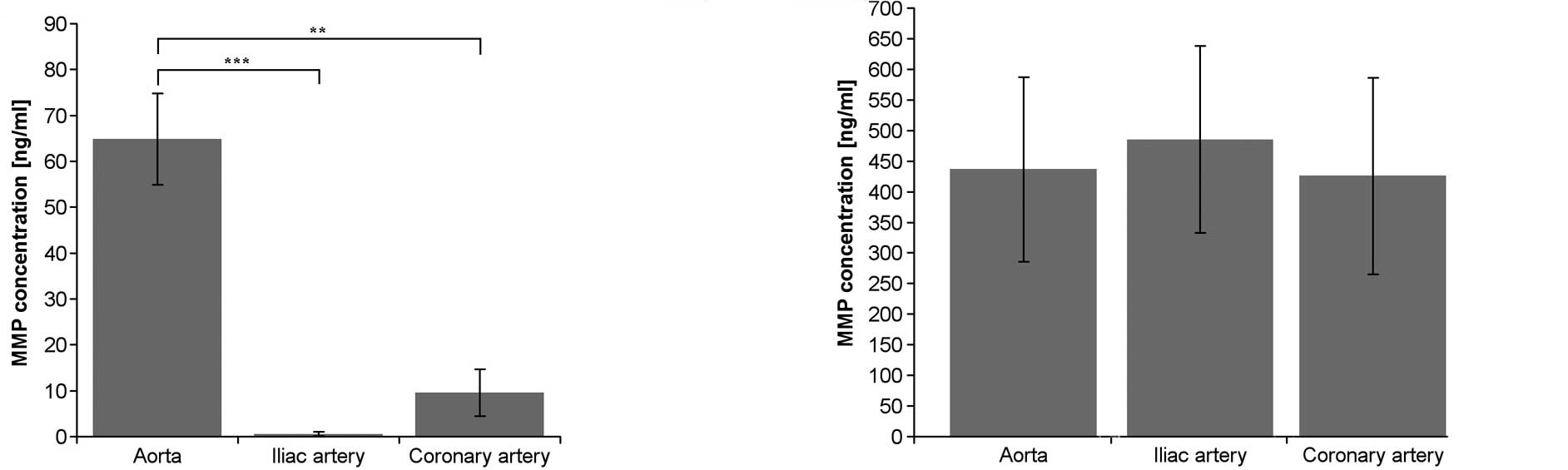
The production of MMP-1 was statistically
significantly greater in endothelial cells derived from the aorta
compared to the production of MMP-1 in endothelium from the
coronary and iliac arteries (Fig.
1A). There were no statistically significant differences in the
production of MMP-2 among the studied endothelium cell lines
(Fig. 1B).
The addition of κ-elastin at all evaluated
concentrations did not statistically significantly influence the
concentration of MMP-1 in the cultured coronary artery endothelium.
Additionally, no statistically significant differences were
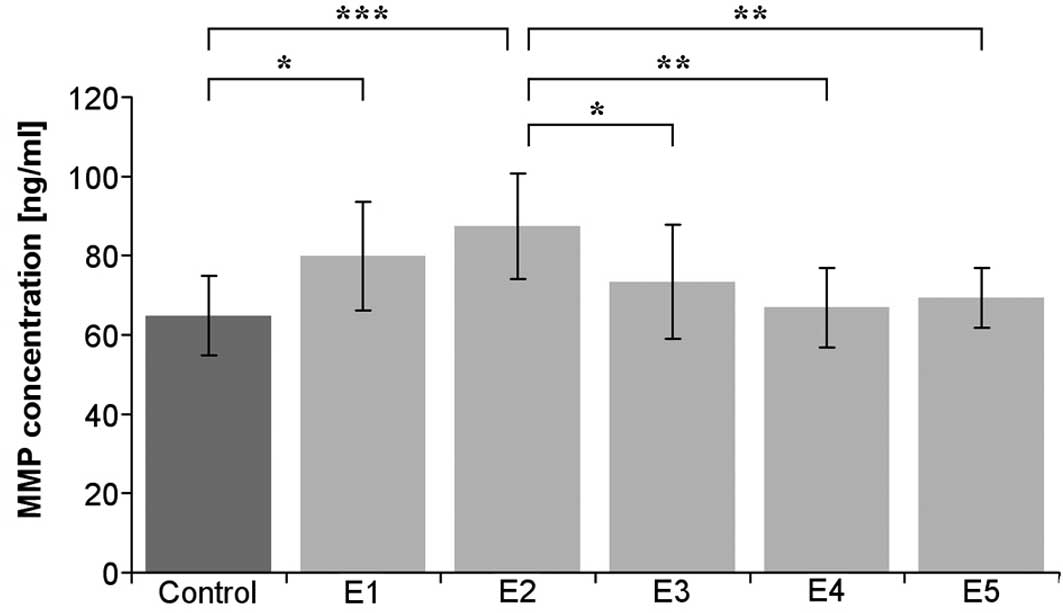
observed in the cultured iliac artery endothelium (Table I). In the cultured endothelium
derived from aorta, κ-elastin at concentrations of 0.1 and 0.4
μg/ml statistically significantly increased the amount of
MMP-1 (Fig. 2).
 | Table I.Concentration of MMP-1 (ng/ml) in the
various types of endothelial cell lines. |
Table I.
Concentration of MMP-1 (ng/ml) in the
various types of endothelial cell lines.
| Cell line | Control | E1 | E2 | E3 | E4 | E5 |
|---|
| Aorta | 64.8±10.0 | 79.8±13.7 | 87.4±13.3 | 73.4±14.4 | 66.8±10.0 | 69.3±7.50 |
| Iliac artery | 0.50±0.40 | 0.30±0.30 | 1.10±0.80 | 1.70±1.70 | 2.30±2.60 | 1.50±1.40 |
| Coronary artery | 9.50±5.10 | 9.60±4.30 | 11.1±5.40 | 11.5±4.40 | 10.5±3.30 | 10.5±2.20 |
None of the concentrations of κ-elastin used in our
study influenced the levels of MMP-2 in the three endothelial cell
lines with statistical significance (Table II).
 | Table II.Concentration of MMP-2 (ng/ml) in
various endothelium cell lines. |
Table II.
Concentration of MMP-2 (ng/ml) in
various endothelium cell lines.
| Cell line | Control | E1 | E2 | E3 | E4 | E5 |
|---|
| Aorta | 436.7±150.7 | 365.4±52.90 | 422.4±102.1 | 430.5±106.0 | 406.5±93.90 | 416.6±72.0 |
| Iliac artery | 485.4±152.3 | 481.1±209.0 | 435.5±72.40 | 434.1±79.70 | 423.6±123.3 | 426.5±65.8 |
| Coronary artery | 425.8±160.6 | 386.1±79.00 | 356.8±96.90 | 332.2±89.40 | 326.0±95.40 | 331.4±43.7 |
Discussion
The endothelium represents an extremely biologically
active region of the blood vessel. Human umbilical vein endothelial
cells (HUVECs) are the most commonly used source of endothelium for
cell cultures. Various properties of endothelial cells depend on
the vascular location. Jackson et al (15) observed that HUVECs produced
substantially higher levels of both MMP-1 and MMP-2 compared to
levels in endothelium derived from neonatal foreskin. As
atherosclerosis is the process affecting arteries, it is likely
that experiments performed on arterial endothelial cell lines would
elucidate the pathology of this process more precisely than studies
carried out on HUVECs. Basu et al (16) postulated that various levels of
blood flow in different arteries cause structural and functional
heterogeneity in vascular remodeling, making specific arteries
prone to atherosclerosis. In their study, significantly higher
expression of MMP-9 and MMP-13, but not MMP-2, was noted in the
endothelium from the carotic artery. Burridge and Freidman
(17) compared endothelium from
porcine atheroprone coronary artery with atheroresistant iliac
artery, and observed different gene expression profiles. The
differences observed in their study did not include the
metalloprotease genes evaluated in our study. The experimental
model of cell culture chosen for the present study allowed for the
elimination of the influence of blood flow and sheer stress. As all
endothelial cell lines treated under at the same conditions, the
obtained results were determined solely in regards to genetic
factors. Aboyans et al (18) indicated that, although
atherosclerotic lesions first occur predominately in large vessels,
the more distal arteries may also be affected by aging. In order to
avoid this potential bias, the endothelial cell lines used in our
experiment were derived only from large arteries.
The most significant clinical manifestation of
atherosclerosis is myocardial infarct, which is caused mainly by
the rupture of unstable atherosclerotic plaque. A previous study by
Galis et al (19) revealed
increased expression of both MMP-1 and MMP-2 in a vulnerable region
of the shoulder of an atherosclerotic plaque. Restenosis occurring
after percutaneous angiovascular procedures, including stent
implantation or balloon angioplasty, is a major clinical problem.
Experimental animal studies revealed that endovascular procedures
led to an increased and sustained expression of MMP-2 in injured
arteries, detected 7–60 days after the procedure (20). Tummers et al (21) evaluated the serum levels of MMP-2
in rats undergoing balloon angioplasty of the carotid artery. An
elevation was detected between 7 and 14 days after the procedure.
Tummers et al postulated that an early and persistent
increase in the serum level of MMP-2 may be a useful marker of
vascular basement membrane remodeling and the presence of intimal
hyperplasia. In the present study, the level of active MMP-2 in
endothelial cell culture was evaluated. This concentration depended
not only on the level of MMP-2 gene expression, but also on
posttranslational processes involving the activation of proenzyme
into active metalloprotease. The results of our experiment indicate
that the place of origin of endothelial cells does not influence
MMP-2 production. Elastin-derived peptides, which may be increased
in various pathologies, also do not influence MMP-2 levels. The
experiments of Feldman et al (20) and Tummers et al (21) were performed on animals, whereas
our experiment was carried out on human arterial endothelial cells.
Our results support the hypothesis of Tummers et al, and
eliminate the potential bias caused by the influence of different
sites of vascular pathology and EDP on MMP-2 levels.
The serum level of MMP-2 may be a marker of a
broader spectrum of processes affecting the cardiovascular system.
Yasmin et al (22) observed
increased serum levels of MMP-2 in patients with systolic
hypertension and arterial stiffening. Friese et al (23) reported that elevation of the serum
concentration of MMP-2 occurs when hypertension is accompanied by
end-stage renal disease. In the arterial wall, metalloproteases are
produced not only by endothelial cells, but also by smooth muscle
cells and inflammatory cells (4,26).
When the endothelium is not damaged, vascular smooth muscle cells
have no contact with the blood stream, and the serum level of
metalloproteases reflects their production by the endothelium.
MMP-2 is also involved in aorta calcification, as well as the
calcification of atherosclerotic plaques in coronary arteries
(24,25).
The aorta is the largest arterial vessel in the
body. It is the site of several vascular pathologies, including
atherosclerotic plaques, calcification and aneurysms. Abdominal
aortic aneurysm (AAA) is a complex and multifactorial disease
(5), and several metalloproteases
are involved in its pathogenesis. Annabi et al (8) observed an increased activity of MMP-1
in AAA. Nishimura et al (6)
found that MMP-2 activity was higher in small AAAs of a diameter
between 30 and 45 mm. In another study, MMP-1 was detected in the
endothelium of AAA, whereas MMP-2 was present in endothelial cells
of matured neovessels within AAAs (27).
MMP-1 and MMP-2 produced by endothelial cells
participate in various (both physiological and pathological)
processes. Our results indicate that the production of MMP-2, in
contrast to MMP-1, is similar in endothelial cells derived from
various parts of the arterial vascular system. We also demonstrated
that elastin-derived peptides, which can be released in various
pathologies, influence the concentration of MMP-1, but not MMP-2.
Until measurement of the serum level of MMP-2 as a marker of
certain vascular pathologies is introduced, the influence of other
cardiovascular risk factors on its production in various
endothelial cells must be evaluated.
References
|
1.
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Nagase H and Woessner JF: Matrix
metalloproteases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar
|
|
3.
|
Ross R: Atherosclerosis – an inflammatory
disease. N Engl J Med. 340:115–126. 1999.
|
|
4.
|
Palombo D, Maione M, Cifiello BI, Udini M,
Maggio D and Lupo M: Matrix metalloproteinases. Their role in
degenerative chronic diseases of abdominal aorta. J Cardiovasc
Surg. 40:257–260. 1999.PubMed/NCBI
|
|
5.
|
Pearce WH and Shively VP: Abdominal aortic
aneurysm as a complex multifactorial disease: interactions of
polymorphisms of inflammatory genes, features of autoimmunity, and
current status of MMPs. Ann NY Acad Sci. 1085:117–132. 2006.
View Article : Google Scholar
|
|
6.
|
Nishimura K, Ikebuchi M, Kanaoka Y, Ohgi
S, Ueta E, Nanba E and Ito H: Relationships between matrix
metalloproteinases and tissue inhibitors of metalloproteinases in
the wall of abdominal aortic aneurysms. Int Angiol. 22:229–238.
2006.PubMed/NCBI
|
|
7.
|
Ishii T and Asuwa N: Collagen and elastin
degradation by matrix metalloproteinases and tissue inhibitors of
matrix metalloproteinases in aortic dissection. Hum Pathol.
31:640–646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Annabi B, Shedid D, Ghosm P, Kenigsberg
RL, Desrosiers RR, Bojanowski MW, Beaulieu E, Nassif E, Moumdjian R
and Béliveau R: Differential regulation of matrix metalloproteinase
activities in abdominal aortic aneurysms. J Vasc Surg. 35:539–546.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Grundy SM, Cleeman JI, Merz CNB, Brewer
HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC and Stone NJ;
for the Coordinating Committee of the National Cholesterol
Education Program: Implications of recent clinical trials for the
National Cholesterol Education Program Adult Treatment Panel III
Guidelines. J Am Coll Cardiol. 44:720–732. 2004. View Article : Google Scholar
|
|
10.
|
Hornebeck W and Robert L: Elastase-like
enzymes in aortas and human breast carcinomas: quantitative
variations with age and pathology. Adv Exp Med Biol. 79:145–156.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gminski J and Drozdz M: Succinyl
trialanine p-nitroanilide hydrolytic activities in plasma and the
aorta of rabbits experimentally immunized with soluble elastin. Exp
Pathol. 43:37–40. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Faury G, Ristori MT, Verdetti J, Jacob MP
and Robert L: Effect of elastin peptides on vascular tone. J Vasc
Res. 32:112–119. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Faury G, Garnier S, Weiss AS, Wallach J,
Fülöp T Jr, Jacob MP, Mecham RP, Robert L and Verdetti J: Action of
tropoelastin and synthetic elastin sequences on vascular tone and
on free Ca2+ level in human vascular endothelial cells.
Circ Res. 82:328–336. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Robert L, Labat-Robert J and Robert AM:
Genetic, epigenetic and posttranslational mechanisms of aging.
Biogerontology. 11:387–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Jackson CJ and Nguyen M: Human
microvascular endothelial cells differ from macrovascular
endothelial cells in their expression of matrix metalloproteinases.
Int J Biochem Cell Biol. 29:1167–1177. 1997. View Article : Google Scholar
|
|
16.
|
Basu P, Sen U, Tyagi N and Tyagi SC: Blood
flow interplays with elastin: collagen and MMP: TIMP ratios to
maintain healthy vascular structure and function. Vasc Health Risk
Manag. 6:215–228. 2010.PubMed/NCBI
|
|
17.
|
Burridge KA and Friedman MH: Environment
and vascular bed origin influence differences in endothelial
transcriptional profiles of coronary and iliac arteries. Am J
Physiol Heart Circ Physiol. 299:H837–H846. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Aboyans V, Lacroix P and Criqui MH: Large
and small vessel atherosclerosis: similarities and differences.
Prog Cardiovasc Dis. 50:112–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Galis ZS, Sukhova GK, Lark MW and Libby P:
Increased expression of matrix metalloproteinases and matrix
degrading activity in vulnerable regions of of human
atherosclerotic plaques. J Clin Invest. 94:2493–2503. 1994.
View Article : Google Scholar
|
|
20.
|
Feldman LJ, Mazighi M, Scheuble A, Deux
JF, De Benedetti E, Badier-Comander C, Brambilla E, Henin D, Steg
PG and Jacob MP: Differential expression of matrix
metalloproteinases after stent implantation and balloon angioplasty
in the hypercholesterolemic rabbit. Circulation. 103:3117–3122.
2001. View Article : Google Scholar
|
|
21.
|
Tummers AM, Mountain DJ, Mix JW,
Kirkpatric SS, Cassada DC, Stevens SL, Freeman MB, Goldman MH and
Grandas OH: Serum levels of matrix metalloproteinase-2 as a marker
of intimal hyperplasia. J Surg Res. 160:9–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yasmin, McEniery CM, Wallace S, Dakham Z,
Pulsalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR and Wilkinson
IB: Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase
activity are associated with systolic hypertension and arterial
stiffness. Arterioscler Thromb Vasc Biol. 25:3722005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Friese RS, Rao F, Khandrika S, Thomas B,
Zielgler MG, Schmid-Schönbein GW and O'Connor DT: Matrix
metalloproteinases: discrete elevations in essential hypertension
and hypertensive end-stage renal disease. Clin Exp Hypertens.
31:521–533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Qin X, Corriere MA, Matrisian LM and
Guzman RJ: Matrix metalloproteinase inhibition attenuates aortic
calcification. Arterioscler Thromb Vasc Biol. 26:1510–1516. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Kieffer P, Giummelly P, Schjoth B,
Carteaux JP, Villemot JP, Hornebeck W and Atkinson J: Activation of
metalloproteinase-2, loss of matrix scleroprotein content and
coronary artery calcification. Atherosclerosis. 157:251–254. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Pauly RR, Passaniti A, Bilato C, et al:
Migration of cultured vascular smooth muscle cells through a
basement membrane barrier requires type IV collagenase activity and
is inhibited by cellular differentiation. Circ Res. 75:41–54. 1994.
View Article : Google Scholar
|
|
27.
|
Reeps C, Pelisek J, Seidl S, Schuster T,
Zimmermann A, Kuehnl A and Eckstein HH: Inflammatory infiltrates
and neovessels are relevant sources of MMPs in abdominal aortic
aneurysm wall. Pathobiology. 76:243–252. 2009.PubMed/NCBI
|