Introduction
Polycystic ovary syndrome (PCOS) is the most common
female endocrinopathy, affecting 5–10% of the female population. It
is an etiologically heterogeneous condition that involves
overproduction of ovarian androgens leading to a heterogeneous
range of symptoms, including hirsutism, acne, anovulation and
infertility (1).
There are many reports pointing to causal links
between PCOS and cardiovascular disease (CVD). Although the level
of risk for CVD remains uncertain in PCOS, there is substantial
evidence that insulin resistance, obesity, dyslipidemia,
hypertension, hypercoagulable state and markers of abnormal
vascular function possibly contribute to increased CVD risk
(2).
Platelets are important in bleeding and coagulation
disorders. The mean platelet volume (MPV), an accurate measure of
platelet size, is considered a marker and determinant of platelet
function. Larger platelets with higher MPV values are
hemostatically more reactive and produce higher amounts of the
prothrombotic factor Thromboxane A2, increasing propensity to
thrombosis (3). MPV has been
reported to be increased in patients with coronary heart disease,
diabetes, atherosclerosis, hypertension and PCOS (4–8).
This study is the first to investigate the
relationship between ovarian volume (OV) and MPV in women with
PCOS. Hormonal parameters and lipid profile of cases, and their
relationship to OV were also assessed.
Patients and methods
This prospective study was performed between January
2008 and August 2010 at the Department of Obstetrics and
Gynecology, Fatih University, Faculty of Medicine. One hundred
regularly menstruating healthy non-hirsute, normoovulatory women
and 210 newly diagnosed PCOS patients were enrolled into the study
for determination of the threshold value of OV for diagnosis of
PCOS in Turkish women. All the participants were healthy women
without any systemic disease. All women gave informed consent.
Approval for this study was obtained from the Local Institutional
Review Board of the Faculty of Medicine, Fatih University.
Rotterdam criteria were used for the diagnosis of
PCOS (9). The presence of two of
three of the following criteria were used for the diagnosis of
PCOS: i) oligo and/or anovulation, ii) clinical and/or biochemical
signs of hyperandrogenism, and iii) echographic PCO, after the
exclusion of other pathologies with a similar clinical
presentation. Any patient known to have hypertension, diabetes,
using anti-coagulant therapy or having a propensity to thrombotic
or bleeding disorders was excluded from the study group. Cases with
a history of ovarian surgery, having received hormonal treatment in
the previous 3 months or for PCOS-related treatment before this
research were excluded from the study.
Transvaginal ultrasound examination was performed to
evaluate the ovaries using a Logic 200 Pro (GE Healthcare, UK) with
a 6.5-MHz transvaginal probe. Regularly menstruating women were
scanned in the early follicular phase (cycle days 3–5).
Oligomenorrheic or amenorrheic women were scanned between days 3
and 5 after a progestin-induced withdrawal bleeding. Three
diameters of ovaries were measured. OV was estimated using a
simplified formula for the volume of a prolate ellipsoid: V = 0.523
× length × height × width. The mean volume of bilateral ovaries was
recorded for study.
Cutoff values for OV were determined by receiver
operating characteristics (ROC) curve analysis. Sensitivity against
(1 - specificity) was plotted at each level, and the area under the
curve (AUC), which reflects the probability of correctly
identifying controls and PCOS patients, was calculated. After
determination of the cutoff point for a Turkish population,
patients with PCOS were divided into three subgroups according to
OV. Age, weight and height of all of the cases were recorded and
body mass index (BMI) was calculated.
Platelet count and MPV were performed as part of
each full blood count. All samples were analyzed on a
Beckman/Coulter MAXM Hematology Analyzer (Beckman Coulter, CA, USA)
2–6 h after collection, to minimize changes in platelet size. The
MPV reference range was 7.8–11.0 fL. The other parameters measured
in fasting blood samples were glucose, total cholesterol,
high-density lipoprotein cholesterol (HDLC), low-density
lipoprotein cholesterol (LDL-C), triglyceride, FSH, LH, E2, PRL,
TSH, total testosterone and DHEAS.
A power analysis based on MPV values was conducted
before recruitment. Using a level of 0.05, a power of 80% and
effect size of 0.40, a sample size of 100 individuals per group was
required to detect a 10% difference between groups. All the data
were analyzed on a personal computer using the SPSS 13.0 for
Windows statistical package (SPSS Inc., Chicago, IL, USA). Normal
distribution of the measurement values as a convenience were
examined graphically using the Shapiro-Wilk test. The data are
presented as proportion and the means ± SD. The three PCOS
subgroups that were formed according to OV were compared using the
Kruskal-Wallis test. Significant differences that were detected in
certain parameters were compared using a Bonferoni corrected Mann
Whitney U test. Two-tailed p<0.05 was considered statistically
significant.
Results
The cutoff value for OV was found to be 6.43
cm3 in the Turkish population, with 95% sensitivity and
81.2% specificity. The PCOS group was divided into three subgroups
according to OV. Forty-two cases having OV <6.43 cm3
were included into Group 1, 101 cases with OV 6.43–10
cm3 were taken into Group 2 and 67 cases with larger
volumes, >10 cm3, were included into Group 3.
The mean age and BMI of the PCOS subgroups were
similiar. The BMI of the control group was significantly lower than
that of the other groups. Demographic features and mean OV of the
groups are shown in Table I.
 | Table I.Demographic features, hematologic
findings and mean ovarian volume of the patient groups. |
Table I.
Demographic features, hematologic
findings and mean ovarian volume of the patient groups.
| Control | PCOS (total) | Group 1 | Group 2 | Group 3 | p-value |
|---|
| Age (years) | 26.7±5.6 | 26.3±5.4 | 27.4±5.3 | 25.4±7.0 | 26.2±4.9 | 0.117 |
| BMI
(kg/m2) | 20.8±2.4 | 26.5±5.3 | 27.1±5.5 | 26.6±4.9 | 25.7±5.1 | <0.001a |
| Volume
(cm3) | 3.5±1.3 | 8.7±1.0 | 5.5±0.8 | 8.2±1.0 | 12.2±1.4 | <0.001a |
| Hb (g/dl) | 12.6±1.0 | 12.5±1.2 | 12.7±1.4 | 12.4±1.1 | 12.5±1.4 | 0.245 |
| Htc (%) | 38.1±1.7 | 38.0±2.4 | 37.7±2.2 | 38.1±2.0 | 38.2±1.9 | 0.670 |
| Plt
(x103/l) | 282.1±53.4 | 275.0±65.9 | 292.5±58.1 | 285.2±53.8 | 290.1±59.7 | 0.254 |
| RDW (%) | 13.7±2.1 | 13.6±2.7 | 13.3±3.0 | 13.9±2.9 | 13.6±2.7 | 0.791 |
| PDW (%) | 13.6±1.0 | 13.3±2.8 | 12.8±3.0 | 13.6±2.7 | 13.1±2.6 | 0.144 |
| MPV (fL) | 8.2±1.28 | 9.0±1.2 | 8.46±0.83 | 9.04±1.51 | 9.45±0.89 | <0.001b |
Hematologic parameters other than MPV were similiar
in all subgroups and the control group (Table I). MPV values were 8.20±1.28,
8.46±0.83, 9.04±1.51 and 9.45±0.89 in the control group, Groups 1,
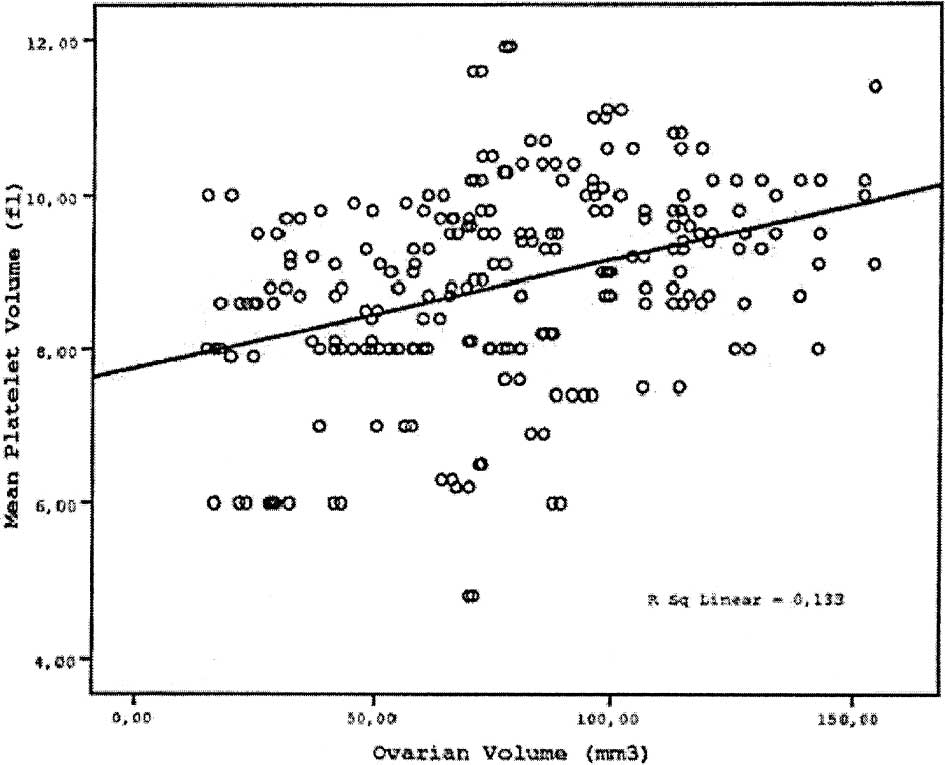
2 and 3, respectively. The MPV value increased gradually as OV
increased, starting from the control group up to Group 3 (Fig. 1). There were significant
differences between the control group vs. Groups 2 and 3. Although
the MPV of Group 1 was higher than the control group, this
difference was not statistically significant (Table I). Comparison of PCOS subgroups to
each other revealed significant difference in MPV between Groups 2
and 3 vs. Group 1 (p<0.001) (Table
I).
Hormonal parameters and the lipid profiles of the
three PCOS subgroups were similiar. When compared to the control
group, there was a significant difference in terms of total
testosterone level between Groups 2 and 3 vs. the control group
(p=0.004 and p<0.001, respectively) (Table II). Comparison of PCOS subgroups to
the control group for lipid profiles revealed a significant
difference in terms of LDL-C and HDL-C levels. For total
cholesterol and trigylceride levels, a significant difference was
observed between Groups 1 and 3 vs. the control group (Table III).
 | Table II.Hormonal parameters of groups (means ±
SD). |
Table II.
Hormonal parameters of groups (means ±
SD).
| Control | Group 1 | Group 2 | Group 3 | p-value |
|---|
| DHEAS (μg/dl) | 223.6±45.8 | 218.3±51.6 | 230.8±47.8 | 234.4±56.5 | 0.145 |
| T.Testest
(ng/dl) | 33.4±7.1 | 49.3±10.3 | 50.9±11.2 | 52.6±12.6 | <0.001a |
| Prolactin
(ng/ml) | 15.2±4.5 | 14.0±5.1 | 14.5±4.6 | 14.7±4.4 | 0.464 |
| TSH (μIU/ml) | 2.2±0.6 | 2.4±0.5 | 2.4±0.5 | 2.5±0.4 | 0.284 |
| LH (mIU/ml) | 5.9±1.4 | 5.7±1.3 | 6.2±1.5 | 6.1±1.4 | 0.322 |
| FSH (mIU/ml) | 5.6±1.2 | 5.9±1.4 | 5.5±1.5 | 6.0±1.3 | 0.158 |
| Estradiol
(pg/ml) | 37.2±8.2 | 36.7±8.5 | 40.2±9.5 | 39.8±8.9 | 0.256 |
 | Table III.Lipid profiles of the groups. |
Table III.
Lipid profiles of the groups.
| Control | PCOS (total) | Group 1 | Group 2 | Group 3 | p-value |
|---|
| Total cholesterol
(mg/dl) | 145.7±14.1 | 168.1±36.5 | 176.9±26.6 | 153.9±30.9 | 172.7±31.9 | 0.005a |
| LDL-C (mg/dl) | 75.4±13.5 | 97.5±28.2 | 102.3±19.8 | 88.3±22.0 | 99.1±28.6 | 0.001b |
| HDL-C (mg/dl) | 60.8±12.9 | 51.5±12.3 | 49.7±12.9 | 51.5±11.0 | 50.5±14.3 | 0.011b |
| Triglyceride
(mg/dl) | 71.4±41.7 | 97.9±51.9 | 114.7±46.8 | 83.5±32.1 | 104.3±41.9 | 0.009a |
Discussion
The association between amenorrhoea and polycystic
ovaries was first described by Stein and Leventhal in 1935
(10). Since then, many studies of
PCOS have been conducted to better understand the ethiopathogenesis
and the near/future complications of the disease. It is known that
several hormonal parameters and symptoms of the disease worsen as
ovarian diameter increases, which has been confirmed by studies
using 3D ultrasound and conventional 2D ultrasound (11–13).
There are many reports showing the causal links
between PCOS and CVD. Several studies suggest an association
between PCOS and hypertension, markers of subclinical
atherosclerosis and vascular dysfunction, including platelet
dysfunction (2,14–19).
In recent years, MPV has gained great attention due
to its role in coronary heart disease, diabetes, atherosclerosis,
hypertension and other vascular insufficiency states (4–8).
Although direct evidence for increased thrombotic risk is lacking
in PCOS patients, increased propensity to thrombosis is suggested
by many studies. A propensity to thrombosis can be corrected by
treatment of cases with oral contraceptives or anti-diabetic drugs
(20). In the study of Kebapcilar
et al (20), treatment of
PCOS cases with ethinyl estradiol plus cyproterone acetate and
metformin regimens resulted in a significant decrease in MPV. Only
decreased insulin levels also significantly predicted the reduction
of the MPV level. Thus, they speculated that decreased insulin
levels may contribute to an additional improvement to a possible
thrombosis risk by reducing MPV. The improvement in
hypercoagulability was observed in all patients after
treatment.
Women with PCOS have abnormalities in the
coagulation cascade and the fibrinolytic system (21). They have low-grade systemic
coagulation and fibrinolytic activation. Several studies have
demonstrated that subjects with PCOS have markedly impaired
platelet responsiveness to NO, irrespective of the presence/absence
of obesity. This is a completely distinct additional abnormality of
platelet function to the previously described hyperaggregability,
which is linked to endothelial dysfunction in such patients
(22).
In a recent study of 48 patients with non-diabetic
PCOS and 30 control subjects, it was shown that MPV, WBC and
D-dimer levels were increased in PCOS patients, suggesting
increased risk for atherosclerosis and CVD in these women (23). They also found that MPV was
positively correlated with insulin levels, DHEAS and free
testosterone levels in PCOS patients.
However, it remains uncertain whether
PCOS-associated CVD risk is related to OV, similar to the link
between androgen levels and OV. To our knowledge, there is no study
in the literature concerning the relationship of OV and MPV levels.
In this study, a significant relationship was found between OV and
MPV in PCOS cases. The larger the ovaries, the higher the MPV,
meaning a higher risk of hypercoagulability, and so an increased
risk of future CVD. We also detected that in PCOS cases with high
OV, total testesterone levels also increased. The increase in MPV
value may be attributed to an increase in androgen levels or an
increase in the severity of insulin resistance as OV increases. A
similiar relationship between OV and lipid profile was not
detected. There may be other factors modifying lipid profiles in
PCOS cases.
There are certain limitations of this study. One is
the moderate sample size and the other is the absence of a
comparison of different parameters related to PCOS that may effect
MPV and coagulation tests. There is evidence for a pathophysiology
link between MPV and insulin resistance; therefore, an important
limitation of the study is the lack of measurements of serum
glucose and insulin concentrations.
In conclusion, increased OV in PCOS patients may be
an early sign of cardiovascular or metabolic disease later in life.
Further studies are required to clarify the contribution of PCOS
and increased OV to the pathogenetic process of the hematologic and
coagulation system, and its direct or indirect effects on the
cardiovascular system.
References
|
1.
|
Homburg R: Polycystic ovary syndrome. Clin
Obstet Gynaecol. 2:261–274. 2008.
|
|
2.
|
Lo JC, Feigenbaum SL, Yang J, Pressman AR,
Selby JV and Go AS: Epidemiology and adverse cardiovascular risk
profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol
Metab. 91:1357–1363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Martin JF, Trowbridge EA, Salmon G and
Plumb J: The biological significance of platelet volume: its
relationship to bleeding time, platelet thromboxane B2 production
and megakaryocyte nuclear DNA concentration. Thrombosis Res.
1:443–460. 1983.PubMed/NCBI
|
|
4.
|
Senaran H, Ileri M, Altinbas A, Kosar A,
Yetkin E, Ozturk M, Karaaslan Y and Kirazli S: Thrombopoietin and
mean platelet volume in coronary artery disease. Clin Cardiol.
24:405–408. 2001.PubMed/NCBI
|
|
5.
|
Schneider DJ: Abnormalities of
coagulation, platelet function, and fibrinolysis associated with
syndromes of insulin resistance. Coron Artery Dis. 16:473–476.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Greisenegger S, Endler G, Hsieh K,
Tentschert S, Mannhalter C and Lalouschek W: Is elevated mean
platelet volume associated with a worse outcome in patients with
acute ischemic cerebrovascular events? Stroke. 35:1688–1691.
2004.PubMed/NCBI
|
|
7.
|
Endler G, Klimesch A, Sunder-Plassmann H,
et al: Mean platelet volume is an independent risk factor for
myocardial infarction but not for coronary artery disease. Br J
Haematol. 117:399–404. 2002.
|
|
8.
|
Gursoy A, Ertugrul DT, Pamuk B, et al:
Mean platelet volume in patients with polycystic ovary disease.
Platelets. 17:505–506. 2006.PubMed/NCBI
|
|
9.
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
Consensus Workshop Group: Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome. Fertil Steril. 81:19–25. 2004. View Article : Google Scholar
|
|
10.
|
Stein IF and Leventhal ML: Amenorrhoea
associated with bilateral polycystic ovaries. Am J Obst Gynecol.
29:181–191. 1935.
|
|
11.
|
Puzigaca Z, Prelevic GM, Stretenovic Z and
Balint-Peric L: Ovarian enlargement as a possible marker of
androgen activity in polycystic ovary syndrome. Gynecol Endocrinol.
5:167–174. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Watkin KL, Tulandi T, Mathur S and LeJeune
A: Three-dimensional visualisation of the polycystic ovary: effect
of ovarian drilling. Hum Reprod Update. 2(5): Item 18 (video),.
1996.
|
|
13.
|
Balen AH, Conway GS, Kaltsas G,
Techatraisak K, Manning P, West C and Jacobs HS: Polycystic ovary
syndrome: the spectrum of the disorder in 1741 patients. Hum
Reprod. 10:2107–2111. 1995.PubMed/NCBI
|
|
14.
|
Yildiz BO, Haznedaroglu IC, Kirazli S and
Bayraktar M: Global fibrinolytic capacity is decreased in
polycystic ovary syndrome, suggesting a prothrombotic state. J Clin
Endocrinol Metab. 87:3871–3875. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Talbott EO, Zborowski JV, Rager JR,
Boudreaux MY, Edmundowicz DA and Guzick DS: Evidence for an
association between metabolic cardiovascular syndrome and coronary
and aortic calcification among women with polycystic ovary
syndrome. J Clin Endocrinol Metab. 89:5454–5461. 2004. View Article : Google Scholar
|
|
16.
|
Kelly CJ, Speirs A, Gould GW, Petrie JR,
Lyall H and Connell JM: Altered vascular function in young women
with polycystic ovary syndrome. J Clin Endocrinol Metab.
87:742–746. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Palmert MR, Gordon CM, Kartashov AI, Legro
RS, Emans SJ and Dunaif A: Screening for abnormal glucose tolerance
in adolescents with polycystic ovary syndrome. J Clin Endocrinol
Metab. 87:1017–1023. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Apridonidze T, Essah PA, Iuorno MJ and
Nestler JE: Prevalence and characteristics of the metabolic
syndrome in women with polycystic ovary syndrome. J Clin Endocrinol
Metab. 90:1929–1935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Legro RS, Urbanek M, Kunselman AR, Leiby
BE and Dunaif A: Self-selected women with polycystic ovary syndrome
are reproductively and metabolically abnormal and undertreated.
Fertil Steril. 78:51–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kebapcilar L, Taner CE, Kebapcilar AG,
Alacacioglu A and Sari I: Comparison of four different treatment
regimens on coagulation parameters, hormonal and metabolic changes
in women with polycystic ovary syndrome. Arch Gynecol Obstet.
281:35–42. 2010. View Article : Google Scholar
|
|
21.
|
Thompson CB, Jakubowski JA, Quinn PG,
Deykin D and Valeri CR: Platelet size and age determine platelet
function independently. Blood. 63:1372–1375. 1984.PubMed/NCBI
|
|
22.
|
Rajendran S, Willoughby S, Chan W, et al:
Polycystic ovary syndrome is associated with severe platelet and
endothelial dysfunction in both obese and lean subjects.
Atherosclerosis. 204:509–514. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kebapcilar L, Taner CE, Kebapcilar AG and
Sari I: High mean platelet volume, low-grade systemic coagulation
and fibrinolytic activation are associated with androgen and
insulin levels in polycystic ovary syndrome. Arch Gynecol Obstet.
280:187–193. 2009.PubMed/NCBI
|