Introduction
Patients with high-risk central nervous system
tumors commonly refuse surgery or receive partial resection due to
the difficulty and risk of surgery, and the rapid recurrence of
tumors (1–3). Palliative treatment is often applied
because of the inability to perform surgery, regular radiation or
chemotherapy due to the invasion of central organs with important
functions by tumor infiltration and multiple metastases (4–6).
Direct involvement or activation of local nociceptors as well as
adjacent nerves, vessels, central nervous tissue as suppressed by
tumors may lead to paralysis or paraplegia accompanied by pain and
tumor compression complex (7–9).
Using CyberKnife to control or reduce local lesions, we attempted
to improve the internal environment, to reduce adverse reactions,
to control tumor progression and to alleviate clinical
symptoms.
Materials and methods
Subjects
A total of 139 central nervous system tumor lesions
in 60 patients who received palliative treatment at the Center for
Non-Traumatic Treatment and Diagnosis of Tumor, Binzhou Medical
College Affiliated with the PLA 107th Hospital, from October 2010
to May 2011, were selected for the study according to the following
standards. i) Diagnosis of tumors was confirmed by pathology or
imaging tests, such as CT and MRI, and were ranked as stages III–IV
according to the WHO clinical staging system. ii) Patients had an
ECOG Performance status 2–4. iii) Patients exhibited crudescence
following surgery, invasion of central organs with vital functions,
infiltration of lymph and neurons or multiple metastases and had no
indications for surgery or were unable to undergo standard chemical
or radiotherapy. iv) Patients provided informed consent. The
exclusion criteria included i) associated Tb, undetermined
diagnosis, early stage disease with surgical indications; ii)
intractable increased intracranial pressure; iii) recent repeated
radiotherapy or suspected radiotherapy complications.
A total of 38 males and 22 females, 6–63 years of
age, with an average age of 50 years, were enrolled. There were 81
targeted areas from 139 lesions including 9 gliomas, 23 brain
metastases, 8 meningiomas, 11 pituitary tumors, 8 intramedullary
spinal cord tumors and 1 spinal meningioma. Between 1 and 24
lesions were noted in each patient, 1–6 lesions were planned to be
targeted, and 31 cases suffered from brain swelling.
Methods
CT slices (1.25-mm) were performed after
hospitalization. IMR, PET-CT or DSA image fusion was conducted when
necessary to determine the target areas. Centrum or skull 6D
tracking outlined target CTV. Treatment plans and treatment doses
were determined according to the number of tumor lesions and tumor
size. Treatment was carried out 1–6 times, and complete treatment
spanned 1 week.
Evaluation
Stereotactic radiowave surgery platform
specifications: CyberKnife System (Accuray Group Co., Ltd., USA).
The output dose rate was 400–600 cGy. The US FDA granted market
access for systemic treatment as certified in August 2001. Tumor
dose coverage was 85–95%. For patients with small and single-tumor
lesions, the general dose was 1–3 F; for those with large and
multiple tumor lesions the general dose was 4–6 F. The tumor dose
DT was 18–60 Gy, with a single dose of 4–18 Gy.
Criteria
Evaluation: i) imaging examination, shrinkage; ii)
RIA and endocrine hormone detection, decrease or recover; iii)
Zubrod-ECOG-WHO (ZPS) (10) scale
1–4: 0, normal activity; 1, some symptoms but still almost fully
ambulatory; 2, <0% of daytime spent in bed; 3, >50% of
daytime spent in bed; 4, completely bedridden; 5, deceased.
WHO objective criteria of curative effect
(11)
CR, complete remission with symptoms and physical
signs totally disappeared for 4 weeks; PR, partial remission with
tumor volume shrinkage >50% for at least 4 weeks; NC, no
significant change observable for at least 4 weeks with a tumor
volume increase of ≤25% or shrinkage ≥50%; PD, progressive disease
with new lesions appearing or the original lesion increasing
>25% in size. The total effective rate was calculated with the
following equation: (CR + PR)/total cases × 100%.
Statistical analysis
All values are expressed as the means ± standard
deviation (SD). Differences were evaluated using Statistical
Package for Social Science 13.0 (SPSS13.0). Statistical analysis
was performed using the two-sided Student’s t-test. Differences
were considered statistically significant at the level of
p<0.05.
Results
Case statistics
A total of 63 cases were assessed. Three cases were
excluded; 2 cases could not follow the study instructions because
of age and 1 case experienced financial problems. A total of 60
cases were involved in the final analysis.
Imaging tests
High-risk patients with at least one of the
following conditions (tumor recurrence after surgery, multiple
metastases, disease uncontrollable by either radiation or
chemotherapy, invalid conservative therapy, or diagnosis at an
advanced stage) exhibited significant shrinkage or disappearance of
lesions as confirmed by imaging compared to 120 advanced patients
who received only conservative therapy by conformal radiation
therapy.
RIA monitoring and endocrine hormone
condition
Compared to the control group, 5 of 24 patients with
increased tumor markers or endocrine hormone became negative, 10
cases were degraded to varying degrees, 6 cases had no obvious
changes and 3 cases increased following CyberKnife treatment.
ZPS scores were improved to various degrees in all
the patients after treatment, accompanied by alleviation or
disappearance of tumor suppression syndrome and pain syndrome.
Objective effect assessment
Patients with advanced cancer who received repeated
treatment also showed a high rate of alleviation of symptoms after
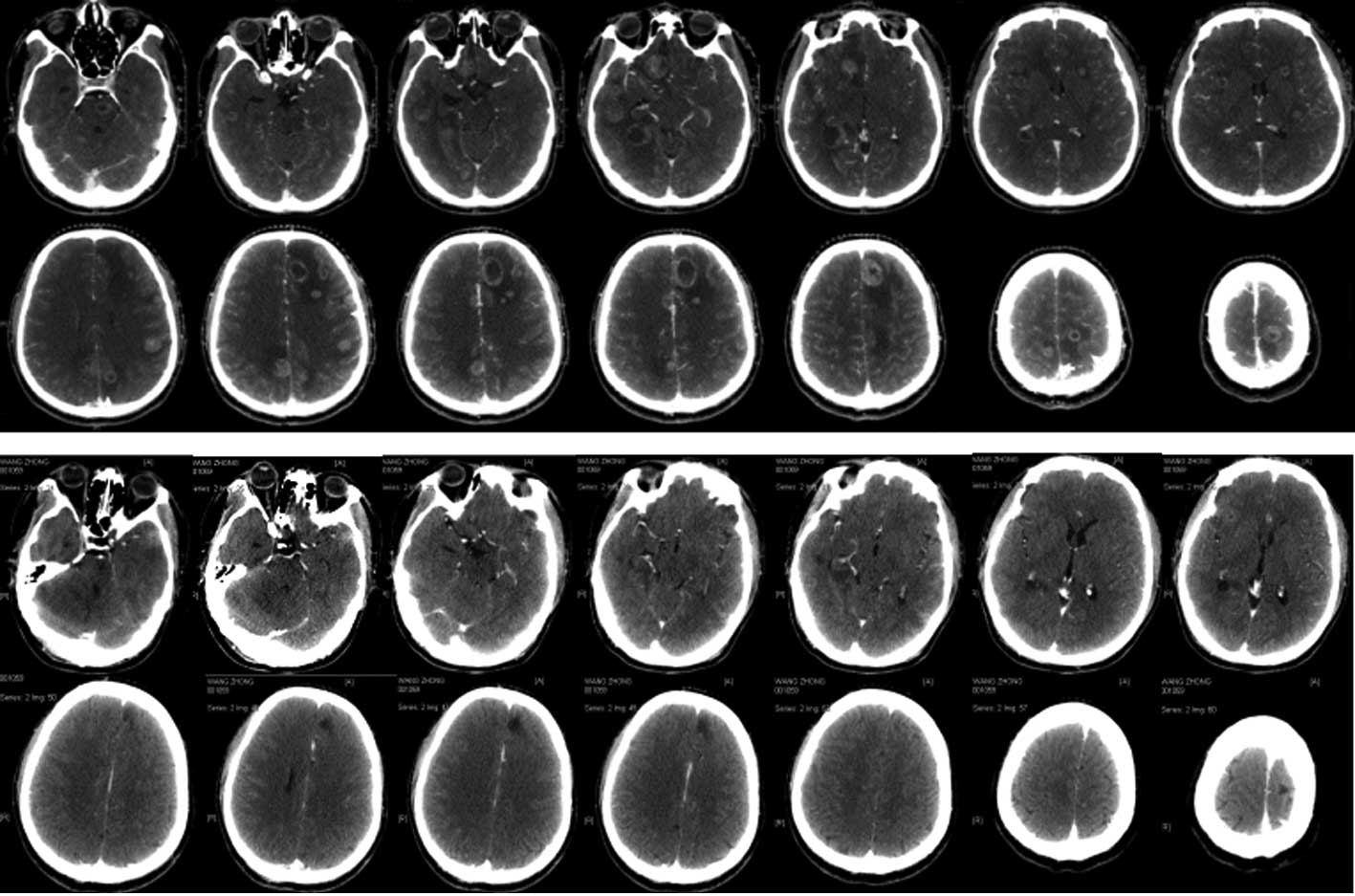
CyberKnife treatment. The brain stem invaded by intracranial
multiple metastases (24 lesions) caused severe central nervous
system suppression syndrome, and the treatment plans were divided
into six parts with at most one target for six tumor lesions. The
patients were able to take care of themselves after completion of
treatment in 19 days (Fig. 1).
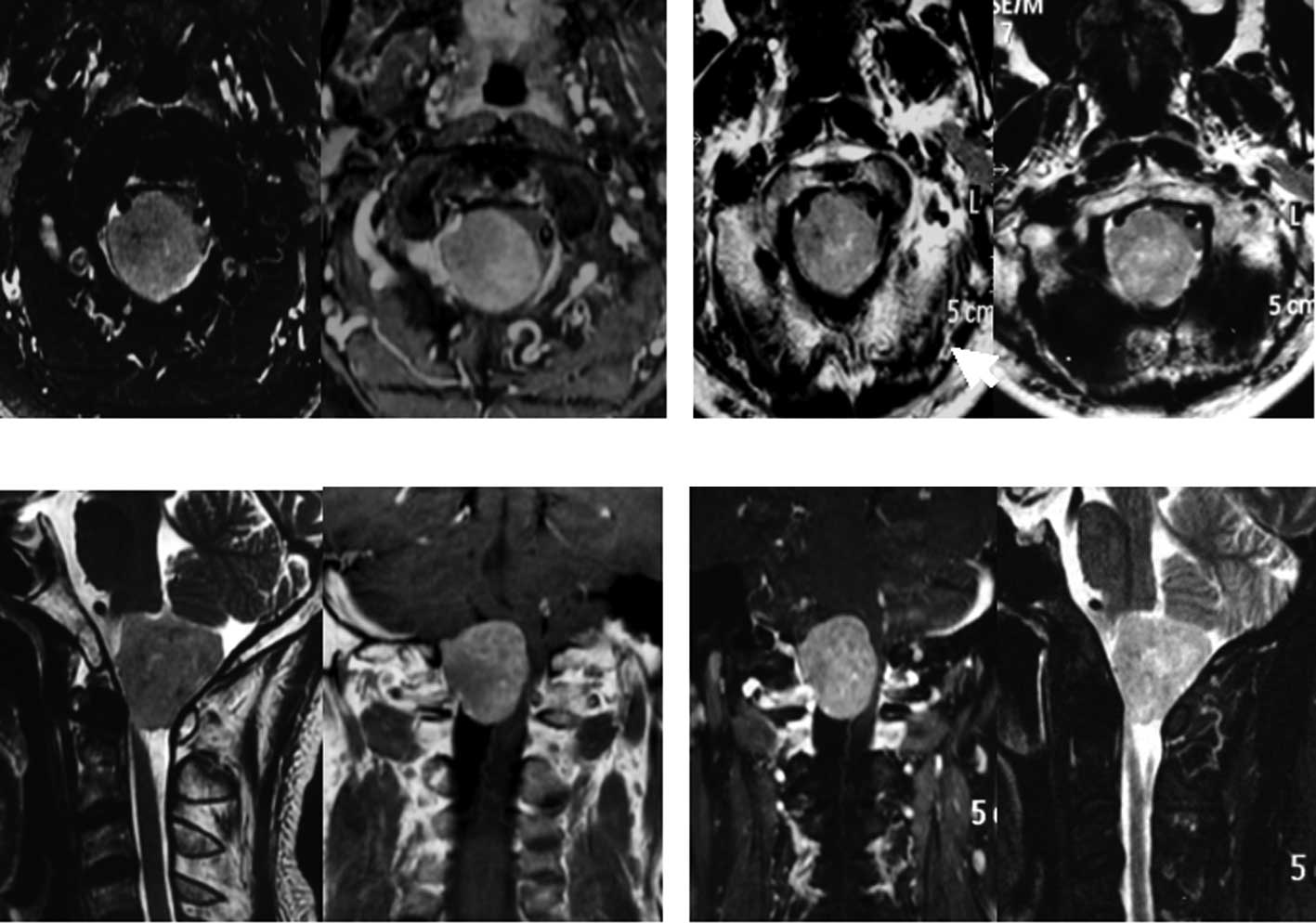
Patients with meningiomas (3.4×3.0×2.7 cm3) who refused
surgery due to the high risk by severe medulla oblongata
placeholder successfully accomplished therapeutic treatment
utilizing CyberKnife (Fig. 2).
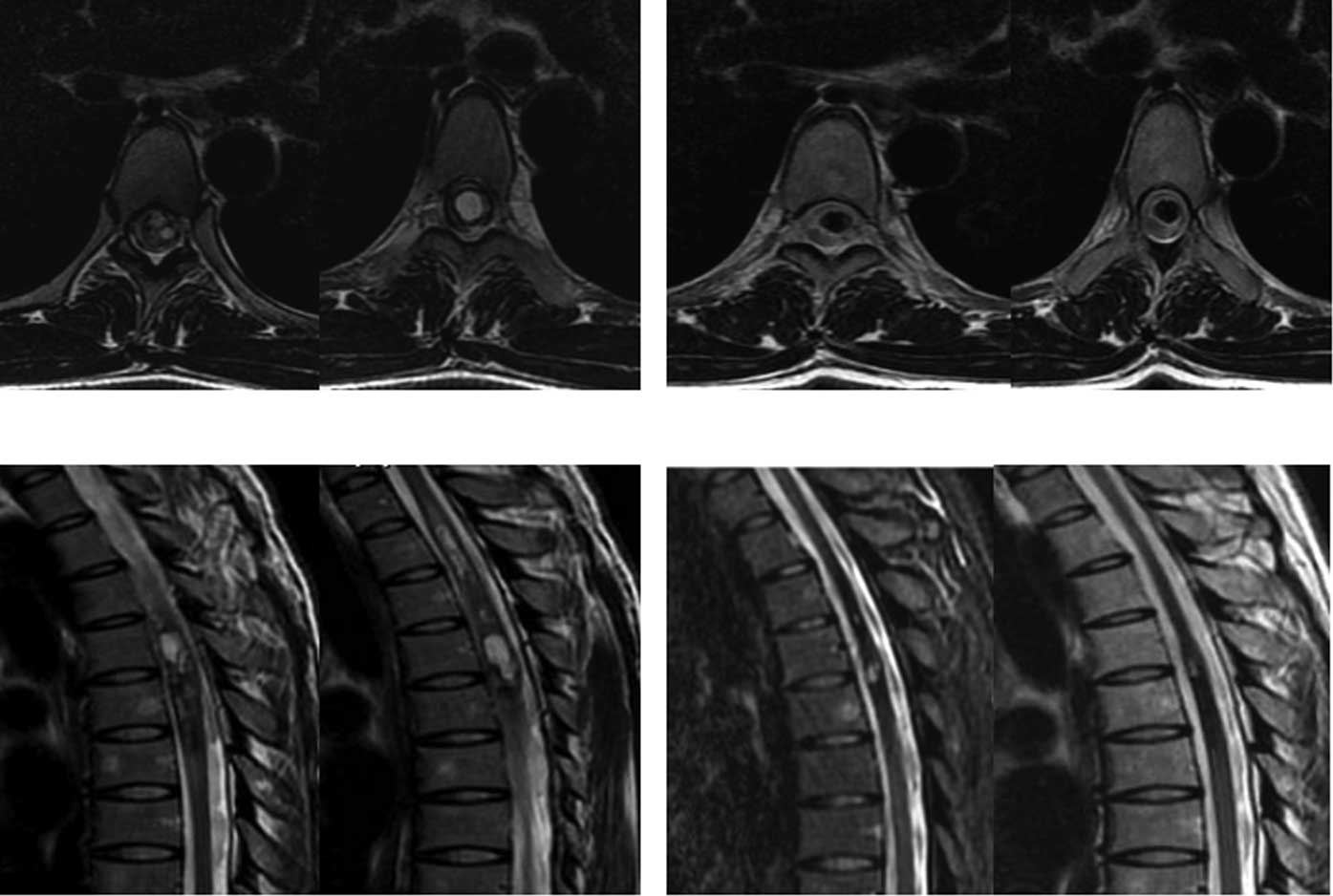
Another case with chest intramedullary hemangiomas (reaching a
length of 14 cm) who was paralyzed in bed and could not care of
himself recovered after CyberKnife treatment and was able to walk
following 4 administrations of CyberKnife treatment (Fig. 3).
Discussion
Palliative treatment is the major treatment for
patients suffering from central nervous system tumors, particularly
brain glioma, metastatic tumors and spinal cord tumors (12–18).
Surgery or conventional radiotherapy is unable to provide a
complete cure due to the late diagnosis of these advanced tumors,
the rapid growth of the tumors, and compression or infiltration of
important organs (13,19–22).
CyberKnife is a flexible method having the advantage of high
accuracy, timely tracking, shorter treatment times, a high-dose,
short treatment course, easy patient access to treatment, improved
local cure rate and favorable results (22,23).
Statistical results from 60 patients with advanced complex tumors
and from 120 patients receiving advanced palliative radiotherapy
showed that, after treatment, in some cases CyberKnife even
achieved complete remission; the effective rate was 71.7%,
suggesting an effective noninvasive treatment for cancer.
CyberKnife technology facilitates cancer treatment
and improves the local control rate, especially in high-risk
patients (24–27). Most patients receiving CyberKnife
do not require hospitalization (28–31),
treatment time is short (32,33),
and no toxic side effects occur (34–36).
We believe that treatment time, frequency and dose should be based
on the number of tumors, size, severity of the disease, repeated
during the length of the treatment time. This means that treatment
should reflect the individual needs and not blindly pursue a rapid
treatment. CyberKnife can be carried out without hospitalization or
early discharge. At the end of treatment, normal routine blood
examination should be carried out. However, after 1–2 weeks of
treatment, if grain dump, brain edema, intracranial pressure and
orthostatic hypotension occurs, it is necessary for the patients to
be hospitalized for observation, consolidation effect, thus they
can be excluded from CyberKnife ‘surgery’ which may cause potential
adverse reactions (37).
CyberKnife also incorporates the merits of the Gamma
(X) knife while overcoming its shortcomings, such as the need for
tumor shape and oversized target areas (38). It works effectively in the cancer
therapy of central nervous system tumors. CyberKnife could
interface with a variety of imaging examinations, including CT,
MRI, PET-CT and DSA, and obtain three-dimensional image to offer
the most direct evidence for gross targeted region delineation
(39). CyberKnife requires more
strict target delineation than ordinary radiotherapy, but not as
traditional as conformal radiation therapy (40). Careful forethought should be given
due to the CyberKnife portrait of fewer but larger doses.
In conclusion, using CyberKnife therapy, the quality
of life of patients with high-risk central nervous system cancer
markedly improves, symptoms are rapidly alleviated and patients
need not undergo surgery (41).
CyberKnife is a new effective therapeutic method for treating
advanced high-risk central nervous system cancers.
Acknowledgements
This study was supported, in part, by
grants from the National Special Issue of the Ministry of Health of
China (no. 2009BX042), the Foundation of Military Scientific and
Technological Issue (no. 06G034), and the Project of Key Program of
Jinan Military Region (no. 02Z07).
References
|
1.
|
J ThariatS BolleY DemizuNew techniques in
radiation therapy for head and neck cancer: IMRT, CyberKnife,
protons, and carbon ions, Improved effectiveness and safety? Impact
on survival?Anticancer Drugs213201021051993
|
|
2.
|
X QiaoO TullgrenI LaxThe role of
radiotherapy in treatment of stage I non-small cell lung cancerLung
Cancer41111200310.1016/S0169-5002(03)00152-112826306
|
|
3.
|
RC McGarryL PapiezM WilliamsStereotactic
body radiation therapy of early-stage non-small-cell lung
carcinoma: phase I studyInt J Radiat Oncol Biol
Phys6310101015200510.1016/j.ijrobp.2005.03.07316115740
|
|
4.
|
J WulfU HaedingerU OppitzStereotactic
radiotherapy for primary lung cancer and pulmonary metastases: a
noninvasive treatment approach in medically inoperable patientsInt
J Radiat Oncol Biol
Phys60186196200410.1016/j.ijrobp.2004.02.06015337555
|
|
5.
|
H YamazakiH ShiomiT TsubokuraQuantitative
assessment of inter-observer variability in target volume
delineation on stereotactic radiotherapy treatment for pituitary
adenoma and mentingioma optic tractRadiat Oncol6102011
|
|
6.
|
CP HasneyRG SwantonPL
FriendlanderCyberKnife stereotactic radiosurgery for recurrent
squamous cell carcinoma of the head and neck following salvage
surgery with close or positive
marginsLaryngoscope4152201010.1002/lary.2161621225750
|
|
7.
|
YS WangQW WangXF JiaThermal dose effect of
regional radiofrequency hyperthermia on metaphase and advanced
stage tumorJ Clin Rehab Tissue Engineering Res11901190152007
|
|
8.
|
S DewasC Dewas-VautraversV ServentResults
and special considerations when treating elderly patients with
CyberKnife: a review of 345 casesCrit Rev Oncol
Hematol7617201021146423
|
|
9.
|
Y XieD DjajaputraCR KingIntrafractional
motion of the prostate during hypofractionated radiotherapyInt J
Radiat Oncol Biol
Phys72236246200810.1016/j.ijrobp.2008.04.05118722274
|
|
10.
|
F UngerK DominikusK
Haselsberger[Stereotactic radio-surgery and fractionated
stereotactic radiotherapy of acoustic neuromas.]HNO5931372011(In
German).
|
|
11.
|
S SharmaJ OttJ WilliamsDose calculation
accuracy of the Monte Carlo Algorithm for CyberKnife compared with
other commercially available dose calculation algorithmsMed
Dosim3514201021144731
|
|
12.
|
YS WangXD XuQW WangClinical research of
partial IMCRT treated malignant ascites combined with Chinese
medicine RFChin Med Front1885872007
|
|
13.
|
VA MarcialTF PajakS KramerRadiation
Therapy Oncology Group (RTOG) studies in head and neck cancerSem
Oncol15396019883278390
|
|
14.
|
BA Jereczed-FossaG BeltramoL
FariselliRobotic image-guided stereotactic radiotherapy, for
isolated recurrent primary, lymph node or metastatic prostate
cancerInt J Radiat Oncol Biol Phys7919201121277113
|
|
15.
|
JW DegenGJ GagnonJM VoyadzisCyberKnife
stereotactic radiosurgical treatment of spinal tumors for pain
control and quality of lifeJ Neurosurg
Spine2540549200510.3171/spi.2005.2.5.054015945428
|
|
16.
|
A SchweikardH ShiomiJ AdlerRespiration
tracking in radiosurgeryMed Phys3127382741200410.1118/1.1774132
|
|
17.
|
BT CollinsK EricksonCA ReichnerRadical
stereotactic radiosurgery with real-time tumor motion tracking in
the treatment of small peripheral lung tumorsRadiat
Oncol239200710.1186/1748-717X-2-3917953752
|
|
18.
|
C ReichnerB CollinsG GagnonThe placement
of gold fiducials for CyberKnife stereotactic radiosurgery using a
modified transbronchial needle aspiration techniqueJ
Bronchol12193195200510.1097/01.lab.0000186345.85025.41
|
|
19.
|
P TherasseSG ArbuckEA EisenhauerNew
guidelines to evaluate the response to treatment in solid tumorsJ
Natl Cancer Inst92205216200010.1093/jnci/92.3.20510655437
|
|
20.
|
L PapiezR TimmermanC
DesRosiersExtracranial stereotactic radioablation: physical
principlesActa
Oncol42882894200310.1080/0284186031001349014968949
|
|
21.
|
JR SimpsonME FrancisR
Perez-TamayoPalliative radiotherapy for inoperable carcinoma of the
lung: final report of a RTOG multi-institutional trialInt J Radiat
Oncol Biol Phys11751758198510.1016/0360-3016(85)90307-42579938
|
|
22.
|
KW RohJS JangMS KimFractionated
stereotactic radiotherapy as reirradiation for locally recurrent
head and neck cancerInt J Radiat Oncol Biol
Phys7413481355200910.1016/j.ijrobp.2008.10.013
|
|
23.
|
HJ ChenSW LeungCY SuLinear accelerator
based radiosurgery as a salvage treatment for skull base and
intracranial invasion of recurrent nasopharyngeal carcinomaJ Clin
Oncol24255258200110.1097/00000421-200106000-0000911404496
|
|
24.
|
SC SharmaClinical implications of adopting
Monte Carlo treatment planning for CyberKnifeJ Appl Clin Med
Phys1170175201020160699
|
|
25.
|
WT BrownX WuF FayadApplication of robotic
stereotactic radiotherapy to peripheral stage I nonsmall cell lung
cancer with curative intentClin
Oncol21623631200910.1016/j.clon.2009.06.00619682875
|
|
26.
|
WT BrownX WuBC WenEarly results of
CyberKnife image-guided robotic stereotactic radiosurgery for
treatment of lung tumorsComput Aided
Surg12253261200710.3109/1092908070168475417957532
|
|
27.
|
LE ErrettJ WilsonRC ChiuWedge resection as
an alternative procedure for peripheral bronchogenic carcinoma in
poor-risk patientsJ Thorac Cardiovasc Surg9065666119853932783
|
|
28.
|
BK ChangRD TimmermanStereotactic body
radiation therapy: a comprehensive reviewAm J Clin
Oncol30637644200710.1097/COC.0b013e3180ca7cb118091059
|
|
29.
|
W HaraBW LooDR GoffinetExcellent local
control with stereotactic radiotherapy boost after external beam
radiotherapy in patients with nasopharyngeal carcinomaInt J Radiat
Oncol Biol Phys71393400200810.1016/j.ijrobp.2007.10.02718164839
|
|
30.
|
A VavassoriBA Jereczek-FossaG
BeltramoImageguided robotic radiosurgery as salvage therapy for
locally recurrent prostate cancer after external beam irradiation:
retrospective feasibility study on six casesTumori9671752010
|
|
31.
|
BA Jereczek-FossaL FariselliG
BeltramoLinac-based or robotic image-guided stereotactic
radiotherapy for isolated lymph node recurrent prostate
cancerRadiother Oncol931417200910.1016/j.radonc.2009.04.001
|
|
32.
|
JF FowlerJS WelshSP HowardLoss of
biological effect in prolonged fraction deliveryInt J Radiat Oncol
Biol Phys159242249200410.1016/j.ijrobp.2004.01.00415093921
|
|
33.
|
J ThariatG LiG AngellierCurrent
indications and ongoing clinical trials with CyberKnife
stereotactic radiotherapy in France in 2009Bull
Cancer96853864200919736172
|
|
34.
|
N Calcerrada Daz-SantosJA Blasco AmaroGA
CardielThe safety and efficacy of robotic image-guided radiosurgery
system treatment for intra- and extracranial lesions: a systematic
review of the literatureRadiother Oncol892452532008
|
|
35.
|
DB FullerJ NaitohC LeeVirtual HDR
CyberKnife treatment for localized prostatic carcinoma: dosimetry
comparison with HDR brachytherapy and preliminary clinical
observationsInt J Radiat Oncol Biol
Phys7015881597200810.1016/j.ijrobp.2007.11.067
|
|
36.
|
SC SharmaJT OttJB WilliamsD
DickowCommissioning and acceptance testing of a CyberKnife linear
acceleratorJ Appl Clin Med
Phys82473200710.1120/jacmp.v8i3.247317712305
|
|
37.
|
RD BucholzKA LaycockLE CuffCyberKnife
stereotactic radiosurgery for intracranial neoplasms, with a focus
on malignant tumorsTechnol Cancer Res
Treat9541550201010.1177/15330346100090060221070076
|
|
38.
|
CR KingJD BrooksH GillT PawlickiC
CotrutzJC Presti JrStereotactic body radiotherapy for localized
prostate cancer: interim results of a prospective phase II clinical
trialInt J Radiat Oncol Biol
Phys7310431048200910.1016/j.ijrobp.2008.05.05918755555
|
|
39.
|
B WowraA MuacevicJC TonnQuality of
radiosurgery for single brain metastases with respect to treatment
technology: a matched-pair analysisJ
Neurooncol946977200910.1007/s11060-009-9802-y19184641
|
|
40.
|
HU AhmedA IshaqE ZacharakisRectal fistulae
after salvage high-intensity focused ultrasound for recurrent
prostate cancer after combined brachytherapy and external beam
radiotherapyBJU
Int103321323200910.1111/j.1464-410X.2008.08026.x19021611
|
|
41.
|
BO ChoiIB ChoiHS JangStereotactic body
radiation therapy with or without transarterial chemoembolization
for patients with primary hepatocellular carcinoma: preliminary
analysisBMC Cancer8351200810.1186/1471-2407-8-351
|