Introduction
Second to cardiovascular disease, cancer is the
leading cause of death throughout the world. Cancer cells evade
self-demise through apoptosis by the accumulation of several
genetic and epigenetic changes (1). Therefore, agents that trigger
apoptosis in cancer cells are regarded as important for the
development of anticancer chemotherapeutics (2). In addition, cancer cells, including
leukemia cells, exhibit a defect in their capacity to mature to
non-replicating adult cells, therefore remaining in a highly
proliferative state and outgrowing their normal cellular
counterparts (3,4). Induction of terminal differentiation,
leading to the eventual elimination of tumorigenic cells and
reestablishment of normal cellular homeostasis, represents an
alternative approach to cancer treatment using conventional
antitumor agents.
Essential oils diluted from several plants have been
used for a long time in perfumery, aromatherapy, food and flavors.
Many essential oils are known to be potent antibacterial and
antifungal agents. Certain essential oil components have been
reported to have anticancer activity (5). The advantage of these components in
anticancer therapy is their low or negligible toxicity. Therefore,
such components can be used for the long-term treatment of
chemoprevention and chemotherapy of cancer (6).
Kuromoji (Lindera umbellata) is a deciduous
shrubby tree that is native to cool or warm temperate areas of
Japan. It is known as a spicebush because it contains aromatic
components in its twigs and leaves. Consequently, Kuromoji branches
have been used for indoor decorations and to produce shavings for
aromatic baths. Furthermore, Kuromoji essential oil (KEO) obtained
through steam distillation has been used traditionally as a
medicine for neuralgia, stiff neck and back pain. The main KEO
components are terpenes and alcohol esters; linalool is the most
abundant component. Geranyl acetate, an ester contained in KEO
contributes a relaxing and calming effect on humans. Other terpenes
(limonene and α-pinene) and alcohols (geraniol and cineol) in
essential oil have beneficial activities for various diseases,
supporting anti-inflammatory and anti-obesity effects.
Linalool reportedly possesses strong activity
against U937 histiocytic lymphoma cells and P3HR1 Burkitt lymphoma
cells (7). Moreover, several
recent reports have revealed that linalool reverses doxorubicin
resistance in human breast adenocarcinoma cells (8) and exhibits antiproliferative activity
against certain solid tumor cells, such as melanoma and renal cell
adenocarcinoma cells (9), and
HepG2 (10). Particularly,
linalool induces apoptosis in human leukemia cells without
affecting normal hematopoietic cell growth (11). These terpenoid components become
ligands in nuclear receptors, regulate expression of various genes
and ameliorate conditions related to these diseases (12).
Human myeloid leukemia cells, specifically HL-60
cells, are known to differentiate into granulocytes or monocytes
when treated with a variety of compounds, such as
all-trans-retinoic acid (ATRA), dimethyl sulfoxide and vitamin D3
(13,14). In particular, ATRA is a class of
chemical compounds that are structurally related to vitamin A; ATRA
is one class of compounds known as retinoids, which are used for
clinical therapy for acute promyelocytic leukemia (APL) (13). These retinoid components become
ligands of nuclear receptors; they regulate cell proliferation and
differentiation (15).
This study was undertaken to investigate whether KEO
induces apoptosis and promotes differentiation in human leukemia
HL-60 cells. The results suggest that KEO may be available for use
as a natural therapeutic agent of APL.
Materials and methods
Chemical reagents and cells
For use in this study, HL-60 cells (ATCC CCL240)
were obtained from DS Pharma Biomedical Co., Ltd. (Osaka, Japan).
Fetal bovine serum (FBS) was purchased from Biological Industries
Ltd. (Kibbutz, Beit, Israel). RPMI-1640 medium was purchased from
Sigma-Aldrich Japan (Tokyo, Japan). ATRA was purchased from Wako
Pure Chemical Industries, Ltd. (Osaka, Japan). A sandwich ELISA kit
used to estimate the levels of histone-associated DNA fragments was
purchased from Roche Diagnostics GmbH (Mannheim, Germany). All
other chemicals, guaranteed to be of reagent or tissue-culture
grade, were obtained from Sigma-Aldrich Japan or Wako Pure Chemical
Industries, Ltd.
Isolation of Kuromoji essential oil
KEO was donated from Senjyakudo (Aomori, Japan).
Fresh Kuromoji leaves and branches were collected in the mountains
near Aomori city. These ingredients were heated to boiling
temperature. The hydro-distilled volatile part was subsequently
collected and separated (upper layer) from the aqueous portion.
Analysis of KEO constituents was carried out using
gas chromatography-MS at the Aomori Prefectural Industrial
Technology Research Center. The GC-MS system (GC-17A/QP-5000;
Shimadzu Corp.) was equipped with a DB-1701 column (30 mm × 0.25 mm
× 0.25 μm; J&W Scientific Inc.). The carrier gas was
helium. The program was operated with the following oven
temperature program: 50°C, held for 5 min, rising at 5°C/min to
100°C, rising at 2°C/min to 150°C, rising at 10°C/min to 250°C; the
injection temperature and volume were 270°C and 1.0 μl,
respectively. Constituents were identified by computer matching of
mass spectral data with data from the NIST62 mass spectral database
(Shimadzu Corp.). The major constituents of KEO were linalool
(65.78%), geranyl acetate (17.59%), geraniol (5.29%), cineol
(2.34%), limonene (2.11%), 3-carene (1.78%), α-pinene (1.43%) and
carvone (1.18%).
Cell culture
HL-60 cells were cultured in RPMI-1640 medium
supplemented with 10% heat-inactivated FBS, 100 μg/ml
streptomycin and 100 IU/ml penicillin at 37°C in a 5%
CO2 humidified incubator. For each experiment, HL-60
cells were seeded at a density of 5×104 cells/ml. As an
ethanol solution, KEO, ATRA and linalool were added to the culture
medium. The final concentration of ethanol was <0.1% (v/v).
Proliferation of HL-60 cells
HL-60 cells (5×104 cells/ml) were
incubated in the culture medium containing KEO, ATRA or linalool.
The cell number was determined using a hematocytometer, and cell
viability was determined with a dye exclusion test using trypan
blue.
ELISA analysis
DNA fragmentation was estimated using a
biotin-labeled anti-histone antibody and a peroxidase-conjugated
anti-DNA-antibody in a sandwich ELISA kit. First, HL-60 cells
(5×104 cells/ml) were treated with KEO or linalool.
Then, cells were washed with PBS and lysed with 200 μl or
lysis buffer for 30 min at room temperature. The lysate was
centrifuged at 200 × g for 10 min, and 20 μl of supernatant
was transferred onto a streptavidin-coated microtiter plate. The
presence of histone-associated DNA fragments in the cell lysates
was determined using a sandwich ELISA kit. The level of DNA
fragmentation of mononucleosomes and oligonucleosomes in HL-60
cells was expressed as an enrichment factor, as calculated using
the following formula: Enrichment factor = mU of the sample (HL-60
cells treated with sample)/mU of the corresponding control (HL-60
cells without sample treatment); mU = absorbance
(A405nm).
Staining and nitroblue tetrazolium
reduction assay
As an indicator of HL-60 cell differentiation, a
nitroblue tetrazolium (NBT) reduction assay was utilized (16). After HL-60 cells were treated with
the samples for 72 h, they were suspended in PBS; Giemsa stain
results were then observed using microscopy (×400). Subsequently,
the cell pellet was centrifuged at 500 × g for 5 min and rinsed
three times with PBS. The cells were suspended in 0.5 ml of NBT
solution containing 2 mg/ml of NBT and 200 ng/ml TPA in PBS. They
were then incubated for 30 min at 37°C. The cells were washed with
aliquots of PBS and resuspended in 0.1 ml of PBS. HL-60 cells do
not produce superoxide anions (O2−), but when they
differentiate, they start to produce O2−. They then form
blue-black formazan deposits by reducing the NBT reagent. For each
determination, at least 200 cells were counted using a
hematocytometer. The number of NBT-positive cells containing
intracellular formazan deposits was expressed as a percentage of
the viable cell number.
Statistical analysis
The results are expressed as the means ± standard
deviation (SD). Statistical analyses between multiple groups were
performed using ANOVA.
Statistical comparisons were carried out using
Dunnet’s multiple comparison test. Differences were considered
significant at p<0.05 or p<0.01. Analyses were performed
using StatView-J software ver. 5.0 (Abacus Concepts, Inc.,
Berkeley, CA, USA).
Results
Kuromoji essential oil inhibits cell
proliferation
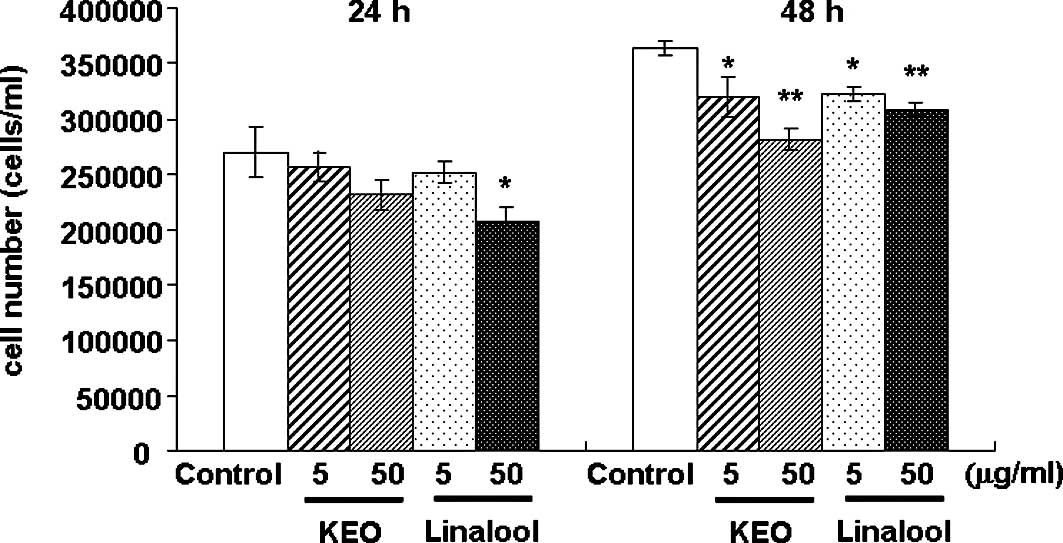
The effect of KEO on the growth of HL-60 cells was
examined (Fig. 1). The HL-60 cells
were treated with 5 or 50 μg/ml KEO or linalool for 24 or 48
h, and cell proliferation was assessed using a dye exclusion test
using trypan blue. The number of viable cells treated with linalool
at 50 μg/ml for 24 h was significantly (p<0.05)
suppressed compared to the control cells. After 48 h of treatment
with KEO or linalool, the cell growth was inhibited significantly
in a dose-dependent manner.
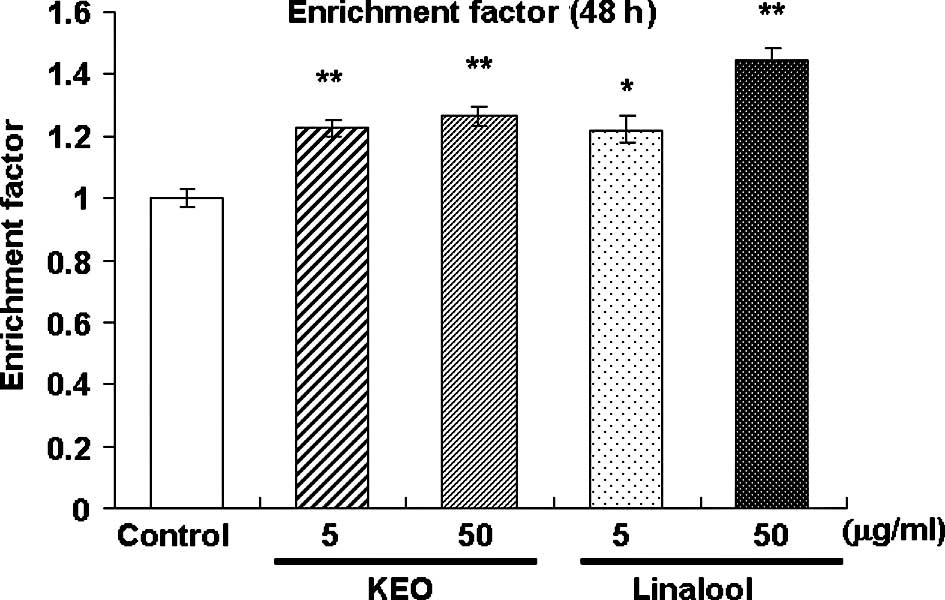
Fig. 2 presents an
illustration of the apoptosis-inducing activity in the HL-60 cells
treated with KEO or linalool for 48 h. Enrichment of
mononucleosomes and oligonucleosomes in the HL-60 cells was
estimated using sandwich ELISA after treatment with KEO or linalool
for 48 h. The fragmented DNA content, designated as an enrichment
factor, increased (p<0.05 or p<0.01) in the HL-60 cells
treated with KEO and linalool compared to the control cells. These
results suggest that KEO and linalool suppress HL-60 cell
proliferation by inducing apoptosis.
Effect of KEO or linalool on the
differentiation of HL-60 cells
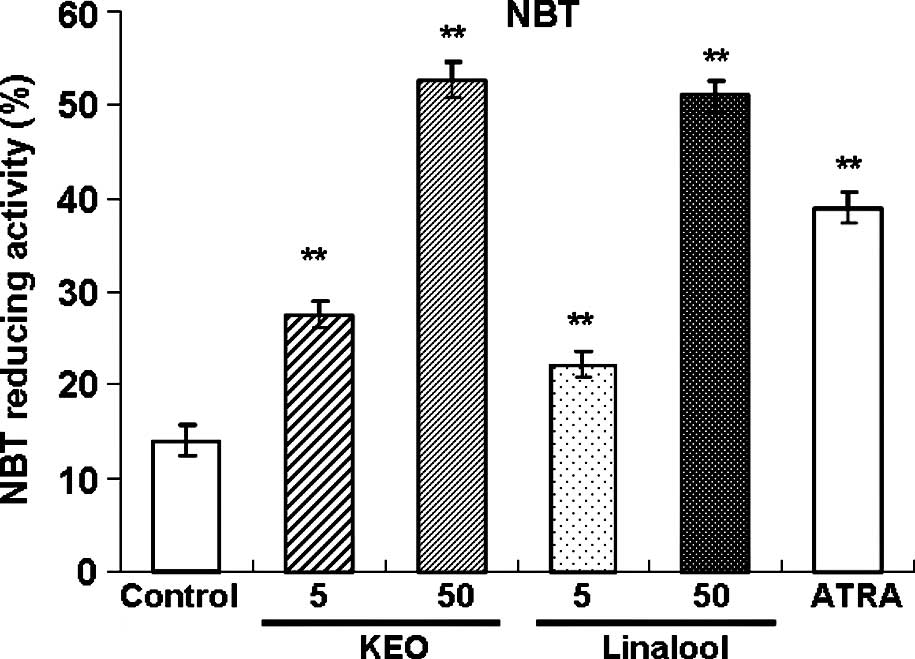
Treatment with KEO or linalool enhanced the
NBT-reducing activity in the HL-60 cells (Fig. 3). Treatment with 1 μM ATRA
increased the NBT-reducing activity. Treatment of the HL-60 cells
with KEO at 5 and 50 μg/ml promoted the NBT-reducing
activity in a dose-dependent manner. Effects of KEO and linalool
treatment on the HL-60 cells were almost equal for equivalent
concentrations.
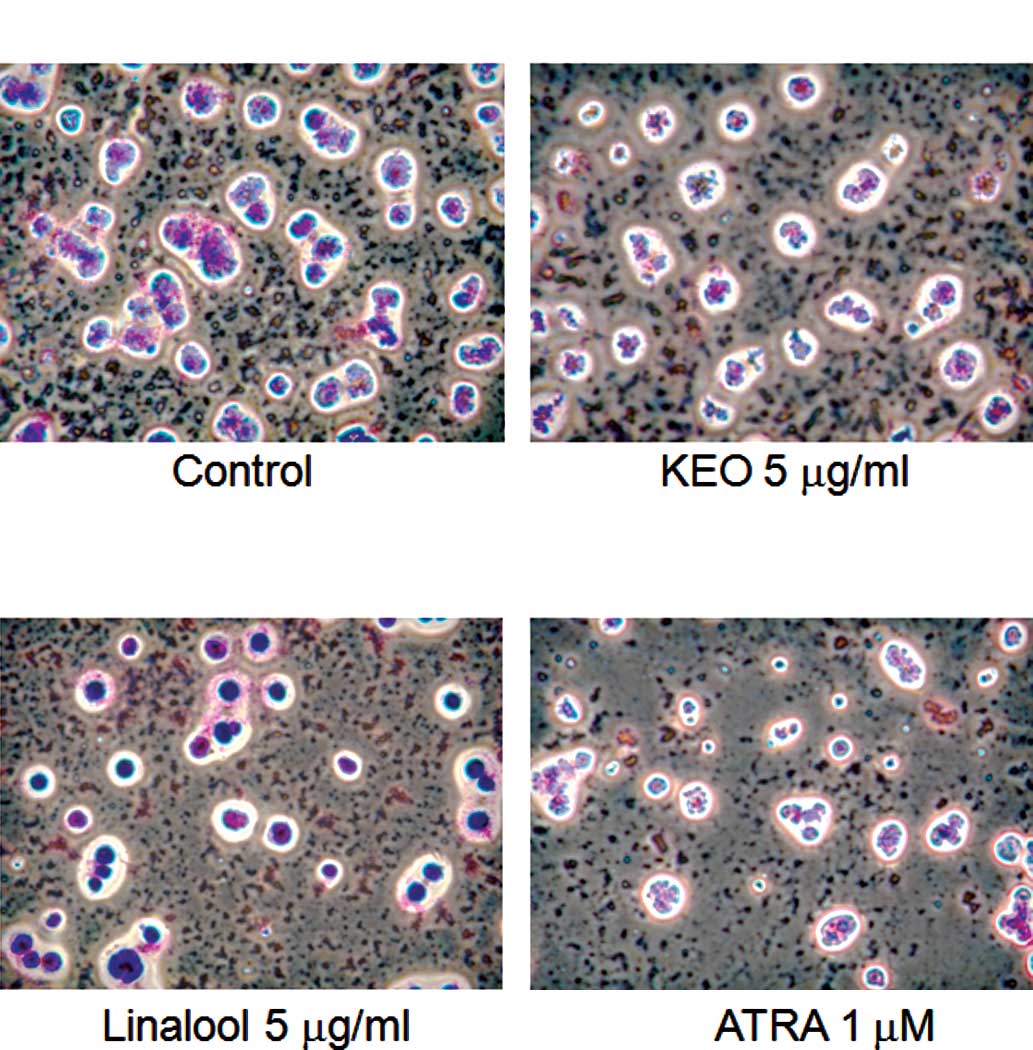
Furthermore, the morphology of cells treated with
KEO was observed using the Giemsa staining method (Fig. 4). Control cells were round and the
ratio of the nucleus to cytoplasm was high (Fig. 4A). By contrast, irregular and small
nuclei, which are typical shapes of differentiation-induced cells,
were observed in cells treated with KEO (5 μg/ml), linalool
(5 μg/ml) or ATRA (1 μM) (Fig. 4B–D).
Discussion
This study demonstrated that KEO and its major
chemical constituent linalool exhibit anticancer therapeutic
effects in HL-60 cells. Treatment with 5 or 50 μg/ml KEO
suppressed the growth of HL-60 cells after 48 h. Treatment with
linalool cells at the same dose of KEO showed an almost identical
effect on HL-60 cells. Cell proliferation is a key to the promotion
and progression of carcinogenesis. Therefore, we investigated the
apoptosis-inducing activity of KEO and linalool. The fragmented DNA
content increased in cells treated with KEO and linalool for 48 h.
This result suggests that KEO has leukemia cell apoptosis-inducing
activity. Gu et al observed that linalool arrested growth
arrest and caused apoptosis of various human leukemia cells, but
noted that it spared normal hematopoietic cells (11). Linalool is the predominant
component of KEO (65.78%). Therefore, it was presumed that linalool
was the most active component of KEO in inducing apoptosis of HL-60
cells. Reportedly, cineole, another KEO component, was also found
to exhibit a suppressive effect on leukemia cell lines resulting
from the induction of apoptosis (17). Consequently, co-efficiency of these
components has been considered. Thus, further studies are necessary
to clarify the active component.
The present study demonstrated that KEO exhibits
apoptotic effects in HL-60 cells. However, the effect was not
strong when compared to effects reported in a previous study
(11). Reportedly, many natural
compounds enhance the effects of leukemia cell differentiation
(18). Treatment with KEO
increased the differentiation of mature cells, similar to that
noted in ATRA-treated cells. Nuclear receptors are known to mediate
the actions of ATRA on cell differentiation (19). Nuclear receptor families of
retinoic acid receptors (RARs) and retinoid X receptors (RXRs) are
associated with the differentiation transcription of various genes
related to the differentiation of HL-60 cells. Actually, ATRA is
known to induce the differentiation of HL-60 cells to granulocytes
as mediated by RARs (20).
Presumably, KEO and linalool regulate the expression of related
differentiation genes as mediated by RARs or RXRs. Therefore,
detailed mechanisms of the differentiation effects of KEO and
linalool should be examined further.
ATRA was found to induce complete remission in a
high percentage of patients with APL, although the response is
sometimes quite slow (21).
Furthermore, relapse and resistance to treatment often occur
despite continued treatment with ATRA (22). Moreover, chemotherapy with ATAR
infrequently induces Sweet’s syndrome, which is characterized by
acute onset of inflammatory skin nodules associated with systemic
features which include malaise, fever and neutrophilia (23). Consequently, combination treatment
strategies have been suggested to circumvent these problems.
In conclusion, results of this study revealed that
KEO as well as its major chemical linalool induce apoptosis and
differentiation in human leukemia HL-60 cells. The possibility
exists that KEO and linalool, currently applied as an essential oil
in aromatherapy, can be developed into an anticancer therapeutic
product.
Acknowledgements
This study was supported by a
grant-in-aid for Young Scientists (21780119) from the Japan Society
for the Promotion of Science (JSPS) Fellows from the Ministry of
Education, Culture, Sports, Science and Technology of Japan. The
authors are grateful to the Aomori Prefectural Industrial
Technology Research Center and Senjyakudo for preparing the
KEO.
References
|
1.
|
G KleinCancer, apoptosis, and nonimmune
surveillanceCell Death
Differ11317200410.1038/sj.cdd.440134214615800
|
|
2.
|
KH LeeAnticancer drug design based on
plant-derived natural productsJ Biomed Sci4236250199910420081
|
|
3.
|
GB PierceWC SpeersTumors as caricatures of
the process of tissue renewal: prospects for therapy by directing
differentiationCancer Res481996200419882450643
|
|
4.
|
HM BeereJA HickmanDifferentiation: a
suitable strategy for cancer chemotherapy?Anticancer Drug
Des429932219938240658
|
|
5.
|
AE EdrisPharmaceutical and therapeutic
potentials of essential oils and their individual volatile
constituents: a reviewPhytother
Res4308323200710.1002/ptr.207217199238
|
|
6.
|
A KumarF MalikS BhushanVK SethiAK ShahiJ
KaurSC TanejaGN QaziJ SinghAn essential oil and its major
constituent isointermedeol induce apoptosis by increased expression
of mitochondrial cytochrome c and apical death receptors in human
leukaemia HL-60 cellsChem Biol
Interact3332347200810.1016/j.cbi.2007.10.003
|
|
7.
|
LC ChiangW ChiangMY ChangLT NgCC
LinAntileukemic activity of selected natural products in TaiwanAm J
Chin Med313746200310.1142/S0192415X0300082512723753
|
|
8.
|
R RavizzaMB GariboldiR MolteniE
MontiLinalool, a plant-derived monoterpene alcohol, reverses
doxorubicin resistance in human breast adenocarcinoma cellsOncol
Rep20625630200818695915
|
|
9.
|
MR LoizzoR TundisF MenichiniAM SaabGA
StattiF MenichiniAntiproliferative effects of essential oils and
their major constituents in human renal adenocarcinoma and
amelanotic melanoma cellsCell
Prolif4110021012200810.1111/j.1365-2184.2008.00561.x19040575
|
|
10.
|
SY PaikKH KohSM BeakSH PaekJA KimThe
essential oils from Zanthoxylum schinifolium pericarp induce
apoptosis of HepG2 human hepatoma cells through increased
production of reactive oxygen speciesBiol Pharm
Bull28802807200515863882
|
|
11.
|
Y GuZ TingX QiuX ZhangX GanY FangX XuR
XuLinalool preferentially induces robust apoptosis of various
leukemia cells via upregulating p53 and cyclin-dependent kinase
inhibitorsToxicology2681924201010.1016/j.tox.2009.11.01319922762
|
|
12.
|
T GotoN TakahashiS HiraiT KawadaVarious
terpenoids derived from herbal and dietary plants function as PPAR
modulators and regulate carbohydrate and lipid metabolismPPAR
Res2010483958201010.1155/2010/48395820613991
|
|
13.
|
S CastaigneC ChomienneMT DanielP
BalleriniR BergerP FenauxL DegosAll-trans retinoic acid as a
differentiation therapy for acute promyelocytic leukemia. I.
Clinical resultsBlood7691704170919902224119
|
|
14.
|
S MishimaY InohY NaritaS OhtaT SakamotoY
ArakiKM SuzukiY AkaoY NozawaIdentification of caffeoylquinic acid
derivatives from Brazilian propolis as constituents involved in
induction of granulocytic differentiation of HL-60 cellsBioorg Med
Chem1358145818200510.1016/j.bmc.2005.05.04415993085
|
|
15.
|
HC KuoWH KuoYJ LeeCJ WangTH
TsengEnhancement of acid phenethyl ester on all-trans retinoic
acid-induced differentiation in human leukemia HL-60 cellsToxicol
Appl Pharmacol2168088200610.1016/j.taap.2006.04.00716766008
|
|
16.
|
TR BreitmanSE SelonickSJ CollinsInduction
of differentiation of the human promyelocytic leukemia cell line
(HL-60) by retinoic acidProc Natl Acad Sci
USA7729362940198010.1073/pnas.77.5.2936
|
|
17.
|
H MotekiH HibasamiY YamadaH KatsuzakiK
ImaiT KomiyaSpecific induction of apoptosis by 1,8-cineole in two
human leukemia cell lines, but not in a human stomach cancer cell
lineOncol Rep9757760200212066204
|
|
18.
|
JA SokoloskiWF HodnickST MayneC CinquinaCS
KimAC SartorelliInduction of the differentiation of HL-60
promyelocytic leukemia cells by vitamin E and other antioxidants in
combination with low levels of vitamin D3: possible relationship to
NF-kappaBLeukemia1115461553199710.1038/sj.leu.24007869305611
|
|
19.
|
E LinneyRetinoic acid receptors
transcription factors modulating gene regulation, development, and
differentiationCurr Top Dev
Biol27309350199210.1016/S0070-2153(08)60538-41330444
|
|
20.
|
KA RobertsonB EmamiL MuellerSJ
CollinsMultiple members of the retinoic acid receptor family are
capable of mediating the granulocytic differentiation of HL-60
cellsMol Cell Biol123743374919921324405
|
|
21.
|
P FenauxS CastaigneC ChomienneH DombretL
DegosAll trans retinoic acid treatment for patients with acute
promyelocytic leukemiaLeukemia6646619921548938
|
|
22.
|
M CornicC ChomienneInduction of retinoid
resistance by all-trans retinoic acid in acute promyelocytic
leukemia after remissionLeuk
Lymphoma18249257199510.3109/104281995090596158535190
|
|
23.
|
J JagdeoR CampbellT LongJ MugliaG TelangL
Robinson-BostomSweet’s syndrome-like neutrophilic lobular
panniculitis associated with all-trans-retinoic acid chemotherapy
in a patient with acute promyelocytic leukemiaJ Am Acad
Dermatol566906932007
|