Introduction
Vitamin B6 is well known as a water-soluble vitamin
essential for normal growth, development and metabolism. Natural
vitamin B6 consists of six interconvertible compounds: pyridoxine,
pyridoxal, pyridoxamine and their phosphorylated forms, pyridoxine
5′-phosphate, pyridoxal 5′-phosphate (PLP) and pyridoxamine
5′-phosphate. In particular, PLP is a biologically active form of
vitamin B6 and functions as a cofactor for numerous enzymes
involved in amino acid and cellular metabolisms (1). Vitamin B6 has been classically
classified as a coenzyme; however, recent reports have prompted us
to consider other physiological functions for vitamin B6. Vitamin
B6 has been reported to play an important protective role against
several types of diseases (2–4). We
previously demonstrated a preventive effect of dietary vitamin B6
in moderate dosage against tumorigenesis in the colon of mice which
were administered azoxymethane (AOM) (5). Further studies have demonstrated that
the anti-tumorigenesis effect of vitamin B6 in the colon of
AOM-injected mice may be mediated, in part, via the suppression of
cell hyperproliferation, inducible nitric oxide synthase (iNOS)
expression and oxidative stress (5,6).
Matsubara et al found an anti-angiogenic effect of vitamin
B6 in an ex vivo serum-free matrix culture using rat aortic
ring models (7). Furthermore, our
recent study revealed an anti-inflammatory effect of vitamin B6,
showing that treatment with vitamin B6 inhibits lipopolysaccharide
(LPS)-induced iNOS and cyclooxygenase-2 (COX2) expression in RAW
264.7 cells through suppression of NF-κB activation, the
pro-inflammatory transcription factor (8). In addition to this in vitro
experiment, dietary vitamin B6 inhibited nitric oxide (NO)
production in response to LPS administration in vivo
(8). Since angiogenesis and
inflammation have been considered to play critical roles in the
pathogenesis of colon cancer, the anti-angiogenic and
anti-inflammatory effects of vitamin B6 may elucidate the
mechanisms underlying the anti-tumor properties of vitamin B6.
Peroxisome proliferator-activated receptor-γ (PPARγ)
is one such protein that may explain, in part, the anti-tumor
behavior of vitamin B6. PPARγ, one of three PPAR subtypes (PPARα,
PPARγ and PPARδ/β), is a ligand-dependent transcription factor that
belongs to the nuclear hormone receptor superfamily (9). PPARγ is found highly expressed in
adipose tissue, and to a lesser extent in the colon and immune
system (10). PPARγ enhances
adipocyte-specific gene expression via the formation of a
heterodimeric DNA-binding complex with the retinoid X receptor. To
date, overexpression and knockout studies with mice strongly
suggest that PPARγ is an essential transcription factor in
adipogenesis in vitro and in vivo (11,12).
Furthermore, the most extensively employed insulin-sensitizing
drugs, thiazolidinedione derivatives (TZDs), such as troglitazone,
pioglitazone and rosiglitazone, have been found to possess a high
affinity for PPARγ (13),
suggesting that the pharmacological actions of TZDs are mediated
through PPARγ activation in adipocytes. It has been reported that
improvement in insulin resistance by PPARγ activation is due to an
increase in the number of differentiating adipocytes, which
promotes adipose-secreted hormone expression and, eventually,
glucose homeostasis (9).
Furthermore, several substances including the prostaglandin J2
derivative are potent natural ligands of PPARγ (14) which control a variety of
physiological functions through the PPARγ signaling pathway.
Activation of PPARγ via TZDs has been found to
elicit both anti-neoplastic and anti-inflammatory effects.
Therefore, PPARγ is expected to be a pharmacological target for
several types of cancers and cardiovascular diseases (15,16).
In colon carcinogenesis, troglitazone, one of the TZDs, has been
shown to inhibit tumor growth (17) and to reduce the number of aberrant
crypt foci, which are precursor lesions of colon cancer in the
colon of mice administered AOM (18,19).
In addition, recent reports have demonstrated that the
anti-atherosclerotic effect of TZDs is partially explained by the
anti-inflammatory functions of PPARγ, and that TZDs inhibit the
expression of various inflammatory proteins including iNOS and COX2
in macrophages. To date, several mechanisms underlying the
anti-inflammatory effects of PPARγ ligands have been proposed,
including the inhibition of NF-κB activity (20).
Kawada et al previously tested the effects of
vitamins and their analogues on the terminal differentiation of
3T3-L1 cells and found that vitamin B6 derivatives regulated
adipogenesis of 3T3-L1 adipocyte cells (21). As the pharmacological properties of
TZDs appear to be similar to the biological effects of vitamin B6,
we hypothesized that vitamin B6 regulates PPARγ activation similar
to TZDs. To examine this hypothesis, we investigated whether
vitamin B6 affects adipogenesis in 3T3-L1 adipocytes, which are
able to be efficiently differentiated in the presence of TZDs and
support PPARγ target gene mRNA expression. The present study shows
that treatment with PLP, one of the vitamin B6 derivatives,
accelerates adipogenesis in 3T3-L1 adipocytes and that PLP
up-regulates the expression of PPARγ target genes in NIH3T3 cells
transfected with PPARγ. Furthermore, an acute administration of
vitamin B6 was shown to enhance the expression of a PPARγ target
gene in vivo. These observations suggest that vitamin B6
plays an important role as an activator of PPARγ in vitro
and in vivo.
Materials and methods
Chemical reagents
Pyridoxal hydrochloride, PLP and pyridoxine
hydrochloride were obtained from Nacalai Tesque (Osaka, Japan).
T-174
[5-[2-(naphthalenylmethyl)-5-benzoxazolyl]-methyl]-2,4-thiazolidinedione),
a specific ligand for PPARγ, was kindly provided by Tanabe
Mitsubishi Pharma Co. (Osaka, Japan).
Cell culture
Mouse 3T3-L1 preadipocytes, NIH3T3 cells and Phoenix
293 cells were cultured in a maintenance medium (10% fetal calf
serum, 100 U/ml penicillin and 100 μg/ml streptomycin in Dulbecco’s
modified Eagle’s medium at 37°C in 5% CO2/95% air under
a humidified condition. High titer retroviruses harboring PPARγ
were produced in Phoenix 293 cells and used to infect NIH3T3 cells,
as reported previously (22).
Adipocyte differentiation and Oil-Red-O
staining
For differentiation assays, confluent 3T3-L1 cells
were treated with differentiation medium [maintenance medium plus
0.5 mM 3-isobutyl-1-methylxanthine (IBMX)], 5 μg/ml insulin, and 1
μM dexamethasone (DEX) and incubated for 2 days. Differentiation
medium was then replaced with adipocyte growth medium (maintenance
medium supplemented with 5 μg/ml insulin) with or without PLP,
which was refreshed every 2 days. Differentiated 3T3-L1 cells were
fixed with 4% buffered paraformaldehyde for 15 min. A stock
solution of 0.3% Oil-Red-O (Sigma) in isopropanol (w/v) was diluted
6:4 prepared in water, filtered and added to the fixed cells. Cells
were washed twice in phosphate-buffered saline (PBS) and
photographed.
Glycerol-3-phosphate dehydrogenase
activity
Differentiated 3T3-L1 cells were washed twice with
ice-cold PBS and suspended in 25 mM Tris-HCl, pH 7.5, 1 mM EDTA,
and 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml pepstatin, and 1
mg/ml leupeptin. Membranes were disrupted by sonication, and
supernatants were collected following centrifugation at 13,000 × g
for 10 min. The reaction was initiated by the addition of
supernatants to a standard mixture containing 100 mM
triethanolamine/HCl buffer (pH 7.5), 2.5 mM EDTA, 0.12 mM
β-nicotinamide adenine dinucleotide disodium salt (NADH), 0.2 mM
dihydroxyacetone-phosphate and 0.1 mM β-mercaptoethanol. The change
in absorbance at 340 nm was measured using a spectrophotometer at
25°C. One unit of enzyme activity was determined to correspond to
the oxidation of 1 nmol of NADH/min.
Experimental animals
Male ICR mice (5-weeks old) (Charles River Japan
Inc., Japan) were housed in groups of 6 animals in plastic cages in
a room with controlled temperature (24°C) and a 12-h light/dark
cycle. The animals were provided with free access to the diet and
water and were maintained according to the ‘Guide for the Care and
Use of Laboratory Animals’ established by Hiroshima University. The
experimental diet (vitamin B6-free) consisted of the following
components (in g/kg diet): α-corn starch, 402; casein, 200;
sucrose, 200; corn oil, 100; cellulose, 50; AIN-93G salt mixture,
35; AIN-93 vitamin mixture (pyridoxine-free), 10; L-cystine, 3. The
experimental feeding period was 2 weeks, and the mice were divided
into 3 groups of 8 mice each. The animals received an oral
administration of pyridoxine hydrochloride (200 or 500 mg/kg body
weight) or physiological saline. Six hours after oral
administration, epididymal fat pads were removed.
Northern blot hybridization
Total RNA from differentiated 3T3-L1 cells was
isolated using Isogen (Nippon Gene). Total RNA from mouse adipose
tissues was isolated using RNeasy lipid tissue kit (Qiagen). Total
RNA (10 μg) was subjected to Northern blot hybridization. Briefly,
RNA was fractionated in 1% agarose gel containing 0.66 M
formaldehyde and 0.02 M MOPS (pH 7.0). Fractionated RNAs were
transferred onto a Hybond-N+ nylon filter (GE
Healthcare) by capillary blotting and then cross-linked by
ultraviolet irradiation. cDNA fragments of mouse adipocyte fatty
acid-binding protein (aP2) and mouse glycerol kinase (GyK) were
amplified using specific primers. Primers were as follows: aP2
forward primer, 5′-GAAGACAGCTCCTCCTCGAAGGTT-3′; aP2 reverse primer,
5′-GGAAGTCACGCCTTTCATAACA-3′; Gyk forward primer,
5′-TGGTGTCAGCAACCAGAGGGA-3′; Gyk reverse primer,
5′-GGCCATAGATCTCAGAAGAAC-3′. 32P-labeled cDNA fragments
encoding aP2, GyK, human β-actin and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) were used for Northern blot hybridization as
probes. Hybridization was performed in PerfectHyb (Toyobo) at 65°C
for 20 h. The membrane was finally washed with 0.2X SSC and 0.1%
SDS at 65°C for 30 min, and the hybridization signals were analyzed
using the BAS system (Fuji Film).
Statistical analyses
Values are presented as the means ± SE. Statistical
significance among the means was estimated at P<0.05 according
to the Student’s t-test.
Results
The effect of vitamin B6 on adipogenesis
in 3T3-L1 adipocytes
To investigate the effect of vitamin B6 on
adipogenesis, 3T3-L1 preadipocytes, which are well characterized as
an in vitro model of adipocyte differentiation were used.
3T3-L1 cells differentiated into mature adipocytes upon exposure to
a hormonal stimulus (0.5 mM IBMX, 5 μg/ml insulin and 1 μM DEX).
After a 2-day incubation with the hormonal mixture, 3T3-L1 cells
were cultured in a medium containing vitamin B6 for 12 days and
then subjected to Oil-Red-O staining for lipid droplet
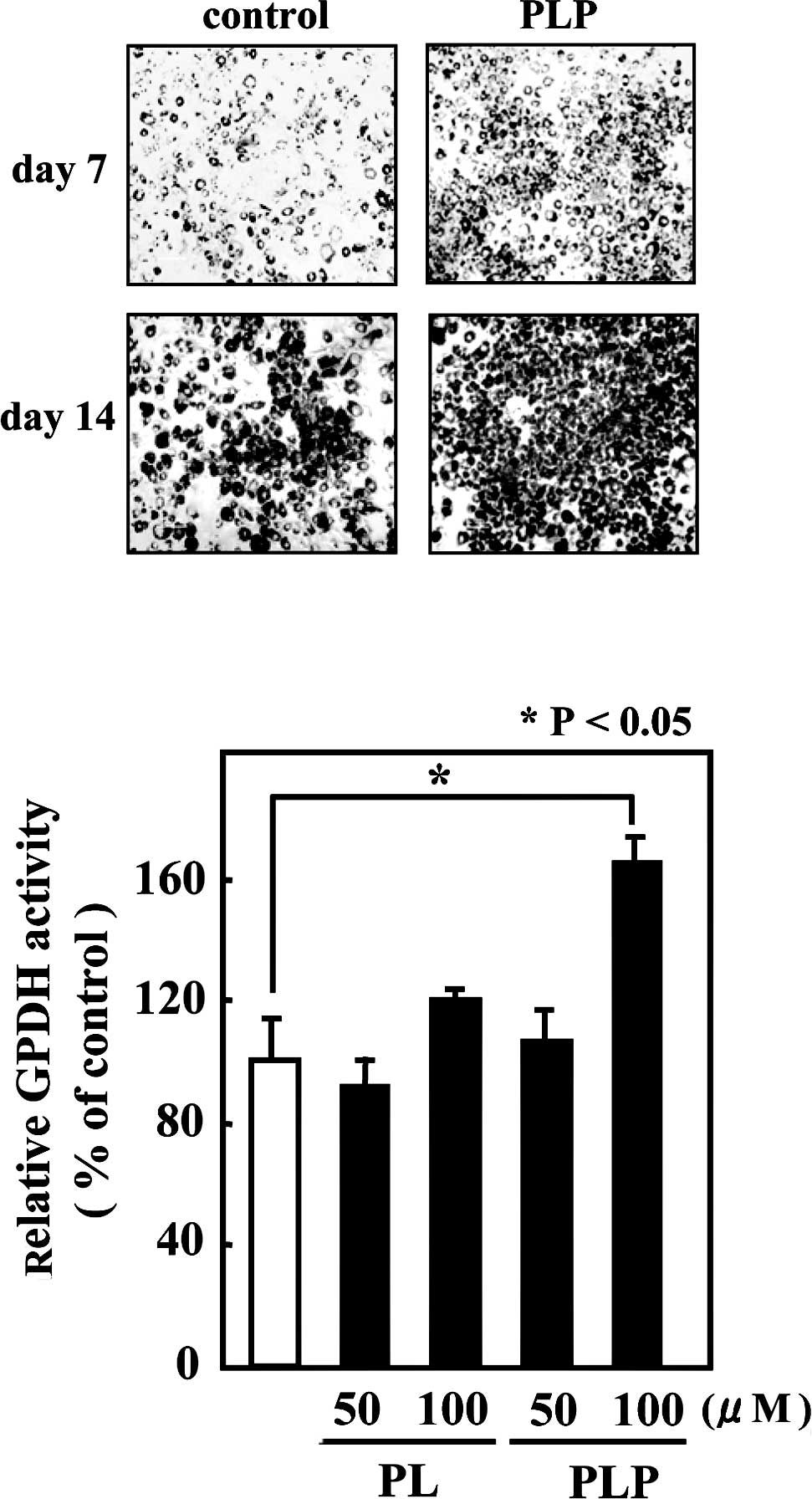
visualization. As shown in Fig.
1A, 3T3-L1 cells treated with PLP accumulated larger and a
greater number of lipid droplets than the control cells. Since
glycerol-3-phosphate dehydrogenase (GPDH) occupies a central
position in the pathway of triglyceride synthesis, GPDH enzyme
activity was measured as an adipocyte differentiation marker. In
agreement with the Oil-Red-O staining, treatment with 100 μM PLP
resulted in a significant increase (by 60%) in GPDH enzyme activity
compared to untreated cells (Fig.
1B).
PLP induces the expression of PPARγ
target genes in 3T3-L1 adipocytes
As described above, PLP promoted adipogenesis in
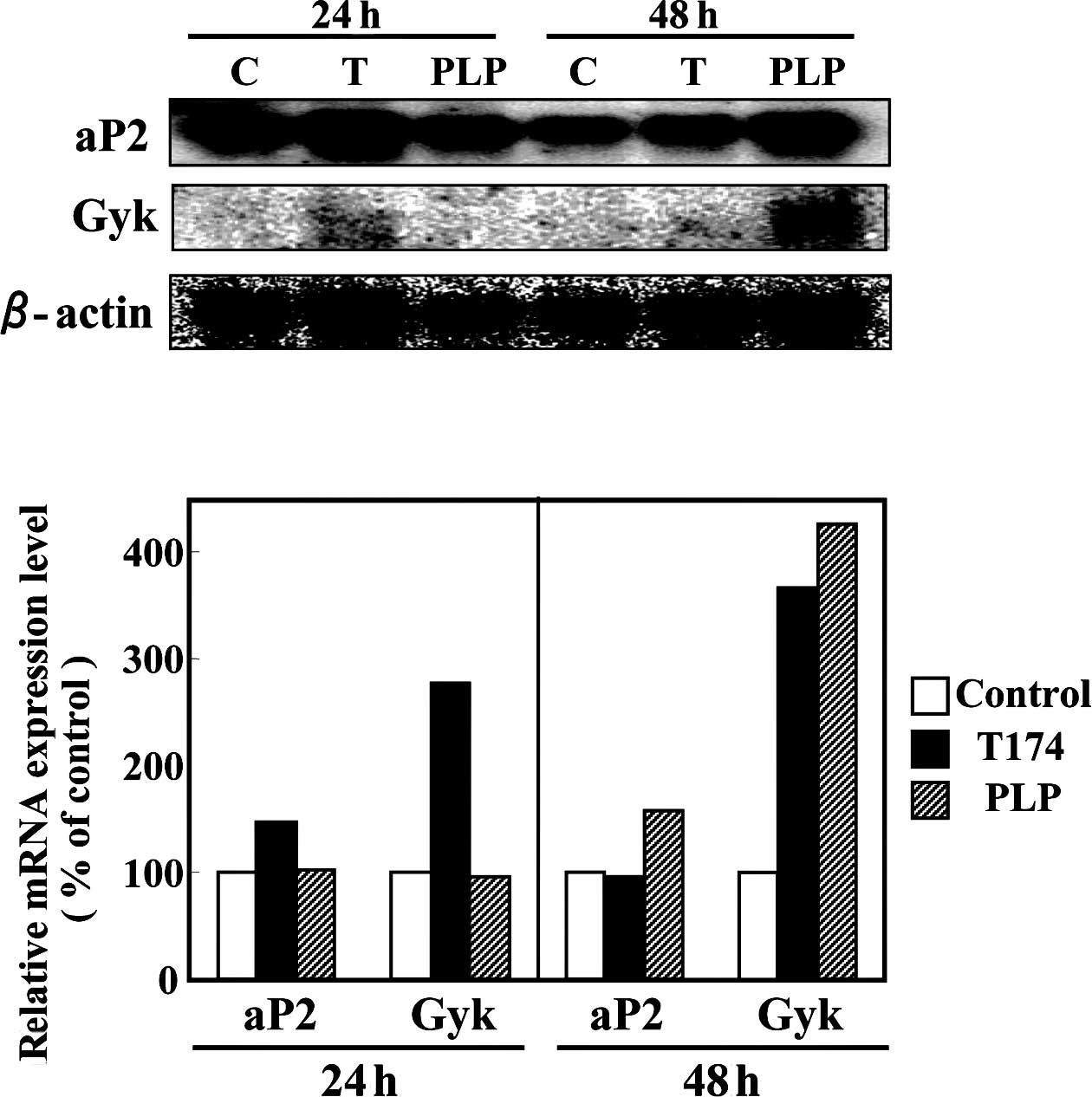
3T3-L1 cells. Subsequently, mRNA expression of PPARγ target genes
was investigated in the differentiated 3T3-L1 adipocytes. On day 6
after differentiation was induced with the hormonal stimulus,
3T3-L1 cells were treated with either a vehicle control (DMSO), 10
µM T-174 or 100 µM PLP for 24 or 48 h. After 24 h, the addition of
10 µM T-174 resulted in an increase in the mRNA expression levels
of PPARγ target genes, aP2 and Gyk (Fig. 2). Although the addition of 100 μM
PLP for 24 h had no influence on the mRNA expression levels of
PPARγ target genes, PLP treatment for 48 h demonstrated 1.6- and
4.3-fold increases in the mRNA expression levels of aP2 and
Gyk genes, respectively (Fig.
2).
PLP induces the expression of a PPARγ
target gene in NIH3T3 cells transfected with PPARγ
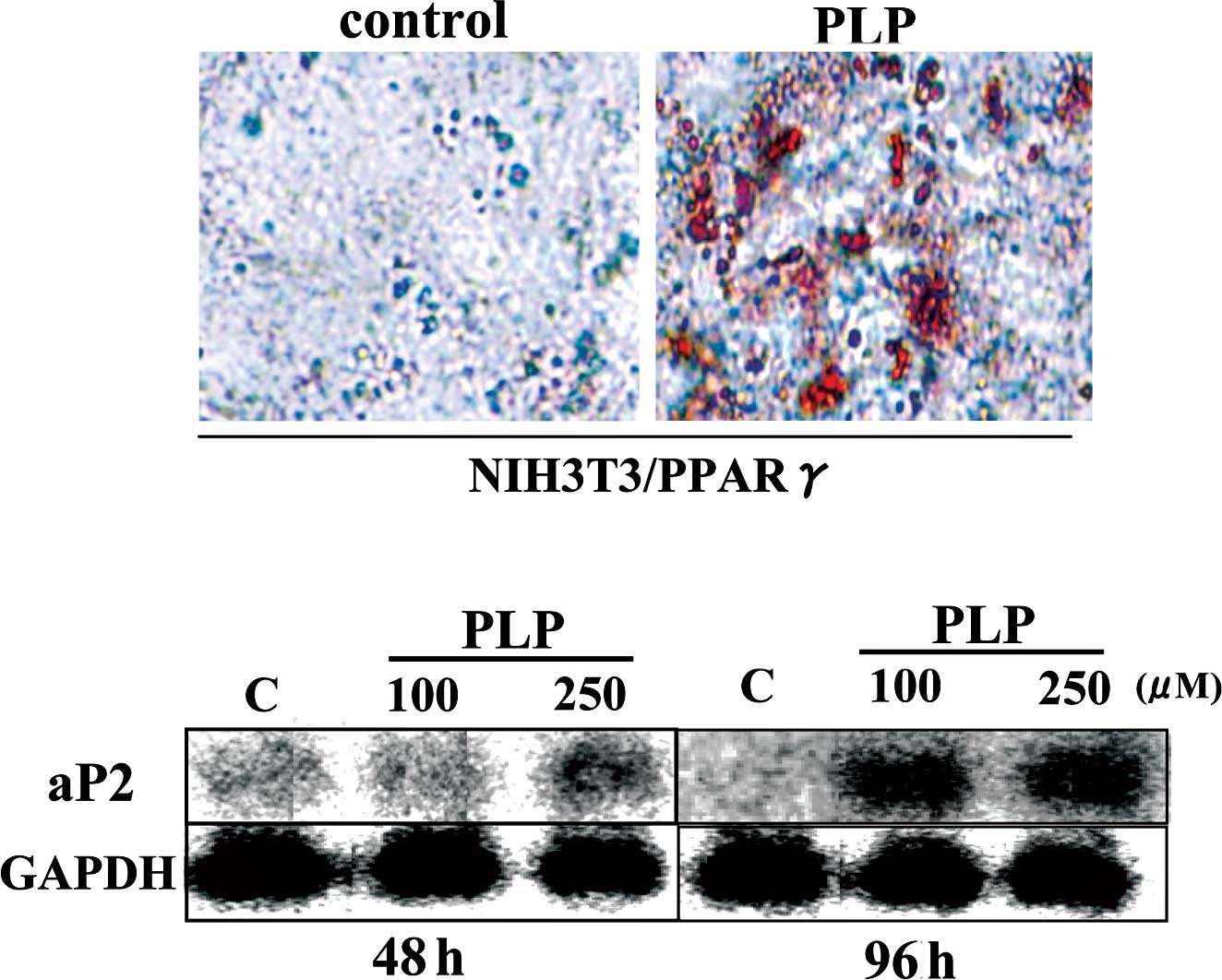
PLP was shown to up-regulate mRNA expression in
PPARγ target genes in differentiated 3T3-L1 adipocytes. To examine
whether PLP activates PPARγ-dependent transcription in cultured
cells, retroviruses harboring PPARγ were used to infect NIH3T3
cells. In PPARγ-transfected NIH3T3 cells, PLP enhanced aP2
mRNA expression and lipid accumulation (Fig. 3). Taken together, these results
showed that PLP activates PPARγ-dependent transcription in cultured
cells.
The effect of acute administration of
vitamin B6 on expression of a PPARγ target gene in mouse adipose
tissues
The present study demonstrated that PLP promotes
adipogenesis in 3T3-L1 cells and the mRNA expression of PPARγ
target genes in differentiated 3T3-L1 adipocytes. Therefore, we
aimed to ascertain whether vitamin B6 regulates the expression of a
PPARγ target gene in vivo and to examine the effect of
vitamin B6 on the transactivation activity of PPARγ in vivo.
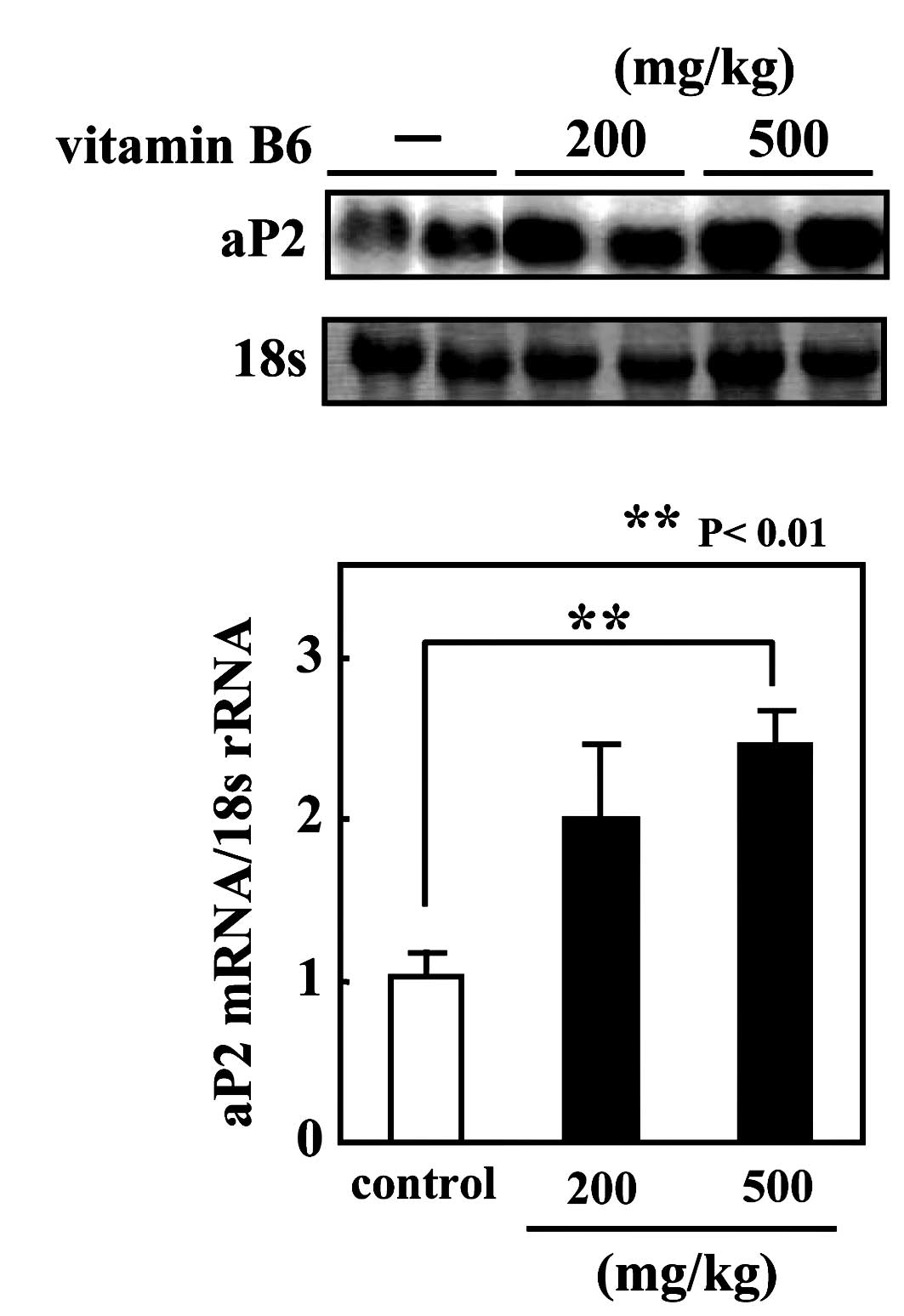
ICR mice were fed a vitamin B6-free diet for 2 weeks, and the
animals subsequently received an oral administration of pyridoxine
hydrochloride (either 200 or 500 mg/kg body weight). Six hours
after oral administration, total RNA was isolated from the
epididymal fat pads. The administration of vitamin B6 significantly
increased aP2 mRNA level in the adipose tissues in a
dose-dependent manner (Fig.
4).
Discussion
In the present study, PLP, one of the vitamin B6
derivatives, was found to promote adipogenesis in 3T3-L1 cells and
up-regulate PPARγ-dependent gene expression in vitro and
in vivo. To address whether the effect of PLP on
adipogenesis is dependent on the transactivation of PPARγ, NIH3T3
cells stably infected with retroviruses encoding PPARγ were
constructed. NIH3T3 cells, which are not committed to adipocytes,
have been well studied and show a similar phenotype to 3T3-L1 cells
when PPARγ is expressed. In fact, adipocyte differentiation of
PPARγ-infected cells was induced in response to T-174 treatment
(data not shown), indicating that NIH3T3 cells stably expressing
PPARγ are well suited for monitoring adipogenesis simply for PPARγ
activation capabilities. In this stable cell line, PLP was shown to
promote lipid accumulation, strongly suggesting that PLP stimulates
PPARγ transactivation.
Two molecular mechanisms by which PLP affects the
expression of PPARγ target genes in a direct or indirect manner
were considered for study. First, as it was previously reported
that thiazolidine compounds are produced by condensation of
aminothiols, such as cysteine, with PLP under a physiological
condition (23), PLP may act as a
PPARγ ligand by conversion to thiazolidine compounds. Indeed, PLP
treatment alone was shown to require more time to induce mRNA
expression of PPARγ target genes compared to T-174 treatment. In
general, PPARγ ligands are well characterized as lipophilic
substances, which contain long-chain polyunsaturated fatty acids
and fatty acid metabolites, such as 15-deoxy-Δ12,14-prostaglandin
J2. PPARγ is thought to have a larger ligand-binding pocket
compared to that of other nuclear receptors (24), which may allow PLP-derived
compounds to act as ligands. However, observations in this study
raise a critical question concerning the selectivity of PLP for
PPARγ target genes. As shown in Fig.
2, PLP was shown to up-regulate aP2 and Gyk mRNA
expression, but not lipoprotein lipase (LPL) (data not shown),
which is also a well-known PPARγ target gene (25), in 3T3-L1 cells. There is increasing
evidence that transcriptional regulation varies even among the
PPARγ-responsive genes via distinct mechanisms (26–28),
and, in fact, several isoprenols were found to induce the
expression of aP2 mRNA, but not LPL mRNA in 3T3-L1 cells
(29), suggesting that PLP may act
as a partial (or weak) agonist similar to the isoprenols.
Second, PLP may influence the interaction of
cofactors with PPARγ, as it has been proposed that ligand-induced
transcriptions of PPARγ target genes are mediated via the
recruitment of distinct cofactors (26–28).
Recently, Huq et al found an important role for vitamin B6
in gene regulation by direct PLP conjugation to receptor
interacting protein 140 (RIP140) (30). RIP140 was originally identified as
a ligand-dependent co-repressor which binds to estrogen receptors,
and, to date, has been shown to interact with and repress a number
of other nuclear receptors, including thyroid hormone receptors and
estrogen-related receptors (31).
Leonardsson et al reported that RIP140-null mice show
defects in fat accumulation and energy expenditure in adipose
tissues, suggesting an important role of RIP140 in regulating the
balance between energy storage and energy expenditure (32). Furthermore, in RIP140-null
adipocytes, energy expenditure was reportedly elevated with high
expression levels of uncoupling protein 1 (Ucp1) and carnitine
palmitoyltransferase 1b mRNAs (33). Debevec et al proposed that
the absence of RIP140 leads to the recruitment of PPARγ, together
with PPARα and estrogen receptor α, to the UCP1 enhancer to allow
activation of Ucp1 gene transcription (34). These observations prompted us to
further consider the physiological significance of the conjugation
between PLP and RIP140 for PPARγ activation in adipocytes.
In summary, these observations presented here show
that vitamin B6 up-regulates the expression of PPARγ target genes
in vitro and in vivo. This study indicates a novel
physiological function of vitamin B6 which may be, in part,
mediated by PPARγ activation. The anti-tumor and anti-inflammatory
effects of vitamin B6, furthermore, may relate to PPARγ activation.
Further study is necessary to elucidate the molecular mechanism
underlying the action of vitamin B6 on PPARγ.
Abbreviations:
|
PPARγ,
|
peroxisome proliferator-activated
receptor-γ;
|
|
TZD,
|
thiazolidinedione;
|
|
PLP,
|
pyridoxal 5′-phosphate;
|
|
PBS,
|
phosphate-buffered saline
|
Acknowledgements
We are grateful to Drs Yasutomi Kamei
and Junko Mizukami for their technical advice. This study was
supported, in part, by a Grant-in-Aid for Scientific Research from
the Ministry of Education, Culture, Sports, Science and Technology
of Japan.
References
|
1.
|
Moon WH and Kirksey A: Cellular growth
during prenatal and early postnatal periods in progeny of
pyridoxine-deficient rats. J Nutr. 103:123–133. 1973.PubMed/NCBI
|
|
2.
|
Haynes WG: Hyperhomocysteinemia, vascular
function and atherosclerosis: effects of vitamins. Cardiovasc Drugs
Ther. 16:391–399. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Cattaneo M, Lombardi R, Lecchi A,
Bucciarelli P and Mannucci PM: Low plasma levels of vitamin B(6)
are independently associated with a heightened risk of deep-vein
thrombosis. Circulation. 104:2442–2446. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Onorato JM, Jenkins AJ, Thorpe SR and
Baynes JW: Pyridoxamine, an inhibitor of advanced glycation
reactions, also inhibits advanced lipoxidation reactions. Mechanism
of action of pyridoxamine. J Biol Chem. 275:21177–21184. 2000.
View Article : Google Scholar
|
|
5.
|
Komatsu SI, Watanabe H, Oka T, Tsuge H,
Nii H and Kato N: Vitamin B-6-supplemented diets compared with a
low vitamin B-6 diet suppress azoxymethane-induced colon
tumorigenesis in mice by reducing cell proliferation. J Nutr.
131:2204–2207. 2001.
|
|
6.
|
Komatsu S, Watanabe H, Oka T, Tsuge H and
Kat N: Dietary vitamin B6 suppresses colon tumorigenesis,
8-hydroxyguanosine, 4-hydroxynonenal, and inducible nitric oxide
synthase protein in azoxymethane-treated mice. J Nutr Sci
Vitaminol. 48:65–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Matsubara K, Mori M, Matsuura Y and Kato
N: Pyridoxal 5′-phosphate and pyridoxal inhibit angiogenesis in
serum-free rat aortic ring assay. Int J Mol Med. 8:505–508.
2001.
|
|
8.
|
Yanaka N, Koyama TA, Komatsu S, Nakamura
E, Kanda M and Kato N: Vitamin B6 suppresses NF-κB activation in
LPS-stimulated mouse macrophages. Int J Mol Med. 16:1071–1075.
2005.
|
|
9.
|
Kersten S, Desvergne B and Wahli W: Roles
of PPARs in health and disease. Nature. 405:421–424. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Braissant O, Foufelle F, Scotto C, Dauca M
and Wahli W: Differential expression of peroxisome
proliferator-activated receptors (PPARs): tissue distribution of
PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology.
137:354–366. 1996.
|
|
11.
|
Chawla A, Schwarz EJ, Dimaculangan DD and
Lazar MA: Peroxisome proliferator-activated receptor (PPAR) gamma:
adipose-predominant expression and induction early in adipocyte
differentiation. Endocrinology. 135:798–800. 1994.
|
|
12.
|
Tontonoz P, Hu E, Graves RA, Budavari AI
and Spiegelman BM: mPPAR gamma 2: tissue-specific regulator of an
adipocyte enhancer. Genes Dev. 8:1224–1234. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lehmann JM, Moore LB, Smith-Oliver TA,
Wilkison WO, Willson TM and Kliewer SA: An antidiabetic
thiazolidinedione is a high affinity ligand for peroxisome
proliferator-activated receptor gamma (PPAR gamma). J Biol Chem.
270:12953–12956. 1995. View Article : Google Scholar
|
|
14.
|
Forman BM, Tontonoz P, Chen J, Brun RP,
Spiegelman BM and Evans RM: 15-Deoxy-delta 12, 14-prostaglandin J2
is a ligand for the adipocyte determination factor PPAR gamma.
Cell. 83:803–812. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Grommes C, Landreth GE and Heneka MT:
Antineoplastic effects of peroxisome proliferator-activated
receptor gamma agonists. Lancet Oncol. 5:419–429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sarraf P, Mueller E, Jones D, King FJ,
DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C
and Spiegelman BM: Differentiation and reversal of malignant
changes in colon cancer through PPARgamma. Nat Med. 4:1046–1052.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tanaka T, Kohno H, Yoshitani S, Takashima
S, Okumura A, Murakami A and Hosokawa M: Ligands for peroxisome
proliferator-activated receptors alpha and gamma inhibit chemically
induced colitis and formation of aberrant crypt foci in rats.
Cancer Res. 61:2424–2428. 2001.
|
|
18.
|
Osawa E, Nakajima A, Wada K, Ishimine S,
Fujisawa N, Kawamori T, Matsuhashi N, Kadowaki T, Ochiai M,
Sekihara H and Nakagama H: Peroxisome proliferator-activated
receptor gamma ligands suppress colon carcinogenesis induced by
azoxymethane in mice. Gastroenterology. 124:361–367. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Welch JS, Ricote M, Akiyama TE, Gonzalez
FJ and Glass CK: PPARgamma and PPARdelta negatively regulate
specific subsets of lipopolysaccharide and IFN-gamma target genes
in macrophages. Proc Natl Acad Sci USA. 100:6712–6717. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Appel S, Mirakaj V, Bringmann A, Weck MM,
Grunebach F and Brossart P: PPAR-gamma agonists inhibit toll-like
receptor-mediated activation of dendritic cells via the MAP kinase
and NF-kappaB pathways. Blood. 106:3888–3894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kawada T, Aoki N, Kamei Y, Maeshige K,
Nishiu S and Sugimoto E: Comparative investigation of vitamins and
their analogues on terminal differentiation, from preadipocytes to
adipocytes, of 3T3-L1 cells. Comp Biochem Physiol A. 96:323–326.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yanaka N, Nogusa Y, Fujioka Y, Yamashita Y
and Kato N: Involvement of membrane protein GDE2 in retinoic
acid-induced neurite formation in Neuro2A cells. FEBS Lett.
581:712–718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Terzuoli L, Leoncini R, Pagani R,
Guerranti R, Vannoni D, Ponticelli F and Marinello E: Some chemical
properties and biological role of thiazolidine compounds. Life Sci.
63:1251–1267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Uppenberg J, Svensson C, Jaki M,
Bertilsson G, Jendeberg L and Berkenstam A: Crystal structure of
the ligand binding domain of the human nuclear receptor PPARgamma.
J Biol Chem. 273:31108–31112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Schoonjans K, Peinado-Onsurbe J, Lefebvre
AM, Heyman RA, Briggs M, Deeb S, Staels B and Auwerx J: PPARalpha
and PPARgamma activators direct a distinct tissue-specific
transcriptional response via a PPRE in the lipoprotein lipase gene.
EMBO J. 15:5336–5348. 1996.PubMed/NCBI
|
|
26.
|
Kodera Y, Takeyama K, Murayama A, Suzawa
M, Masuhiro Y and Kato S: Ligand type-specific interactions of
peroxisome proliferator-activated receptor gamma with
transcriptional coactivators. J Biol Chem. 275:33201–33204. 2000.
View Article : Google Scholar
|
|
27.
|
Robinson CE, Wu X, Nawaz Z, Onate SA and
Gimble JM: A corepressor and chicken ovalbumin upstream promoter
transcriptional factor proteins modulate peroxisome
proliferator-activated receptor-gamma2/retinoid X receptor
alpha-activated transcription from the murine lipoprotein lipase
promoter. Endocrinology. 140:1586–1593. 1990.
|
|
28.
|
Guan HP, Ishizuka T, Chui PC, Lehrke M and
Lazar MA: Corepressors selectively control the transcriptional
activity of PPARgamma in adipocytes. Genes Dev. 19:453–461. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Takahashi N, Kawada T, Goto T, Yamamoto T,
Taimatsu A, Matsui N, Kimura K, Saito M, Hosokawa M, Miyashita K
and Fushiki T: Dual action of isoprenols from herbal medicines on
both PPARgamma and PPARalpha in 3T3-L1 adipocytes and HepG2
hepatocytes. FEBS Lett. 514:315–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Huq MD, Tsai NP, Lin YP, Higgins L and Wei
LN: Vitamin B6 conjugation to nuclear corepressor RIP140 and its
role in gene regulation. Nat Chem Biol. 3:161–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Fritah A, Christian M and Parker MG: The
metabolic coregulator RIP140: an update. Am J Physiol endocrinol
Metab. 299:E335–E340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Leonardsson G, Steel JH, Christian M,
Pocock V, Milligan S, Bell J, So PW, Medina-Gomez G, Vidal-Puig A,
White R and Parker MG: Nuclear receptor corepressor RIP140
regulates fat accumulation. Proc Natl Acad Sci USA. 101:8437–8442.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Christian M, Kiskinis E, Debevec D,
Leonardsson G, White R and Parker MG: RIP140-targeted repression of
gene expression in adipocytes. Mol Cell Biol. 25:9383–9391. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Debevec D, Christian M, Morganstein D,
Seth A, Herzog B, Parker M and White R: Receptor interacting
protein 140 regulates expression of uncoupling protein 1 in
adipocytes through specific peroxisome proliferator activated
receptor isoforms and estrogen-related receptor alpha. Mol
Endocrinol. 21:1581–1592. 2007. View Article : Google Scholar
|