Introduction
Curative resection of functional pancreatic
neuroendocrine tumors (PNETs) is increasingly being performed due
to advances in early diagnosis based on hormone-induced symptoms
and blood hormone measurement, as well as advances in localization
techniques, such as the selective arterial secretagogue injection
test and secretin receptor scintigraphy (1). In addition, sustained-release
octreotide preparations have been found to alleviate the symptoms
of functional PNETs and suppress tumor growth (2,3). On
the other hand, non-functional PNETs are often detected
incidentally by imaging studies, such as CT, or by symptoms due to
pressure of the tumor on surrounding organs. These tumors usually
exhibit slow growth, but some of them may be pancreatic
neuroendocrine cell carcinoma (PNEC), a malignant tumor which
metastasizes to the liver, even when the primary tumor is small.
The survival time of patients with hepatic metastasis of PNEC has
been reported to be within 5 years, and almost no chemotherapy is
effective for metastatic unresectable PNEC (2,4).
Both functional and non-functional PNETs, including PNEC, are
hypervascular tumors and are known to express angiogenic molecules
(5). In addition, it has been
reported that the serum levels of angiogenic cytokines increase in
PNET patients (6–8). For these reasons, anti-angiogenic
therapy is expected to be effective against PNEC (9–12).
By contrast, anti-angiogenic therapy is ineffective in many
patients with common ductal cell carcinoma (DCC) (13,14),
since DCC is a hypovascular tumor and because pancreatitis
associated with pancreatic duct obstruction due to pancreatic
cancer induces the secretion of growth and adhesion factors, making
anti-angiogenic therapy alone insufficient (15).
In this study, we compared PNEC and DCC cell lines
regarding their vascular endothelial growth factor (VEGF)
expression levels and the effects of treatment with the anti-VEGF
antibody bevacizumab (Avastin®; Genentech, Inc., San
Francisco, CA, USA). In addition, we investigated the influence of
bevacizumab administration on pancreatitis and considered the
possibility of anti-angiogenic therapy for PNEC.
Materials and methods
Cell lines and assays
The QGP-1 pancreatic neuroendocrine cell carcinoma
cell line was purchased from the Japanese Collection of Research
Bioresources (Osaka, Japan) (16–18),
and the AsPC-1 and BxPC-3 human pancreatic ductal carcinoma cell
lines were purchased from the American Type Culture Collection
(Manassas, VA, USA). Cells were cultured at 37°C in RPMI-1640
(Gibco, Life Technologies Japan Ltd., Tokyo, Japan) supplemented
with 10% fetal calf serum (Sigma, St. Louis, MO, USA) in a
humidified atmosphere containing 5% CO2. For the cell
viability assay, cells were cultured in 96-well microplates for 24
h at a volume of 100 μl (10,000 cells/well) at 37°C in a humidified
atmosphere of 5% CO2. When the cells became adherent to
the plates, the plates were transferred to an environment of either
1 or 20% O2, and incubated for 1, 2 and 3 days. To
evaluate the cell viability in a hypoxic atmosphere,
methyl-tetrazolium (MTT;
3[4,5-dimethyl-thiazoyl-2-yl]2,5-diphenyl-tetrazolium bromide;
Sigma) was used. Cells were cultured in 96-well microplates and
irradiated for 24 h; 10 μl of MTT solution (5 mg of MTT/1 ml of
phosphate-buffered saline) was added to each well, followed by
incubation for 4 h. Finally, 100 μl of acid-isopropanol was added
to each well to solubilize MTT formazan. After complete
solubilization of the dye by vortexing the plate, the absorbance
was read on an Immunoreader (Powerscan HT; DS Pharma Biomedical Co.
Ltd., Osaka, Japan) at an optical density of 570 nm. For the human
VEGF (h-VEGF) enzyme (protein)-linked immunosorbent assay (ELISA),
when the cells became adherent to the plate, the plates were
transferred to an environment of either 1 or 20% O2, and
incubated for 1, 2 and 3 days. To evaluate the h-VEGF protein
expression of these cancer cells in a hypoxic atmosphere, a
cell-based ELISA of human total VEGF (R&D Systems Inc.,
Minneapolis, MN, USA) was used. The fluorescence of h-VEGF protein
expression in the cells was normalized to that of the quantity of
h-VEGF protein of a known level.
Pancreatitis and assays
Female ICL mice, weighing 20–25 g, obtained from
Clea Japan Inc. (Tokyo, Japan), were treated with caerulein
(Sigma), 50 μg/kg intraperitoneally (i.p.) every hour for 6–7 h
twice a week, to induce edematous pancreatitis (19,20).
At predetermined time points during a 4-week period after the first
caerulein injection, 5–9 mice were treated with bevacizumab or
human IgG (Sigma) as a control. Mice were i.p. injected with
pimonidasole hydrochloride (60 mg/kg) (Sigma), and the pancreases
were removed 120 min later. Immunostaining for the detection of
hypoxic cells was carried out with anti-pimonidasol Ab
(Hypoxyprobe-1 Plus; Natural Pharmacia International, Inc.,
Belmont, MA, USA) (21). Initial
weight of the whole pancreas was recorded, and a fresh sample
<10 mg was obtained from the pancreatic portion. The fresh
pancreas sample was incubated to release the collagen at a pepsin
concentration of 1 mg/ml of 0.5 M acetic acid at 4°C. The amount of
collagen of the pancreas was measured using Sircol Soluble Collagen
Assay (Biocolor Ltd., Carrickfergus, Northern Ireland, UK). To
evaluate mouse-VEGF (m-VEGF) protein and mouse-VEGF receptor 1
(m-VEGFR1) expression in the mice under a pancreatitis condition,
Quantikine mouse VEGF immunoassay and mouse VEGF-R1 immunoassay
(R&D Systems) were used. The fluorescence of m-VEGF and
m-VEGFR1 protein expression in the cells was normalized to that of
the quantity of these proteins of a known level.
Xenograft model and assay
Athymic female Balb/c-nu/nu nude mice (4-6 weeks
old) were purchased from Clea Japan. The cell suspension of each
cell line which was adjusted to a cell suspension (2×107
°cell/ml) in phenol red-free RPMI-1640 (Gibco) was mixed with
Matrigel matrix (BD Biosciences, San Jose, CA, USA) on ice at a 1:4
ratio. The mixture was implanted subcutaneously into the back of
the mice. The negative control group was injected with Matrigel
alone (n=2). Bevacizumab or human IgG (Sigma) was i.p.
administrated twice a week from 7 to 28 days after cancer cell
implantation. On day 28, each Matrigel and cancer cell mixture was
removed and weighed, and the remaining gel was treated with Dispase
II (1.5 mg/ml; Roche Diagnostics, Tokyo, Japan), followed by
determination of the hemoglobin content using the Quantichrom
hemoglobin assay kit (Gentaur, Kampenhout, Belgium). The remaining
gel was immersed in 10% paraformaldehyde and embedded in paraffin.
Sections were processed for immunostaining with H&E, Ki-67
(MIB1; Dako Cytomation, Glostrup, Denmark) and CD34 (Abbiotec LLC,
San Diego, CA, USA). Nine different fields representing areas with
maximum microvessel content and number of Ki-67-positive cells were
selected from each tumor. Necrotic areas were avoided. Regarding
tumor characteristics, the number of microvessels with
CD34-positive vascular endothelial cells and the proliferative
tumor cell ratio as determined by Ki-67-positivity [Ki-67 labeling
index (LI)] were evaluated.
Statistical analysis
Statistical analyses were performed using Stat View
(Abacus Concepts Inc., Berkely, CA, USA). The weight of the
pancreas and the collagen weight/pancreas, as well as the number of
microvessels, Ki-67 LI, hemoglobin content, volume and weight of
the tumors were compared using the Mann-Whitney U test.
Results
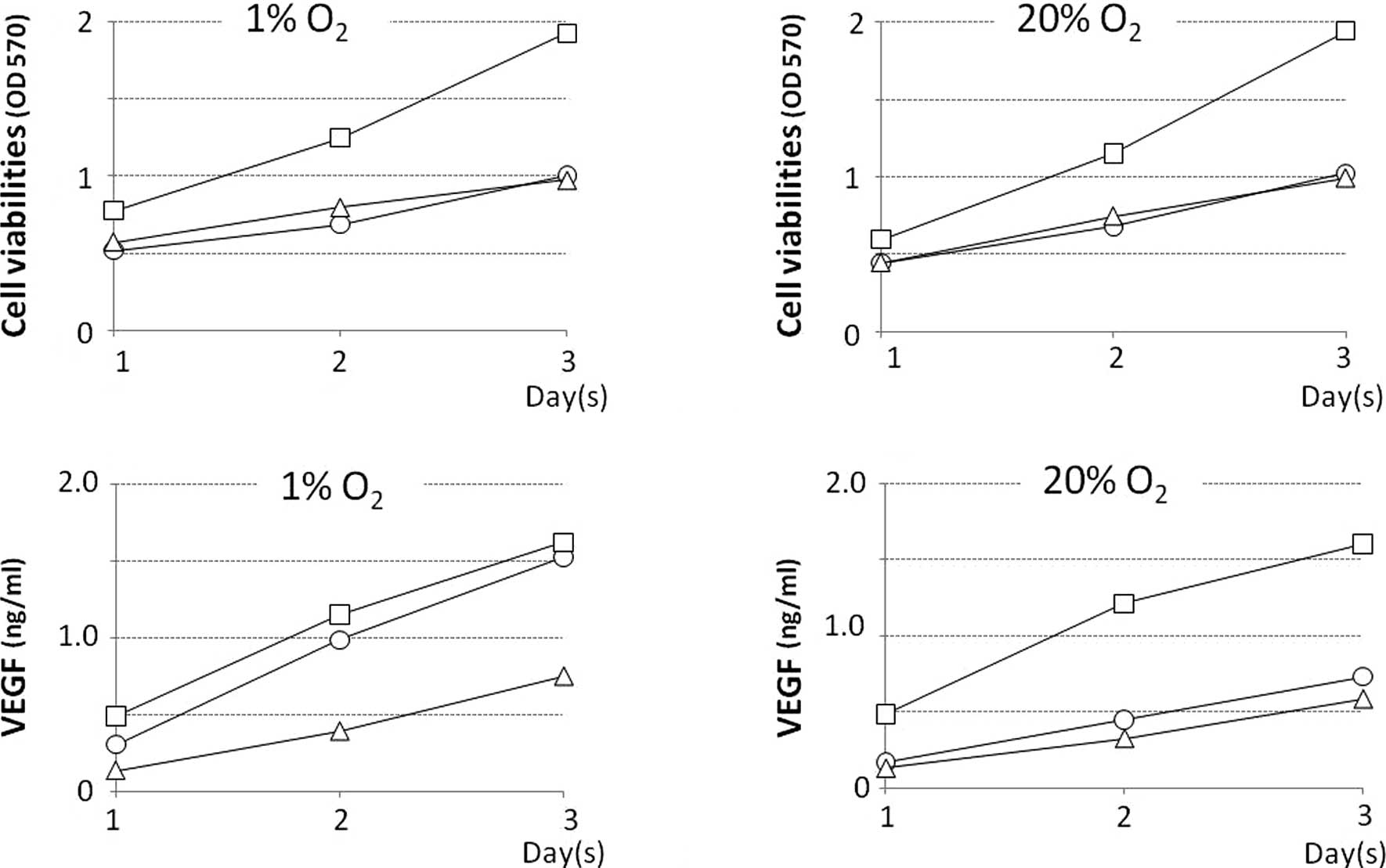
Among the QGP-1, BxPC-3 and AsPC-1 cell lines,
BxPC-3 exhibited the highest proliferation rate. The growth rate of
all cell lines in normoxic and hypoxic environments was
approximately the same (Fig. 1 A
and B). The level [accumulated during day(s) 1, 2 and 3] of VEGF
protein secreted in the culture supernatant of the BxP-3 cell line
under normoxia was the highest, and these levels in the QGP-1 and
AsPC-1 cell lines were approximately half. The levels of VEGF
protein secreted in the culture supernatant of the QGP-1 cell line
under normoxia were 135.3, 359.1 and 585.7 pg/ml on day(s) 1, 2 and
3, respectively, but these levels were much higher under hypoxia,
at 242.9, 789.8 and 1221.1 pg/ml on day(s) 1, 2 and 3, respectively
(Fig. 1C and D). The properties of
the three cell lines are shown in Table I.
 | Table I.Properties of the pancreatic cell
lines. |
Table I.
Properties of the pancreatic cell
lines.
| Variables | QGP-1 | BxPC-3 | AsPC-1 |
|---|
| MTT | | | |
| Hypoxia | + | ++ | + |
| Normoxiaa | +++ | + | |
| VEGF protein
expression | | | |
| Hypoxia | ++ | ++ | + |
| Normoxiaa | + | ++ | + |
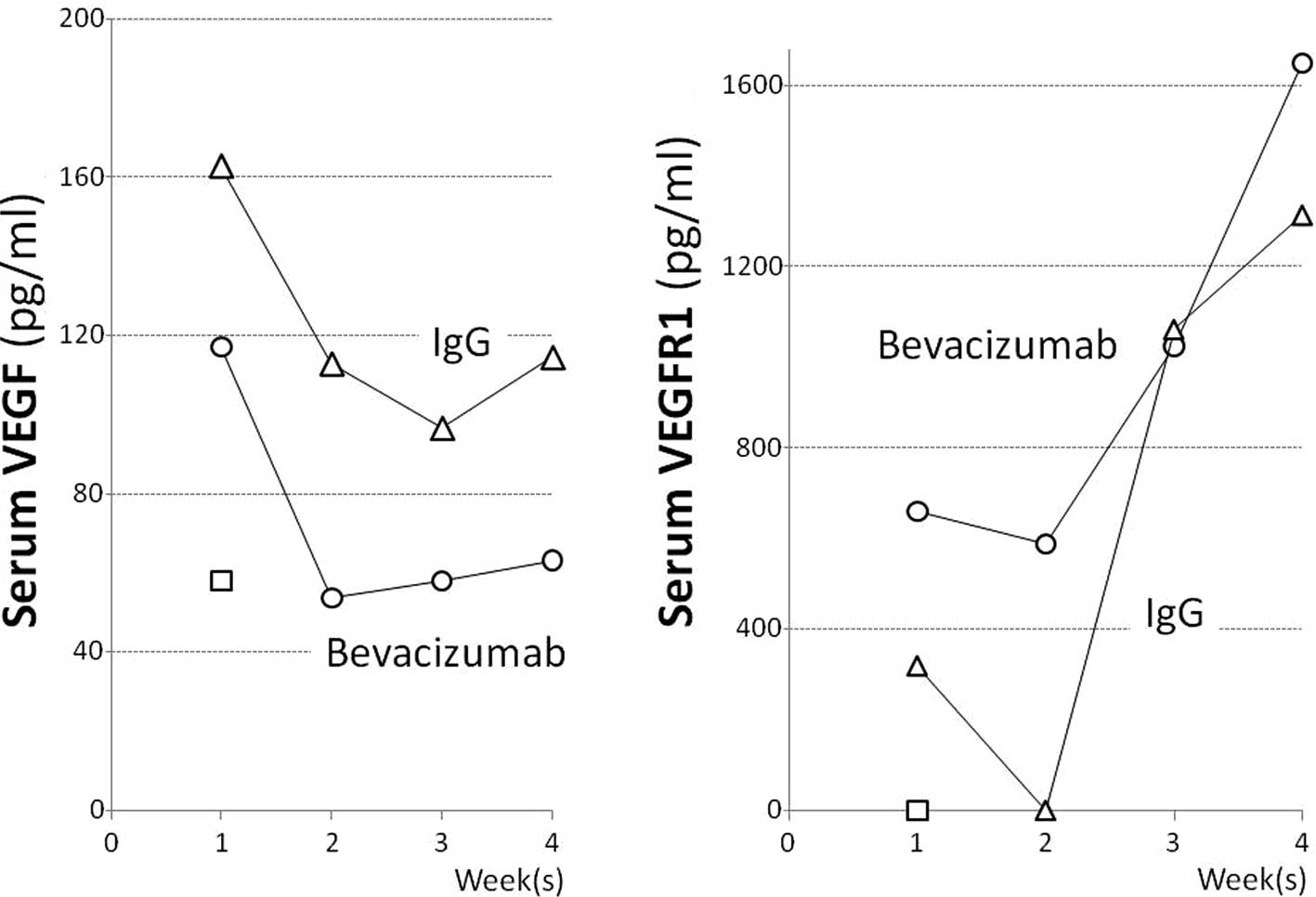
In mice with caerulein-induced pancreatitis, the
serum VEGF levels at 1, 2, 3 and 4 weeks after the start of
caerulein administration were 162.7, 112.9, 96.7 and 114.5 pg/ml,
respectively, and those in the pancreatitis mice that received
bevacizumab were 63.1, 57.9, 53.7 and 117.1 pg/ml, respectively.
The serum VEGF level in the pancreatitis mice administered
bevacizumab decreased to approximately half that (57.9 pg/ml) in
the normal mice in the second week or later (Fig. 2A). The serum VEGFR1 levels in the
pancreatitis mice at 1, 2, 3 and 4 weeks after the start of
caerulein administration were 319.4, below the detection limit,
1,062.2 and 1,314.8 pg/ml, respectively, and those in the
pancreatitis mice receiving bevacizumab were somewhat higher, at
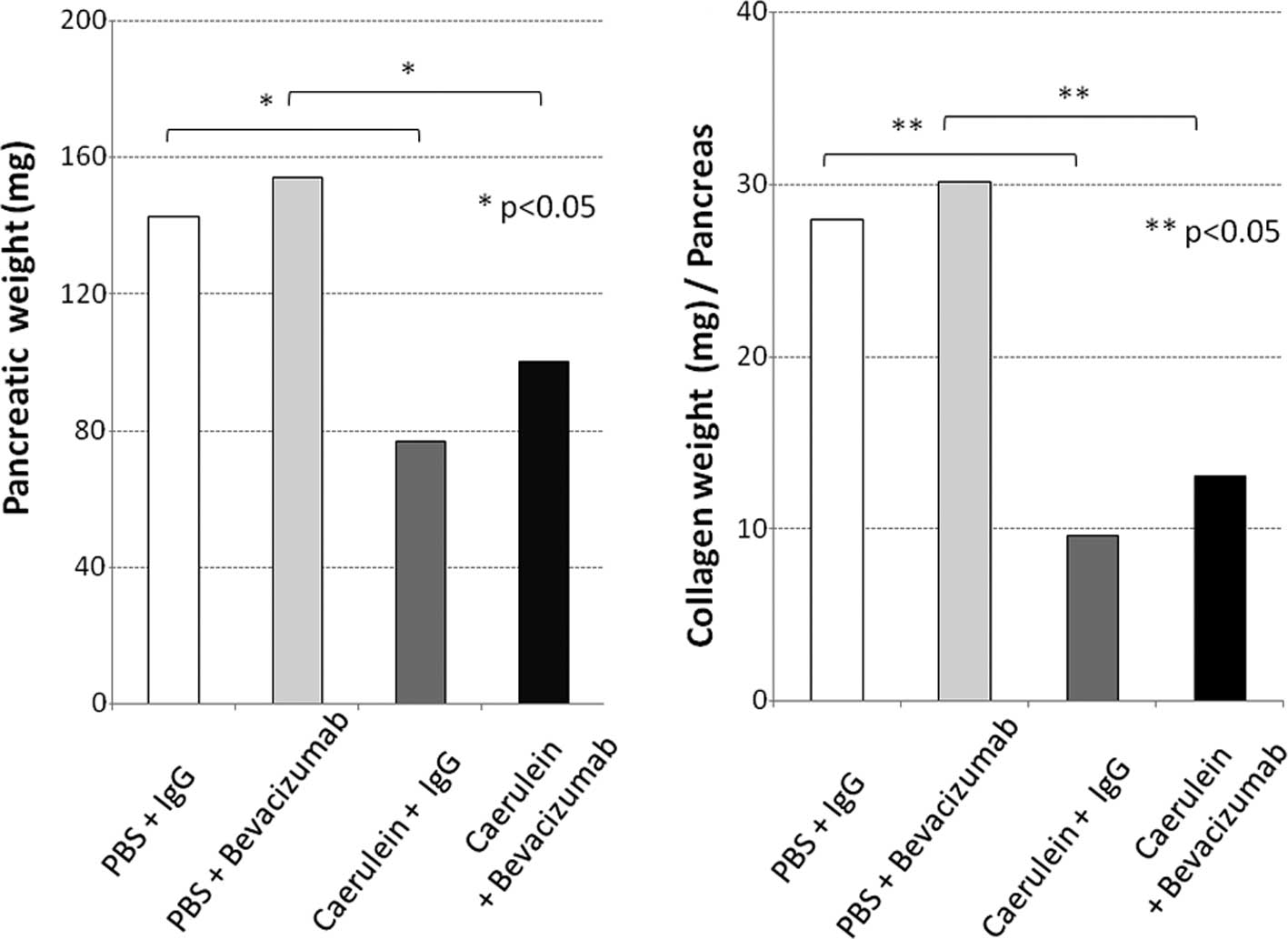
659.5, 587.0, 1,024.7 and 1,649.9 pg/ml, respectively (Fig. 2B). Grossly, the pancreas of normal
mice appeared yellowish, whereas that of pancreatitis mice was
whitish and atrophic. The pancreas of the normal and pancreatitis
mice (both groups were administered IgG for comparison to the group
administered bevacizumab) weighed 142.5±13.5 and 76.7±20.1 (mg ±
SD), respectively, and the latter value was significantly lower
than the former (Fig. 3A). The
collagen content in the pancreatitis mice was significantly lower,
at 9.6 μg/mg, compared to that of the normal mice (28 μg/mg;
p=0.037; Fig. 3B). Thus, edematous
pancreatitis was identified. The mean pancreatic weights of the
bevacizumab-administered normal and pancreatitis mice were
154.1±45.9 and 100.2±46.6 (mg ± SD), respectively (Fig. 3A). The collagen content in these
mice was significantly lower; 13.0±6.0 compared to that of the
normal mice [30.2±8.9 (μg/mg ± SD); p=0.027; Fig. 3B]. Thus, the pancreatic weight of
the bevacizumab-administered mice was somewhat higher, but not
significantly, suggesting that bevacizumab does not exacerbate
pancreatitis. No areas of positive pimonidazole staining were
observed in the pancreas of the pancreatitis or normal mice, or the
bevacizumab-administered mice (Fig.
4A–C). These results suggest that bevacizumab does not increase
the area of ischemia in pancreatitis.
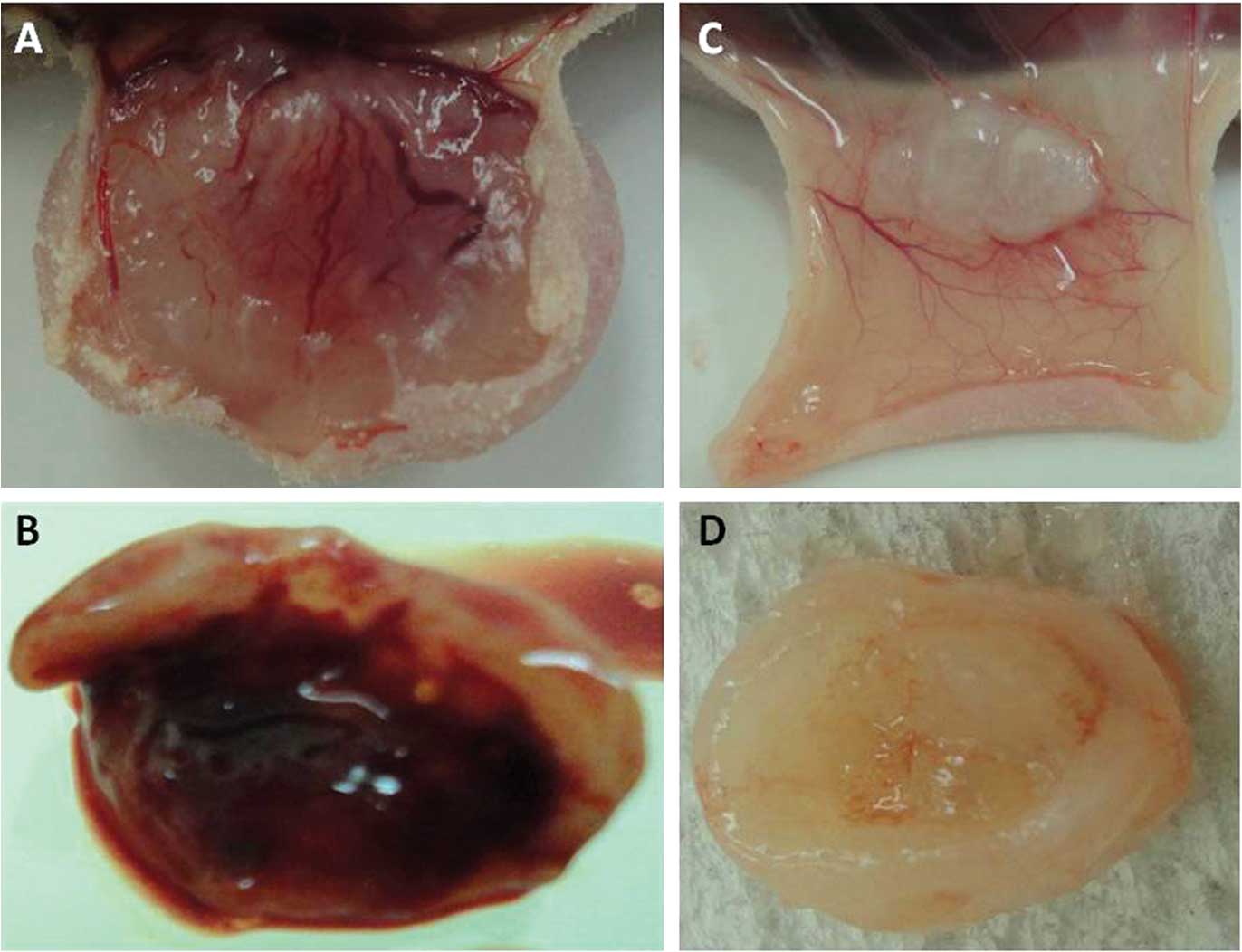
At autopsy, subcutaneous blood vessels overlying the
tumor that were transplanted into the mice were grossly examined.
Numerous subcutaneous blood vessels were overlying the reddish
tumor in the IgG-administered mice bearing the QGP-1, BxPC-3 or
AsPC-1 cell lines (Fig. 5A and B),
while few blood vessels were observed in the
bevacizumab-administered mice (Fig. 5C
and D). Intratumoral bleeding was noted in the
IgG-administered, but not in the bevacizumab-administered mice
bearing BxPC-3 cells (Fig.
5B).
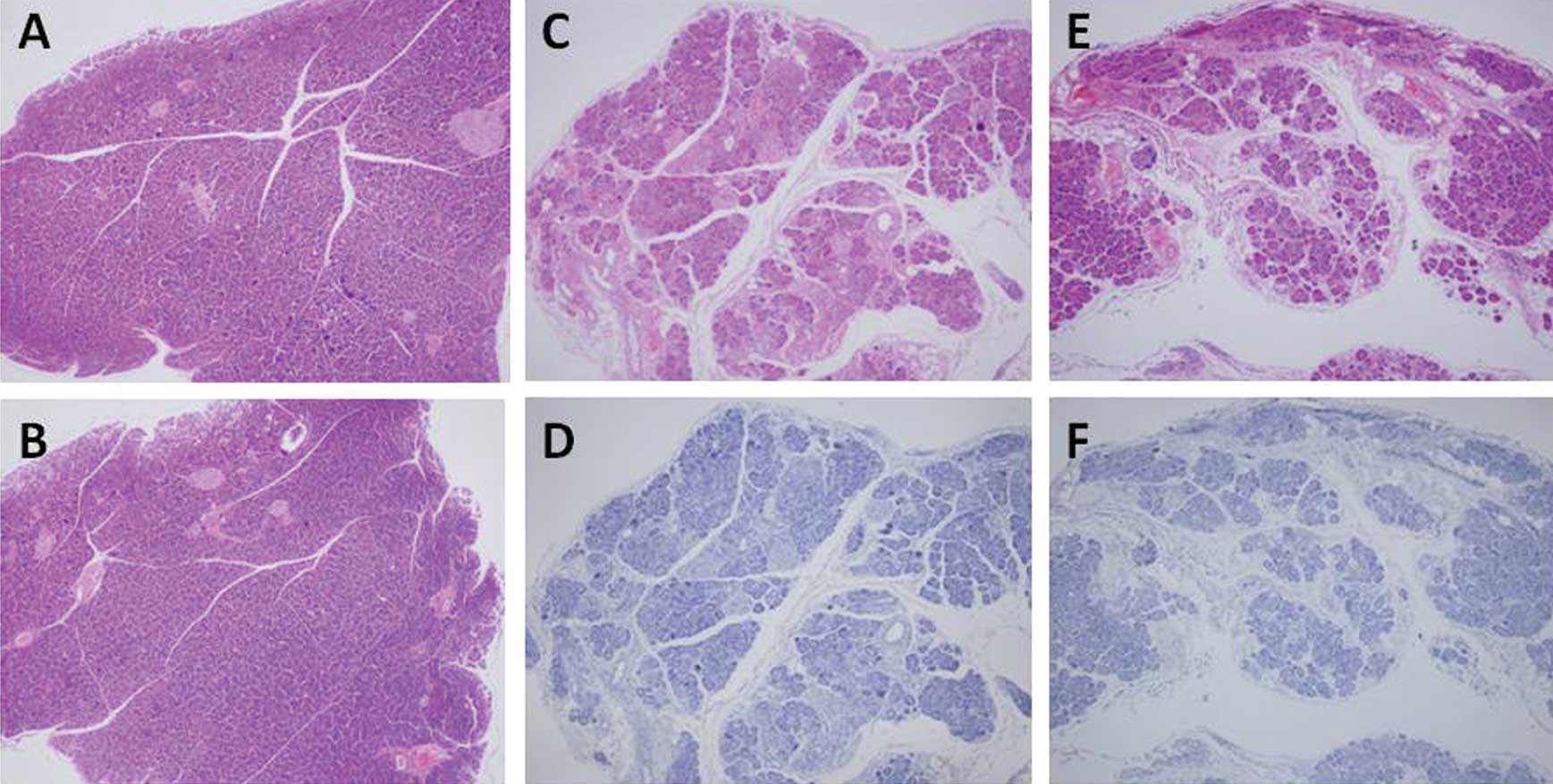
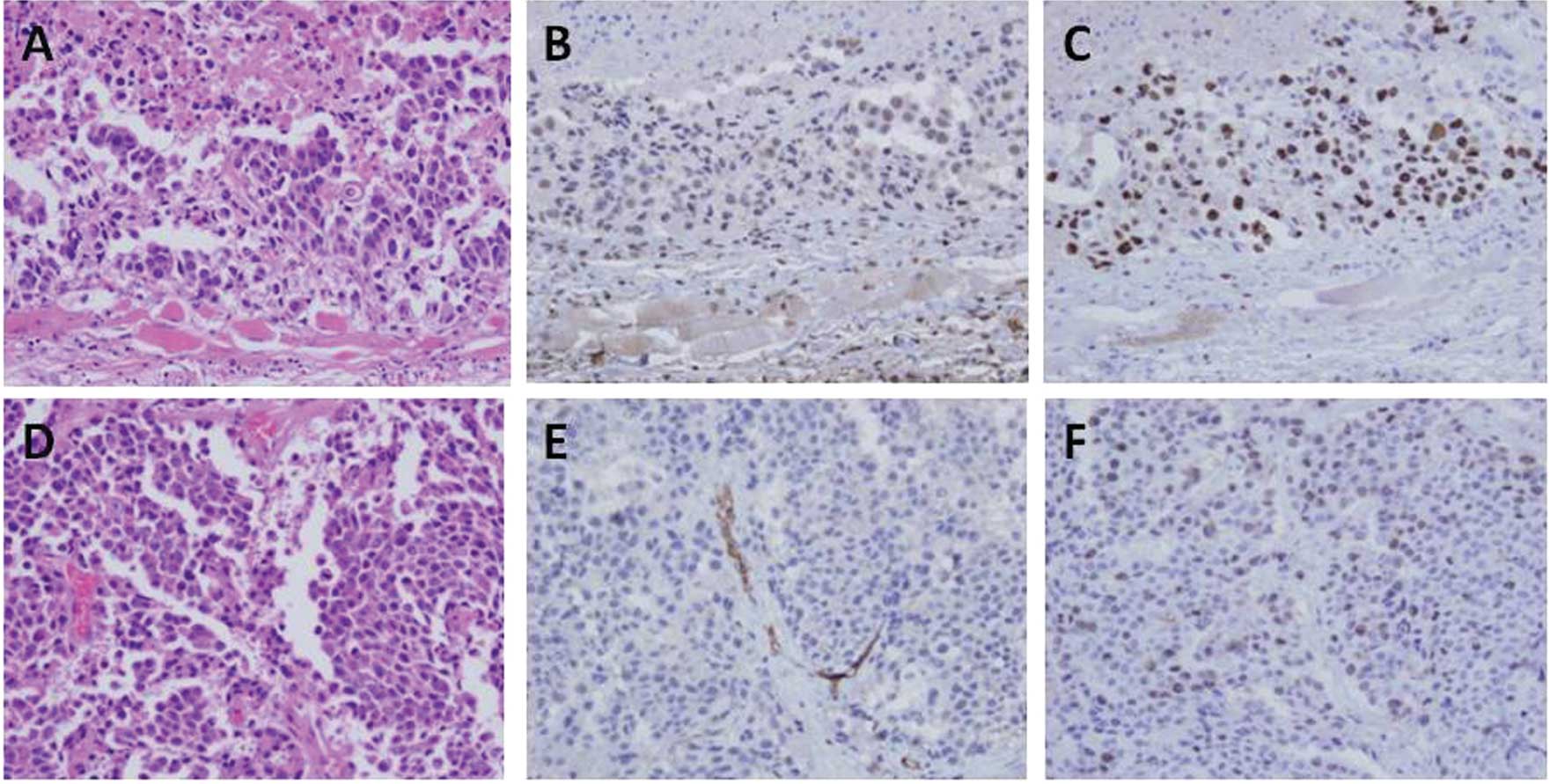
H&E, CD34 and Ki-67 immunostaining of QGP-1
cells is shown in Fig. 6A–F. The
tumor characteristics are described in detail in Table II. A significant difference in the
number of CD34-positive microvessels for tumors composed of the
QGP-1 and BxPC-3 cells was observed between the IgG- and
bevacizumab-administered groups while no significant difference was
noted in the tumors consisting of AsPC-1 cells (Fig. 6; Table
II). By contrast, there was no significant difference in the
Ki-67 LI between the IgG- and bevacizumab-administered groups for
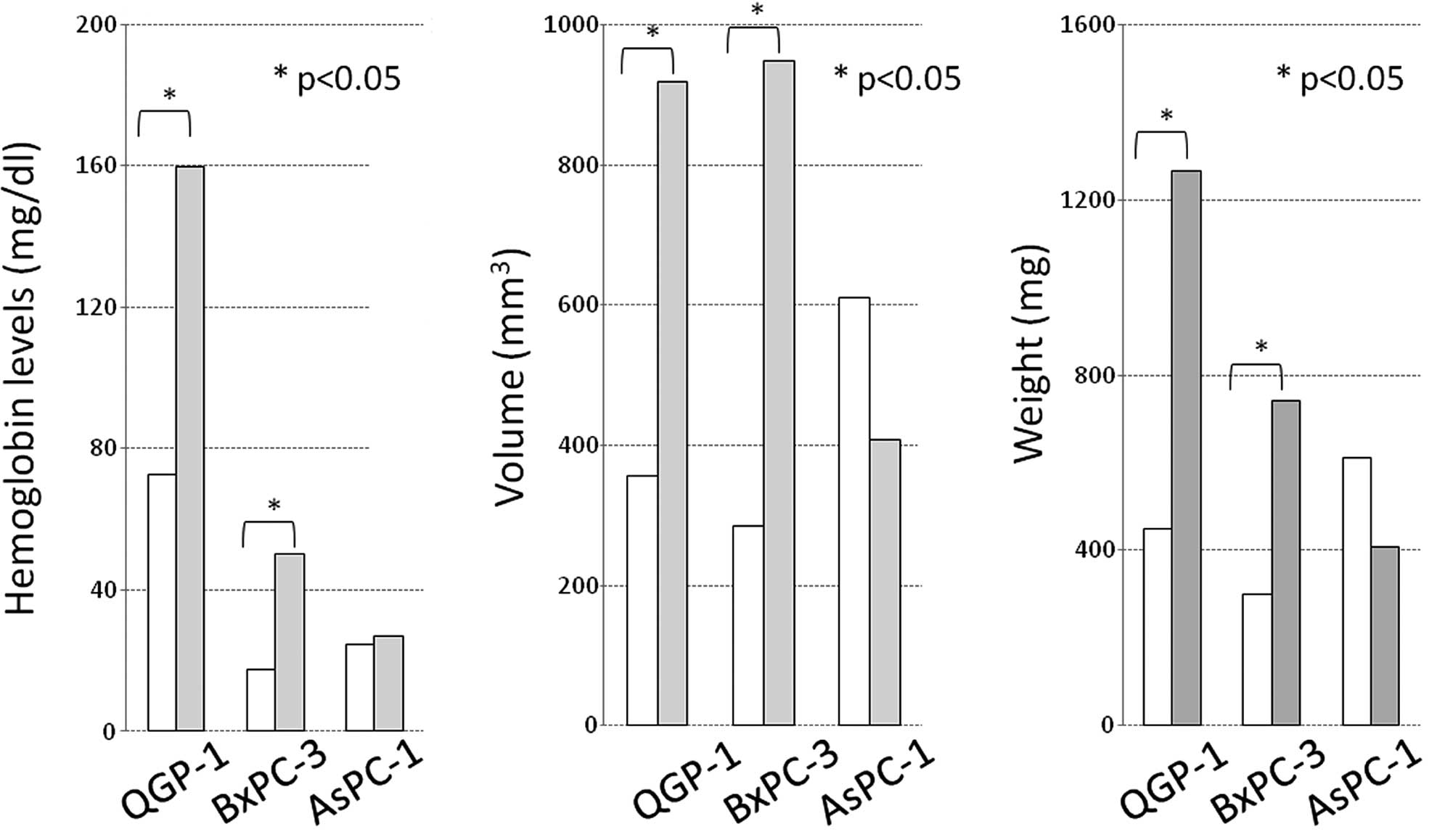
all cell lines (Fig. 6; Table II). The hemoglobin levels in
Matrigel (reflecting the intratumoral vascular bed) in the
IgG-administered mice bearing QGP-1, BxPC-3 and AsPC-1 cells were
159.8, 50.4 and 27.1 mg/dl, respectively, showing inter-cell-line
differences, while these levels in their bevacizumab-administered
counterparts were 72.7, 17.7 and 24.6 mg/dl, respectively, which
were lower than those in the IgG-administered mice (Fig. 7A; p=0.027, p=0.037 and no
significant difference). The volume (mm3) and weight
(mg) of the tumors composed of each cell line significantly
decreased in response to therapy. The QGP-1 and BxPC-3 cell lines
responded more strongly than the AsPC-1 cell line (Fig. 7B and C).
 | Table II.Tumor characteristics. |
Table II.
Tumor characteristics.
| Variables | QGP-1 | BxPC-3 | AsPC-1 |
|---|
| No. of CD34-positive
cells/9 high-power fields | | | |
| Bevacizumab | 5.0a | 12.5a | 1.6 |
| IgG | 27.0a | 28.0a | 3.2 |
| Ki-67 labeling
index | | | |
| Bevacizumab | 34.4% | 26.4% | 35.2% |
| IgG | 37.3% | 33.3% | 47.0% |
Discussion
Bevacizumab is a recombinant human IgG1 monoclonal
antibody against VEGF; it specifically binds to VEGF in the
bloodstream and inhibits the binding of VEGF to VEGF receptors
(VEGFRs) in vascular endothelial cells, thereby inhibiting
angiogenesis. The interstitial pressure around a tumor is usually
increased, inhibiting the delivery of anticancer drugs to tumor
tissue. Bevacizumab normalizes tumor blood vessels, reduces the
interstitial pressure and thereby improves the delivery of
anticancer drugs to tumor tissue (10,11).
In expectation of the added effect of combining bevacizumab with
gemicitabine, a randomized controlled trial of gemicitabine +
placebo vs. gemicitabine + bevacizumab for the treatment of
advanced unresectable pancreatic cancer (CALGB80303) was conducted.
However, no significant differences were observed between the
gemicitabine + placebo and gemicitabine + bevacizumab groups in the
therapeutic response rates (11 vs. 10%, respectively), median
progression-free survival times (4.9 vs. 4.7 months, respectively;
p=0.99) and median survival times (5.8 vs. 6.1 months,
respectively; p=0.78). Thus, gemicitabine + bevacizumab therapy did
not prolong the survival time compared to gemicitabine therapy
(13,14).
K-ras mutations are found in nearly all pancreatic
cancers. Possible reasons for the unresponsiveness of pancreatic
cancers to drugs include K-ras gene mutations. However, in a
randomized controlled trial of chemotherapy vs. chemotherapy +
bevacizumab for the treatment of colorectal cancer with K-ras
mutations, chemotherapy + bevacizumab prolonged the
progression-free survival time by 69% in patients with K-ras
mutations (5.5 vs. 9.4 months), and by 82% in those with the
wild-type K-ras gene, that is without K-ras mutations (7.4 vs. 13.5
months) (22). No standard
chemotherapy has been established for gastrointestinal
neuroendocrine tumors. Somatostatin analogues are often used to
alleviate the symptoms of neuroendocrine tumors (3), and their antitumor effects have been
reported (23). In a randomized
phase II trial of bevacizumab vs. interferon-α for the treatment of
patients (n=44) with unresectable carcinoid tumors treated with
octreotide, a somatostatin analogue, the added effect of combining
bevacizumab with the somatostatin analogue was reported. The
therapeutic response rates were 18 vs. 0%, and the 8-week
progression-free survival rates were 95 vs. 68% (12). The effect of bevacizumab on PNEC
alone has not been reported.
In many cases, pancreatic cancer is associated with
pancreatitis, often with acute pancreatitis. Since bevacizumab has
been reported to cause various adverse reactions by its vascular
endothelial cell growth-inhibiting activity, there is concern that
it may exacerbate the pancreatitis and promote fibrosis, as
concomitant pancreatitis induces the release of various cytokines
and clustering of fibroblasts, thereby resulting in the local
invasion and distant metastasis of pancreatic cancer cells
(15). In pancreatic cancer, VEGF
and VEGFR are overexpressed (7,8), but
high serum VEGF levels are not only due to its secretion by cancer
cells, but also often due to coexisting pancreatitis. Indeed, in
the present mouse model of pancreatitis (19,20),
serum VEGF levels were higher than in normal mice. On the other
hand, the weight and collagen content of the pancreas decreased,
indicating that marked fibrosis as in pancreatitis coexisting with
pancreatic cancer was not induced. The duration of caerulein
administration was 4 weeks and the environment produced by
pancreatitis may have been very limited; however, in this model
histopathological examination showed no evidence of exacerbation of
pancreatitis or promotion of fibrosis in the
bevacizumab-administered group. These results mean that in
pancreatitis coexisting with pancreatic cancers, including PNEC,
bevacizumab administration does not induce pancreatitis or the
associated hypoxic environment, or is not negatively involved.
A previous study confirmed the antitumor effect of
ZM447439, which was found to simultaneously inhibit two members of
the Aurora kinase family, Aurora A and B, in the QGP-1 PNEC cell
line (17). Also, the use of
cyclin-dependent kinase and the low-molecular-weight VEGFR
inhibitor ZK304709 induced apoptosis (18). These are anticancer agents that
target the cell cycle. In the present study, bevacizumab itself
showed no cytotoxic effect, but only reduced the rate of tumor
growth by inhibiting host angiogenesis. However, this effect was
extremely strong, suggesting that drugs with potent cytotoxic
activity, including the above-described anticancer agents, are
important.
In conclusion, the PNEC cell line showed a higher
secretion of VEGF than the DCC cell lines. Single therapy with the
anti-VEGF antibody bevacizumab inhibited the induction of host
angiogenesis in vivo, resulting in significant tumor growth
inhibition, but not in tumor angiogenesis inhibition. In addition,
bevacizumab reduced serum VEGF levels, but did not increase the
area of ischemia in the pancreas. These results show that
bevacizumab does not affect the pancreas itself, and it is expected
to exert a potent growth-inhibitory effect on PNEC alone.
References
|
1
|
Eriksson B and Oberg K: Neuroendocrine
tumours of the pancreas. Br J Surg. 87:129–131. 2000. View Article : Google Scholar
|
|
2
|
Modlin IM, Oberg K, Chung DC, et al:
Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol.
9:61–72. 2008. View Article : Google Scholar
|
|
3
|
Oberg KE, Reubi JC, Kwekkeboom DJ and
Krenning EP: Role of somatostatins in gastroenteropancreatic
neuroendocrine tumor development and therapy. Gastroenterology.
139:742–753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kazanjian KK, Reber HA and Hines OJ:
Resection of pancreatic neuroendocrine tumors: results of 70 cases.
Arch Surg. 141:765–769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi Y, Akishima-Fukasawa Y,
Kobayashi N, et al: Prognostic value of tumor architecture,
tumor-associated vascular characteristics, and expression of
angiogenic molecules in pancreatic endocrine tumors. Clin Cancer
Res. 13:187–196. 2007. View Article : Google Scholar
|
|
6
|
Pavel ME, Hassler G, Baum U, Hahn EG,
Lohmann T and Schuppan D: Circulating levels of angiogenic
cytokines can predict tumour progression and prognosis in
neuroendocrine carcinomas. Clin Endocrinol. 62:434–443. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karayiannakis AJ, Bolanaki H, Syrigos KN,
Asimakopoulos B, Polychronidis A, Anagnostoulis S and Simopoulos C:
Serum vascular endothelial growth factor levels in pancreatic
cancer patients correlate with advanced and metastatic disease and
poor prognosis. Cancer Lett. 194:119–124. 2003. View Article : Google Scholar
|
|
8
|
Chang YT, Chang MC, Wei SC, et al: Serum
vascular endothelial growth factor/soluble vascular endothelial
growth factor receptor 1 ratio is an independent prognostic marker
in pancreatic cancer. Pancreas. 37:145–150. 2008. View Article : Google Scholar
|
|
9
|
Pàez-Ribes M, Allen E, Hudock J, et al:
Antiangiogenic therapy elicits malignant progression of tumors to
increased local invasion and distant metastasis. Cancer Cell.
15:220–231. 2009.PubMed/NCBI
|
|
10
|
Willett CG, Boucher Y, di Tomaso E, et al:
Direct evidence that the VEGF-specific antibody bevacizumab has
antivascular effects in human rectal cancer. Nat Med. 10:145–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Pract Oncol. 3:24–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao JC, Phan A, Hoff PM, et al: Targeting
vascular endothelial growth factor in advanced carcinoid tumor: a
random assignment phase ll study of depot octreotide with
bevacizumab and pegylated interferon alpha-2b. Clin Oncol.
26:1316–1323. 2008. View Article : Google Scholar
|
|
13
|
Kindler HL, Friberg G, Singh DA, et al:
Phase II trial of bevacizumab plus gemcitabine in patients with
advanced pancreatic cancer. J Clin Oncol. 23:8033–8040. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kindler HL, Niedzwiecki D, Hollis D, et
al: A double-blind, placebo-controlled, randomized phase III trial
of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus
placebo (P) in patients (pts) with advanced pancreatic cancer (PC):
a preliminary analysis of Cancer and Leukemia Group B (CALGB). J
Clin Oncol. 25(Suppl 18): 45082007.
|
|
15
|
Kuehn R, Lelkes PI, Bloechle C, Niendorf A
and Izbicki JR: Angiogenesis, angiogenic growth factors, and cell
adhesion molecules are upregulated in chronic pancreatic diseases:
angiogenesis in chronic pancreatitis and in pancreatic cancer.
Pancreas. 18:96–103. 1999. View Article : Google Scholar
|
|
16
|
Takahashi K, Hirano F, Matsumoto K, Aso K
and Haneda M: Homeobox gene CDX2 inhibits human pancreatic cancer
cell proliferation by down-regulating cyclin D1 transcriptional
activity. Pancreas. 38:49–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Georgieva I, Koychev D, Wang Y, Holstein
J, Hopfenmüller W, Zeitz M and Grabowski P: ZM447439, a novel
promising aurora kinase inhibitor, provokes antiproliferative and
proapoptotic effects alone and in combination with bio- and
chemotherapeutic agents in gastroenteropancreatic neuroendocrine
tumor cell lines. Neuroendocrinology. 91:121–130. 2010. View Article : Google Scholar
|
|
18
|
Scholz A, Wagner K, Welzel M, et al: The
oral multitarget tumour growth inhibitor, ZK 304709, inhibits
growth of pancreatic neuroendocrine tumours in an orthotopic mouse
model. Gut. 58:261–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neuschwander-Tetri BA, Burton FR, Presti
ME, et al: Repetitive self-limited acute pancreatitis induces
pancreatic fibrogenesis in the mouse. Dig Dis Sci. 45:665–674.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carrière C, Young AL, Gunn JR, Longnecker
DS and Korc M: Acute pancreatitis markedly accelerates pancreatic
cancer progression in mice expressing oncogenic Kras. Biochem
Biophys Res Commun. 382:561–565. 2009.PubMed/NCBI
|
|
21
|
Liu J, Qu R, Ogura M, Shibata T, Harada H
and Hiraoka M: Real-time imaging of hypoxia-inducible factor-1
activity in tumor xenografts. J Radiat Res. 46:93–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hurwitz HI, Yi J, Ince W, Novotny WF and
Rosen O: The clinical benefit of bevacizumab in metastatic
colorectal cancer is independent of K-ras mutation status: analysis
of a phase III study of bevacizumab with chemotherapy in previously
untreated metastatic colorectal cancer. Oncologist. 14:22–28. 2009.
View Article : Google Scholar
|
|
23
|
Panzuto F, di Fonzo M, Iannicelli E, et
al: Long-term clinical outcome of somatostatin analogues for
treatment of progressive, metastatic, well-differentiated
entero-pancreatic endocrine carcinoma. Ann Oncol. 17:461–466. 2006.
View Article : Google Scholar
|