Introduction
Although the gene encoding DBC1 was identified as a
candidate breast tumor-suppressor gene (1), the expression of DBC1 is postulated
to be a poor prognostic factor in gastric (2) and breast cancer (3,4).
Currently, the molecular and cellular functions of DBC1 are being
extensively investigated to reveal its precise physiological role
(5–9). The endogenous DBC1 is a nuclear
protein and the amino-terminus of DBC1 has been shown to be a
protein-interaction surface and DBC1 serves as a transcriptional
factor to repress transcriptional activation function, such as
BRCA1 (8) and estrogen receptor β
(9). During TNF-α induced
apoptosis, DBC1 is translocated to the cytoplasm with loss of the
nuclear localization signal by caspase-dependent cleavage, and this
cleavage promotes apoptosis due to the death-promoting activity of
its carboxyl-terminal coiled-coil domain (7). Previous studies have demonstrated
that DBC1 promotes p53-mediated apoptosis through specific
inhibition of SIRT1 (5,6). However, the functions of DBC1 in
living cells remain largely unknown and it should be determined
whether DBC1 plays a pivotal role in tumor suppression or
promotion.
SIRT1, the mammalian homologue of yeast silent
information regulator 2 (Sir2), functions as an
NAD+-dependent class III histone deacetylase (10). SIRT1 deacetylates multiple targets
in mammalian cells. By regulating these molecules, SIRT1 functions
as a master regulator of energy homeostasis, gene silencing,
metabolism, genomic stability and cell survival. Recent reports
have revealed that SIRT1 may be involved in both tumorigenesis and
anti-tumorigenesis. The expression of SIRT1 has been shown to be
increased in human prostate (11),
gastric (2), colon (12), ovarian (13) and breast cancer (3,4,14),
and SIRT1 was found to promote cellular survival by deacetylating
key cell cycle molecules and apoptosis regulatory proteins
(15,16). SIRT1 inactivates p53 by
deacetylation and then allows cells to proliferate in the presence
of damaged DNA and subsequently promotes tumor progression
(15). In contrast to these
tumorigenic activities, SIRT1 inactivates β-catenin by
deacetylation and protects colonic tissue from tumor formation
(17). Collectively, these studies
implicate that the DBC1 and SIRT1 expression axis may play an
important role in the development of malignant tumors.
Therefore, we aimed to identify the role of DBC1 and
SIRT1 expression in breast cancer specimens obtained as core biopsy
specimens prior to surgery. We revealed that DBC1-positive cells
may constitute an unfavorable environment for breast cancer. These
results suggest the pivotal role of the DBC1 and SIRT1 expression
axis in patients with breast cancer.
Patients and methods
Patients and tissue sampling
A total of 52 patients who underwent primary
systemic chemotherapy followed by definitive surgery during
December 2005 and April 2008 at the Tokyo Metropolitan Cancer and
Infectious Diseases Center, Komagome Hospital (Tokyo, Japan) were
consecutively enrolled in this study. Informed consent was obtained
from all patients and approval of the Institutional Review Board of
Komagome Hospital was also obtained. All patients were diagnosed
with invasive breast carcinoma by core needle biopsy and 4 patients
were excluded since they were treated with primary hormonal
therapy. Thus, 48 of the originally enrolled patients were
evaluated. Four cycles of FEC (fluorouracil 500 mg/m2,
epirubicin 100 mg/m2 and cyclophosphamide 500
mg/m2) administered intravenously (i.v.) on day 1 every
21 days were followed by four cycles of docetaxel i.v. (75
mg/m2) every 21 days, prior to surgery. None of the
patients were administered with trastsuzumab prior to surgery.
Pathological response evaluation
The Japanese Pathological Response Criteria were
applied, defined as follows: grade 0, no chemotherapeutic change in
remnant cancer cells; grade 1a, 0–1/3 of remnant cancer cells in
degeneration or necrosis; grade 1b, 1/3–2/3; grade 2, >2/3;
grade 3, no viable cancer cells in duct and stroma (18,19).
Immunohistochemical staining of DBC1- and
SIRT1-positive cell
Immunohistochemistry was performed to visualize the
signal. Paraffin sections (4 μm) mounted on organosilane-coated
glass slides were dewaxed in xylene and rehydrated through a graded
ethanol series. The tissue sections were treated with a microwave
antigen retrieval procedure in sodium citrate buffer (pH 6.0) for
20 min. They were subsequently treated with 0.3% hydrogen peroxide
in methanol for 15 min to quench endogenous peroxidase activity.
The primary antibodies were anti-DBC1 rabbit polyclonal antibody
(produced in our laboratory) (8,9) and
anti-SIRT1 rabbit polyclonal antibodies (H-300; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). These primary antibodies
were diluted (DBC1 1/100,000; SIRT1 1/200), and the tissue sections
were incubated for 30 min at room temperature using reagents
provided with the ChemMate EnVision™ Detection system (Dako,
Carpinteria, CA, USA). Cells were visualized using the chromogen
diaminobenzidine and counterstained with Mayer’s hematoxylin.
Appropriate positive and negative controls were included.
Evaluation of DBC1 and SIRT1
expression
For semi-quantitative evaluation of DBC1/SIRT1
expression, immunohistochemical scoring was performed, and nuclear
staining of DBC1 and SIRT1 was evaluated according to the Allred
score (20). The immunostaining
results were interpreted as positive when at least 5% of cells were
stained. No expression or expression of <5% of tumor cells was
considered negative. The semi-quantification for immunostaining
intensity was scored on a scale of: 0, negative; 1, weak; 2,
moderate and 3, intense. Average numbers of immunopositive cells
within the neoplastic tissues were determined in at least five
areas at x400 magnification. The semi-quantification of the
percentage of immunopositive cells was scored on a scale of 0
(0–5%), 1 (6–25%), 2 (26–50%), 3 (51–75%) and 4 (>75%). The
percentage of the staining intensity and positive tumor cells were
multiplied to produce a weighted score for each case. These scores
were determined as the positive index. Immunohistochemical analysis
was performed by three authors by consensus without knowledge of
the clinicopathological information.
Breast cancer subtyping according to
estrogen receptor (ER), progesterone receptor (PR) and human
epidermal growth factor receptor-2 (HER2) status
For ER, PR and HER2 evaluation, immunohistochemical
staining was performed using anti-ER mouse monoclonal antibody
(clone 1D5; Dako), anti-PR mouse monoclonal antibody (clone PgR636;
Dako) and a HercepTest kit (Dako), respectively. Hormone receptor
status was evaluated as the percentage of positive nuclear staining
among cancer cells, and the cut-off value was set to 10%. HER2
scoring was carried out according to the standard HercepTest
guidelines. HER2 expression status was further confirmed by
fluorescence in situ hybridization using Vysis Path Vision
HER2/neu DNA probe kit (Abott, Chicago, IL, USA), when the tumor
was evaluated as 2+ by the HercepTest. The breast tumor
samples were classified into four subtypes, namely luminal A
(ER+ and/or PR+, and HER2−),
luminal B (ER+ and/or PR+, and
HER2+), HER2+ (ER− and
PR−, and HER2+) and triple-negative
(ER−, PR− and HER2−), according to
the system for the immunohistochemical subtyping of breast cancer
(21).
Statistical analysis
The association between DBC1 and SIRT1 expression
and clinicopathological features was examined by the Mann-Whitney
U-test. The correlation test was used to analyze correlations
between SIRT1 and DBC1 using Spearman’s rank correlation test. All
tests were two-tailed, and a P-value <0.05 was considered
statistically significant.
Results
Patient background
The clinical characteristics and pathological data
of the 48 breast cancer patients are shown in Table I. All patients were female and the
median age of the enrolled patients was 53 years (range 32–75).
Lymph node involvement was found in 18 patients and metastasis
occurred in 22 patients. The TNM staging of the tumors ranged from
stage 0 to stage IV: stage 0 (n=1), stage I (n=5), stage II (n=31),
stage III (n=6) and stage IV (n=5). All tumors were graded
according to the modified Bloom-Richardson system (22): grade 1 (n=12), grade 2 (n=10) and
grade 3 (n=26). ER, PR and HER2 were positive in 28, 16 and 35
patients, respectively. The breast cancer subtypes in the present
study included luminal A (n=8, 16.7%), luminal B (n=20, 41.7%),
HER2+ (n=5, 10.3%) and triple-negative (n=15, 31.3%)
subtypes.
 | Table IPatient clinical and pathological
characteristics. |
Table I
Patient clinical and pathological
characteristics.
| No. of patients
(%) |
|---|
| Age (years) | |
| Median | 53 |
| Range | 32–75 |
| Menopausal
status | |
| Pre-menopause | 21 (43.8) |
| Postmenopause | 27 (56.3) |
| Tumor stage | |
| 0 | 1 (2.1) |
| 1 | 5 (10.4) |
| 2 | 31 (64.6) |
| 3 | 6 (12.5) |
| 4 | 5 (10.4) |
| Nodal stage | |
| N0 | 29 (60.5) |
| N1 | 17 (35.5) |
| N2 | 1 (2.0) |
| Unknown | 1 (2.0) |
| Nuclear grade | |
| 1 | 12 (25.0) |
| 2 | 10 (20.8) |
| 3 | 26 (54.2) |
| ER | |
| Positive | 28 (58.3) |
| Negative | 20 (41.7) |
| PR | |
| Positive | 16 (33.3) |
| Negative | 32 (66.7) |
| HER2 (IHC) | |
| 0 | 12 (25.0) |
| 1+ | 22 (45.8) |
| 2+ | 6 (12.5) |
| 3+ | 7 (14.6) |
| Unknown | 1 (2.1) |
| Subtypes of breast
cancer | |
| Luminal A | 8 (16.7) |
| Luminal B | 20 (41.7) |
|
HER2+ | 5 (10.3) |
| Triple
negative | 15 (31.3) |
| Pathological
response | |
| Grade 0 | 3 (6.3) |
| Grade 1a | 22 (45.8) |
| Grade 1b | 10 (20.8) |
| Grade 2 | 6 (12.5) |
| Grade 3 | 5 (10.4) |
| Unknown | 2 (4.2) |
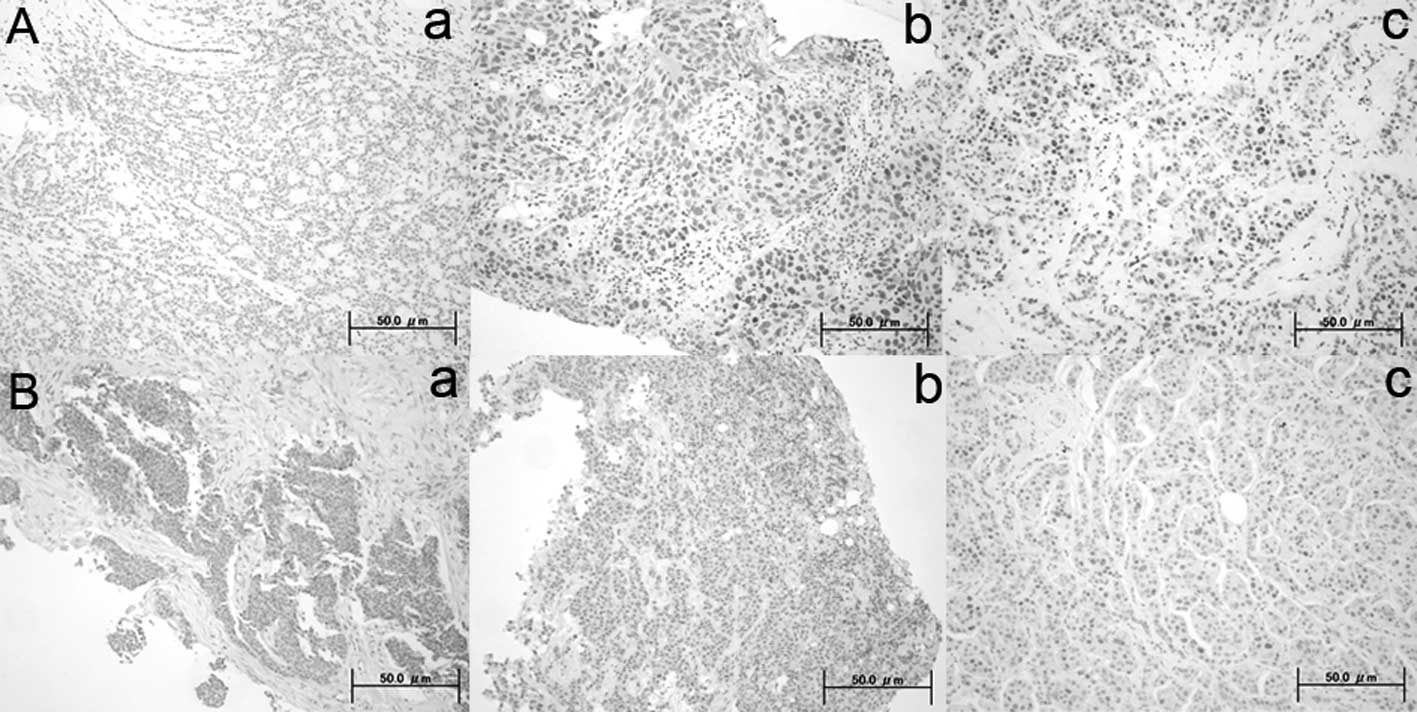
DBC1 and SIRT1 expression in core needle
biopsy specimens
DBC1 and SIRT1 immunoreactivity was present in the
nuclei of normal and tumor cells. Positive expression of DBC1 and
SIRT1 was noted in 85% (41 of 48) and in 98% (47 of 48) of
patients, respectively. Representative immunostained tissues are
shown in Fig. 1. Positive indices
of DBC1 and SIRT1 judged by immunohistochemistry were calculated,
and the association between the positive indices and the
clinicopathological characteristics of the patients was further
investigated. Elevated DBC1 expression was significantly associated
with nuclear grade as determined by the modified Bloom-Richardson
system (P= 0.019). Expression of DBC1 was inversely correlated with
the HER2 expression status (P=0.026), while other clinical factors
exhibited no significant correlation with the DBC1-positive index.
Expression of SIRT1 was also negatively correlated with the HER2
expression status (P=0.003). However, in contrast to other studies,
SIRT1 expression showed no relation with hormone receptor status
and luminal subtype. We also analyzed the correlation coefficient
between DBC1 and SIRT1 in breast cancer tissue and a marginal
correlation was detected between the expression of DBC1 and the
expression of SIRT1 (P=0.047, r=0.34).
Correlation between DBC1 and SIRT1
expression and pathological response
Three patients were diagnosed with a grade 0
pathological response, and 22, 10, 6 and 5 patients were diagnosed
with grades 1a, 1b, 2 and 3, respectively. To investigate whether
DBC1 and SIRT1 expression is associated with chemotherapeutic
response of the patients, the correlation between
immunohistochemical positive index and pathological response of the
invasive component of the breast carcinoma was evaluated, as
pathological response is proposed to provide accurate information
for prognosis. Expression of DBC1 and SIRT1 judged by
immunohistochemistry was compared to the pathological response of
the patients. Patients who exhibited a favorable response to
neoadjuvant chemotherapy showed lower positive indices for DBC1 and
SIRT1 rather than those who exhibited an unfavorable response, but
this inverse relationship was not statistically significant
(Table II).
 | Table IICorrelation between positive index
(PI) score (DBC1 and SIRT1) and clinicopathological features of the
48 specimens. |
Table II
Correlation between positive index
(PI) score (DBC1 and SIRT1) and clinicopathological features of the
48 specimens.
| DBC1 PI score
| SIRT1 PI score
|
|---|
| (mean, 95% CI) | P-value | (mean, 95% CI) | P-value |
|---|
| Menopausal
status | | NS | | NS |
| Premenopause | 2.91
(2.06–3.77) | | 3.65
(3.27–4.03) | |
|
Postmenopause | 3.32
(2.54–4.10) | | 3.72
(3.16–4.29) | |
| Tumor stage
(T) | | NS | | NS |
| I–II (n=37) | 3.19
(2.55–3.83) | | 3.68
(3.27–4.08) | |
| III–IV
(n=11) | 2.91
(1.55–4.27) | | 3.73
(3.05–4.41) | |
| Nodal status | | NS | | NS |
| Negative
(n=29) | 3.00
(2.19–3.81) | | 3.62
(3.16–4.08) | |
| Positive
(n=19) | 3.32
(2.06–4.07) | | 3.79
(3.27–4.31) | |
| Nuclear grade | | 0.0189a | | NS |
| 1 (n=12) | 2.08
(1.39–2.77) | | 3.33
(2.42–4.25) | |
| 2 and 3
(n=36) | 3.47
(2.79–4.16) | | 3.81
(3.45–4.16) | |
| ER status | | NS | | NS |
| Negative
(n=20) | 3.11
(1.91–4.32) | | 3.89
(3.47–4.30) | |
| Positive
(n=28) | 3.13
(2.54–3.73) | | 3.57
(3.08–4.05) | |
| PR status | | NS | | NS |
| Negative
(n=32) | 3.31
(2.57–4.05) | | 3.56
(3.11–4.01) | |
| Positive
(n=16) | 2.75
(1.89–3.61) | | 3.94
(3.44–4.43) | |
| HER2 status | | 0.0264a | | 0.0028b |
| 0 (n=12) | 4.08
(2.86–5.31) | | 4.50
(4.17–4.83) | |
| 1–3 (n=36) | 2.80
(2.19–3.43) | | 3.42
(3.02–3.82) | |
| Luminal
subtype | | NS | | NS |
| Luminal
(n=28) | 3.32
(2.66–3.98) | | 3.64
(3.13–4.15) | |
| Non-luminal
(n=20) | 2.85
(1.82–3.88) | | 3.75
(3.32–4.18) | |
| Pathological
response | | NS | | NS |
| 0-1a (n=25) | 3.32
(2.54–4.01) | | 3.80
(3.37–4.23) | |
| 1b-3 (n=21) | 2.91
(2.06–3.77) | | 3.57
(3.02–4.12) | |
Discussion
Recently, the physiological significance and
tumor-promoting functions of DBC1 have been revealed. DBC1 has been
found to associate with unliganded-ERα and to manipulate
ligand-independent growth of breast cancer cells (23). Our previous data also indicated the
possible tumorigenic role of DBC1 (8,9).
However, given that DBC1 inhibits the deacetylase activity of SIRT1
and promotes p53-dependent apoptosis (5,6),
DBC1 expression may not be directly associated with tumorigenesis.
DBC1 expression has been shown to be elevated in breast cancer
(14,24). In patients with breast cancer,
expression of DBC1 and SIRT1 was significantly associated with
distant metastatic relapse, shorter relapse-free survival and
reduced overall survival (4).
These findings suggest the possibility that the expression of DBC1
is a clinically significant prognostic indicator for breast
carcinoma patients. However, another recent study found that
overexpression of DBC1 in tumor tissue had no significant
correlations with clinicopathological factors of breast cancer, but
overexpression of SIRT1 was significantly correlated with luminal
subtypes, ER and PR expression by immunohistochemistry (3). Further studies are required to define
DBC1 as a tumor promoter, since DBC1 was originally identified
during a genetic search for candidate breast tumor-suppressor genes
(1).
In the present study, the expression of DBC1 was
significantly associated with nuclear grade, which is considered an
unfavorable prognostic factor. Histological and nuclear grades have
almost the same prognostic relevance, and a high nuclear grade is a
significant prognostic factor for the development of ipsilateral
breast recurrences in numerous retrospective and prospective
studies. Since histological grade has a strong correlation with
HER2 status, inactivation of p53, hormone receptor negativity and
accumulation of chromosomal alterations, we may expect that the
elevated expression of DBC1 has certain clinical significance in
breast cancer patients. The fact that DBC1 and SIRT1 expression
correlates with ErbB2/HER2 status may simply be translated that
DBC1 and SIRT1 expression affects cellular homeostasis because the
overexpression of ErbB2/HER2 has been reported as an adverse
prognostic factor in invasive breast cancer and is considered to be
a marker of aggressive biology.
It remains questionable why the overall expression
of SIRT1 and DBC1 simultaneously increases in breast tumor tissues.
Since SIRT1 and DBC1 possess simultaneous roles both in tumor
promotion and tumor suppression, their individual expression is not
sufficient to determine the fate of tumorigenic cells. Therefore,
it is not surprising that the correlation between SIRT1 and DBC1
was not lost in tumor tissue, in contrast to a previous study
(3). However, beyond the balance
between SIRT1 and DBC1, a more exquisite determinant of
tumorigenesis may exist between these two proteins. Although our
data were insufficient to show that lower expression of DBC1 and
SIRT1 suggests favorable pathological response to chemotherapy, we
expect that the expression of DBC1 and SIRT1 in breast tissues
reflects baseline tumor characteristics. Due to the limited sample
size of patients in our investigation, further studies are required
to verify our findings and establish the role of DBC1 and SIRT1 as
a reliable clinical predictor for the outcome of breast cancer
patients.
In conclusion, our study demonstrated that the
expression levels of DBC1 and SIRT1 may constitute important tumor
characteristics for patients with breast cancer. Our study suggests
that DBC1 may be a more useful prognostic factor in breast cancer
rather than SIRT1. In clinical practice, considering that the
activation of SIRT1 by small molecules have been extensively
investigated for the treatment of diabetes, our study may implicate
that these molecules have certain roles in the pathophysiology of
breast cancer.
Acknowledgements
This study was supported by a
Grant-in-Aid for Scientific Research from the Ministry of
Education, Science and Culture, the JMS Bayer Schering Pharma
Grant, the Kowa Life Science Foundation and the Kanzawa Medical
Research Foundation, Japan.
References
|
1
|
Hamaguchi M, Meth JL, von Klitzing C, et
al: DBC2, a candidate for a tumor-suppressor gene involved in
breast cancer. Proc Natl Acad Sci USA. 99:13647–13652. 2002.
View Article : Google Scholar
|
|
2
|
Cha EJ, Noh SJ, Kwon KS, et al: Expression
of DBC1 and SIRT1 is associated with poor prognosis of gastric
carcinoma. Clin Cancer Res. 15:4453–4459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung JY, Kim R, Kim JE and Lee J: Balance
between SIRT1 and DBC1 expression is lost in breast cancer. Cancer
Sci. 101:1738–1744. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee H, Kim KR, Noh SJ, et al: Expression
of DBC1 and SIRT1 is associated with poor prognosis for breast
carcinoma. Hum Pathol. 42:204–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim JE, Chen J and Lou Z: DBC1 is a
negative regulator of SIRT1. Nature. 451:583–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao W, Kruse JP, Tang Y, et al: Negative
regulation of the deacetylase SIRT1 by DBC1. Nature. 451:587–590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sundararajan R, Chen G, Mukherjee C and
White E: Caspase-dependent processing activates the proapoptotic
activity of deleted in breast cancer-1 during tumor necrosis
factor-alpha-mediated death signaling. Oncogene. 24:4908–4920.
2005. View Article : Google Scholar
|
|
8
|
Hiraike H, Wada-Hiraike O, Nakagawa S, et
al: Identification of DBC1 as a transcriptional repressor for
BRCA1. Br J Cancer. 102:1061–1067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koyama S, Wada-Hiraike O, Nakagawa S, et
al: Repression of estrogen receptor beta function by putative tumor
suppressor DBC1. Biochem Biophys Res Commun. 392:357–362. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blander G and Guarente L: The Sir2 family
of protein deacetylases. Annu Rev Biochem. 73:417–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huffman DM, Grizzle WE, Bamman MM, et al:
SIRT1 is significantly elevated in mouse and human prostate cancer.
Cancer Res. 67:6612–6618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kabra N, Li Z, Chen L, et al: SirT1 is an
inhibitor of proliferation and tumor formation in colon cancer. J
Biol Chem. 284:18210–18217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jang KY, Kim KS, Hwang SH, et al:
Expression and prognostic significance of SIRT1 in ovarian
epithelial tumours. Pathology. 41:366–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Zhang M, Dong H, et al:
Deacetylation of cortactin by SIRT1 promotes cell migration.
Oncogene. 28:445–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaziri H, Dessain SK, Ng Eaton E, et al:
hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell.
107:149–159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong S and Weber JD: Deacetylation of the
retinoblastoma tumour-suppressor protein by SIRT1. Biochem J.
407:451–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oberdoerffer P, Michan S, McVay M, et al:
SIRT1 redistribution on chromatin promotes genomic stability but
alters gene expression during aging. Cell. 135:907–918. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Japanese Pathological Response Criteria of
Breast Cancer: General Rules for Clinical and Pathological
Recording of Breast Cancer (15th edition). 75–76. 2008.
|
|
19
|
Kurosumi M, Akashi-Tanaka S, Akiyama F, et
al: Histopatho-logical criteria for assessment of therapeutic
response in breast cancer (2007 version). Breast Cancer. 15:5–7.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harvey JM, Clark GM, Osborne CK and Allred
DC: Estrogen receptor status by immunohistochemistry is superior to
the ligand-binding assay for predicting response to adjuvant
endocrine therapy in breast cancer. J Clin Oncol. 17:1474–1481.
1999.
|
|
21
|
Carey LA, Perou CM, Livasy CA, et al:
Race, breast cancer subtypes, and survival in the Carolina Breast
Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsuda H, Akiyama F, Kurosumi M, et al:
Establishment of histological criteria for high-risk node-negative
breast carcinoma for a multi-institutional randomized clinical
trial of adjuvant therapy. Japan National Surgical Adjuvant Study
of Breast Cancer (NSAS-BC) Pathology Section. Jpn J Clin Oncol.
28:486–491. 1998. View Article : Google Scholar
|
|
23
|
Trauernicht AM, Kim SJ, Kim NH and Boyer
TG: Modulation of estrogen receptor alpha protein level and
survival function by DBC-1. Mol Endocrinol. 21:1526–1536. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Richardson AL, Wang ZC, de Nicolo A, et
al: X chromosomal abnormalities in basal-like human breast cancer.
Cancer Cell. 9:121–132. 2006. View Article : Google Scholar : PubMed/NCBI
|