Introduction
Breast cancer is one of the leading causes of
cancer-related morbidity and mortality in women worldwide, and its
incidence has been rapidly increasing in China (1). Most breast cancers are sporadic and
develop in response to the cumulative effect of environmental risk
factors and genetic susceptibility of individuals (2). A family history of breast or ovarian
cancer (BOC) and early age at diagnosis are considered potential
indicators of an underlying genetic predisposition for breast
cancer (BC), and approximately 20% of breast cancers are associated
with a clear family history of the disease (3). The association of a family history
with presentation and outcome of breast cancer has been
investigated in several studies, but the results are conflicting
and few studies were population-based using highly reliable family
history and clinical data.
Several of these studies have reported that tumors
in women with a family history of BC are more likely to be smaller,
poorly differentiated, oestrogen receptor
(ER)-negative/progesterone receptor (PR)-negative, and often
exhibit lymphovasular invasion, which is associated with a less
favorable prognosis (4,5). However, other studies have yielded
mixed results. They did not find evidence that a family history of
BC is associated with tumor size, nodal status, hormone receptor
status or grade (6). Therefore,
there is no consensus regarding the association of
clinicopathological characteristics and outcomes of familial breast
cancer (FBC), and whether this association differs from sporadic
breast cancer (SBC) in regards to prognostic factors.
In the present study, the association of the
prognosis of FBC with clinical and pathological factors, treatment,
recurrence/metastasis-free survival (RFS) and overall survival (OS)
was retrospectively investigated in a Chinese population.
Patients and methods
Patients and follow-up
A total of 693 patients, who were diagnosed with BC
through histopathology and treated at the Department of Breast
Surgery at the Cancer Hospital/Institute, Fudan University
(Shanghai, China) during the period January 1, 1994 to December 31,
2004 were enrolled in the present study. These patients were
divided into an FBC group (348 cases) and an SBC group (345 cases),
and all cases were females without distant metastasis at initial
diagnosis, and with infiltrative carcinoma according to the
inclusion criteria. In addition, all FBC cases were from a family
with ≥2 patients with BOC among the first-degree relatives,
including the proband, regardless of age. The family histories
concerning three-generation pedigrees of the eligible cases were
retrieved from the medical records and standard questionaires,
ascertained by the families and/or the patients personally.
According to the numbers of FBC patients who were
enrolled every year during the study period, similar numbers of SBC
patients were randomly selected in the corresponding year, and a
total of 345 SBC cases without a family history of BOC or other
malignancies in three generations recruited from the Cancer
Hospital of Fudan University were enrolled as controls. All
patients were required to undergo a complete physical examination,
bilateral mammography, chest radioscopy, ECG, ultrasonography of
the breasts, axillary fossa, cervical regions, abdomen and pelvis,
and routine blood and biochemical tests before surgery and
accompanying adjuvant therapy, according to surgical standards. All
patients who presented a risk of relapse received adjuvant
chemotherapy of different regimens for 4–6 cycles followed by local
radiotherapy (if required) and/or hormone therapy (if required)
according to the standard therapy at the time of surgery. This
research project was approved by the Scientific and Ethics
Committee of the Cancer Hospital of Fudan University.
Follow-up data were collected annually from medical
records for breast cancer recurrence, new primary cancers and
death. Personal contact with the patient, through routine
correspondence or telephone visits, was used for follow-up. The
patient follow-up was carried out at the Cancer Hospital of Fudan
University every 3 months during the first 2 years, every 6 months
during the next 2 years and once a year thereafter.
Methods for biological
characteristics
Immunohistochemical status of each postoperative
paraffin-embedded tumor sample was defined through
immunohistochemical staining, including ER, PR and HER2/neu. All of
the primary monoclonal antibodies were purchased from Dako,
Hamburg, Germany. The detailed staining procedures were strictly
followed according to the instructions provided with the reagents.
Negative controls were obtained by incubation of parallel slides
omitting the primary antibodies. Sections known to be stained
positively in each run served as positive controls. The percentage
and intensity score of the stained tumor cells (ER, PR, HER2/neu,
P53, cathepsin-D and PCNA) were determined by at least two
independent pathologists. The percentage was interpreted as
follows: 0, no staining; 1, ≤25% positively stained cells; 2,
25–50% positively stained cells; 3, 50–75% positively stained
cells; and 4, >75% positively stained cells. Regarding the
intensity, the score was as follows: 0, negative; 1, weakly
positive; 2, moderately positive; 3, strongly positive. The
percentage and intensity scores were combined to produce a final
score. For all markers except HER2/neu, a score of 0 was defined as
negative and 1–12 as positive, while merely scores of 9–12 for
strong membranous staining (Dako score 3+) were defined as
positive.
Statistical analysis
The association of clinicopathological factors was
evaluated using Pearson's Chi-square or Fisher's exact tests. The
primary clinical outcomes for this study were RFS and OS. OS was
defined as the time from initial diagnosis of primary BC to death
from any cause, and RFS was defined as the time from initial
diagnosis to local recurrence or metastasis. Survival time was
calculated from the date of surgery to these endpoints, censoring
at the date of last contact and non-breast primaries. The 5-year
survival rate was calculated by the Life Tables method, and
survival curves were obtained by the Kaplan-Meier method. The
log-rank test was used to determine the statistical significance in
comparative survival for a variety of patient and tumor
characteristics. All the statistically significant variables
observed in the univariate analysis were investigated by means of
multivariate analysis using the Cox proportional hazards model. All
P-values <0.05 were considered statistically significant. All
P-values were two-sided. SPSS 15.0 software package (SPSS Inc.) was
used for statistical analysis.
Results
Characteristics of the patients
The FBC group was comprised of 348 cases who were
between 26 and 87 years of age (mean 49.3). The SBC group was
comprised of 345 cases who were between 27 and 87 years of age
(mean 56.3). The mean age of the FBC cases was significantly
younger compared to the SBC cases (51.1±10.4 vs. 53.7±11.0 years),
likely reflecting that the patients with a genetic risk exhibited
an early-onset of disease. The age distributions and
characteristics of the two groups are listed in Table I. The FBC group exhibited a
relatively lower T stage (P=0.00), while the FBC cases did not
differ from the SBC group in terms of the lymph node-positive rate
and grade of tumor differentiation, and there was no significant
differences in ER or PR status when the unknowns were excluded
between the two groups. Regarding hormone receptor (ER or PR)
status, the SBC group had a relatively higher hormone
receptor-positive rate (P=0.05). As for HER2/neu, cathepsin-D and
PCNA, the two groups had a similar amplification status (P=0.0.47
for HER2/neu, P=0.33 for cathepsin-D and P=0.14 for PCNA). With
regard to the method of adjuvant treatment, the SBC individuals
were more likely to receive adjuvant chemotherapy or hormone
therapy compared to the FBC cases (P=0.000 and P=0.000,
respectively), while a similar radiotherapy proportion (P=0.168)
was noted. In addition, no statistically significant differences in
terms of surgery were noted between the two groups.
 | Table I.Clinicopathological characteristics of
the FBC and SBC patients. |
Table I.
Clinicopathological characteristics of
the FBC and SBC patients.
| Characteristics | FBC n=348
| SBC n=345
| P-value |
|---|
| No. (%) | No. (%) |
|---|
| Age (years) | | | 0.054 |
| ≤40 | 54 (18.4) | 36 (10.4) | |
| >40 | 294 (81.6) | 309 (89.6) | |
| Surgery | | | 0.447 |
| Mastectomy | 310 (89.1) | 314 (91.0) | |
| BCS | 38 (10.9) | 31 (9.0) | |
| Tumour size (cm) | | | 0.000 |
| T≤2 | 117 (33.6) | 66 (19.1) | |
| 2<T≤5 | 180 (51.8) | 214 (62.0) | |
| T>5 | 14 (4.0) | 23 (6.7) | |
| Tx | 37 (10.6) | 42 (12.2) | |
| Lymph node
status | | | 0.144 |
| 0 | 180 (51.8) | 174 (50.4) | |
| 1–3 | 74 (21.2) | 52 (15.1) | |
| 4–10 | 45 (12.9) | 26 (7.5) | |
| >10 | 10 (2.9) | 11 (3.2) | |
| Unknown | 39 (11.2) | 82 (23.8) | |
| Grade of
differentiation | | | 0.592 |
| I | 5 (1.4) | 8 (2.3) | |
| II | 142 (40.8) | 175 (50.7) | |
| III | 33 (9.5) | 32 (9.3) | |
| Unknown | 168 (48.3) | 130 (37.7) | |
| ER status | | | 0.388 |
| Negative | 94 (27.0) | 121 (35.1) | |
| Positive | 170 (48.9) | 188 (54.5) | |
| Unknown | 84 (24.1) | 36 (10.4) | |
| PR status | | | 0.108 |
| Negative | 110 (31.6) | 152 (44.1) | |
| Positive | 147 (42.2) | 154 (44.6) | |
| Unknown | 91 (26.2) | 39 (11.3) | |
| HER2/neu status | | | 0.470 |
| Negative | 200 (57.5) | 232 (67.2) | |
| Positive | 51 (14.6) | 69 (20.0) | |
| Unknown | 97 (27.9) | 44 (12.8) | |
| Chemotherapy | | | 0.000 |
| No | 63 (18.1) | 32 (9.3) | |
|
MTX-containing | 93 (26.7) | 56 (16.2) | |
|
Anthrocyclin-containing | 160 (46.0) | 219 (63.5) | |
|
Taxane-containing | 6 (1.7) | 3 (0.9) | |
| Others | 12 (3.5) | 15 (4.3) | |
| Unknown | 14 (4.0) | 20 (5.8) | |
| Radiation
therapy | | | 0.168 |
| No | 314 (90.2) | 291 (84.3) | |
| Yes | 24 (6.9) | 33 (9.6) | |
| Unknown | 10 (2.9) | 21 (6.1) | |
| Hormone
therapy | | | 0.000 |
| No | 258 (74.2) | 142 (41.2) | |
| Yes | 85 (24.4) | 198 (57.4) | |
| Unknown | 5 (1.4) | 5 (1.4) | |
| P53 | | | 0.000 |
| Negative | 94 (27.0) | 172 (49.9) | |
| Positive | 138 (39.7) | 127 (36.8) | |
| Unknown | 116 (33.3) | 46 (13.3) | |
| Cathepsin-D | | | 0.332 |
| Negative | 52 (15.0) | 63 (18.3) | |
| Positive | 156 (44.8) | 237 (68.7) | |
| Unknown | 140 (40.2) | 45 (13.0) | |
| PCNA | | | 0.141 |
| Negative | 219 (62.9) | 163 (47.2) | |
| Positive | 80 (23.0) | 43 (12.5) | |
| Unknown | 49 (14.1) | 139 (40.3) | |
The median follow-up was 55.7 months (range 6–120);
52.3 months for the FBC group vs. 61.1 months for the SBC group. A
total of 46 patients were lost during the follow-up period.
Univariate survival analysis
The 5-year OS was 94 vs. 98%, and the 5-year RFS was
85 vs. 87% in the FBC and SBC groups, respectively. Upon comparison
between the two groups, different outcomes were revealed.
The patients with a family history of BOC had a
significantly higher risk of recurrence/metastasis (P=0.04) and a
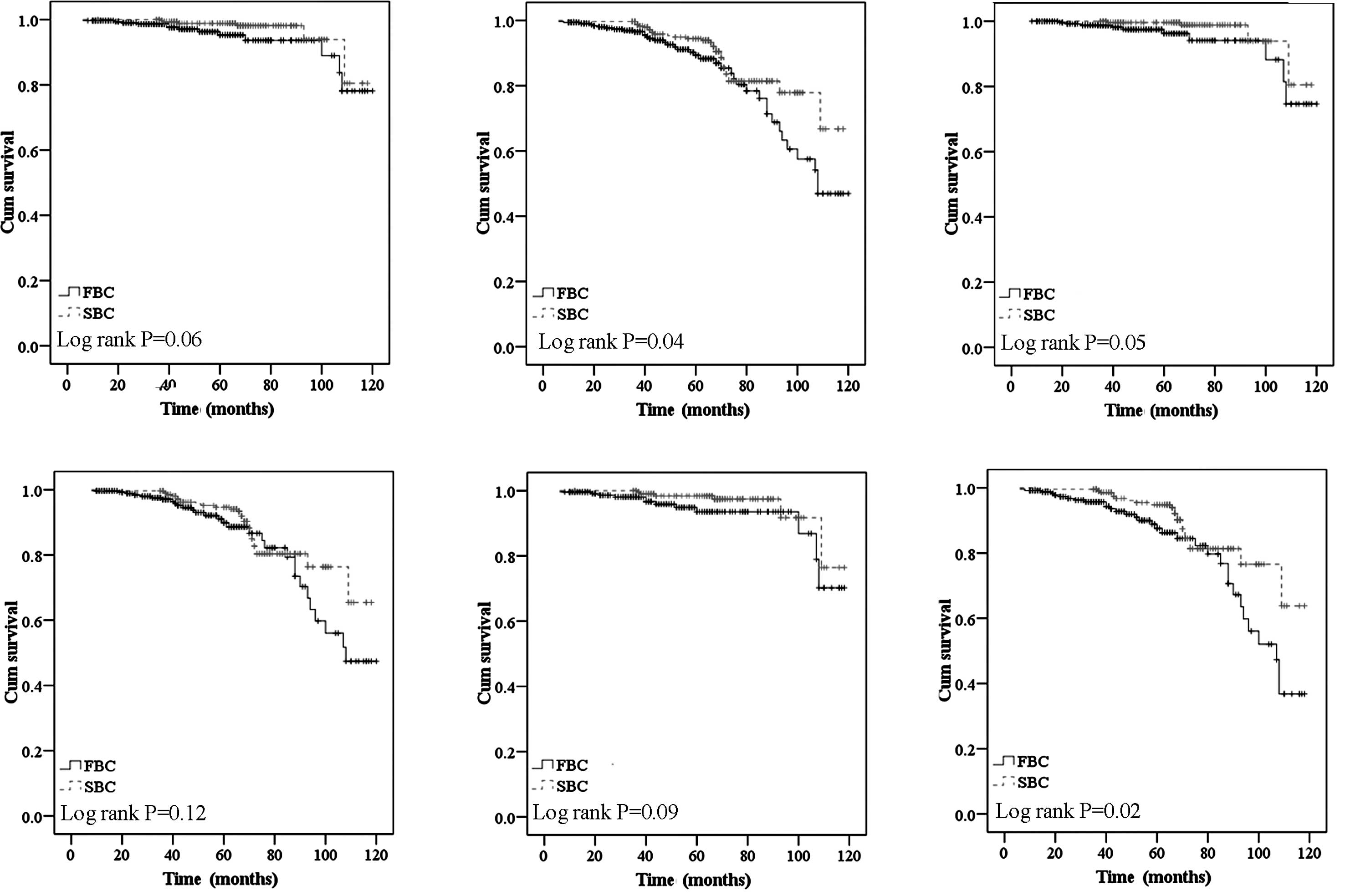
non-significantly increased risk of death (P=0.06) (Fig. 1A and B) regardless of age and TNM
stage. Family history was one of the major predictors of worse
survival in the elder (>40 years of age) group, the higher TNM
stage group and the hormone receptor-positive group. After
adjustment for age, a borderline significant difference was
observed in the elder subgroup (>40 years) (P=0.06 for OS and
P=0.12 for RFS) (Fig. 1C and D).
When TNM stage was included in the analysis, the FBC patients
exhibited a significantly increased risk for RFS and decreased OS
compared to the SBC cases in the higher TNM stage subgroup (P=0.09
for OS and P=0.02 for RFS) (Fig. 1E
and F). In univariate analysis, there was also an increased
risk of recurrence/metastasis for the FBC women when compared to
the SBC cases in the subgroup with hormone receptor-positive tumors
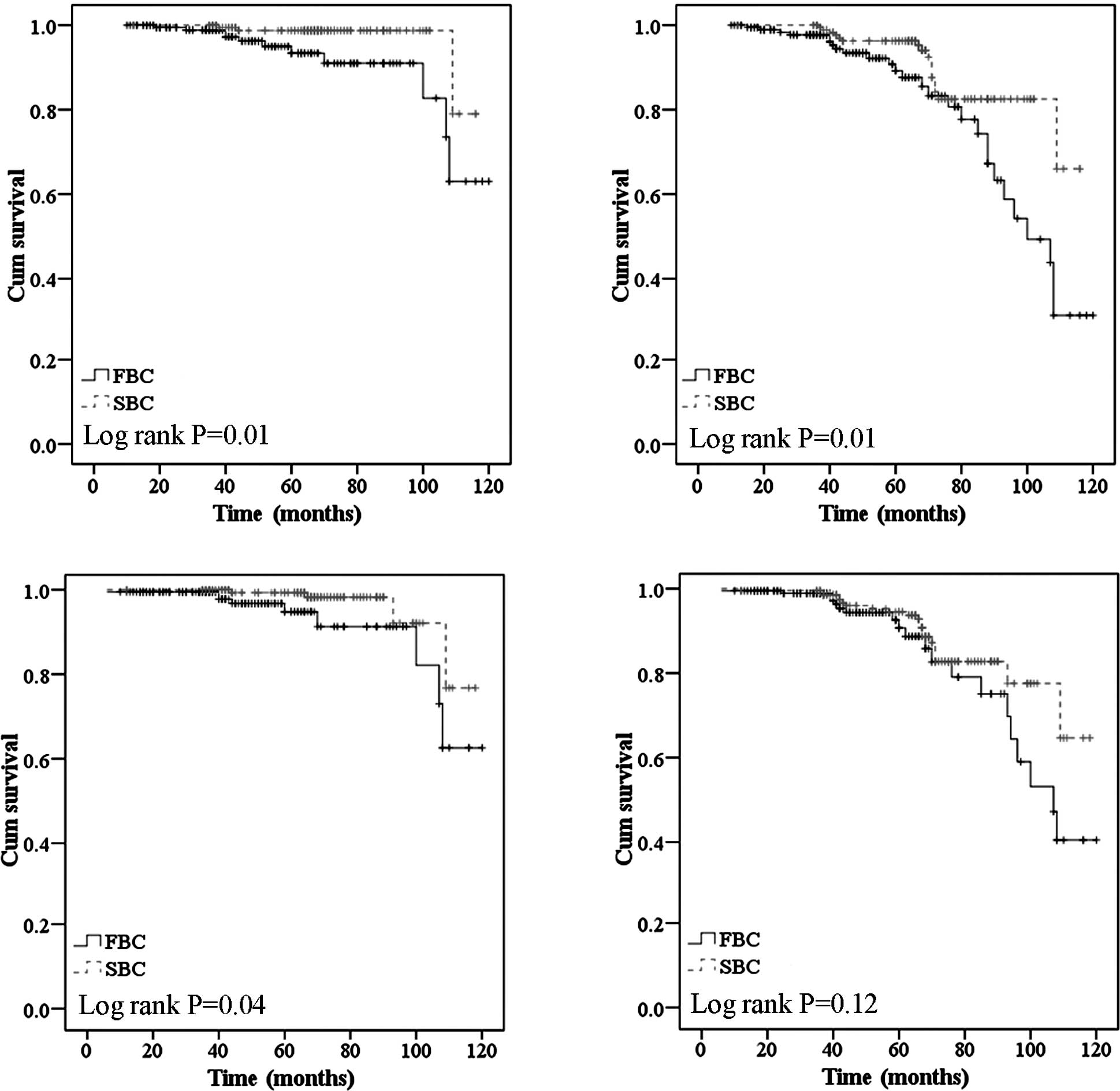
(P=0.01 for OS, and P=0.01 for RFS) (Fig. 2A and B). Overexpression of HER2/neu
is considered to be an unfavorable predictor for RFS or OS in
breast cancer patients, but the difference was more significant in
the FBC patients with HER2/neu-negative status (P=0.04 for OS and
P=0.12 for RFS) in the present analysis (Fig. 2C and D).
The response rate of systemic adjuvant therapy in
the FBC patients was lower than that of the SBC cases, particularly
for chemotherapy and hormone therapy. The familial BC women who
received chemotherapy had a higher rate of death and
recurrence/metastasis (P=0.03) compared to their counterparts
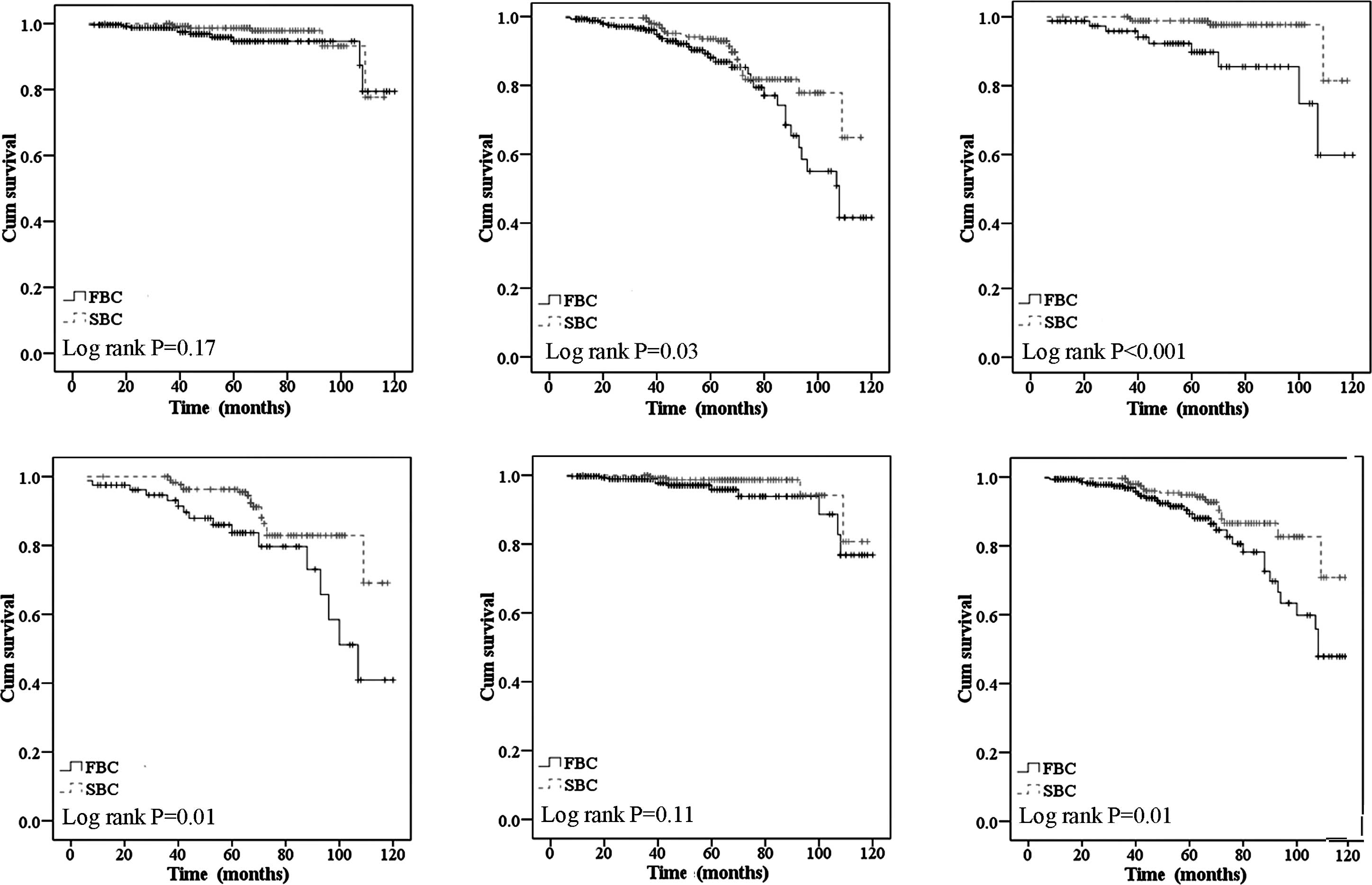
(Fig. 3A and B). Regarding the
patients who received hormone therapy, the difference was more
significant (P=0.00 for OS and P=0.01 for RFS) (Fig. 3C and D). Additionally, when
administered hormone therapy after chemotherapy, the FBC group
experienced more recurrence/metastasis events, and the OS
difference reached statistical significance (data not shown).
Multivariate survival analysis
Upon univariate survival analysis, a significantly
increased risk for RFS and decreased OS was observed for the
familial cases compared to the SBC group in the hormone
receptor-positive population (P=0.01 for OS and P=0.01 for RFS) in
contrast to the hormone receptor-negative population (P=0.82 for OS
and P=0.23 for RFS); therefore, multivariate survival analysis was
further performed in the hormone receptor-positive population.
Family history of BOC was identified to be an independent
predictive factor for both the recurrence/metastasis rate (P=0.01,
HR=0.012, 95% CI 0.02–0.57) and mortality (P=0.044, HR=0.43, 95% CI
0.19–0.98) (Table II).
 | Table II.Cox's proportional hazards regression
model for the general hormone receptor-positive population. |
Table II.
Cox's proportional hazards regression
model for the general hormone receptor-positive population.
| Variables | P-value | HR | 95% CI for hormone
receptor |
|---|
| For general OS | | | |
| Without family
history | 0.044 | 0.43 | 0.19–0.98 |
| Age (years)
≥40 | 0.099 | 0.28 | 0.06–1.27 |
| Higher TNM
stage | 0.023 | 5.31 | 0.60–8.88 |
| HER2/neu
negative | 0.187 | 0.33 | 0.06–1.72 |
| Chemotherapy | 0.947 | 0.94 | 0.18–5.04 |
| Radiotherapy | 0.275 | 0.26 | 0.02–2.92 |
| Hormone
therapy | 0.137 | 0.78 | 0.23–2.60 |
| For general
RFS | | | |
| Without family
history | 0.010 | 0.32 | 0.02–0.57 |
| Age (years)
≥40 | 0.612 | 0.79 | 0.32–1.97 |
| Higher TNM
stage | 0.040 | 1.81 | 0.75–4.39 |
|
HER2/neu-negative | 0.737 | 0.87 | 0.39–1.94 |
| Chemotherapy | 0.874 | 1.11 | 0.32–3.85 |
| Radiotherapy | 0.190 | 1.05 | 0.42–2.65 |
| Hormone
therapy | 0.299 | 0.67 | 0.32–1.42 |
Higher TNM stage (P=0.023, HR=5.31, 95% CI 0.60–8.88
for OS and P=0.04, HR=1.81, 95% CI 0.75–4.39 for RFS) indicated a
poor prognosis for OS, no matter whether the patients had a family
history of BOC (P=0.012, HR=4.42, 95% CI 0.45–6.70 in the FBC group
and P=0.03, HR=3.33, 95% CI 1.39–4.79 in the SBC group; Table III), whereas HER2/neu amplification
did not predict a worse survival (P=0.19 for OS and P=0.74 for
RFS).
 | Table III.Cox's proportional hazards regression
model for the FBC and SBC hormone receptor-positive groups. |
Table III.
Cox's proportional hazards regression
model for the FBC and SBC hormone receptor-positive groups.
| Variables | P-value | HR | 95% CI for hormone
receptor |
|---|
| For FBC | | | |
| Age (years)
≥40 | 0.436 | 0.48 | 0.08–3.01 |
| Higher TNM
stage | 0.019 | 4.42 | 0.45–6.70 |
|
HER2/neu-negative | 0.408 | 0.49 | 0.09–2.67 |
| Chemotherapy | 0.824 | 0.83 | 0.16–4.40 |
| Radiotherapy | 0.440 | 0.38 | 0.03–4.51 |
| Hormone
therapy | 0.932 | 1.04 | 0.44–2.46 |
| For SBC | | | |
| Age (years)
≥40 | 0.561 | 0.52 | 0.06–4.72 |
| Higher TNM
stage | 0.032 | 3.33 | 1.39–4.79 |
|
HER2/neu-negative | 0.759 | 0.78 | 0.16–3.90 |
| Chemotherapy | 0.059 | 0.10 | 0.01–1.09 |
| Radiotherapy | 0.071 | 0.35 | 0.01–1.11 |
| Hormone
therapy | 0.035 | 0.31 | 0.08–1.22 |
Moreover, adjuvant therapy had a tendency to reduce
the risk of recurrence/metastasis (P=0.060, HR=0.69, 95% CI
0.4–1.02) in the SBC group, but not in the FBC group. Although
there was similar rate (P=0.10) of receiving chemotherapy between
the two groups in the hormone receptor-positive population, a
borderline difference was still observed in the SBC individuals
(P=0.06), while in the FBC group the P-value became 0.82. A similar
consequence was noted in the patients who received radiotherapy
(P=0.07). Hormone therapy had a tendency to reduce the risk of
recurrence/metastasis (P=0.229, HR=0.67, 95% CI 0.32–1.42) in the
general hormone receptor-positive population, which was
demonstrated in the SBC group (P=0.035, HR=0.31, 95% CI 0.08–1.22),
but not in the FBC group (P=0.93, HR=1.04, 95% CI 0.44–2.46).
In our cohort, HER2/neu overexpression did not
demonstrate the opposite prognostic value in both groups (P=0.41 in
the FBC group and P=0.076 in the SBC group; Table III).
As expected, the Cox's proportional hazard
regression model for the FBC and SBC groups was consistent with the
findings revealed in the univariate analysis.
Discussion
Several risk factors for the etiology of breast
cancer have also been correlated with the prognosis of breast
cancer (7). As compared to SBCs, a
positive family history of BOC appears to be a risk factor for the
development of breast cancer, and in medical practice, a family
history of cancers is vital information. Most familial cases are
thought to be caused by an inherited genetic disorder (8). Although many studies have focused on
germline mutations of breast cancer susceptibility genes which may
contribute to FBCs, knowledge concerning the outcome of FBC remains
to be elucidated.
Several studies have reported that individuals with
a family history of BOC are more likely to present with smaller
tumors and a higher grade of malignancy than those without a family
history (9), while others studies
have failed to find these associations (10,11).
Women with a family history of BOC in our study were characterized
by early disease onset and relatively smaller tumor sizes. Two
possible explanations may be i) that these women were affected by
genetic factors and predisposed to an early-onset of breast cancer
possibly attributable in part to more frequent BRCA1 or BRCA2
mutations in the familial women (8); and ii) that individuals with a
familial history of BOC prefer to undergo early and regular routine
screening of the breasts and present with a small tumor size at the
initial diagnosis (12).
Additionally, our results also showed that the familial tumors had
a similar lymph node-positive rate, differentiation grade and
hormone receptor expression status as the sporadic ones. There were
no differences in clinical management when a family history of BOC
was present. The two groups had a similar rate of receiving
breast-conserving (BCS) therapy, and we did not observe more
frequent recurrence/metastasis or death events in the BCS
population (data not shown). This suggests that a family history of
BOC did not have an impact on the treatment decisions.
The Chinese women with a family history of BOC were
significantly younger of diagnosis, and appeared to have a worse
RFS in contrast to their SBC counterparts, suggesting that a family
history had a negative effect on RFS, but not OS. However, this
prognostic impact of family history was more significant after
stratification for age, TNM stage and different adjuvant therapies.
In multivariate analysis using the Cox regression model with
factors such as age, TNM stage, HER2/neu status and adjuvant
treatment methods, age ≥40 years became a favorable factor in this
model, with a relative risk ratio of 0.28 for OS and 0.78 for RFS,
and no obvious effect of adjuvant chemotherapy or hormone therapy
was detectable in the hormone receptor-positive population.
Breast cancer in young women often presents with
more unfavourable characteristics and a lower response rate to
systemic adjuvant therapy, which results in a poor prognosis. These
observations have been validated by several large population-based
studies (13–17). Upon univariate, adverse prognostic
effects of family history appeared to be restricted to women with
hormone-responsive breast cancer, the majority of whom received
adjuvant chemotherapy. As the FBC women in the hormone
receptor-positive population did not gain as much benefit from
hormone therapy as their SBC counterparts, this poor outcome may
have been due to the more young breast cancer cases and more
inherent aggressive tumor phenotypes (such as HER2/neu
overexpression) were present in the FBC group, which would probably
lead to their resistance to tamoxifen treatment (18,19).
Although we found that p53 had a significantly
abnormal expression in the FBC group, the levels of p53 did not
become an independent prognostic factor in the present study (data
not shown). Finally, HER2/neu overexpression is believed to be an
important factor in breast cancer survival prediction (20), but we failed to identify its effect
in both groups. Considering that our findings were achieved in a
limited sample of hormone receptor-positive tumors and the
introduction of trastuzumab and similar targeted drugs against
HER2/neu has dramatically altered the prognosis in patients with
tumors expressing HER2/neu, the worse prognosis for patients with
HER2-expressing tumors in our series during a period before the
widespread use of trastuzumab should be taken into account.
In our retrospective study, we constructed
three-generation cancer pedigrees and verified cases of BOC in
relatives where possible, and then we focused on first-degree
family history which has been shown to be valid and reproducible
(21). Even though a detailed
investigation in Chinese women was carried out, many more patients
should be recruited in this cohort, and a longer follow-up time
exceeding 10 years is required to improve the statistical power. In
addition, the mutation frequency of BRCA1/BRCA2 in the Chinese FBC
cases was reported to be more than 10% (1), and histopathological studies of
BRCA-associated breast cancers have revealed features generally
associated with a poor prognosis (22,23).
However we were unable to assess the BRCA1/BRCA2 mutation status in
our cohort in view of that few FBC cases had undergone BRCA
mutation testing; further studies should be carried out on
BRCA1/BRCA2 mutation and prognosis of familial breast cancer
patients in China as well as the targeted therapy related to this
point.
We found that Chinese women with a family history of
BOC had a significantly worse prognosis, but this should be further
validated in larger datasets having greater statistical power to
see whether a modest survival benefit is present in SBC cases. A
prognostic effect of a family history identified in our analysis
was not consistent with a number of other studies. Although it has
never been shown that family history is related to shorter survival
in a single population-based study (24), a significantly longer survival was
demonstrated in two other studies (4,25).
However, the latter studies were restricted to a small sample of
young pre-menopausal Caucasian women. In addition, there were
differences in the analyzed ethnic groups, data quality and
statistical power, even in the definition of family history.
Although various studies have reported their own
observations, there is still no worldwide consensus with regard to
the relationship of family history and RFS in breast cancer, and
also the specific risk factors for familial women (26,27).
It is of interest to attempt to elucidate the possible differences
in the genetic alterations in tumors of familial patients. Then, a
comprehensive knowledge of the potential clinical importance of
various aspects of genetic risk will help improve our understanding
of the biological heterogeneity in breast cancer, and thus may lead
to better clinical management of breast cancer patients.
Acknowledgements
The authors thank the patients for
their willingness to cooperate with our study. This study was
supported in part by grants from the Shanghai Science and
Technology Committee (06DJ14004, 06DZ19504).
References
|
1.
|
Cao AY, Hu Z and Shao ZM: Mutation
screening of breast cancer susceptibility genes in Chinese
high-risk families: the results will develop the genetic testing
strategy in China. Breast Cancer Res Treat. 120:271–272. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Campeau PM, Foulkes WD and Tischkowitz MD:
Hereditary breast cancer: new genetic developments, new therapeutic
avenues. Hum Genet. 124:31–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Easton DF: Familial risks of breast
cancer. Breast Cancer Res. 4:179–181. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Mohammed SN, Smith P, Hodgson SV, Fentiman
IS, Miles DW, Barnes DM, Millis RR and Rubens RD: Family history
and survival in premenopausal breast cancer. Br J Cancer.
77:2252–2256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Verkooijen HM, Chappuis PO, Rapiti E,
Vlastos G, Fioretta G, Sarp S, Sappino AP, Schubert H and Bouchardy
C: Impact of familial risk factors on management and survival of
early-onset breast cancer: a population-based study. Br J Cancer.
94:231–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Russo A, Herd-Smith A, Gestri D, Bianchi
S, Vezzosi V, Rosselli Del Turco M and Cardona G: Does family
history influence survival in breast cancer cases? Int J Cancer.
99:427–430. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Chun J, Pocock B, Joseph KA, El-Tamer M,
Klein L and Schnabel F: Breast cancer risk factors in younger and
older women. Ann Surg Oncol. 16:96–99. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Meiser B, Tucker K, Friedlander M,
Barlow-Stewart K, Lobb E, Saunders C and Mitchell G: Genetic
counselling and testing for inherited gene mutations in newly
diagnosed patients with breast cancer: a review of the existing
literature and a proposed research agenda. Breast Cancer Res.
10:2162008. View
Article : Google Scholar
|
|
9.
|
Nomizu T, Tsuchiya A, Kanno M, Katagata N,
Watanabe F, Yamaki Y, Abe R and Miki Y: Clinicopathological
features of hereditary breast cancer. Breast Cancer. 4:239–242.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Atchley DP, Albarracin CT, Lopez A, Valero
V, Amos CI, Gonzalez-Angulo AM, Hortobagyi GN and Arun BK: Clinical
and pathologic characteristics of patients with BRCA-positive and
BRCA-negative breast cancer. J Clin Oncol. 26:4282–4288. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hamann U and Sinn HP: Survival and tumor
characteristics of German hereditary breast cancer patients. Breast
Cancer Res Treat. 59:185–192. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Moller P, Evans DG, Reis MM, Gregory H,
Anderson E, Maehle L, Lalloo F, Howell A, Apold J, Clark N,
Lucassen A and Steel CM: Surveillance for familial breast cancer:
differences in outcome according to BRCA mutation status. Int J
Cancer. 121:1017–1020. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Anders CK, Hsu DS, Broadwater G, Acharya
CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG,
Nevins JR, Potti A and Blackwell KL: Young age at diagnosis
correlates with worse prognosis and defines a subset of breast
cancers with shared patterns of gene expression. J Clin Oncol.
26:3324–3330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Han W, Kim SW, Park IA, Kang D, Kim SW,
Youn YK, Oh SK, Choe KJ and Noh DY: Young age: an independent risk
factor for disease-free survival in women with operable breast
cancer. BMC Cancer. 4:822004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Livi L, Meattini I, Saieva C, Borghesi S,
Scotti V, Petrucci A, Rampini A, Marrazzo L, Di Cataldo V, Bianchi
S, Cataliotti L and Biti G: The impact of young age on breast
cancer outcome. Eur J Surg Oncol. 36:639–645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Maggard MA, O'Connell JB, Lane KE, Liu JH,
Etzioni DA and Ko CY: Do young breast cancer patients have worse
outcomes? J Surg Res. 113:109–113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Jobsen JJ, Meerwaldt JH and van der Palen
J: Family history in breast cancer is not a prognostic factor?
Breast. 9:83–87. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Figueiredo JC, Ennis M, Knight JA,
McLaughlin JR, Hood N, O'Malley F, Andrulis IL and Goodwin PJ:
Influence of young age at diagnosis and family history of breast or
ovarian cancer on breast cancer outcomes in a population-based
cohort study. Breast Cancer Res Treat. 105:69–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tang LC, Yin WJ, Di GH, Shen ZZ and Shao
ZM: Unfavourable clinicopathologic features and low response rate
to systemic adjuvant therapy: results with regard to poor survival
in young Chinese breast cancer patients. Breast Cancer Res Treat.
122:95–104. 2010. View Article : Google Scholar
|
|
20.
|
Dawood S, Broglio K, Buzdar AU, Hortobagyi
GN and Giordano SH: Prognosis of women with metastatic breast
cancer by HER2 status and trastuzumab treatment: an
institutional-based review. J Clin Oncol. 28:92–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Murff HJ, Spigel DR and Syngal S: Does
this patient have a family history of cancer? An evidence-based
analysis of the accuracy of family cancer history. JAMA.
292:1480–1489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Marcus JN, Watson P, Page DL, Narod SA,
Lenoir GM, Tonin P, Linder-Stephenson L, Salerno G, Conway TA and
Lynch HT: Hereditary breast cancer: pathobiology, prognosis, and
BRCA1 and BRCA2 gene linkage. Cancer. 77:697–709. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Robson M, Gilewski T, Haas B, Levin D,
Borgen P, Rajan P, Hirschaut Y, Pressman P, Rosen PP, Lesser ML,
Norton L and Offit K: BRCA-associated breast cancer in young women.
J Clin Oncol. 16:1642–1649. 1998.PubMed/NCBI
|
|
24.
|
Slattery ML, Berry TD and Kerber RA: Is
survival among women diagnosed with breast cancer influenced by
family history of breast cancer? Epidemiology. 4:543–548. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Malone KE, Daling JR, Weiss NS, McKnight
B, White E and Voigt LF: Family history and survival of young women
with invasive breast carcinoma. Cancer. 78:1417–1425. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Rapiti E, Fioretta G, Verkooijen HM,
Vlastos G, Schäfer P, Sappino AP, Kurtz J, Neyroud-Caspar I and
Bouchardy C: Survival of young and older breast cancer patients in
Geneva from 1990 to 2001. Eur J Cancer. 41:1446–1452. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Schouten LJ, Hupperets PS, Jager JJ,
Volovics L, Wils JA, Verbeek AL and Blijham GH: Prognostic
significance of etiological risk factors in early breast cancer.
Breast Cancer Res Treat. 43:217–223. 1997. View Article : Google Scholar : PubMed/NCBI
|